The Genus Curcuma and Inflammation: Overview of the Pharmacological Perspectives
Abstract
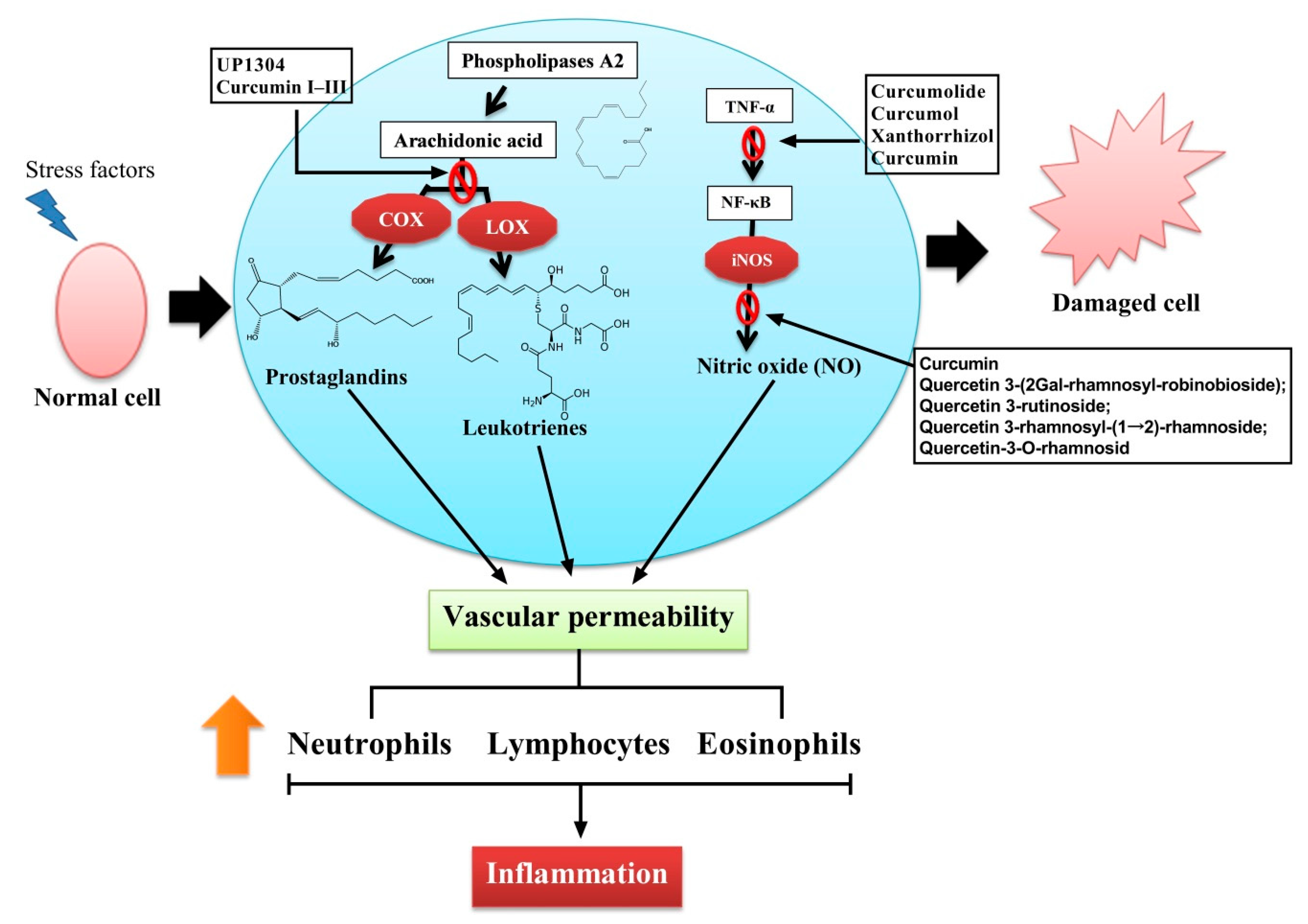
1. Introduction
2. The Genus Curcuma
3. Anti-inflammatory Activity of the Genus Curcuma
3.1. Curcuma longa L.
3.2. Curcuma xanthorrhiza Roxb.
3.3. Curcuma zedoaria (Christm.) Roscoe
3.4. Curcuma phaeocaulis Val.
3.5. Curcuma wenyujin Y.H.
3.6. Curcuma mangga Val.
3.7. Curcuma amada Roxb.
3.8. Curcuma aeruginosa Roxb.
3.9. Curcuma aromatica Salisb.
4. Inflammation and Natural Bioactive Compounds
5. Commercial Uses of the Genus Curcuma
6. Clinical and Preclinical Studies of Bioactive Compounds of Curcuma Species Based on the Anti-inflammatory Properties
- Use as liposomal curcumin via liposomal technology.
- Curcumin as nanoparticles.
