1. Introduction
Although most soils contain enough iron (Fe) to satisfy the needs of crops, in the presence of oxygen, an alkaline medium, and an excess of soil moisture, this ion precipitates as the insoluble hydroxide, which is a scarcely soluble Fe form that, therefore, causes serious problems of iron chlorosis for plants. Thus, in alkaline soils such as those in the Mediterranean basin, high levels of bicarbonate ions are the main cause of iron chlorosis in citrus plantations. The most effective way to combat this deficiency is by applying iron chelates. However, their use markedly increases operating costs, accounting for 14% of the total cost of fertilization [
1].
The importance of Fe in plants is well known, as it is involved in a series of metabolic processes that are fundamental to their development. This is the case of photosynthesis, since Fe participates in several of the steps of the chlorophyll biosynthetic pathway regulating the activity of the enzyme system for the formation of protochlorophyll and other pigments, besides being responsible for the morphology, structure, and maintenance of chloroplasts [
2]. So, the deficiency of Fe impairs the photosynthetic capacity of the tree decreasing photoassimilated compounds such as sugar levels and starch. This ion is also part of a good number of enzyme systems important for the metabolism of the plants, such as the protein ferredoxin that acts as a final acceptor of electrons and whose high redox potential allows to reduce substances as NADP
+, nitrate, oxygen, and sulfate [
2,
3]. Finally, free Fe can interact with oxygen to form superoxide anions, which damage membranes by degrading unsaturated lipid components, especially in the leaf. Excess of Fe is stored in an Fe-protein complex called phytoferritin, which contains between 5400 and 6200 atoms of Fe (III) as a ferric oxide-phosphate complex [
4]. Therefore, Fe deficiency (or Fe chlorosis) is one of the main abiotic stresses in the citrus and fruit trees of the Mediterranean basin, mainly as a consequence of the predominance of calcareous soils. Although the response may vary from one species to another, iron chlorosis reduces the longevity of the plantation, as it affects the development of new shoots due to the low translocation of the element from the adult leaves to the new tissues. Therefore, the vegetative shoots are less vigorous, and their leaves are smaller, even giving rise to an early defoliation of the shoots and their progressive death from the apical zone. Fruit production and quality are also reduced, and at levels of acute deficiency, mature fruits are usually smaller in size, with soft skin and lack of color. The flavedo of oranges and mandarins characteristically acquire a yellowish color, without reaching the orange-reddish tonalities of the normal fruits, and it diminishes the content of soluble solids in the juice [
