Abstract
Plants are often exposed to abiotic stresses such as drought, salinity, heat, cold, and heavy metals that induce complex responses, which result in reduced growth as well as crop yield. Phytohormones are well known for their regulatory role in plant growth and development, and they serve as important chemical messengers, allowing plants to function during exposure to various stresses. Seed priming is a physiological technique involving seed hydration and drying to improve metabolic processes prior to germination, thereby increasing the percentage and rate of germination and improving seedling growth and crop yield under normal and various biotic and abiotic stresses. Seed priming allows plants to obtain an enhanced capacity for rapidly and effectively combating different stresses. Thus, seed priming with phytohormones has emerged as an important tool for mitigating the effects of abiotic stress. Therefore, this review discusses the potential role of priming with phytohormones to mitigate the harmful effects of abiotic stresses, possible mechanisms for how mitigation is accomplished, and roles of priming on the enhancement of crop production.
1. Introduction
Due to the consequences of global warming, crop production and productivity are hampered in many localities. Different environmental constraints, such as drought, salinity, heat, cold, and heavy metals, can seriously affect plant growth and development [1,2,3,4]. The early stages of plants, such as seed germination and seedling establishment, are susceptible to these environmental constraints [5,6]. In this era, much attention has been given to developing approaches to alleviate the constraints of abiotic stresses on seed germination. Different physiological and non-physiological techniques are available for enhancing seed germination as well as alleviating abiotic stresses. Seed priming is a low-cost and effective physiological and biochemical process that stimulates seed germination, enhances morphological parameters, and improves plant growth and development under abiotic stress [7,8,9,10,11].
Plant hormones are known as phytohormones or plant growth regulators (PGRs). Phytohormones are chemical molecules produced by plants and have important roles in regulating plant growth and development. Auxins (IAAs), cytokinins (CKs), gibberellins (GAs), abscisic acid (ABA), salicylic acid (SA), and ethylene (ET) are well-known phytohormones that are essential for plant growth and development [8,12]. Phytohormones function as important chemical messengers and modulate many cellular processes in plants, and they can coordinate different signaling pathways during exposure to abiotic stresses [13,14]. Several studies have reported that phytohormones can interact with each other and manage the physiology of plants exposed to different biotic and abiotic stresses [15,16,17,18].
Seed priming with hormone solutions is referred to as hormonal priming, and hormonal seed priming plays an important role in seed metabolism [9]. Currently, hormonal seed priming is a commonly used technique to improve seed germination, seedling growth, and crop yield in adverse conditions [19,20,21]. Ensuring better germination and seedling vigor by seed priming would result in healthy and productive plants under adverse conditions (Figure 1).
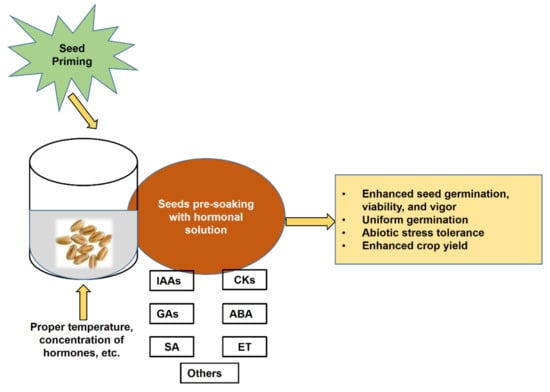
Figure 1.
Schematic model showing possible effects of seed priming with phytohormones.
In hormonal seed priming, seeds are pre-soaked with an optimal concentration of phytohormone, which enhances germination, seedling growth, and yield by increasing nutrient uptake through enhanced physiological activities and root production [22,23]. Seed priming with phytohormones has been studied in a range of crop species, and it modulates many physiological processes such as growth and development, respiration, and transpiration [24,25,26]. Phytohormones have a significant role in the biochemical, defense, and signaling pathways of plants [12]. Many researchers are working to develop effective approaches to alleviate abiotic stresses and enhance crop production. Seed priming with phytohormones can modulate the biochemical and molecular mechanisms making plants capable of tolerating these abiotic stresses, and these techniques are now very promising. Thus, the purpose of this review is to summarize the current understanding of the regulation of abiotic stresses through phytohormone priming and its future promise. Therefore, this review discusses hormonal seed priming and its role in stress mitigation, mechanisms of action of the hormones, and benefits for crop production in the future.
2. Commonly Used PGRs in Seed Priming
Among the plant growth regulators, IAAs, CKs, GAs, ABA, SA, and ET are commonly used in seed priming. In addition, methyl jasmonate (MeJA) and strigolactone have also been used in seed priming.
2.1. Auxin
The IAAs were the first identified and are most well-known phytohormone that demonstrates a vital role in modulating plant growth and developmental processes such as root growth, cell elongation, vascular differentiation, and apical dominance [27,28]. IAAs promote plant growth not only under normal conditions but also under different stress conditions [29] (Figure 2). In higher plants, IAAs mainly exist in the form of IAA conjugates, and they are the primary free endogenous auxin involved in plant developmental processes such as lateral root formation. The exogenous application of IAAs induces the formation of adventitious roots [30,31].
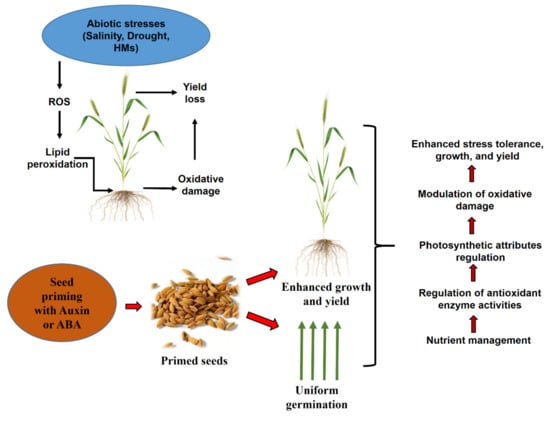
Figure 2.
Proposed possible mechanisms used by auxin-and abscisic acid (ABA)-priming and their roles on the germination, growth, and development of plants under different stresses.
Plant growth and development are hampered by different abiotic stresses, and seed priming with IAAs has been reported as an effective tool to reduce the effects of these stresses [23,32]. Seed priming with IAAs enhances cell division, photosynthetic activities, and translocation of carbohydrates, which results in lateral root initiation, flowering, and good stand establishment [33,34,35]. Seed priming with IAAs (1 ppm) enhanced the seedling establishment of Bouteloua gracilis [36], and in wheatgrass (Agropyron elongatum), seeds priming with IAAs at 50 ppm improved tolerance to drought stress by enhancing antioxidant enzyme activities such as catalase (CAT), superoxide dismutase (SOD), and peroxidase (POD) [7]. Under salinity stress, wheat seeds priming with IAAs (100, 150, and 200 mg L−1) regulated hormonal homeostasis, which enhanced the CO2 assimilation rate and ultimately resulted in increased grain yield [32]. Also, seed priming with IAAs improved the germination and growth of different species, such as rice (Oryza Sativa) and pigeon pea (Cajanus cajan), under arsenic or cadmium (Cd) stress [26,37].
Iqbal and Ashraf [32] reported that seed priming with IAAs ameliorated salt stress via modulation of ion homeostasis in wheat and induced salicylic acid biosynthesis in leaves. Also, seed priming with IAAs activates calcium anion channels and inhibits K+ inward, rectifying channels during salt stress, which results in a reduction of transpirational water loss from plants. Modulation of the stomatal opening and closing helps plants reduce water loss via transpiration [38,39]. Consequently, plant growth is improved under stressful conditions. It is well known that exogenous and endogenous IAAs play an important role in stomatal movement and function as a positive regulator in the stomatal opening, but high concentrations of IAAs have a negative effect [40].
2.2. Cytokinin
CKs are the major plant hormones that regulate numerous aspects of plant growth and development, such as cell division, apical dominance, root formation, stomatal behavior, and chloroplast development [41,42]. It is well known that CKs application promotes crop production. For example, the application of CKs to cotton seedlings increased cotton yield by 5–10% [43]. CKs play an important role in plant pathogenesis, and CK application induced resistance against Pseudomonas syringae in Arabidopsis thaliana [44,45] and Nicotiana tabacum [45]. CKs may act as a biological agent to control diseases. For instance, Pseudomonas fluorescens G20-18 produces CKs, which controls Pseudomonas syringae infection in Arabidopsis and enhances biomass yield [46]. The exogenous application of CKs can mitigate the abiotic stresses on crop plants, which ultimately results in increased growth, development, and yield. Likewise, supplementation of CKs also reduces salinity stress in plants [47,48], and it increases starch accumulation in salt-stressed rice plants [49]. In addition, exogenously applied CKs increased net C-assimilation, net photosynthesis, and dry matter accumulation in Epipremnum aureum, which resulted in enhanced plant growth [50,51]. However, Zahir et al. [52] reported that exogenous application of CKs significantly increased the growth and yield of rice.