- Use as a curcumin–phospholipid complex.
- New formulation (micelles, exosomes) to increase the bioavailability.
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petrovska, B.B. Historical review of medicinal plants’ usage. Pharmacogn. Rev. 2012, 6, 1. [Google Scholar] [CrossRef]
- Lichterman, B.L. Aspirin: The Story of a Wonder Drug. BMJ 2004, 329, 1408. [Google Scholar] [CrossRef]
- Sumner, J. The Natural History of Medicinal Plants; Timber Press: Portland, OR, USA, 2000. [Google Scholar]
- Krawinkel, M.B. Global Healthy Diet Approach to Nutrition. Development 2014, 57, 234–239. [Google Scholar] [CrossRef]
- Ferlazzo, N.; Cirmi, S.; Calapai, G.; Ventura-Spagnolo, E.; Gangemi, S.; Navarra, M. Anti-Inflammatory Activity of Citrus bergamia Derivatives: Where Do We Stand? Molecules 2016, 21, 1273. [Google Scholar] [CrossRef] [PubMed]
- Kress, W.J.; Prince, L.M.; Williams, K.J. The phylogeny and a new classification of the gingers (Zingiberaceae): Evidence from molecular data. Am. J. Bot. 2002, 89, 1682–1696. [Google Scholar] [CrossRef] [PubMed]
- Larsen, K. A preliminary checklist of the Zingiberaceae of Thailand. Thai For. Bull. 1996, 24, 35–49. [Google Scholar]
- Leong-Skornicková, J.; Sída, O.; Jarolímová, V.; Sabu, M.; Fér, T.; Trávnícek, P.; Suda, J. Chromosome numbers and genome size variation in Indian species of Curcuma (Zingiberaceae). Ann. Bot. 2007, 100, 505–526. [Google Scholar] [CrossRef] [PubMed]
- Maknoi, C. Taxonomy and Phylogeny of the Genus Curcuma L. (Zingiberaceae) with Particular Reference to Its Occurrence in Thailand. Ph.D. Thesis, Prince of Songkla University, Songkhla, Thailand, 2006. [Google Scholar]
- Xia, Q.; Zhao, K.J.; Huang, Z.G.; Zhang, P.; Dong, T.T.; Li, S.P.; Tsim, K.W. Molecular genetic and chemical assessment of Rhizoma Curcumae in China. J. Agric. Food Chem. 2005, 53, 6019–6026. [Google Scholar] [CrossRef]
- Anamthawat-Jónsson, K.; Umpunjun, P. Polyploidy in the Ginger Family from Thailand. In Chromosomal Abnormalities; IntechOpen: London, UK, 2020. [Google Scholar]
- Dũng, N.X.; Truong, P.X.; Ky, P.T.; Leclercq, P.A. Volatile Constituents of the Leaf, Stem, Rhizome, Roof and Flower Oils of Curcuma harmandii Gagnep. from Vietnam. J. Essent. Oil Res. 1997, 9, 677–681. [Google Scholar] [CrossRef]
- Cuéllar, M.J.; Giner, R.M.; Recio, M.C.; Just, M.J.; Máñez, S.; Cerdá, S.; Ríos, J.L. Screening of antiinflammatory medicinal plants used in traditional medicine against skin diseases. Phyther. Res. 1998, 12, 18–23. [Google Scholar] [CrossRef]
- Dũng, N.X.; Tuyêt, N.T.B.; Leclercq, P.A. Characterization of the Leaf Oil of Curcuma aeruginosa Roxb. from Vietnam. J. Essent. Oil Res. 1995, 7, 657–659. [Google Scholar] [CrossRef]
- Sirirugsa, P. Thai Zingiberaceae: Species diversity and their uses. Pure Appl. Chem. 1999, 70, 23–27. [Google Scholar]
- Malek, S.N.; Seng, C.K.; Zakaria, Z.; Ali, N.A.; Ibrahim, H.; Jalil, M.N. The Essential Oil of Curcuma inodora aff. Blatter from Malaysia. J. Essent. Oil Res. 2006, 18, 281–283. [Google Scholar] [CrossRef]
- Xiang, Z.; Wang, X.-Q.; Cai, X.-J.; Zeng, S. Metabolomics Study on Quality Control and Discrimination of Three Curcuma Species based on Gas Chromatograph–Mass Spectrometry. Phytochem. Anal. 2011, 22, 411–418. [Google Scholar] [CrossRef]
- Prasad, S.; Aggarwal, B.B. Turmeric, the golden spice: From traditional medicine to modern medicine. Herb. Med. Biomol. Clin. Asp. 2011, 13, 263–288. [Google Scholar]
- Srivilai, J.; Waranuch, N.; Tangsumranjit, A.; Khorana, N.; Ingkaninan, K. Germacrone and sesquiterpene-enriched extracts from Curcuma aeruginosa Roxb. increase skin penetration of minoxidil, a hair growth promoter. Drug Deliv. Transl. Res. 2018, 8, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Jiang, B.; Wang, H.; Shen, C.; Chen, H.; Zeng, L. Curcumin Suppresses Intestinal Fibrosis by Inhibition of PPARγ-Mediated Epithelial-Mesenchymal Transition. Evid. Based Complement. Altern. Med. 2017, 2017, 7876064. [Google Scholar] [CrossRef] [PubMed]