5].
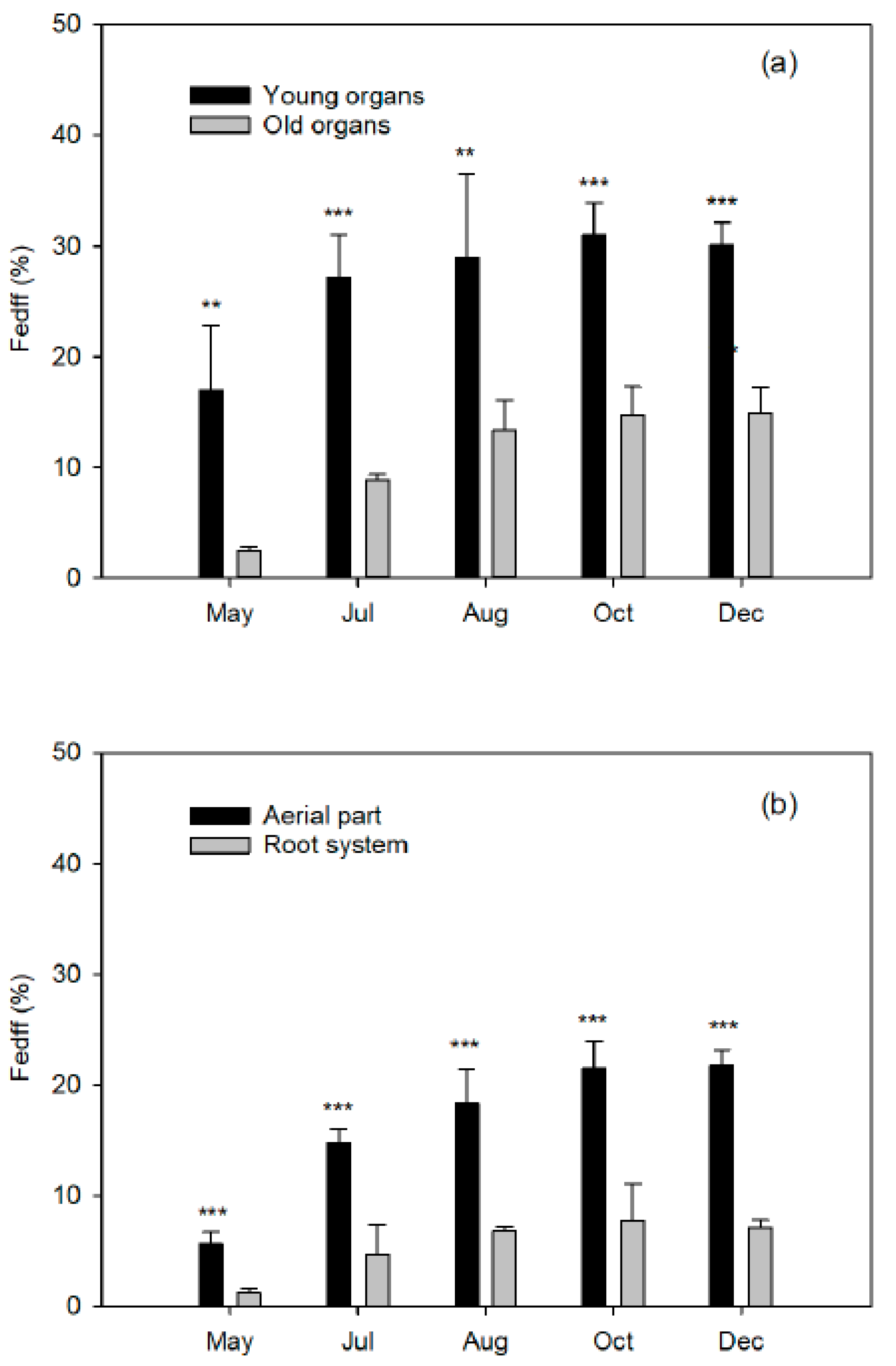
As a dicotyledonous plant, citrus develop a strategy I mechanism for mobilization and acquisition of Fe from the soil or soil solution [
6,
7]. Regarding the mobility of Fe in the plant, it is known that this hardly remobilizes from the old leaves to the young developing tissues, since the Fe is transported from the roots to the aerial part via xylem, to the cells of the mesophyll mainly bound to the citrate ion. Nevertheless, once fixed in the aerial organs, Fe remains immobile due to its retention in organic acids (mainly citric and malic) of the foliar parenchyma tissue. Thus, leaves accumulate Fe as they age and do not remobilize it to new shoots. This fact was confirmed when applying 3 doses of Fe via foliar (Rayplex) and soil (Fe-EDDHA) to orange trees of the "Navelina" variety cultivated in a calcareous soil and with flood irrigation [
8]. The Fe applied on the leaves of the spring flush did not translocate to the shoots of the following seasonal flushes of the same year, or to the fruits undergoing development. At the next vegetative cycle, the leaves of the spring flush of the treated trees and the control leaves showed identical Fe concentrations. In addition, the foliar levels increased correlatively to the dose applied by foliar treatment. This confirms the low mobility of this nutrient between leaf tissues, at least in citrus trees. However, we have no information on its translocation from the woody organs (old branches, trunk, and root system) to the new tissues.
Several factors are involved in the absorption of Fe. In addition to those intrinsic to the plant (vigor of the variety and pattern and age), other examples include the culture medium (calcareous soils rich in bicarbonate ions and with low pH that cause an excessive release of ion phosphate and other metals), as well as irrigation management and fertilization practices. Despite the importance of this latter, few studies have focused on Fe dose, its time of application or seasonal distribution, and Fe fractionation (number of applications) in citrus.
The Fe dose depends on the characteristics of the plantation (tree size, variety, rootstock, plantation frame, production, soil type, cropping system, etc.), as well as the nutritional status of the plant evaluated by leaf analysis. However, there are a few studies on the response of citrus fruits to differential doses of Fe. The application of two doses of Fe-EDDHA to adult mandarin trees increased the total leaf chlorophyll content and improved their yield, with an increase in the number of fruit sets [
9]. The lower dose (24 kg ha
−1) could be considered the most convenient from a nutritional and economic point of view.