Seed priming with CKs or a combination of CKs and other plant hormones has resulted in the mitigation of abiotic stresses in various plant species (Table 1). Priming with CKs enhances chlorophyll (Chl) formation and biomass accumulation in plants, and it increases photosynthetic rate, promotes membrane stability, and maintains stable ionic levels. It has been reported that wheat seeds priming with kinetin (100 mg L−1, 150 mg L−1, and 200 mg L−1) enhanced germination and tolerance against salt by decreasing ABA and increasing IAAs concentrations [53]. Likewise, Mangena [54] reported that soybean seed priming with CKs (Benzyl adenine; 4.87 mg L−1) increased soybean root biomass, flowering, and fruiting under drought stress. Priming of aged groundnut (Arachis hypogaea L.) seeds with CKs (150 ppm) enhanced germination and seedling indices by enhancing antioxidant enzyme activities and decreasing oxidative damage [55]. However, the detailed mechanisms of how priming with CKs mitigate abiotic stress have not been investigated. CKs play a significant role in stomatal movement, and when applied exogenously, this PGR inhibits ABA-induced stomatal closure [56,57]. However, seed priming with CKs and its effects on stomatal movement are still unclear.

Table 1.
Seed-priming with cytokinin adopted for developing abiotic stress tolerance in plants.
2.3. Gibberellin
GAs are plant growth hormones and have positive effects on seed germination, stem elongation, flowering initiation, and flower and fruit development [66,67]. GAs regulate plant growth and development during the entire life cycle of plants [68]. Demir et al. [68] reported that the application of GA3 significantly increased the germination speed of eggplant (Solanum melongena) seeds. Also, GAs can interact with other plant hormones and mediate many developmental processes in plants [69].
Different abiotic stresses, such as salinity, drought, chilling, heat, and heavy metals, inhibit proper nutrient uptake and photosynthesis, which ultimately results in stunted plant growth [70,71]. The exogenous application of GAs can mitigate abiotic stresses and enhance plant growth and development. The application of GAs in combination with poultry manure improved the growth of pepper (Capsicum annuum) plants and increased their salinity tolerance [72]. Moumita et al. [73] reported that exogenous application of GAs improved the growth of wheat (Triticum aestivum) plants and mitigated drought-induced oxidative damage by maintaining relative water content, balancing the antioxidant mechanism system, and conserving the Chl concentration. Foliar application of GA3 to tomato (Solanum lycopersicum) plants increased relative leaf water content, stomatal density, and Chl content by mitigating salinity stress [74]. Besides, GA3 stimulated plant growth and yield leaf of lettuce (Lactuca sativa) by enhancing biomass accumulation, leaf expansion, stomatal conductance, water use efficiency, and nitrogen use efficiency [75].
GAs are used as important seed priming agents to mitigate abiotic stresses in different crops (Table 2). Guangwu and Xuwen [76] reported that GAs (5 × 10−5 M) promoted seed respiration and lowered the ABA level and stimulated IAAs and GAs biosynthesis. In addition, wheat seeds treated with GA3 (100 mg L−1, 150 mg L−1, and 200 mg L−1) exhibited a decrease in the concentration of polyamines, ABA, and Na+ and an increase in the concentration of Ca2+ and K+ [23]. Moreover, wheat seeds primed with GAs (150 ppm) enhanced germination and seedling parameters under salt stress [77]. In the case of salt stress, maize seed priming with GAs (5 mg L−1) increased the shoot and root length and tissue water content [78]. Recently, Ma et al. [79] reported that seeds priming with GAs (50 µM) increased the germination rate, plant growth, and biomass production in Leymus chinensis. Likewise, seed priming with GAs increased the percentage and rate of seed germination and enhanced growth, yield, and yield-contributing characters of different crops species such as wheat, maize, and lentil [80,81,82,83]. However, more research is required to find the mechanisms of GA priming in abiotic stress mitigation.

Table 2.
Seed-priming with gibberellin and response of plant species.
2.4. Abscisic Acid
ABA is one of the major plant hormones and is also known as a stress hormone. It plays a vital role in mediating plant responses to various abiotic stresses, such as salt, heat, and drought [98,99,100]. ABA not only plays a role in abiotic stress mitigation but also plays a significant role in plant growth and development [101,102].
ABA is a potent seed priming hormone for the enhancement of germination as well as increased tolerance to various stresses by different crop species [103]. Rice seeds primed with ABA exhibited enhanced seedling growth and yield in saline soil by balancing nutrient uptake [103,104,105,106]. Likewise, priming rice seeds with ABA at 10 µM reduced alkaline stress by enhancing antioxidant enzyme activities and the activity of stress tolerance-related genes in the roots of rice seedlings [107]. Moreover, Wei et al. [108] reported that ABA (10 µM and 50 µM) priming of rice seeds improved the growth rate, survival rate, biomass accumulation, and root formation under alkaline stress. Also, seed priming with ABA enhanced salinity tolerance and increased the growth of rice, wheat, and sorghum [104,109]. Fricke et al. [110] reported that ABA priming promoted barley leaves growth by reducing transpirational water loss under saline conditions. Rice seeds primed with ABA at 10−5 M showed increased osmoregulation by reduced cellular Na concentration and increased proline and sugar accumulation in salt-stressed rice leaves [104]. The deterioration of Agropyron elongatum seeds was prevented by priming them with ABA at 50 ppm, which enhanced antioxidant enzyme activities [8]. Under saline soils, good stand establishment of sesame (Sesamum indicum) was achieved by ABA seed priming [111]. Zongshuai et al. [112] reported that the salt tolerance of wheat plants was enhanced by seed priming with ABA.
It has been reported that phytohormones are effective in the mitigation of heavy metal stress [12,26]. ABA biosynthetic gene expressions are induced by heavy metal stresses, which results in increased levels of endogenous ABA [12,18]. Under Cd stress, the germination of pigeon pea was improved by ABA (100 µM) priming [26]. However, the mechanism is still not clear, and the effects of seed priming with ABA on mitigation of heavy metal stress remain to be explored. Although seed priming with ABA enhances germination, many studies have reported that ABA inhibits seed germination which is dose dependent (10–30 µM) [113,114]. These differences may come from the endogenous and exogenous concentrations of ABA, whereas Srivastava et al. [115] reported the priming of mustard seeds with ABA (100 µM) increased the germination rate by 25% compared to the control under salt stress. In other words, an exogenous concentration may have an effect, and may enhance the germination at higher concentrations. However, how seed priming with ABA promotes germination needs more clarification with molecular studies.
ABA facilitates growth improvement via modulation of ion transport and regulation of stomatal movement in plants [116]. ABA is synthesized in plants under water-deficit conditions, and this induces stomatal closure via modulation of reactive oxygen species, reactive carbonyl species, cytosolic alkalization, and elevation of cytosolic calcium [38,117,118,119]. Exogenous application of ABA to plants also stimulates the regulation of stomatal movements, which helps reduce transpirational water loss. Marthandan et al. [120] reported priming Arabidopsis seeds with amino-butyric acid enhanced drought tolerance by accumulation of ABA and the closing of stomata. However, it is not known how seed priming with ABA helps regulate stomatal movements in plants. Based on information in the literature, we created a model showing how seed priming with ABA influences plant growth and development (Figure 2).
2.5. Salicylic Acid
SA is a phenolic plant hormone that regulates growth and development and many physiological processes, such as photosynthesis, respiration, transpiration, and the transportation of ions in plants. SA exhibits a key role in the activation, modulation, and regulation of numerous responses during exposure to abiotic and biotic stresses [102,121,122,123]. It is well known that SA generates a cascade of signaling pathways by interacting with other plant hormones such as ABA, MeJA, and ET and plays an important role in mitigating plant stresses [124,125]. Also, plant resistance to salinity, heat, and cell death under various hypersensitive stresses can be regulated by the presence of SA [126,127]. The exogenous application of SA enhanced maize (Z. mays) productivity under low temperature stress, as well as the germination and growth parameters of garden cress (Lepidium sativum) seedlings under salinity stress [128], and mitigated drought stress and enhanced the vegetative growth of safflower (Carthamus tinctorius) [129].
Seed priming with SA mitigates the effects of abiotic stresses and enhances yield in a range of crop species (Table 3).

Table 3.
Seed priming with salicylic acid (SA) and response of plant species.