- Saikia, B.; Borthakur, S.K. Use of medicinal plants in animal healthcare-A case study from Gohpur, Assam. Indian J. Tradit. Knowl. 2010, 9, 49–51. [Google Scholar]
- Ayati, Z.; Ramezani, M.; Amiri, M.S.; Moghadam, A.T.; Rahimi, H.; Abdollahzade, A.; Sahebkar, A.; Emami, S.A. Ethnobotany, Phytochemistry and Traditional Uses of Curcuma spp. and Pharmacological Profile of Two Important Species (C. longa and C. zedoaria): A Review. Curr. Pharm. Des. 2019, 25, 871–935. [Google Scholar] [CrossRef]
- Anuchapreeda, S.; Khumpirapang, N.; Chiampanichayakul, S.; Nirachonkul, W.; Saiai, A.; Usuki, T.; Okonogi, S. Characterization and Biological Properties of Zederone and Zedoarondiol from Rhizomes of En-Lueang (Curcuma cf. amada). Nat. Prod. Commun. 2018, 13, 1615–1618. [Google Scholar] [CrossRef]
- Borah, A.; Paw, M.; Gogoi, R.; Loying, R.; Sarma, N.; Munda, S.; Kumar Pandey, S.; Lal, M. Chemical composition, antioxidant, anti-inflammatory, anti-microbial and in-vitro cytotoxic efficacy of essential oil of Curcuma caesia Roxb. leaves: An endangered medicinal plant of North East India. Ind. Crops Prod. 2019, 129, 448–454. [Google Scholar] [CrossRef]
- Mukunthan, K.S.; Satyan, R.S.; Patel, T.N. Pharmacological evaluation of phytochemicals from South Indian Black Turmeric (Curcuma caesia Roxb.) to target cancer apoptosis. J. Ethnopharmacol. 2017, 209, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Akter, J.; Hossain, M.A.; Takara, K.; Islam, M.Z.; Hou, D.-X. Antioxidant activity of different species and varieties of turmeric (Curcuma spp): Isolation of active compounds. Comp. Biochem. Physiol. Part. C Toxicol. Pharmacol. 2019, 215, 9–17. [Google Scholar] [CrossRef] [PubMed]
- De, B.; Karak, S.; Das, S.; Begum, S.; Gupta, P.; De Pradhan, I.; Acharya, J.; Subramani, E.; Choudhury, K. Profiling non-polar terpenes of rhizomes for distinguishing some Indian Curcuma species. J. Appl. Res. Med. Aromat. Plants 2019, 13, 100207. [Google Scholar] [CrossRef]
- Vella, F.M.; Calandrelli, R.; Cautela, D.; Fiume, I.; Pocsfalvi, G.; Laratta, B. Chemometric Screening of Fourteen Essential Oils for Their Composition and Biological Properties. Molecules 2020, 25, 5126. [Google Scholar] [CrossRef]
- Devi, N.B.; Singh, P.K.; Das, A.K. Ethnomedicinal utilization of Zingiberaceae in the valley districts of Manipur. IOSR J. Environ. Sci. Toxicol. Food Technol. 2014, 8, 21–23. [Google Scholar] [CrossRef]
- Yadav, R.P.; Tarun, G. Versatility of turmeric: A review the golden spice of life. J. Pharmacogn. Phytochem. 2017, 6, 41–46. [Google Scholar]
- Kaliyadasa, E.; Samarasinghe, B.A. A review on golden species of Zingiberaceae family around the world: Genus Curcuma. Afr. J. Agric. Res. 2019, 14, 519–531. [Google Scholar]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O. Biological activities of essential oils: From plant chemoecology to traditional healing systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef]
- Mollik, A.H.; Haq, W.M.; Bachar, S.C.; Jahan, R.; Rahmatullah, M. Anti-inflammatory effect of Curcuma longa (turmeric) rhizome when administered topically in gel form. Planta Med. 2009, 75, PH31. [Google Scholar] [CrossRef]
- Sun, W.; Wang, S.; Zhao, W.; Wu, C.; Guo, S.; Gao, H.; Tao, H.; Lu, J.; Wang, Y.; Chen, X. Chemical constituents and biological research on plants in the genus Curcuma. Crit. Rev. Food Sci. Nutr. 2017, 57, 1451–1523. [Google Scholar] [CrossRef] [PubMed]
- Bagad, A.S.; Joseph, J.A.; Bhaskaran, N.; Agarwal, A. Comparative Evaluation of Anti-Inflammatory Activity of Curcuminoids, Turmerones, and Aqueous Extract of Curcuma longa. Adv. Pharmacol. Sci. 2013, 2013, 805756. [Google Scholar] [PubMed]