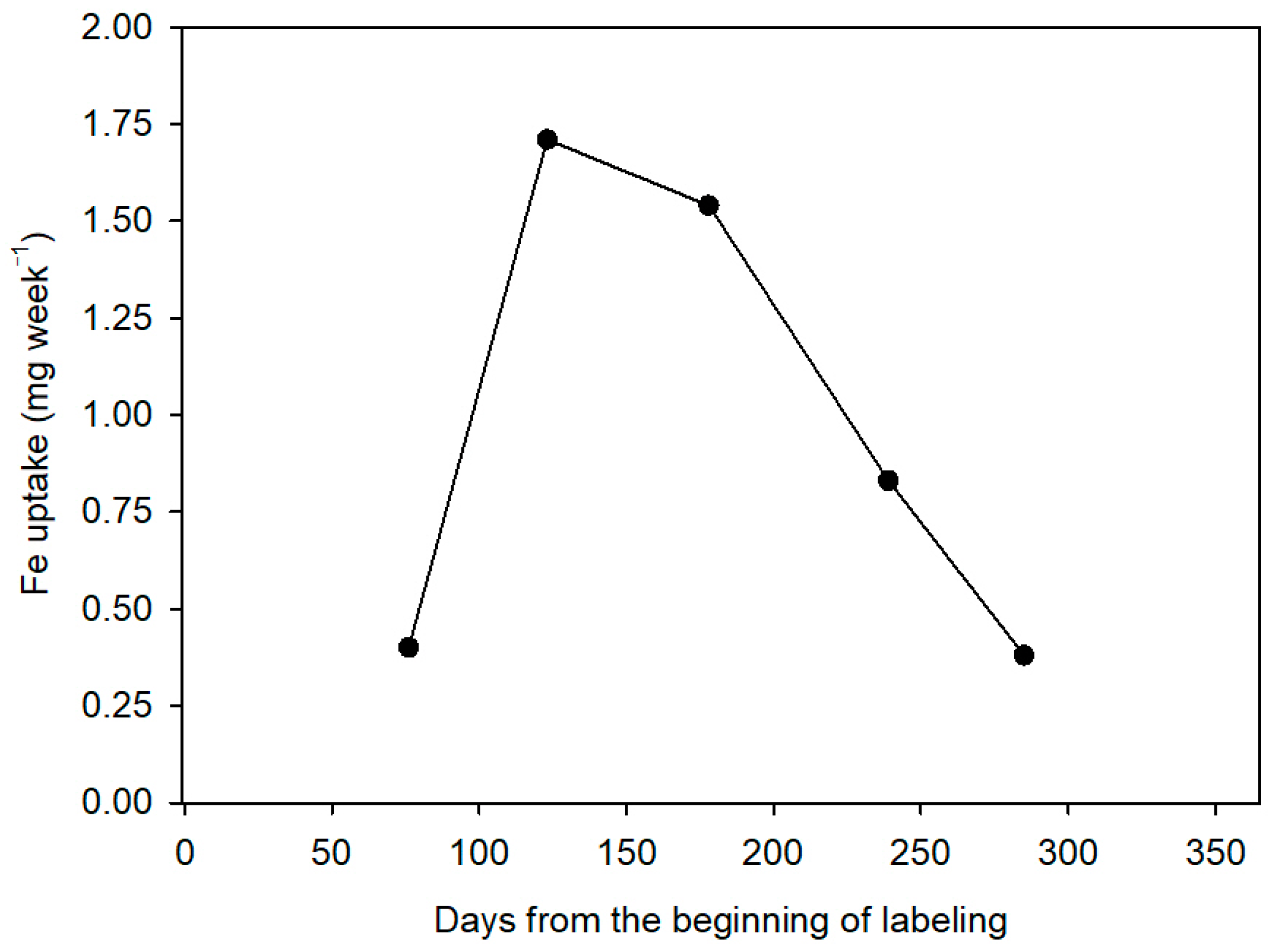
The time of application of nutrients is also important, as it is known that these are absorbed by citrus throughout the year but not steadily throughout the cycle. In general, the time of maximum absorption includes the end of spring, summer, and early fall, and the minimum during winter. The decrease in the absorption at very low temperatures seems to be due to a lower fluidity in the cytoplasmic membrane and, therefore, to a greater resistance to the passage of the ions. Moreover, the growth of the fibrous roots is also considerably reduced. Recent studies have established the distribution pattern of nitrogen fertilizer in citrus throughout the vegetative cycle of the plant [
10], but similar information about Fe is not currently available.
The interest of fractionation of the Fe dose lies in the fact that it fulfills the function of maintaining a level of this micronutrient available to the plant in a constant and prolonged manner, thereby improving the efficiency of its use and reducing the fertilization costs. However, a few results on this aspect can be found in the current literature, and, in general, the studies evaluate the relative distribution between the different organs of the total accumulated Fe in the plant up to the time of extraction, chlorophyll concentration, or increase in production [
8,
11]. Thus, a study carried out in adult trees of the variety “Clementina de Nules” (Nules clementine), where a dose of 3 g Fe (Fe-EDDHA) was applied for 6 months (April to September) and distributed in 3, 6, 12, and 24 applications at intervals of 8, 4, 2, or 1 weeks, respectively, concluded that the application of the chelate increased chlorophyll production and concentration in the leaves [
11]. In addition, these authors noticed that the best responses were achieved when the chelate was added in periods of more than 1 week. However, the use of ferric fertilizers enriched with
57Fe not only determines the uptake of Fe by the crop [
12,
13], but also its destination within the plant, in the culture medium, the seasonal absorption of Fe from the applied fertilizer, and its relative distribution among the different organs of the plant. The use of isotopes as tracers constitutes a powerful research tool that makes it possible to obtain comprehensive information about the Fe dynamics in the plant–soil system that is not accessible by the conventional procedures and techniques of study [
14,
15].