Seed priming with SA mitigated abiotic stresses by enhancing antioxidant enzyme activities such as CAT, APX, and SOD and regulating lipid peroxidation and H2O2 production. Likewise, seed priming with SA also increased the production of osmolytes such as proline and glycine betaine, which play an important role in mitigating different stresses [12,37]. Ion homeostasis and nutrient uptake were regulated by priming with SA at 0.25 mM and 0.50 mM, which enhanced the tolerance of heavy metal stress [130]. In addition, priming with SA at 0.5 mM also enhanced endogenous SA content and α-amylase activity during abiotic stresses [134]. Moreover, priming with SA enhanced the integration affinity among the phytohormones as a result of the GAs biosynthetic gene, and ABA catabolism gene expression enhanced and upregulated the GAs and ABA signaling pathways (Figure 3). In addition to abiotic stress mitigation, priming with SA has an important role in enhancing seed germination and crop productivity. Priming with SA at 100 mg L−1 enhanced emergence and early seedling growth in cucumber [151] and increased germination and productivity of Vicia faba [152] and sesame [153]. It has been reported that rice seeds primed with SA (100 ppm) had increased germination and accelerated seedling growth by ion absorption in PEG-induced water stress [131]. In addition, priming of rice seeds with SA enhanced tolerance to chilling stress by enhancing antioxidant enzyme activities and reducing oxidative damage [132]. SA induced stomatal closure and reduced transpirational water loss from plants [154,155]. Seed priming with SA has a role in the stomatal movement that has not been analyzed, and integration mechanisms with other phytohormones are still unclear.
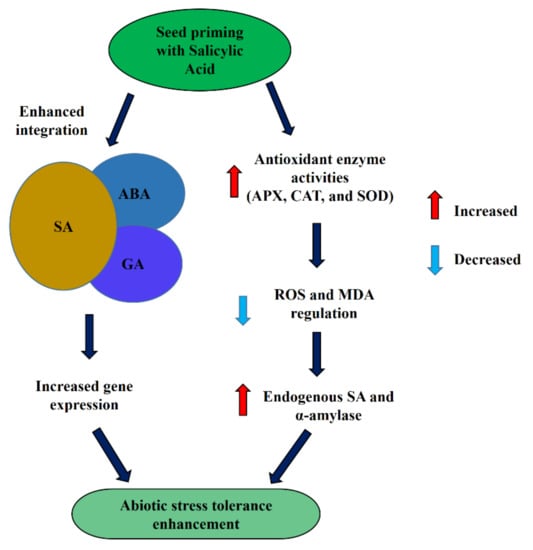
Figure 3.
Mechanisms of SA priming for abiotic stress tolerance enhancement.
2.6. Ethylene
The hydrocarbon ET is an important plant hormone and it is widely used for ripening fruits [156]. For example, the application of 100 μL L−1 of ET for 12 h stimulated the production of 1-amino cyclopropane-1-carboxylic acid (ACC: an ethylene precursor) and increased ACC oxidase activity, which accelerated the ripening of ‘Ataulfo’ mangoes [157]. The exogenous application of 5 mL L−1 ET improved the activity of CAT, APX, and SOD and reduced the activity of polyphenol oxidase (PPO) and POX, which prevented browning of the peel of the ‘Huangguan’ Pear (Pyrus bretschneideri Rehd cv. Huangguan) [158]. The exogenous application of ethephon (source of ethylene) to soybean (Glycine max) plants mitigated waterlogging stress by promoting the initiation of adventitious roots and by increasing root surface area, expression of glutathione transferases, and relative glutathione activity [159].
The exogenous application of ET has been an important player in the mitigation of abiotic stresses, but seed priming with ET has received little research attention. Nascimento et al. [160] reported that priming lettuce seeds with ACC increased germination at a high temperature (36 °C). Priming pigeon pea seeds with 10 mM ET (chloroethylphosphonic acid) increased the germination percentage under Cd stress conditions [26]. The combined application of ethephon and gibberellic acid to rice seeds increased α-amylase activity and sugar content [89]. Manoharlal and Saiprasad [161] reported that priming with ethephon improved the germination of soybean seeds. Further research is necessary to determine the effects of priming seed with ethylene on germination under different abiotic stresses.
2.7. Others
Jasmonic acid derivatives are widely used as a priming agent to ameliorate abiotic stresses. It has been reported that, with rice seed priming with MeJA at 2.5 mM and 5 mM, seeds experienced increased Chl content and photochemical efficiency under PEG stress [162]. Likewise, priming with MeJA improved the growth of broccoli sprouts under salinity stress [163]. In addition, priming with MeJA (1 mM) may function as a biocontrol agent and protect tomato seedlings against fusarium wilt [164]. Another phytohormone, brassinosteroids, has also been used as a priming agent and has been known to regulate plant growth and development and resistance to abiotic stresses [165]. It has been reported that the seed priming of lucerne (Medicago sativa L.) with brassinolide (5 µM L−1) improved seed germination and seedling growth under salinity stress [166]. Likewise, peanut seed priming with brassinosteroids at 0.15 ppm improved drought tolerance and increased the yield of peanut [167]. However, more research is necessary to find out the effects of seed priming with jasmonic acid and brassinosteroids under abiotic stresses.
3. Conclusions with Future Perspectives
Seed priming with phytohormones has emerged as a promising strategy in modern stress management as it protects plants against various abiotic stresses by increasing level of antioxidant enzyme activity, decreasing oxidative damage, and enhancing plant growth. Thus, seed priming with phytohormones improves the tolerance of crop plants to abiotic stress, and this technique can be utilized to maintain sustainable crop production in drought-, saline-, and flood-prone areas of the world. Seed priming with phytohormones not only improves the tolerance to abiotic stresses but also ensures hermonized germination by breaking the dormancy and enhancing viability. This review provides insight into the role of seed priming with phytohormones in mitigating the effects of abiotic stress on seed germination and plant growth. The data compiled in this review can be used for developing further extensive research on abiotic stress mitigation by seed priming with phytohormones. Seed priming with phytohormones has emerged as an effective seed treating tool for many crops, but treating conditions and methods differ from crop to crop, and seed priming with phytohormones has still limitations. For instance, prolonged seed treatment with hormonal solution during priming may cause the loss of seed tolerance to desiccation, which reduces seed viability. However, more research at the molecular level is required to clarify the mechanisms of involvement of phytohormones in seed priming, especially in the application methods, and phytohormones cross-talk and stress-responsive genes.
Author Contributions
Conceptualization, M.S.R. and M.H.; writing—original draft preparation, M.S.R.; S.I.; F.R.; M.K.; and C.C.B.; writing—review and editing, Y.M. and M.H.; visualization, M.S.R.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, H.; Irving, L.J.; McGill, C.; Matthew, C.; Zhou, D.; Kemp, P. The effects of salinity and osmotic stress on barley germination rate: Sodium as an osmotic regulator. Ann. Bot. 2010, 106, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef] [PubMed]
- Dhankher, O.P.; Foyer, C.H. Climate resilient crops for improving global food security and safety. Plant Cell Environ. 2018, 5, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.B.; Nahar, K.; Mohsin, S.M.; Al Mahmud, J.; Parvin, K.; Fujita, M. Exogenous nitric oxide-and hydrogen sulfide-induced abiotic stress tolerance in plants. In Protective Chemical Agents in the Amelioration of Plant Abiotic Stress: Biochemical and Molecular Perspectives; Roychoudary, A., Tripathi, D.K., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2020; Volume 30, p. 174. [Google Scholar] [CrossRef]
- Vishal, B.; Kumar, P.P. Regulation of seed germination and abiotic stresses by gibberellins and abscisic acid. Front. Plant Sci. 2018, 9, 838. [Google Scholar] [CrossRef]