- Kim, D.W.; Lee, S.M.; Woo, H.S.; Park, J.-Y.; Ko, B.S.; Heo, J.D.; Ryu, Y.B.; Lee, W.S. Chemical constituents and anti-inflammatory activity of the aerial parts of Curcuma longa. J. Funct. Foods 2016, 26, 485–493. [Google Scholar] [CrossRef]
- Illuri, R.; Bethapudi, B.; Anandakumar, S.; Murugan, S.; Joseph, J.A.; Mundkinajeddu, D.; Agarwal, A.; Chandrasekaran, C.V. Anti-Inflammatory Activity of Polysaccharide Fraction of Curcuma longa Extract (NR-INF-02). Antiinflamm. Antiallergy. Agents Med. Chem. 2015, 14, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Walter, B.A.; Purmessur, D.; Likhitpanichkul, M.; Weinberg, A.; Cho, S.K.; Qureshi, S.A.; Hecht, A.C.; Iatridis, J.C. Inflammatory Kinetics and Efficacy of Anti-inflammatory Treatments on Human Nucleus Pulposus Cells. Spine 2015, 40, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Oliviero, F.; Scanu, A.; Zamudio-Cuevas, Y.; Punzi, L.; Spinella, P. Anti-inflammatory effects of polyphenols in arthritis. J. Sci. Food Agric. 2018, 98, 1653–1659. [Google Scholar] [CrossRef]
- Yimam, M.; Lee, Y.-C.; Moore, B.; Jiao, P.; Hong, M.; Nam, J.-B.; Kim, M.-R.; Hyun, E.-J.; Chu, M.; Brownell, L.; et al. Analgesic and anti-inflammatory effects of UP1304, a botanical composite containing standardized extracts of Curcuma longa and Morus alba. J. Integr. Med. 2016, 14, 60–68. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A review of its’ effects on human health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Bordoloi, D.; Padmavathi, G.; Monisha, J.; Roy, N.K.; Prasad, S.; Aggarwal, B.B. Curcumin, the golden nutraceutical: Multitargeting for multiple chronic diseases. Br. J. Pharmacol. 2017, 174, 1325–1348. [Google Scholar] [CrossRef]
- Choudhary, M.; Kumar, V.; Malhotra, H.; Singh, S. Medicinal plants with potential anti-arthritic activity. J. Intercult. Ethnopharmacol. 2015, 4, 147. [Google Scholar] [CrossRef]
- Kaushik, M.L.; Jalalpure, S.S. Evaluation of anti-inflammatory effect of ethanolic and aqueous extracts of Curcuma zedoaria Rosc root. Int. J. Drug Dev. Res. 2011, 3, 360–365. [Google Scholar]
- Liu, Y.; Ma, J.; Zhao, Q.; Liao, C.; Ding, L.; Chen, L.; Zhao, F.; Qiu, F. Guaiane-Type Sesquiterpenes from Curcuma phaeocaulis and Their Inhibitory Effects on Nitric Oxide Production. J. Nat. Prod. 2013, 76, 1150–1156. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Lu, C.-L.; Zeng, Q.-H.; Jiang, J.-G. Anti-inflammatory, antioxidant and antitumor activities of ingredients of Curcuma phaeocaulis Val. EXCLI J. 2015, 14, 706–713. [Google Scholar] [PubMed]
- Naik, D.G.; Majumdar, A.M.; Dandge, C.N.; Puntambekar, H.M. Antiinflammatory activity of Curcuma amada roxb. In albino rats. Indian J. Pharmacol. 2000, 32, 375–377. [Google Scholar]
- Dosoky, N.S.; Setzer, W.N. Chemical composition and biological activities of essential oils of Curcuma species. Nutrients 2018, 10, 1196. [Google Scholar] [CrossRef] [PubMed]
- Umar, N.M.; Parumasivam, T.; Aminu, N.; Toh, S.-M. Phytochemical and pharmacological properties of Curcuma aromatica Salisb (wild turmeric). J. Appl. Pharm. Sci. 2020, 10, 180–194. [Google Scholar]
- Dos Santos Filho, E.X.; Ávila, P.H.M.; Bastos, C.C.C.; Batista, A.C.; Naves, L.N.; Marreto, R.N.; Lima, E.M.; Mendonça, E.F.; Valadares, M.C. Curcuminoids from Curcuma longa L. reduced intestinal mucositis induced by 5-fluorouracil in mice: Bioadhesive, proliferative, anti-inflammatory and antioxidant effects. Toxicol. Rep. 2015, 3, 55–62. [Google Scholar] [CrossRef]
- Liju, V.B.; Jeena, K.; Kuttan, R. An evaluation of antioxidant, anti-inflammatory, and antinociceptive activities of essential oil from Curcuma longa L. Indian J. Pharmacol. 2011, 43, 526–531. [Google Scholar]