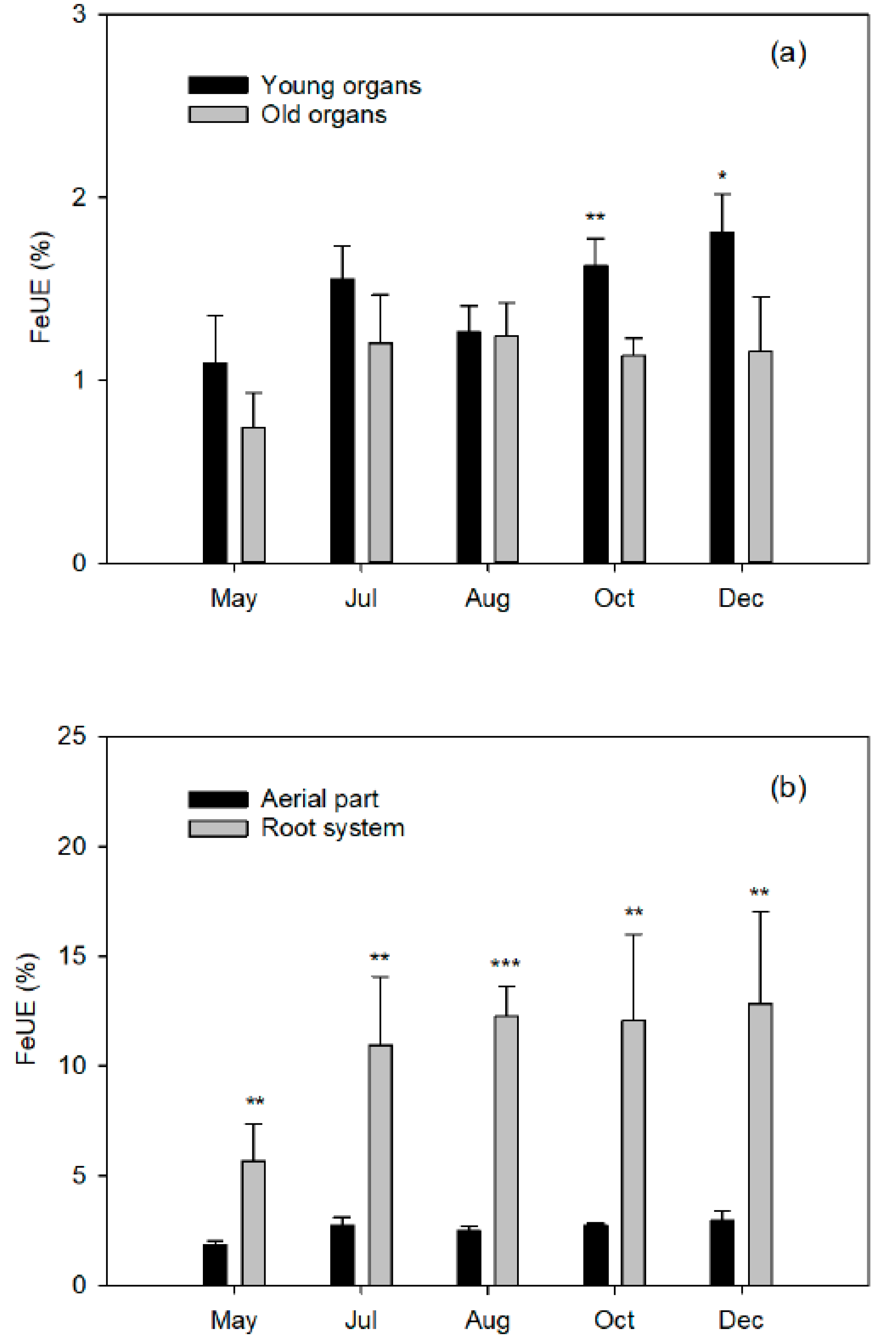
Finally, closely related to the dose of Fe applied is the Fe use efficiency (FeUE), which estimates the proportion of Fe absorbed by the plant in relation to the dose applied. Generally, FeUE decreases with the increase in fertilizer dose, so this parameter will be of agronomic interest as it influences the Fe consumption of the crop. A low efficiency is not always indicative of a low absorption capacity of the crop, but rather of an excessive dose. Therefore, better FeUE values will be obtained when the Fe dose is more matched to the needs of the plant, which will in turn reduce the fertilizer costs.