- Yadav, S.; Modi, P.; Dave, A.; Vijapura, A.; Patel, D.; Patel, M. Effect of Abiotic Stress on Crops. In Sustainable Crop Production; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Eisvand, H.R.; Tavakkol-Afshari, R.; Sharifzadeh, F.; Maddah Arefi, H.; Hesamzadeh Hejazi, S.M. Effects of hormonal priming and drought stress on activity and isozyme profiles of antioxidant enzymes in deteriorated seed of tall wheatgrass (Agropyron elongatum Host). Seed Sci. Technol. 2010, 38, 280–297. [Google Scholar] [CrossRef]
- Muhei, S.H. Seed priming with phytohormones to improve germination under dormant and abiotic stress conditions. Adv. Crop Sci. Technol. 2018, 6, 403–409. [Google Scholar] [CrossRef]
- Rhaman, M.S.; Rauf, F.; Tania, S.S.; Khatun, M. Seed priming methods: Application in field crops and future perspectives. Asian J. Res. Crop Sci. 2020, 5, 8–19. [Google Scholar] [CrossRef]
- Tania, S.S.; Rhaman, M.S.; Hossain, M.M. Hydro-priming and halo-priming improve seed germination, yield and yield contributing characters of okra (Abelmoschus esculentus L.). Trop. Plant Res. 2020, 7, 86–93. [Google Scholar] [CrossRef]
- Jisha, K.C.; Vijayakumari, K.; Puthur, J.T. Seed priming for abiotic stress tolerance: An overview. Acta Physiol. Plant. 2013, 35, 1381–1396. [Google Scholar] [CrossRef]
- Sytar, O.; Kumar, P.; Yadav, S.; Brestic, M.; Rastogi, A. Phytohormone priming: Regulator for heavy metal stress in plants. J. Plant Growth Regul. 2018, 38, 739–752. [Google Scholar] [CrossRef]
- Vob, U.; Bishopp, A.; Farcot, E.; Bennett, M.J. Modelling hormonal response and development. Trends Plant Sci. 2014, 19, 311–319. [Google Scholar] [CrossRef]
- Kazan, K. Diverse roles of jasmonates and ethylene in abiotic stress tolerance. Trends Plant Sci. 2015, 20, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, R.; Watanabe, Y.; Fujita, Y.; Le, D.T.; Kojima, M.; Werner, T.; Vankova, R.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Kakimoto, T.; et al. Analysis of cytokinin mutants and regulation of cytokinin metabolic genes reveals important regulatory roles of cytokinins in drought, salt and abscisic acid responses, and abscisic acid biosynthesis. Plant Cell 2011, 23, 2169–2183. [Google Scholar] [CrossRef] [PubMed]
- Colebrook, E.H.; Thomas, S.G.; Phillips, A.L.; Hedden, P. The role of gibberellin signalling in plant responses to abiotic stress. J. Exp. Biol. 2014, 217, 67–75. [Google Scholar] [CrossRef]
- Xu, Y.X.; Mao, J.; Chen, W.; Qian, T.T.; Liu, S.C.; Hao, W.J.; Li, C.F.; Chen, L. Identification and expression profiling of the auxin response factors (ARFs) in the tea plant (Camellia sinensis (L.) O. Kuntze) under various abiotic stresses. Plant Physiol. Biochem. 2016, 98, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Bucker-Neto, L.; Paiva, A.L.S.; Machado, R.D.; Arenhart, R.A.; Margis-Pinheiro, M. Interactions between plant hormones and HMs responses. Genet. Mol. Biol. 2017, 40, 373–386. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Fotopoulos, V. Priming and Pretreatment of Seeds and Seedlings: Implication in Plant Stress Tolerance and Enhancing Productivity in Crop Plants; Springer: Singapore, 2019. [Google Scholar] [CrossRef]
- Masood, A.; Iqbal, N.; Khan, N.A. Role of ethylene in alleviation of cadmium-induced capacity inhibition by sulphur in mustard. Plant Cell Environ. 2012, 35, 524–533. [Google Scholar] [CrossRef]
- Hu, Y.F.; Zhou, G.; Na, X.F.; Yang, L.; Nan, W.B.; Zhang, Y.O.; Li, J.L.; Bi, Y.R. Cadmium interferes with maintenance of auxin homeostasis in Arabidopsis seedlings. J. Plant Physiol. 2013, 170, 965–975. [Google Scholar] [CrossRef]
- Afzal, I.; Basra, S.M.A.; Ahmad, N.; Cheema, M.A.; Warriach, E.A.; Khaliq, A. Effect of priming and growth regulator treatment on emergence. Int. J. Agric. Biol. 2002, 4, 306. [Google Scholar]
- Akbari, G.; Sanavy, S.A.; Yousefzadeh, S. Effect of auxin and salt stress (NaCl) on seed germination of wheat cultivars (Triticum aestivum L.). Pak. J. Biol. Sci. 2007, 10, 2557–2561. [Google Scholar] [CrossRef]
- Iqbal, M.; Ashraf, M. Gibberellic acid mediated induction of salt tolerance in wheat plants: Growth, ionic partitioning, photosynthesis, yield and hormonal homeostasis. Environ. Exp. Bot. 2013, 86, 76–85. [Google Scholar] [CrossRef]
- Yuan, Z.; Wang, C.; Li, S.; Li, X.; Tai, F. Effects of different plant hormones or PEG seed soaking on maize resistance to drought stress. Can. J. Plant Sci. 2014, 94, 1491–1499. [Google Scholar] [CrossRef]
- Sneideris, L.C.; Gavassi, M.A.; Campos, M.L.; Damico-Damiao, V.; Carvalho, R.F. Effects of hormonal priming on seed germination of pigeon pea under cadmium stress. Anais da Academia Brasileira de Ciências 2015, 87, 1847–1852. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mopper, S.; Hasentein, K.H. Effects of salinity on endogenous ABA, IAA, JA, and SA in Iris hexagona. J. Chem. Ecol. 2001, 27, 327–342. [Google Scholar] [CrossRef] [PubMed]
- Lymperopoulos, P.; Msanne, J.; Rabara, R. Phytochrome and phytohormones: Working in tandem for plant growth and development. Front. Plant Sci. 2018, 9, 1037. [Google Scholar] [CrossRef]
- Kazan, K. Auxin and the integration of environmental signals into plant root development. Ann. Bot. 2013, 112, 1655–1665. [Google Scholar] [CrossRef]
- Ludwig-Muller, J. Auxin conjugates: Their role for plant development and in the evolution of land plants. J. Exp. Bot. 2011, 62, 1757–1773. [Google Scholar] [CrossRef]
- Schiefelbein, J. Cell-fate specification in the epidermis: A common patterning mechanism in the root and shoot. Curr. Opin. Plant Biol. 2003, 6, 74–78. [Google Scholar] [CrossRef]
- Iqbal, M.; Ashraf, M. Seed treatment with auxins modulates growth and ion partitioning in salt stressed wheat plants. J. Integr. Plant Biol. 2007, 49, 1045–1057. [Google Scholar] [CrossRef]
- MacDonald, H. Auxin perception and signal transduction. Physiol. Plant. 1997, 100, 423–430. [Google Scholar] [CrossRef]
- Awan, I.U.; Baloch, M.S.; Sadozai, N.S.; Sulemani, M.Z. Stimulatory effect of GA3 and IAA on ripening process, kernel development and quality of rice. Pak. J. Biol. Sci. 1999, 2, 410–412. [Google Scholar] [CrossRef][Green Version]
- Naeem, M.; Bhatti, I.R.A.M.; Ahmad, R.H.; Ashraf, M.Y. Effect of some growth hormones (GA3, IAA and kinetin) on the morphology and early or delayed initiation of bud of lentil (Lens culinaris Medik). Pak. J. Bot. 2004, 36, 801–809. [Google Scholar]
- Roohi, R.; Jameson, D.A. The effect of hormone, dehulling and seedbed treatments on germination and adventitious root formation in blue grama. J. Range Manag. 1991, 44, 237–241. [Google Scholar] [CrossRef]
- Fahad, S.; Hussain, S.; Bano, A.; Saud, S.; Hassan, S.; Shan, D.; Khan, F.A.; Khan, F.; Chen, Y.T.; Wu, C.; et al. Potential role of phytohormones and plant growth-promoting rhizobacteria in abiotic stresses: Consequences for changing environment. Environ. Sci. Pollut. Res. 2015, 22, 4907–4921. [Google Scholar] [CrossRef] [PubMed]
- Munemasa, S.; Hauser, F.; Park, J.; Waadt, R.; Brandt, B.; Schroeder, J.I. Mechanisms of abscisic acid-mediated control of stomatal aperture. Curr. Opin. Plant Biol. 2015, 28, 154–162. [Google Scholar] [CrossRef]
- Murata, Y.; Mori, I.C.; Munemasa, S. Diverse stomatal signaling and the signal integration mechanism. Annu. Rev. Plant Biol. 2015, 66, 369–392. [Google Scholar] [CrossRef] [PubMed]
- Daszkowska-Golec, A.; Szarejko, I. Open or close the gate–Stomata action under the control of phytohormones in drought stress conditions. Front. Plant Sci. 2013, 4, 138. [Google Scholar] [CrossRef]
- Werner, T.; Motyka, V.; Strnad, M.; Schmülling, T. Regulation of plant growth by cytokinin. Proc. Natl. Acad. Sci. USA 2001, 98, 10487–10492. [Google Scholar] [CrossRef]