- Kim, K.; Park, K.I. A Review of Antiplatelet Activity of Traditional Medicinal Herbs on Integrative Medicine Studies. Evid. Based Complement. Altern. Med. 2019, 2019, 7125162. [Google Scholar] [CrossRef]
- Kim, M.B.; Kim, C.; Song, Y.; Hwang, J.K. Antihyperglycemic and Anti-Inflammatory Effects of Standardized Curcuma xanthorrhiza Roxb. Extract and Its Active Compound Xanthorrhizol in High-Fat Diet-Induced Obese Mice. Evid. Based Complement. Altern. Med. 2014, 2014, 205915. [Google Scholar] [CrossRef]
- Claeson, P.; Panthong, A.; Tuchinda, P.; Reutrakul, V.; Kanjanapothi, D.; Taylor, W.C.; Santisuk, T. Three non-phenolic diarylheptanoids with anti-inflammatory activity from Curcuma xanthorrhiza. Planta Med. 1993, 59, 451–454. [Google Scholar] [CrossRef]
- Song, Y.; Zhou, Y.; Yan, X.T.; Bi, J.B.; Qiu, X.; Bian, Y.; Wang, K.F.; Zhang, Y.; Feng, X.S. Pharmacokinetics and Tissue Distribution of Alnustone in Rats after Intravenous Administration by Liquid Chromatography-Mass Spectrometry. Molecules 2019, 24, 3183. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.Z.; Rana, M.S.; Hossain, S.; Dutta, E.; Ferdous, S.; Dutta, M.; Emran, T.B. In vivo neuroprotective, antinociceptive, anti-inflammatory potential in Swiss albino mice and in vitro antioxidant and clot lysis activities of fractionated Holigarna longifolia Roxb. bark extract. J. Complement. Integr. Med. 2019, 17, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.M.; Zaman, S.; Juhara, F.; Akter, L.; Tareq, S.M.; Masum, E.H.; Bhattacharjee, R. Evaluation of antinociceptive, in-vivo & in-vitro anti-inflammatory activity of ethanolic extract of Curcuma zedoaria rhizome. BMC Complement. Altern. Med. 2014, 14, 346. [Google Scholar]
- Burapan, S.; Kim, M.; Paisooksantivatana, Y.; Eser, B.E.; Han, J. Thai Curcuma Species: Antioxidant and Bioactive Compounds. Foods 2020, 9, 1219. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Qu, F.; Zhang, H.J.; Zhuge, X.H.; Cheng, L.Z. Comparison of anti-inflammatory and anti-nociceptive activities of Curcuma wenyujin Y.H. Chen et C. Ling and Scutellaria baicalensis Georgi. Afr. J. Tradit. Complement. Altern. Med. 2010, 7, 339–349. [Google Scholar] [CrossRef][Green Version]
- Dong, J.; Shao, W.; Yan, P.; Cai, X.; Fang, L.; Zhao, X.; Lin, W.; Cai, Y. Curcumolide, a unique sesquiterpenoid with anti-inflammatory properties from Curcuma wenyujin. Bioorganic Med. Chem. Lett. 2015, 25, 198–202. [Google Scholar] [CrossRef]
- Arablou, T.; Kolahdouz-Mohammadi, R. Curcumin and endometriosis: Review on potential roles and molecular mechanisms. Biomed. Pharmacother. 2018, 97, 91–97. [Google Scholar] [CrossRef]
- Xiang, H.; Zhang, L.; Xi, L.; Yang, Y.; Wang, X.; Lei, D.; Zheng, X.; Liu, X. Phytochemical profiles and bioactivities of essential oils extracted from seven Curcuma herbs. Ind. Crop. Prod. 2018, 111, 298–305. [Google Scholar] [CrossRef]
- Beer, A.M.; Zagorchev, P.; Filipova, D.M.; Lukanov, J. Effects of 1, 8-cineole on the activity of cyclooxygenase and cyclooxygenase 1 and cyclooxygenase 2 isoforms. Nat. Prod. Chem. Res. 2017, 5, 4172. [Google Scholar]
- Rana, M.; Reddy, S.S.; Maurya, P.; Singh, V.; Chaturvedi, S.; Kaur, K.; Agarwal, H.; Ahmad, H.; Naqvi, A.; Dwivedi, A.K.; et al. Turmerone enriched standardized Curcuma longa extract alleviates LPS induced inflammation and cytokine production by regulating TLR4–IRAK1–ROS–MAPK–NFκB axis. J. Funct. Foods 2015, 16, 152–163. [Google Scholar] [CrossRef]
- Rubió, L.; Motilva, M.J.; Romero, M.P. Recent advances in biologically active compounds in herbs and spices: A review of the most effective antioxidant and anti-inflammatory active principles. Crit. Rev. Food Sci. Nutr. 2013, 53, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Ambriz-Pérez, D.L.; Leyva-López, N.; Gutierrez-Grijalva, E.P.; Heredia, J.B. Phenolic compounds: Natural alternative in inflammation treatment. A Review. Cogent Food Agric. 2016, 2, 1131412. [Google Scholar]