Specifically, the objective of the present work is to quantify the effect of the seasonal distribution of a dose of Fe on the following aspects: (a) quantification of Fe absorption along the vegetative cycle and its use efficiency; (b) distribution of Fe absorbed among the different plant organs at different phenological moments; and (c) relative contribution of the Fe absorbed from the fertilizer to the fruit setting, as well as to the vegetative development of the different flushes. The use of a chelate enriched with 57Fe allows them to be quantified exhaustively. In this way, this study will expand the knowledge of the Fe dynamics in plants, in order to establish the fertilizer criteria of this nutrient and to optimize its seasonal application, thus limiting the supply of excessive amounts of ferric fertilizers, which will result in a saving in production costs.
3. Materials and Methods
3.1. Plant Material and Growth Conditions
Four-year-old Nules clementine trees (Citrus clementina Hort ex Tan), grafted on Carrizo citrange (Citrus sinensis × Poncirus trifoliata) rootstock, were grown individually in containers of about 46 l (internal upper and lower diameter of 40 and 45 cm, respectively, and 40 cm high) filled with 65 kg of loamy soil (45% sand, 36% silt, and 19% clay; 27% CaCO3; and pH 8.3). The containers were placed outdoors and covered to protect them from the rain. The area was surrounded by a buffer row of identical trees to those used in the experiment. The trial was conducted in the IVIA experimental field station (Moncada, Valencia, Spain; 39°32′ N, 0°23′ W).
Between March (spring growth resumption) and October (end of fruit development) of 2008, 20 trees were fertilized with 240 mg Fe plant
−1; this rate being consistent with Fe fertilizer guidelines for young citrus trees with a canopy diameter of about 60 cm [
53,
54] (Legaz et al., 2008). The fertilizer was labeled with
57Fe EDDHA, with an abundance of 94.00% (
Table 7) in
57Fe (Cambridge Isotope Laboratories, Inc., Andover, MA, USA) to discriminate Fe fertilizer from other sources of Fe (especially soil and plant tissues).
Table 7 shows the percentage monthly distribution of labeled Fe fertilizer, which was divided into 16 applications and applied every 2 weeks dissolved in a nutrient solution. The rest of the macro- and micronutrients in the nutrient solution were 2 mM N, 0.12 mM P, 0.36 mM K, 0.53 mM Mg, 10 µM Zn, 9 µM Mn, and 0.5 µM Cu, which were provided as KNO
3, Ca(NO
3)
2, H
3PO
4, MgSO
4, Mn chelates, Zn chelates, and CuSO
4, respectively, as proposed for citrus trees [
53,
54].
The experiment, therefore, consisted of 20 trees arranged in a randomized block design with five harvests and four replicates of one tree each. The plants were watered using two drip emitters for each tree (4 l h
−1 emitter
−1). The amount of water applied to each tree was equivalent to the total seasonal crop evapotranspiration (ETc). The volume of water applied weekly was calculated using the expression: ETc = ETo × Kc, where ETo is the reference crop evapotranspiration under standard conditions, determined using the Penman-Monteith approach [
55] and meteorological data from the Agrometeorological Station of the IVIA. Kc (crop coefficient) is a function of canopy size and plantation frame [
56]. The soil humidity was monitored daily using a ThetaProbe PR2 (Delta-T Devices, Cambridge, UK), data were acquired by a Moisture meter HH2 (Delta-T Devices Ltd.), and irrigation was scheduled when the matric potential at a depth of 30 cm reached –10 kPa.
The 57Fe budget contained in each plant was calculated, and 57Fe content in the soil was assumed to be the natural abundance (2.20%) as it was diluted with total Fe and we did not appreciate an increase in 57Fe in the soil at the end of the experiment (data not shown).