- Schaller, G.E.; Street, I.H.; Kieber, J.J. Cytokinin and the cell cycle. Curr. Opin. Plant Biol. 2014, 21, 7–15. [Google Scholar] [CrossRef]
- Yao, S. Plant Hormone Increases Cotton Yields in Drought Conditions. News & Events. Agricultural Research Service (ARS); U.S. Department of Agriculture: Washington, DC, USA, 2010. [Google Scholar]
- Choi, J.; Huh, S.U.; Kojima, M.; Sakakibara, H.; Paek, K.H.; Hwang, I. The cytokinin-activated transcription factor ARR2 promotes plant immunity via TGA3/NPR1-dependent salicylic acid signaling in arabidopsis. Dev. Cell 2010, 19, 284–295. [Google Scholar] [CrossRef]
- Grobkinsky, D.K.; Naseem, M.; Abdelmohsen, U.R.; Plickert, N.; Engelke, T.; Griebel, T.; Zeier, J.; Novák, O.; Strnad, M.; Pfeifhofer, H.; et al. Cytokinins mediate resistance against Pseudomonas syringae in tobacco through increased antimicrobial phytoalexin synthesis independent of salicylic acid signaling. Plant Physiol. 2011, 157, 815–830. [Google Scholar] [CrossRef] [PubMed]
- Grobkinsky, D.K.; Tafner, R.; Moreno, M.V.; Stenglein, S.A.; García de Salamone, I.E.; Nelson, L.M.; Novák, O.; Strnad, M.; van der Graaff, E.; Roitsch, T. Cytokinin production by Pseudomonas fluorescens G20-18 determines biocontrol activity against Pseudomonas syringae in Arabidopsis. Sci. Rep. 2016, 6, 23310. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.; Vankova, R.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.S. Cytokinins: Metabolism and function in plant adaptation to environmental stresses. Trends Plant Sci. 2012, 17, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Taiz, L.; Zeiger, E.; Moller, I.M.; Murphy, A. Plant Physiology and Development, 6th ed.; Sinauer Associates: Sunderland, CT, USA, 2015. [Google Scholar]
- Javid, M.G.; Sorooshzadeh, A.; Sanavy, S.A.M.M.; Allahdadi, I.; Moradi, F. Effects of the exogenous application of auxin and cytokinin on carbohydrate accumulation in grains of rice under salt stress. Plant Growth Regul. 2011, 65, 305–313. [Google Scholar] [CrossRef]
- Di Benedetto, A.; Galmarini, C.; Tognetti, J. Effects of combined or single exogenous auxin and/or cytokinin applications on growth and leaf area development in Epipremnum aureum. J. Hortic. Sci. Biotechnol. 2015, 90, 643–654. [Google Scholar] [CrossRef]
- Di Benedetto, A.; Galmarini, C.; Tognetti, J. Exogenous cytokinin promotes Epipremnum aureum L. growth through enhanced dry weight assimilation rather than through changes in partitioning. Am. J. Exp. Agric. 2015, 5, 419–434. [Google Scholar] [CrossRef]
- Zahir, Z.A.; Asghar, H.N.; Arshad, M. Cytokinin and its precursors for improving growth and yield of rice. Soil Biol. Biochem. 2001, 33, 405–408. [Google Scholar] [CrossRef]
- Iqbal, M.; Ashraf, M.; Jamil, A. Seed enhancement with cytokinins: Changes in growth and grain yield in salt stressed wheat plants. Plant Growth Regul. 2006, 50, 29–39. [Google Scholar] [CrossRef]
- Mangena, P. Effect of hormonal seed priming on germination, growth, yield and biomass accumulation in soybean grown under induced drought stress. Indian J. Agric. Res. 2020. [Google Scholar] [CrossRef]
- Sepehri, A.; Rouhi, H.R. Effect of cytokinin on morphological and physiological characteristics and antioxidant enzymes activity of aged groundnut (Arachis hypogaea L.) seeds under drought stress. Iran. J. Seed Sci. Technol 2016, 5, 181–198. [Google Scholar]
- Stoll, M.; Loveys, B.; Dry, P. Hormonal changes induced by partial root zone drying of irrigated grapevine. J. Exp. Bot. 2000, 51, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Sano, T.; Tamaoki, M.; Naka-jima, N.; Kondo, N.; Hasezawa, S. Cytokinin and auxin inhibit abscisic acid-induced stomatal closure by enhancing ethylene production in Arabidopsis. J. Exp. Bot. 2006, 57, 2259–2266. [Google Scholar] [CrossRef]
- Verma, J.; Srivastava, A.K. Physiological basis of salt stress resistance in pigeon pea (Cajanus cajan L.). II. Pre-sowing seed soaking treatment in regulating early seedling metabolism during seed germination. Plant Physiol. Biochem. 1998, 25, 89–94. [Google Scholar]
- Bagheri, A.; Bagherifard, A.; Saborifard, H.; Ahmadi, M.M.; Safarpoor, M. Effects of drought, cytokinin and GA3 on seedling growth of basil (Ocimum basilicum). Int. J. Adv. Biol. Biomed. Res. 2014, 2, 489–493. [Google Scholar]
- Iqbal, M.; Ashraf, M. Presowing seed treatment with cytokinins and its effect on growth, photosynthetic rate, ionic levels and yield of two wheat cultivars differing in salt tolerance. J. Integr. Plant Biol. 2005, 47, 1315–1325. [Google Scholar] [CrossRef]
- Afzal, I.; Basra, S.M.; Iqbal, A. The effects of seed soaking with plant growth regulators on seedling vigor of wheat under salinity stress. J. Stress Physiol. Biochem. 2005, 1, 6–14. [Google Scholar]
- Angrish, A.; Kumar, B.; Datta, K.S. Effect of gibberellic acid and kinetin on nitrogen content and nitrate reductase activity in wheat under saline conditions. Indian J. Plant Physiol. 2001, 6, 172–177. [Google Scholar]
- Gadallah, M.A.A. Effects of kinetin on growth, grain yield and some mineral elements in wheat plants growing under excess salinity and oxygen deficiency. Plant Growth Regul. 1999, 27, 63–74. [Google Scholar] [CrossRef]
- Datta, K.; Varma, S.; Angrish, R.; Kumar, B.; Kumari, P. Alleviation of salt stress by plant growth regulators in Triticum aestivum L. Biol. Plant. 1997, 40, 269–275. [Google Scholar] [CrossRef]
- Hedden, P.; Sponsel, V. A Century of Gibberellin Research. J. Plant Growth Regul. 2015, 34, 740–760. [Google Scholar] [CrossRef]
- Yamaguchi, S. Gibberellin metabolism and its regulation. Annu. Rev. Plant Physiol. 2008, 59, 225–251. [Google Scholar] [CrossRef] [PubMed]
- Richards, D.E.; King, K.E.; Ait-ali, T.; Harberd, N.P. How gibberellin regulates plant growth and development: A molecular genetic analysis of gibberellin signaling. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 67–88. [Google Scholar] [CrossRef]
- Demir, I.; Ellialtioglu, S.; Tipirdamaz, R. The effect of different priming treatments on reparability of aged eggplant seeds. Acta Hortic. 1994, 362, 205–212. [Google Scholar] [CrossRef]
- Munteanu, V.; Gordeev, V.; Martea, R.; Duca, M. Effect of gibberellin cross talk with other phytohormones on cellular growth and mitosis to endoreduplication transition. Int. J. Adv. Res. Biol. Sci. 2014, 1, 136–153. [Google Scholar]
- Cramer, G.R.; Urano, K.; Delrot, S.; Pezzotti, M.; Shinozaki, K. Effects of abiotic stress on plants: A systems biology perspective. BMC Plant Biol. 2011, 11, 163. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.H.; Parvin, K.; Bhuiyan, T.F.; Anee, T.I.; Nahar, K.; Hossen, M.; Zulfiqar, F.; Alam, M.; Fujita, M. Regulation of ROS metabolism in plants under environmental stress: A review of recent experimental evidence. Int. J. Mol. Sci. 2020, 22, 8695. [Google Scholar] [CrossRef] [PubMed]
- AlTaey, D.K.A. Alleviation of salinity effects by poultry manure and gibberellin application on growth and peroxidase activity in pepper. Int. J. Environ. Agric. Biotechnol. 2017, 2, 1851–1862. [Google Scholar] [CrossRef]
- Moumita, M.; Mahmud, J.A.; Biswas, P.K.; Nahar, K.; Fujita, M.; Hasanuzzaman, M. Exogenous application of gibberellic acid mitigates drought-induced damage in spring wheat. Acta Agrobot. 2019, 72, 1776. [Google Scholar] [CrossRef]
- Jayasinghe, T.; Perera, P.; Wimalasekera, R. Effect of foliar application of gibberellin in mitigating salt stress in tomato (Solanum Lycopersicum), ‘Thilina’ variety. In Proceedings of the 6th International Conference on Multidisciplinary Approaches (iCMA), Faculty of Graduate Studies, University of Sri Jayewardenepura, Nugegoda, Sri Lanka, 26–27 November 2019; Available online: https://ssrn.com/abstract=3497340 (accessed on 3 December 2019).