- Leyva-Jiménez, F.J.; Lozano-Sánchez, J.; Cádiz-Gurrea, M.D.; Arráez-Román, D.; Segura-Carretero, A. Functional Ingredients based on Nutritional Phenolics. A Case Study against Inflammation: Lippia Genus. Nutrients 2019, 11, 1646. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.P.; Sudheer, A.R. Antioxidant and anti-inflammatory properties of curcumin. Adv. Exp. Med. Biol. 2007, 595, 105–125. [Google Scholar]
- Rajkumari, S.; Sanatombi, K. Nutritional value, phytochemical composition, and biological activities of edible Curcuma species: A review. Int. J. Food Prop. 2017, 20, S2668–S2687. [Google Scholar] [CrossRef]
- Anjusha, S.; Gangaprasad, A. Phytochemical and Antibacterial Analysis of Two Important Curcuma species, Curcuma aromatica Salisb. and Curcuma xanthorrhiza Roxb. (Zingiberaceae). J. Pharmacogn. Phytochem. 2014, 3, 50–53. [Google Scholar]
- Sharma, G.; Chirangini, P.; Rajkumar, K. Gingers of Manipur: Diversity and potentials as bioresources. Genet. Resour. Crop. Evol. 2011, 58, 753–767. [Google Scholar] [CrossRef]
- Abas, F.; Lajis, N.H.; Shaari, K.; Israf, D.A.; Stanslas, J.; Yusuf, U.K.; Raof, S.M. A Labdane Diterpene Glucoside from the Rhizomes of Curcuma m angga. J. Nat. Prod. 2005, 68, 1090–1093. [Google Scholar] [CrossRef]
- Suciyati, S.W.; Adnyana, I.K. Red ginger (Zingiber officinale Roscoe var rubrum): A review. Red 2017, 2, 60–65. [Google Scholar]
- Pandey, A.K.; Chowdhury, A.R. Volatile constituents of the rhizome oil of Curcuma caesia Roxb. from central India. Flavour Fragr. J. 2003, 18, 463–465. [Google Scholar] [CrossRef]
- Kulkarni, R.R.; Patki, P.S.; Jog, V.P.; Gandage, S.G.; Patwardhan, B. Treatment of osteoarthritis with herbomineral formulation: A double-blind, placebo-controlled, cross-over study. J. Ethnopharmacol. 1991, 33, 91–95. [Google Scholar] [CrossRef]
- Satoskar, R.R.; Shah, S.J.; Shenoy, S.G. Evaluation of anti-inflammatory property of curcumin (diferuloyl methane) in patients with postoperative inflammation. Int. J. Clin. Pharmacol. Ther. Toxicol. 1986, 24, 651. [Google Scholar]
- Jurenka, J.S. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: A review of preclinical and clinical research. Altern. Med. Rev. 2009, 14, 141–153. [Google Scholar] [PubMed]
- Chen, X.; Zong, C.; Gao, Y.; Cai, R.; Fang, L.; Lu, J.; Liu, F.; Qi, Y. Curcumol exhibits anti-inflammatory properties by interfering with the JNK-mediated AP-1 pathway in lipopolysaccharide-activated RAW264. 7 cells. Eur. J. Pharmacol. 2014, 723, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, A.L. The Problem of Curcumin and Its Bioavailability: Could Its Gastrointestinal Influence Contribute to Its Overall Health-Enhancing Effects? Adv. Nutr. 2018, 9, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Harsha, C.; Banik, K.; Vikkurthi, R.; Sailo, B.L.; Bordoloi, D.; Gupta, S.C.; Aggarwal, B.B. Is curcumin bioavailability a problem in humans: Lessons from clinical trials. Expert Opin. Drug Metab. Toxicol. 2019, 15, 705–733. [Google Scholar] [CrossRef]

| Species of Curcuma | Plant Parts | Extracts/Fractions/Gels | Dose/Concentration | Assay | Study Model | Mechanism of Action | Ref. |
|---|---|---|---|---|---|---|---|
| Curcuma longa | Rhizome | Aqueous | 0.1% | 1 | Rabbits | Significant reduction in the levels of inflammatory cells, proteins, and TNF-α in aqueous humor, thus reducing the severity of clinical signs and histopathologic changes of uveitis | [34] |
| Rhizome | Oil-free aqueous | Three doses (20 mg/kg, 60 mg/kg, 180 mg/kg) | 2, 3 | Albino Swiss mice and Wistar rats | Significant reduction in ear weight and decrease in wet as well as dry weights of cotton pellets in both models | [35] | |