3.2. Plant Uprooting
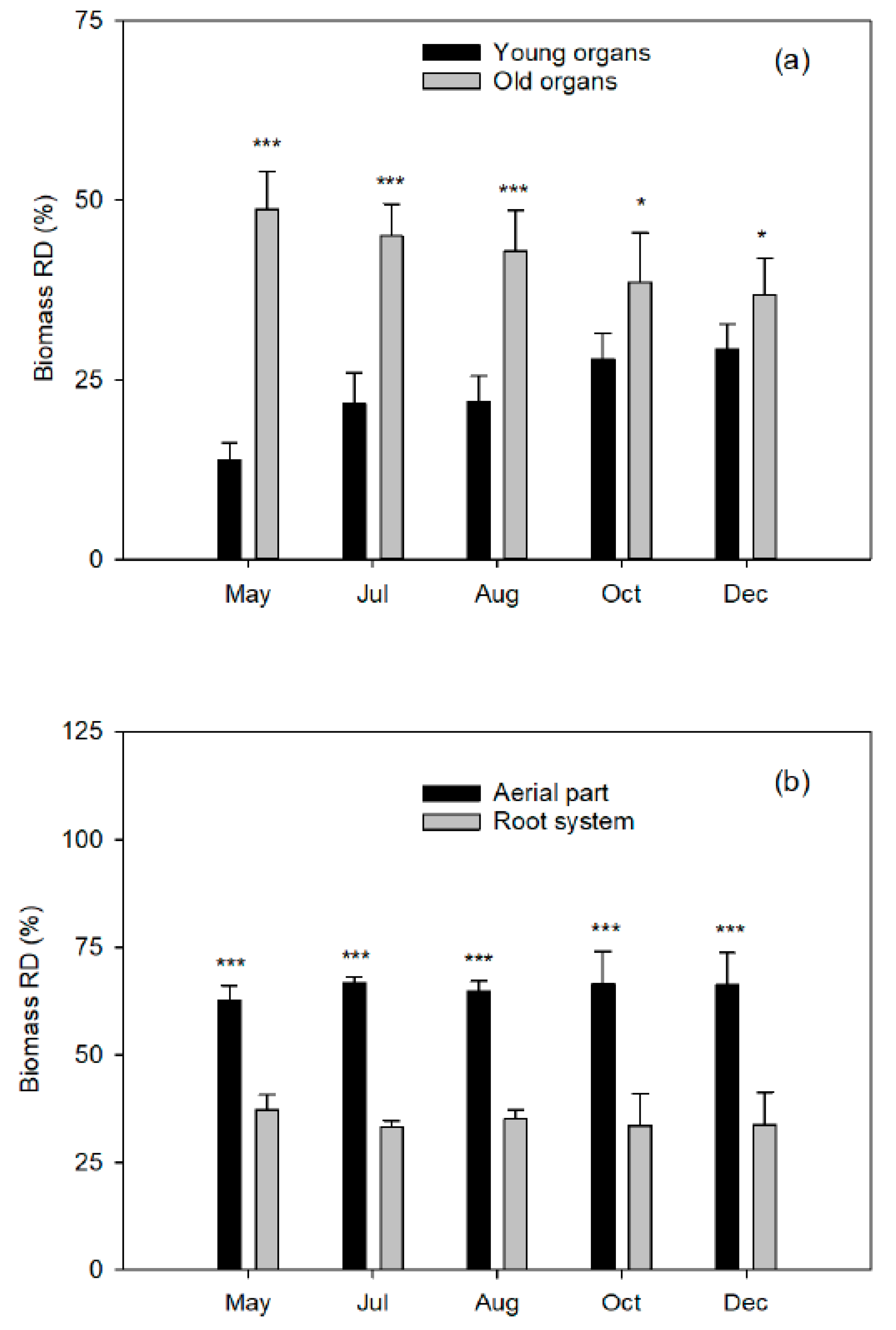
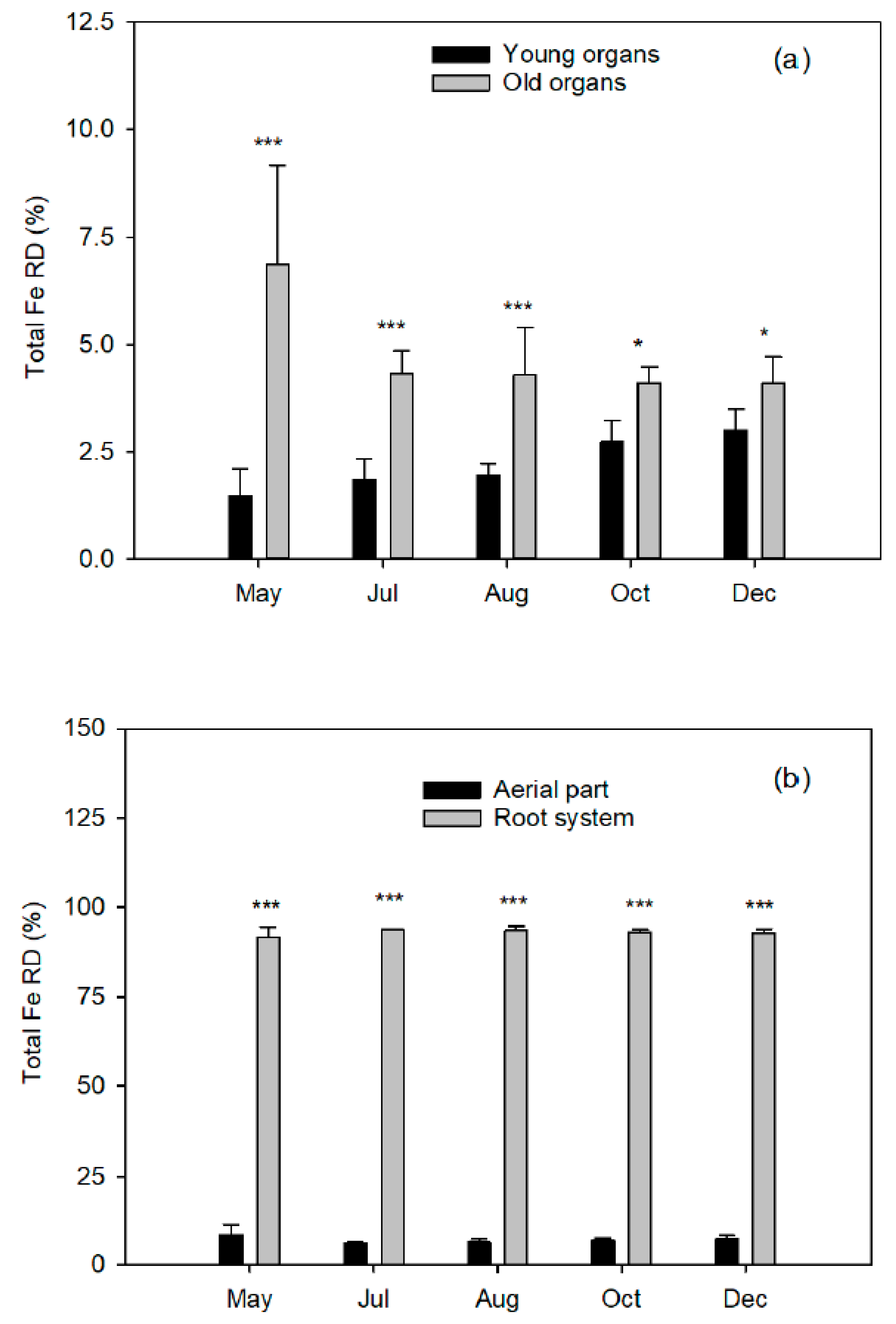
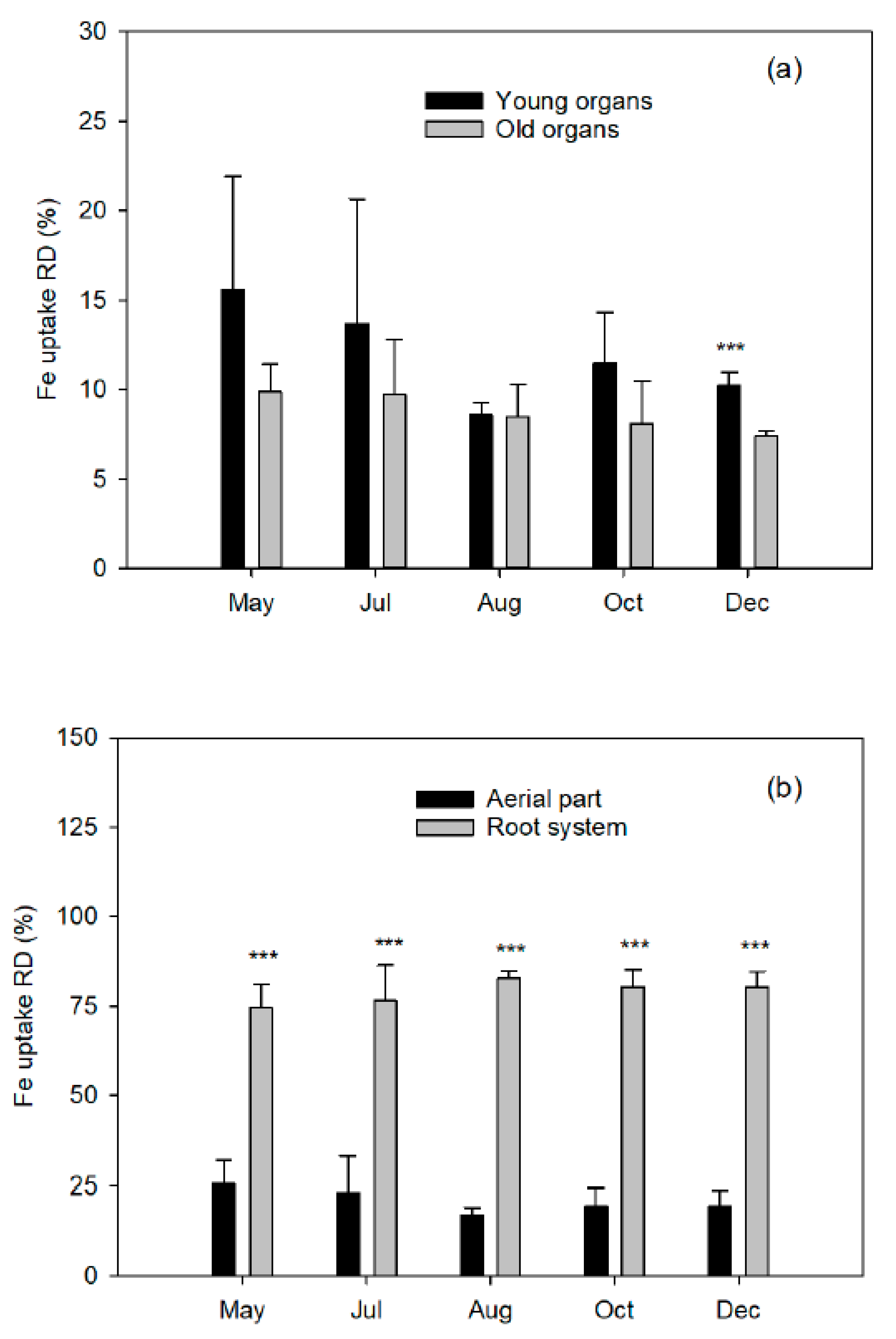
At five different plant phenological states, the end of flowering (15 May 2008), the end of fruit setting and fruit drop (1 July 2008), two fruit growing moments (1 August and 15 October 2008), and at complete fruit maturity (10 December 2008), a block of four labeled trees were destructively harvested to quantify total dry biomass.
The aerial part was divided into young organs (flowers/fruits, leaves, and twigs of the spring, summer, and fall flushes) and old organs (leaves of the previous year, trunk, and branches). The root system was carefully removed from the soil to avoid tissue loss and divided into coarse and fibrous roots (less than 2 mm diameter). Fresh weights of tree organs were determined, and representative samples of each fraction were taken. All samples were washed in nonionic detergent solution followed by several rinses in deionized water, weighed, frozen in liquid nitrogen, freeze-dried (Telstar LyoAlfa 6, Barcelona, Spain), and powdered in a refrigerated ball mill (IKA A10, Staufen, Germany) after dry-weight determination.