- Miceli, A.; Moncada, A.; Sabatino, L.; Vetrano, F. Effect of gibberellic acid on growth, yield, and quality of leaf lettuce and rocket grown in a floating system. Agronomy 2019, 9, 382. [Google Scholar] [CrossRef]
- Guangwu, Z.; Xuwen, J. Roles of gibberellin and auxin in promoting seed germination and seedling vigor in Pinus massoniana. For. Sci. 2014, 60, 367–373. [Google Scholar] [CrossRef]
- Abido, W.A.E.; Allem, A.; Zsombik, L.; Attila, N. Effect of gibberellic acid on germination of six wheat cultivars under salinity stress levels. Asian J. Biol. Sci. 2019, 12, 51–60. [Google Scholar] [CrossRef]
- Ghodrat, V.; Rousta, M.J. Effect of priming with gibberellic acid (GA3) on germination and growth of corn (Zea mays L.) under saline conditions. Int. J. Agric. Crop. Sci. 2012, 4, 883–885. [Google Scholar]
- Ma, H.Y.; Zhao, D.D.; Ning, Q.R.; Wei, J.P.; Li, Y.; Wang, M.M.; Liu, X.L.; Jiang, C.J.; Liang, Z.W. A Multi-year beneficial effect of seed priming with gibberellic acid-3 (GA 3) on plant growth and production in a perennial grass, Leymus chinensis. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Shineeanwarialmas, B.; Menaka, C.; Yuvaraja, A. Effect of different seed priming treatments for improving seedling growth of maize seeds. Int. J. Chem. Stud. 2019, 7, 1777–1781. [Google Scholar]
- Toklu, F. Effects of different priming treatments on seed germination properties, yield components and grain yield of lentil (Lens culinaris Medik.). Not. Bot. Horti. Agrobo. 2015, 43, 153–158. [Google Scholar] [CrossRef]
- Tian, Y.; Guan, B.; Zhou, D.; Yu, J.; Li, G.; Lou, Y. Responses of seed germination, seedling growth, and seed yield traits to seed pretreatment in maize (Zea mays L.). Sci. World J. 2014, 2014, 834630. [Google Scholar] [CrossRef]
- Ghobadi, M.; Abnavi, M.S.; Honarmand, S.J.; Ghobadi, M.E.; Mohammadi, R.M. Effect of hormonal priming (GA3) and osmopriming on behaviour of seed germination in wheat (Triticum aestivum L.). J. Agric. Sci. 2012, 4, 244–250. [Google Scholar] [CrossRef]
- Sedghi, M.; Nemati, A.; Esmaielpour, B. Effect of seed priming on germination and seedling growth of two medicinal plants under salinity. Emir. J. Food Agric. 2010, 22, 130–139. [Google Scholar] [CrossRef]
- Sedghi, M.; Nemati, A.; Amanpour-Balaneji, B.; Gholipouri, A. Influence of different priming materials on germination and seedling establishment of milk thistle (Silybum marianum) under salinity stress. World Appl. Sci. J. 2010, 11, 604–609. [Google Scholar]
- Shariatmadari, M.H.; Parsa, M.; Nezami, A.; Kafi, M. Effects of hormonal priming with gibberellic acid on emergence, growth and yield of chickpea under drought stress. Biosci. Res. 2017, 14, 34–41. [Google Scholar]
- Sheykhbaglou, R.; Rahimzadeh, S.; Ansari, O.; Sedghi, M. The Effect of salicylic acid and gibberellin on seed reserve utilization, germination and enzyme activity of sorghum (Sorghum bicolor L) seeds under drought stress. J. Stress Physiol. Biochem. 2014, 10, 5–13. [Google Scholar]
- Tsegay, B.A.; Andargie, M. Seed priming with gibberellic acid (GA3) alleviates salinity induced inhibition of germination and seedling growth of Zea mays L., Pisum sativum Var. abyssinicum A. Braun and Lathyrus sativus L. J. Crop Sci. Biotechnol. 2018, 21, 261–267. [Google Scholar] [CrossRef]
- Watanabe, H.; Honma, K.; Adachi, Y.; Fukuda, A. Effects of combinational treatment with ethephon and gibberellic acid on rice seedling growth and carbohydrate mobilization in seeds under flooded conditions. Plant Prod. Sci. 2018, 21, 380–386. [Google Scholar] [CrossRef]
- Younesi, O.; Moradi, A. Effect of priming of seeds of Medicago sativa ‘Bami’ with gibberellic acid on germination, seedlings growth and antioxidant enzymes activity under salinity stress. J. Hortic. Res. 2014, 22, 167–174. [Google Scholar] [CrossRef]
- Raheem, S.; Khan, J.; Gurmani, A.R.; Waqas, M.; Hamayun, M.; Khan, A.L.; Kang, S.M.; Lee, I.J. Seed priming with gibberellic acid (GA3) in sponge gourd modulated high salinity stress. Pakhtunkhwa J. Life Sci. 2014, 2, 75–86. [Google Scholar]
- Dai, L.Y.; Zhu, H.D.; Yin, K.D.; Du, J.D.; Zhang, Y.X. Seed priming mitigates the effects of saline-alkali stress in soybean seedlings. Chil. J. Agric. Res. 2017, 77, 118–125. [Google Scholar] [CrossRef]
- Hamza, J.H.; Ali, M.K.M. Effect of seed soaking with GA3 on emergence and seedling growth of corn under salt stress. Iraqi J. Agric. Sci. 2017, 48, 560–566. [Google Scholar] [CrossRef]
- Zhu, G.; An, L.; Jiao, X.; Chen, X.; Zhou, G.; McLaughlin, N. Effects of gibberellic acid on water uptake and germination of sweet sorghum seeds under salinity stress. Chil. J. Agric. Res. 2019, 79, 415–424. [Google Scholar] [CrossRef]
- Nada, H.S.; Hamza, J.H. Priming of maize seed with gibberellin (GA3) to tolerate drought stress. 2. Field emergence and its properties. Iraqi J. Des. Stud. 2019, 9, 1–12. [Google Scholar]
- Yakoubi, F.; Babou, F.Z.; Belkhodja, M. Effects of gibberellic and abscisic acids on germination and seedling growth of okra (Abelmoschus esculentus L.) under salt stress. Pertanika J. Trop. Agric. Sc. 2019, 42, 847–860. [Google Scholar]
- Samad, R.; Karmokar, J.L. Effects of gibberellic acid and kn on seed germination and accumulation of Na+ and K+ in the seedlings of Triticale-I under salinity stress. Bangladesh J. Bot. 2012, 41, 123–129. [Google Scholar] [CrossRef]
- Ye, N.; Jia, L.; Zhang, J. ABA signal in rice under stress conditions. Rice 2012, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Blumwald, E. The roles of ROS and ABA in systemic acquired acclimation. Plant Cell 2015, 27, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Sah, S.K.; Reddy, K.R.; Li, J. Abscisic acid and abiotic stress tolerance in crop plants. Front. Plant Sci. 2016, 7, 571. [Google Scholar] [CrossRef]
- Eyidogan, F.; Oz, M.T.; Yucel, M.; Oktem, H.A. Signal transduction of phytohormones under abiotic stresses. In Phytohormones and Abiotic Stress Tolerance in Plants Khan; Nazar, R., Iqbal, N., Anjum, N.A., Eds.; Springer: Berlin, Germany, 2012; pp. 1–4. [Google Scholar]
- Devinar, G.; Llanes, A.; Masciarelli, O.; Luna, V. Different relative humidity conditions combined with chloride and sulfate salinity treatments modify abscisic acid and salicylic acid levels in the halophyte Prosopis strombulifera. Plant Growth Regul. 2013, 70, 247–256. [Google Scholar] [CrossRef]
- Gurmani, A.R.; Bano, A.; Ullah, N.; Khan, H.; Jahangir, M.; Flowers, T.J. Exogenous abscisic acid (ABA) and silicon (Si) promote salinity tolerance by reducing sodium (Na+) transport and bypass flow in rice (Oryza sativa indica). Aust. J. Crop. Sci. 2013, 7, 1219–1226. [Google Scholar]
- Gurmani, A.; Bano, A.; Khan, S.; Din, J.; Zhang, J. Alleviation of salt stress by seed treatment with abscisic acid (ABA), 6-benzylaminopurine (BA) and chlormequat chloride (CCC) optimizes ion and organic matter accumulation and increases yield of rice (‘Oryza sativa’ L.). Aust. J. Crop. Sci. 2011, 5, 1278–1285. [Google Scholar]
- Gurmani, A.; Bano, A.; Salim, M. Effect of growth regulators on growth, yield and ions accumulation of rice (Oryza sativa L.) under salt stress. Pak. J. Bot. 2006, 38, 1415–1424. [Google Scholar]
- Li, X.J.; Yang, M.F.; Chen, H.; Qu, L.Q.; Chen, F.; Shen, S.H. Abscisic acid pretreatment enhances salt tolerance of rice seedlings: Proteomic evidence. Biochim. Biophys. Acta 2010, 1804, 929–940. [Google Scholar] [CrossRef]