| Rhizome | Administered topically in gel form | 3.33–33.3% of turmeric | 4 | Rats | Significant and dose-dependent inhibition of rat paw edema | [33] | |
| Rhizome | Polysaccharide fraction (F1) of an aqueous-based extract (NR-INF-02) | 11.25, 22.5, and 45 mg/kg | 2, 3, 4, | Rodent | Significant inhibition of paw and ear edema and reduction of wet and dry weights of cotton pellets | [37] | |
| Curcuma zedoaria | Root | Petroleum ether, chloroform, and methanol | Petroleum ether, 200 and 400 mg/kg chloroform extracts | 4, 5 | Albino rats | n.m. | [43] |
| Root | Ethanol | Ethanol at 200 and 400 mg/kg root extracts | 4, 5 | Rats | n.m. | [44] | |
| Curcuma phaeocaulis | Rhizome | Methanol | 500 mg/kg | 6 | Adjuvant arthritis model mice | Reduction in serum haptoglobin concentration | [45] |
| Rhizome | Ethanol | 10 to 80 μg/mL | 7 | Mouse RAW264.7 cells | Inhibition of nitrite production in inflammatory reactions | [46] | |
| Curcuma mangga | Rhizome | Ethanol extract and its aqueous fractions, chloroform, ethyl acetate, hexane | 200 mg/kg | 4, 8 | Rat, mouse | n.m. | [34] |
| Curcuma amada | Rhizome | Ethanol | 40–80 mg/kg | 3, 4 | Albino rats | n.m. | [47] |
| Curcuma aeruginosa | Rhizome | Chloroform, methanol, and water | 3.03 g/kg | 4 | Rats | No anti-inflammatory effect | [48] |
| Curcuma aromatica | Rhizome | Ethanol | 1–2 g | 9 | Albino mice | n.m. | [49] |
| Species | Plant Parts | Bioactive Compounds | Extracts/ Fractions/Oils | Assay | Study Model | Results/Mechanism of Action | Ref. |
|---|---|---|---|---|---|---|---|
| Curcuma longa | Aerial parts | Quercetin 3-(2Gal-rhamnosyl-robinobioside), quercetin 3-rutinoside, quercetin 3-rhamnosyl-(1→2)-rhamnoside, and quercetin-3-O-rhamnoside | Methanol (CH2Cl2 and n-BuOH fractions) | 1 | RAW264.7 macrophage cells | Significantly suppressed the lipopolysaccharide-mediated induction of nitric oxide, prostaglandin E2, and pro-inflammatory cytokines; reduced the levels of nitric oxide synthase and cyclooxygenase-2 in a concentration-dependent manner | [36] |
| n.m. | Curcumin | DMSO | 2 | Human intervertebral disc cells | Reduced levels of IL-1β, IL-6, IL-8, MMP1, MMP3, and MMP13, and up-regulated TNF-α | [38] | |
| Rhizome and root barks | UP1304 (10% curcumin and 2% mulberroside) | Ethanol | 3 | Rat | Inhibition of the enzymatic activities of COX and LOX | [39] | |
| Rhizome | Curcumin | Petroleum ether, alcohol | 4, 5, 6 | Inhibition of AA metabolism, COX, LOX, cytokines (ILs and TNF), and NF-κB | [42] | ||
| Rhizome | Curcuminoids | Mucoadhesive formulation containing curcuminoids (MFC) | 7 | Adult Swiss male mice | Stimulated cell proliferation by ≈90% in the epithelial cells’ lining from villi and crypts, and reduced MPO levels and MDA formation by 60% and 44%, respectively | [50] | |
| n.m. | Ar-turmerone (61.79%), curlone (12.48%), curcumene (6.11%), etc. | Essential oil (turmeric oil) | 3, 8, 9 | Mice | Significant reduction in paw thickness | [51] | |
| n.m. | Curcuminoids, bisdemethoxycurcumin (2.5 to 6.5%), demethoxycurcumin (15 to 25%), and curcumin (70 to 80%) | Turmeric extract (Sabinsa Company, Malaysia) | 10 | Rats | Arrested the degenerative changes in the bone and joints | [32] | |
| Rhizome | Phenolic curcuminoids: curcumin, demethoxycurcumin, and bisdemethoxycurcumin | n.m. | 5 | Rats | Significantly suppressed the incidence and severity of arthritis by increasing/decreasing the production of anti-inflammatory/pro-inflammatory cytokines, respectively | [39] | |
| Rhizome, roots | Curcumin | n.m. | 11 | Animal and human | Inhibition of phospholipase, LOX, COX-2, LTs, Tx, PGs, NO, collagenase, elastase, hyaluronidase, MCP-1, Interferon-inducible protein, TNF-α, and IL-12 | [34] | |
| n.m. | Curcumin | n.m. | 12 | H.-pylori-infected gastritis patients | Limited anti-bactericidal effect on H. pylori and on the production of inflammatory cytokines | [42] | |