3.3. Plant Tissue Analysis
Prior to Fe content analysis, the plant material (0.5 g DW) was calcined in a muffle furnace for 12 h at 550 °C. Subsequently, Fe was extracted with 2% nitric acid (Hiperpur Panreac, Fe < 1 ppb) in an ultrasonic bath (Fungilab S.A., Sant Feliu de Llobregat, Barcelona, Spain) for 30 min at 40 °C and diluted to 50 mL final volume. This extraction was divided into two subsamples to determine total Fe and 57Fe concentrations.
Total Fe concentration was measured with the AAS analyzer (iCAP 6000, Thermo Scientific). The abundance in
57Fe of each sample was determined using a multiple-collector inductively coupled to an isotope-ratio mass spectrometer (MC-ICP MS, Thermo Finnigan Neptune) [
57].
All analyses were carried out with ultrapure water (Ultra Pure Water Systems Milli Q Plus). All determinations were performed in duplicate and a standard was run to ensure accuracy after each set of 10 samples.
3.4. Calculations
The
57Fe content in each plant compartment was calculated by Equation (1):
where atom%
57Fe excess was calculated by subtracting the natural abundance of
57Fe (2.20%, International Atomic Energy Agency [
55]) from the
57Fe abundance of each sample.
The content of Fe absorbed by the organ was calculated by Equation (2):
Iron derived from fertilizer (Fedff) quantifies the contribution of Fe from fertilizer in relation to the total Fe in the organ (Equation (3)):
Total plant recovery of applied
57Fe fertilizer represents the proportion of applied
57Fe that is taken up by the tree and embodies its fertilizer Fe use efficiency (FeUE, Equation (4)).
where
57Fe taken up
plant compartment (µg) = Ʃ
57Fe (µg)
organ.
3.5. Statistical Analysis
Data were subjected to ANOVA, and means were separated by the LSD test at the 5% level to determine the significance of differences among the Fe seasonal distribution within each harvest event, using SAS software (Statistical Analysis System Institute Inc., Cary, NC, USA).