- Liu, X.L.; Zhang, H.; Jin, Y.Y.; Wang, M.M.; Yang, H.Y.; Ma, H.Y.; Jiang, C.J.; Liang, Z.W. Abscisic acid primes rice seedlings for enhanced tolerance to alkaline stress by upregulating antioxidant defense and stress tolerance-related genes. Plant Soil 2019, 438, 39–55. [Google Scholar] [CrossRef]
- Wei, L.X.; Lv, B.S.; Wang, M.M.; Ma, H.Y.; Yang, H.Y.; Liu, X.L.; Jiang, C.J.; Liang, Z.W. Priming effect of abscisic acid on alkaline stress tolerance in rice (Oryza sativa L.) seedlings. Plant Physiol. Biochem. 2015, 90, 50–57. [Google Scholar] [CrossRef]
- Amzallag, G.N.; Lerner, H.R. Physiological adaptation of plants to environmental stresses. In Handbook for Plant and Crop Physiology; Pessarakli, M., Ed.; Marcel Dekker Inc.: Boca Raton, NY, USA, 1990; pp. 557–576. [Google Scholar]
- Fricke, W.; Akhiyarova, G.; Veselov, D.; Kudoyarova, G. Rapid and tissue-specific changes in ABA and in growth rate in response to salinity in barley leaves. J. Exp. Bot. 2004, 5, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Safari, H.; Hosseini, S.M.; Azari, A.; Rafsanjani, M.H. Effects of seed priming with ABA and SA on seed germination and seedling growth of sesame (Sesamum indicum L.) under saline condition. Aust. J. Crop Sci. 2018, 12, 1385. [Google Scholar] [CrossRef]
- Zongshuai, W.; Xiangnan, L.; Xiancan, Z.; Shengqun, L.; Fengbin, S.; Fulai, L.; Yang, W.; Xiaoning, Q.; Fahong, W.; Zhiyu, Z.; et al. Salt acclimation induced salt tolerance is enhanced by abscisic acid priming in wheat. Plant Soil Environ. 2017, 63, 307–314. [Google Scholar] [CrossRef]
- Garciarrubio, A.; Legaria, J.P.; Covarrubias, A.A. Abscisic acid inhibits germination of mature Arabidopsis seeds by limiting the availability of energy and nutrients. Planta 1997, 203, 182–187. [Google Scholar] [CrossRef]
- Nambara, E.; Okamoto, M.; Tatematsu, K.; Yano, R.; Seo, M.; Kamiya, Y. Abscisic acid and the control of seed dormancy and germination. Seed Sci. Res. 2010, 20, 55–67. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Lokhande, V.H.; Patade, V.Y.; Suprasanna, P.; Sjahril, R.; Dsouza, S.F. Comparative evaluation of hydro-, chemo-, and hormonal-priming methods for imparting salt and PEG stress tolerance in Indian mustard (Brassicajuncea L.). Acta Physiol. Plant. 2010, 32, 1135–1144. [Google Scholar] [CrossRef]
- Holbrook, N.M.; Shashidar, V.R.; James, R.A.; Munns, R. Stomatal control in tomato with ABA deficient roots: Response of grafted plants to soil drying. J. Exp. Bot. 2002, 53, 1503–1514. [Google Scholar]
- Islam, M.M.; Ye, W.; Matsushima, D.; Rhaman, M.S.; Munemasa, S.; Okuma, E.; Nakamura, Y.; Biswas, M.S.; Mano, J.I.; Murata, Y. Reactive carbonyl species function as signal mediators downstream of H2O2 production and regulate [Ca2+]cyt elevation in ABA signal pathway in Arabidopsis guard cells. Plant Cell Physiol. 2019, 60, 1146–1159. [Google Scholar] [CrossRef]
- Rhaman, M.S.; Nakamura, T.; Nakamura, Y.; Munemasa, S.; Murata, Y. The myrosinases TGG1 and TGG2 function redundantly in reactive carbonyl species signaling in Arabidopsis guard cells. Plant Cell Physiol. 61, 967–977. [CrossRef]
- Ye, W.; Ando, E.; Rhaman, M.S.; Tahjib-Ul-Arif, M.; Okuma, E.; Nakamura, Y.; Kinoshita, T.; Murata, Y. Inhibition of light-induced stomatal opening by allyl isothiocyanate does not require guard cell cytosolic Ca2+ signaling. J. Exp. Bot. 2020, 71, 2922–2932. [Google Scholar] [CrossRef] [PubMed]
- Marthandan, V.; Geetha, R.; Kumutha, K.; Renganathan, V.G.; Karthikeyan, A.; Ramalingam, J. Seed Priming: A Feasible Strategy to Enhance Drought Tolerance in Crop Plants. Int. J. Mol. Sci. 2020, 21, 8258. [Google Scholar] [CrossRef] [PubMed]
- Khan, W.; Prithiviraj, B.; Smith, D. Photosynthetic response of corn and soybean to foliar application of salicylates. J. Plant Physiol. 2003, 160, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Senaratna, T.; Touchell, D.; Bumm, E.; Dixon, K. Acetyl salicylic acid (aspirin) and salicylic acid induce multiple stress tolerance in bean and tomato plants. Plant Growth Regul. 2000, 30, 157–161. [Google Scholar] [CrossRef]
- Bastam, N.; Baninasab, B.; Ghobadi, C. Improving salt tolerance by exogenous application of salicylic acid in seedlings of pistachio. Plant Growth Regul. 2013, 69, 275–284. [Google Scholar] [CrossRef]
- Jia, C.; Zhang, L.; Liu, L.; Wang, J.; Li, C.; Wang, Q. Multiple phytohormone signalling pathways modulate susceptibility of tomato plants to Alternaria alternate f. sp. lycopersici. J. Exp. Bot. 2013, 64, 637–650. [Google Scholar] [CrossRef]
- Matilla-Vazquez, M.A.; Matilla, A.J. Ethylene: Role in plants under environmental stress. In Physiological Mechanisms and Adaptation Strategies in Plants under Changing Environment; Ahmad, P., Wani, M.R., Eds.; Springer: New York, NY, USA, 2014; Volume 2, pp. 189–222. [Google Scholar]
- Fahad, S.; Bano, A. Effect of salicylic acid on physiological and biochemical characterization of maize grown in saline area. Pak. J. Bot. 2012, 44, 1433–1438. [Google Scholar]
- Khanna, P.; Kaur, K.; Gupta, A.K. Salicylic acid induces differential anti-oxidant response in spring maize under high temperature stress. Indian J. Exp. Biol. 2016, 54, 386–393. [Google Scholar]
- Habibi, A.; Abdoli, M. Influence of salicylic acid pre-treatment on germination, vigor and growth parameters of garden cress (Lepidium sativum) seedlings under water potential loss at salinity stress. Int. Res. J. Basic Appl. Sci. 2013, 4, 1393–1399. [Google Scholar]
- Chavoushi, M.; Najafi, F.; Salimi, A.; Angaji, S.A. Improvement in drought stress tolerance of safflower during vegetative growth by exogenous application of salicylic acid and sodium nitroprusside. Ind. Crops Prod. 2019, 134, 168–176. [Google Scholar] [CrossRef]
- Shinwari, K.I.; Jan, M.; Shah, G.; Khattak, S.R.; Urehman, S.; Daud, M.K.; Naeem, R.; Jamil, M. Seed priming with salicylic acid induces tolerance against chromium (VI) toxicity in rice (Oryza sativa L.). Pak. J. Bot. 2015, 47, 161–170. [Google Scholar]
- Shatpathy, P.; Kar, M.; Dwibedi, S.K.; Dash, A. Seed priming with salicylic acid improves germination and seedling growth of rice (Oryza sativa L.) under PEG-6000 induced water stress. Int. J. Cur. Microbiol. Appl. Sci. 2018, 7, 907–924. [Google Scholar] [CrossRef]
- Pouramir-Dashtmian, F.; Khajeh-Hosseini, M.; Esfahani, M. Improving chilling tolerance of rice seedling by seed priming with salicylic acid. Arch. Agron. Soil Sci. 2014, 60, 1291–1302. [Google Scholar] [CrossRef]
- Theerakulpisut, P.; Kanawapee, N.; Panwong, B. Seed priming alleviated salt stress effects on rice seedlings by improving Na+/K+ and maintaining membrane integrity. Int. J. Plant Biol. 2016, 7, 6402. [Google Scholar] [CrossRef]
- Li, Z.; Xu, J.; Gao, Y.; Wang, C.; Guo, G.; Luo, Y.; Huang, Y.; Hu, W.; Sheteiwy, M.S.; Guan, Y.; et al. The synergistic priming effect of exogenous salicylic acid and H2O2 on chilling tolerance enhancement during maize (Zea mays L.) seed germination. Front. Plant Sci. 2017, 8, 1153. [Google Scholar] [CrossRef]
- Farooq, M.; Aziz, T.; Basra, S.M.; Cheema, M.A.; Rehman, H. Chilling tolerance in hybrid maize induced by seed priming with salicylic acid. J. Agron. Crop. Sci. 2008, 194, 161–168. [Google Scholar] [CrossRef]