| Curcumin I, curcumin II (monodemethoxycurcumin), and curcumin III (bisdemethoxycurcumin) | n.m. | n.m. | Inhibition of lipid peroxidation COX-I and COX-II enzymes | [34] | |||
| Curcumin (diferuloylmethane), demethoxycurcumin, and bisdemethoxycurcumin | Volatile oils (tumerone, atlantone, and zingiberone) | 3, 9, 13 | n.m. | Significant inhibition of paw edema and curcumin has potential as a therapeutic agent against these anti-inflammatory diseases or conditions | [41] | ||
| Curcuma xanthorrhiza | Ehizome | Germacrone | Methanol, ether-soluble fractions, n-hexane soluble fractions | 3, 14 | Rats, mice | Significantly inhibited edema, vascular permeability, and the number of writhes | [52] |
| Rhizome | Xanthorrhizol | Ethanol | 15 | Mice | Significantly inhibited the production of inflammatory cytokines, such as TNF-α, IL-6, IL-1β, and C-reactive protein in adipose tissue, liver, and muscle | [53] | |
| Rhizome | trans,trans-1,7-Diphenyl-1,3-heptadien-4-one (alnustone), trans-1,7-diphenyl-1-hepten-5-ol, and trans,trans-1,7-diphenyl-1,3-heptadien-5-ol | Hexane | 3 | Rats | Significant anti-inflammatory activity | [54,55] | |
| Non-phenolic linear 1,7-diarylheptanoids | n.m. | 16 | Murine model | Potent inhibition of ethyl-phenylpropiolate-induced ear edema | [56] | ||
| Curcuma zedoaria | Rhizome | Tannins, saponins, flavonoids, gums, carbohydrates, steroids, alkaloids, reducing sugars, and terpenoids | Ethanol | 3, 17 | Long Evans rats | Significantly inhibited the carrageenan-induced inflammatory response in a dose-related manner and protein denaturation in vitro | [57] |
| Rhizome | Sesquiterpene: furanodiene (J) and furanodienone (K) | Methanol | 18 | Mouse | Suppressed the TPA-induced inflammation of mouse ears | [58] | |
| Curcuma wenyujin | Curcumol | Aqueous | 3, 4, 14 | Mice, rats | Decrease the levels of TNF-α and IL-6; significant anti-inflammatory effects against all the test and pelvic inflammation | [59] | |
| Curcumolide | n.m. | 1 | RAW 264.7 macrophages | Suppress LPS-induced NF-κB activation, and decreased TNF-α, IL-6, IL-1β, NO, and reactive oxygen species (ROS) production | [60] | ||
| Curcuma mangga | Rhizome | Demethoxycurcumin, 15,16 bisnorlabda-8(17), 11-dien-13-one, (E)-15,15-diethoxylabda-8(17),12-dien-16-al, bisdemethoxycurcumin | Chloroform fraction, n-hexane fraction | 1 | RAW 264.7 cells | Down-regulation of the mRNA expressions of iNOS and COX-2 in a dose-dependent manner | [61] |
| Curcuma aromatica | Leaves and rhizomes | Ar-Turmerone, borneol, curcumin, demethoxycurcumin, linalool, xanthorrhizol, | Ethanol | 19 | Albino mice | n.m. | [49] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahaman, M.M.; Rakib, A.; Mitra, S.; Tareq, A.M.; Emran, T.B.; Shahid-Ud-Daula, A.F.M.; Amin, M.N.; Simal-Gandara, J. The Genus Curcuma and Inflammation: Overview of the Pharmacological Perspectives. Plants 2021, 10, 63. https://doi.org/10.3390/plants10010063
Rahaman MM, Rakib A, Mitra S, Tareq AM, Emran TB, Shahid-Ud-Daula AFM, Amin MN, Simal-Gandara J. The Genus Curcuma and Inflammation: Overview of the Pharmacological Perspectives. Plants. 2021; 10(1):63. https://doi.org/10.3390/plants10010063
Chicago/Turabian StyleRahaman, Md. Moshiur, Ahmed Rakib, Saikat Mitra, Abu Montakim Tareq, Talha Bin Emran, A. F. M. Shahid-Ud-Daula, Mohammad Nurul Amin, and Jesus Simal-Gandara. 2021. "The Genus Curcuma and Inflammation: Overview of the Pharmacological Perspectives" Plants 10, no. 1: 63. https://doi.org/10.3390/plants10010063
APA StyleRahaman, M. M., Rakib, A., Mitra, S., Tareq, A. M., Emran, T. B., Shahid-Ud-Daula, A. F. M., Amin, M. N., & Simal-Gandara, J. (2021). The Genus Curcuma and Inflammation: Overview of the Pharmacological Perspectives. Plants, 10(1), 63. https://doi.org/10.3390/plants10010063