- Zanganeh, R.; Jamei, R.; Rahmani, F. Impacts of seed priming with salicylic acid and sodium hydrosulfide on possible metabolic pathway of two amino acids in maize plant under lead stress. Mol. Biol. Res. Commun. 2018, 7, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.P.; Kumar, J.; Singh, M.; Singh, S.; Prasad, S.M.; Dwivedi, R.; Singh, M.P. Role of salicylic acid-seed priming in the regulation of chromium (VI) and UV-B toxicity in maize seedlings. Plant Growth Regul. 2016, 78, 79–91. [Google Scholar] [CrossRef]
- Afzal, I.; Basra, S.M.; Farooq, M.; Nawaz, A. Alleviation of salinity stress in spring wheat by hormonal priming with ABA, salicylic acid and ascorbic acid. Int. J. Agric. Biol. 2006, 8, 23–28. [Google Scholar]
- Ulfat, A.N.; Majid, S.A.; Hameed, A. Hormonal seed priming improves wheat (Triticum aestivum) field performance under drought and non-stress conditions. Pak. J. Bot. 2017, 49, 1239–1253. [Google Scholar]
- Khamseh, S.R.; Shekari, F.; Zangani, E. The effects of priming with salicylic acid on resistance to osmotic stress in germination stage of wheat. Int. J. Agric. Res. Rev. 2013, 3, 543–558. [Google Scholar]
- Azeem, M.; Abbasi, M.W.; Qasim, M.; Ali, H. Salicylic acid seed priming modulates some biochemical parameters to improve germination and seedling growth of salt stressed wheat (Triticum aestivum L.). Pak. J. Bot. 2018, 51, 385–391. [Google Scholar] [CrossRef]
- El-Shazoly, R.M.; Metwally, A.A.; Hamada, A.M. Salicylic acid or thiamin increases tolerance to boron toxicity stress in wheat. J. Plant Nutr. 2019, 42, 702–722. [Google Scholar] [CrossRef]
- Gul, F.; Arfan, M.; Shahbaz, M.; Basra, S. Salicylic acid seed priming modulates morphology, nutrient relations and photosynthetic attributes of wheat grown under cadmium stress. Int. J. Agric. Biol. 2020, 23, 197–204. [Google Scholar] [CrossRef]
- Namdari, A.; Baghbani, A. Consequences of seed priming with salicylic acid and hydro priming on smooth vetch seedling growth under water deficiency. J. Agric. Sci. 2017, 9, 259–267. [Google Scholar] [CrossRef][Green Version]
- Bahadoori, S.; Behrooz, E.; Mokhtar, H.; Surur, K.; Mosadegh, P.S.; Hassankeloo, N.T.; Alireza, G. Effects of seed priming with salicylic acid and polyamines on physiological and biochemical characteristics of okra (Abelmoschus esculentus) under low temperature stress. J. Plant Process Funct. 2016, 5, 145–156. [Google Scholar]
- Tabatabaei, S.A. Effect of salicylic acid and ascorbic acid on germination indexes and enzyme activity of sorghum seeds under drought stress. J. Stress Physiol. Biochem. 2013, 9, 32–38. [Google Scholar]
- Ghoohestani, A.; Gheisary, H.; Zahedi, S.; Dolatkhahi, A. Effect of seed priming of tomato with salicylic acid, ascorbic acid and hydrogen peroxide on germination and plantlet growth in saline conditions. Int. J. Agron. Plant Prod. 2012, 3, 700–704. [Google Scholar]
- Singh, S.K.; Singh, P.K. Effect of seed priming of tomato with salicylic acid on growth, flowering, yield and fruit quality under high temperature stress conditions. Int. J. Adv. Res. 2016, 4, 723–727. [Google Scholar]
- Rafique, N.; Raza, S.H.; Qasim, M.; Iqbal, N.A. Pre-sowing application of ascorbic acid and salicylic acid to seed of pumpkin and seedling response to salt. Pak. J. Bot. 2011, 43, 2677–2682. [Google Scholar]
- Azooz, M.M. Salt stress mitigation by seed priming with salicylic acid in two faba bean genotypes differing in salt tolerance. Int. J. Agric. Biol. 2009, 11, 343–350. [Google Scholar]
- Rehman, H.; Farooq, M.; Basra, S.M.; Afzal, I. Hormonal priming with salicylic acid improves the emergence and early seedling growth in cucumber. J. Agric. Soc. Sci. 2011, 7, 109–113. [Google Scholar]
- Soliman, M.H.; Al-Juhani, R.S.; Hashash, M.A.; Al-Juhani, F.M. Effect of seed priming with salicylic acid on seed germination and seedling growth of broad bean (Vicia faba L.). Int. J. Agric. Technol. 2016, 12, 1125–1138. [Google Scholar]
- Ahmad, F.; Iqbal, S.; Khan, M.R.; Abbas, M.W.; Ahmad, J.; Nawaz, H.; Shah, S.M.; Iqbal, S.; Ahmad, M.; Ali, M. Influence of seed priming with salicylic acid on germination and early growth of sesame. Pure Appl. Biol. 2019, 8, 1206–1213. [Google Scholar] [CrossRef]
- Mori, I.C.; Pinontoan, R.; Kawano, T.; Muto, S. Involvement of superoxide generation in salicylic acid-induced stomatal closure in Vicia faba. Plant Cell Physiol. 2001, 42, 1383–1388. [Google Scholar] [CrossRef] [PubMed]
- Khokon, M.A.; Okuma, E.I.; Hossain, M.A.; Munemasa, S.; Uraji, M.; Nakamura, Y.; Mori, I.C.; Murata, Y. Involvement of extracellular oxidative burst in salicylic acid-induced stomatal closure in Arabidopsis. Plant Cell Environ. 2011, 34, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.L.; Li, H.; Ecker, J.R. Ethylene biosynthesis and signaling networks. Plant Cell 2002, 14, S131–S151. [Google Scholar] [CrossRef]
- Montalvo, E.; Garcia, H.S.; Tovar, B.; Mata, M. Application of exogenous ethylene on postharvest ripening of refrigerated ‘Ataulfo’ mangoes. LWT-Food Sci. Technol. 2007, 40, 1466–1472. [Google Scholar] [CrossRef]
- Ma, Y.; Yang, M.; Wang, J.; Jiang, C.-Z.; Wang, Q. Application of exogenous ethylene inhibits postharvest peel browning of ‘Huangguan’ pear. Front. Plant Sci. 2017, 7, 2029. [Google Scholar] [CrossRef]
- Kim, Y.; Seo, C.; Khan, A.L.; Mun, B.G.; Shahzad, R.; Ko, J.W.; Yun, B.W.; Park, S.K.; Lee, I.J. Exo-ethylene application mitigates waterlogging stress in soybean (Glycine max L.). BMC Plant Biol. 2018, 18, 254. [Google Scholar] [CrossRef]
- Nascimento, W.M.; Cantliffe, D.J.; Huber, D.J. Ethylene evolution and endo-beta-mannanase activity during lettuce seed germination at high temperature. Sci. Agric. 2004, 61, 156–163. [Google Scholar] [CrossRef]
- Manoharlal, R.; Saiprasad, G.V.S.; Kovařík, A. Gene-specific DNA demethylation changes associates with ethylene induced germination of soybean [Glycine max (L.) Merrill]. Plant Physiol. Rep. 2019, 24, 272–277. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; Gong, D.; Gao, Y.; Pan, R.; Hu, J.; Guan, Y. Priming with methyl jasmonate alleviates polyethylene glycol-induced osmotic stress in rice seeds by regulating the seed metabolic profile. Environ. Exp. Bot. 2018, 153, 236–248. [Google Scholar] [CrossRef]
- Hassini, I.; Martinez-Ballesta, M.C.; Boughanmi, N.; Moreno, D.A.; Carvajal, M. Improvement of broccoli sprouts (Brassica oleracea L. var. italica) growth and quality by KCl seed priming and methyl jasmonate under salinity stress. Sci. Hortic. 2017, 226, 141–151. [Google Scholar] [CrossRef]
- Król, P.; Igielski, R.; Pollmann, S.; Kępczyńska, E. Priming of seeds with methyl jasmonate induced resistance to hemi-biotroph Fusarium oxysporum f. sp. lycopersici in tomato via 12-oxo-phytodienoic acid, salicylic acid, and flavonol accumulation. J. Plant Physiol. 2015, 179, 122–132. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Li, X.; Liu, A.; Chen, S. Brassinosteroids in plant tolerance to abiotic stress. J. Plant Growth Regul. 2020. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, J.; Zhang, Y.; Xie, X.J.; Knapp, A. Seed priming with brassinolide improves lucerne (Medicago sativa L.) seed germination and seedling growth in relation to physiological changes under salinity stress. Aust. J. Agric. Res. 2007, 58, 811–815. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, L.; Zeng, R.; Wang, X.; Zhang, H.; Wang, L.; Liu, S.; Wang, X.; Chen, T. Brassinosteroid priming improves peanut drought tolerance via eliminating inhibition on genes in photosynthesis and hormone signaling. Genes 2020, 11, 919. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).