Phytotoxic Effects of Plant Essential Oils: A Systematic Review and Structure-Activity Relationship Based on Chemometric Analyses
Abstract
1. Introduction
2. Materials and Methods
3. Phytotoxic EOs Derived from Plants Rich in Monoterpenes
4. Monoterpene-Rich EO-Allelopathy Correlation
5. Phytotoxic EOs Derived from Plants Rich in Sesquiterpenes
6. Sesquiterpene-Rich EO-Allelopathy Correlation
7. Phytotoxic EOs Derived from Plants Rich in Non-Terpenoids
8. Structure-Activity Relationship Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jugulam, M. Biology, Physiology and Molecular Biology of Weeds; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Rice, E. Allelopathy, 2nd ed.; Academic Press: New York, NY, USA, 1984. [Google Scholar]
- Abd El-Gawad, A.M.; Elshamy, A.I.; El Gendy, A.E.-N.; Gaara, A.; Assaeed, A.M. Volatiles profiling, allelopathic activity, and antioxidant potentiality of Xanthium strumarium leaves essential oil from Egypt: Evidence from chemometrics analysis. Molecules 2019, 24, 584. [Google Scholar] [CrossRef] [PubMed]
- Assaeed, A.; Elshamy, A.; El Gendy, A.E.-N.; Dar, B.; Al-Rowaily, S.; Abd El-Gawad, A. Sesquiterpenes-rich essential oil from above ground parts of Pulicaria somalensis exhibited antioxidant activity and allelopathic effect on weeds. Agronomy 2020, 10, 399. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O. Biological activities of essential oils: From plant chemoecology to traditional healing systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef]
- Abd El-Gawad, A.M.; Elshamy, A.; El Gendy, A.E.-N.; Al-Rowaily, S.L.; Assaeed, A.M. Preponderance of oxygenated sesquiterpenes and diterpenes in the volatile oil constituents of Lactuca serriola L. revealed antioxidant and allelopathic activity. Chem. Biodivers. 2019, 16, e1900278. [Google Scholar] [CrossRef] [PubMed]
- Elshamy, A.; Abd El-Gawad, A.M.; El-Amier, Y.A.; El Gendy, A.; Al-Rowaily, S. Interspecific variation, antioxidant and allelopathic activity of the essential oil from three Launaea species growing naturally in heterogeneous habitats in Egypt. Flavour Fragr. J. 2019, 34, 316–328. [Google Scholar] [CrossRef]
- Abd El-Gawad, A.M.; El-Amier, Y.A.; Bonanomi, G. Essential oil composition, antioxidant and allelopathic activities of Cleome droserifolia (Forssk.) Delile. Chem. Biodivers. 2018, 15, e1800392. [Google Scholar] [CrossRef]
- Abd El-Gawad, A.M.; El-Amier, Y.A.; Bonanomi, G. Allelopathic activity and chemical composition of Rhynchosia minima (L.) DC. essential oil from Egypt. Chem. Biodivers. 2018, 15, e1700438. [Google Scholar] [CrossRef]
- Deng, W.; Liu, K.; Cao, S.; Sun, J.; Zhong, B.; Chun, J. Chemical composition, antimicrobial, antioxidant, and antiproliferative properties of grapefruit essential oil prepared by molecular distillation. Molecules 2020, 25, 217. [Google Scholar] [CrossRef]
- Elshamy, A.I.; Ammar, N.M.; Hassan, H.A.; Al-Rowaily, S.L.; Raga, T.R.; El Gendy, A.; Abd El-Gawad, A.M. Essential oil and its nanoemulsion of Araucaria heterophylla resin: Chemical characterization, anti-inflammatory, and antipyretic activities. Ind. Crop. Prod. 2020, 148, 112272. [Google Scholar] [CrossRef]
- Arunachalam, K.; Balogun, S.O.; Pavan, E.; de Almeida, G.V.B.; de Oliveira, R.G.; Wagner, T.; Cechinel Filho, V.; de Oliveira Martins, D.T. Chemical characterization, toxicology and mechanism of gastric antiulcer action of essential oil from Gallesia integrifolia (Spreng.) Harms in the in vitro and in vivo experimental models. Biomed. Pharmacother. 2017, 94, 292–306. [Google Scholar] [CrossRef]
- Damtie, D.; Braunberger, C.; Conrad, J.; Mekonnen, Y.; Beifuss, U. Composition and hepatoprotective activity of essential oils from Ethiopian thyme species (Thymus serrulatus and Thymus schimperi). J. Essent. Oil Res. 2019, 31, 120–128. [Google Scholar] [CrossRef]
- Zhang, J.; An, M.; Wu, H.; Li Liu, D.; Stanton, R. Chemical composition of essential oils of four Eucalyptus species and their phytotoxicity on silverleaf nightshade (Solanum elaeagnifolium Cav.) in Australia. Plant. Growth Regul. 2012, 68, 231–237. [Google Scholar] [CrossRef]
- Abd El-Gawad, A.M.; Elshamy, A.; El-Amier, Y.A.; El Gendy, A.; Al-Barati, S.; Dar, B.; Al-Rowaily, S.; Assaeed, A. Chemical composition variations, allelopathic, and antioxidant activities of Symphyotrichum squamatum (Spreng.) Nesom essential oils growing in heterogeneous habitats. Arab. J. Chem. 2020, 13, 237–4245. [Google Scholar] [CrossRef]
- Bozok, F.; Ulukanli, Z. Volatiles from the aerial parts of east Mediterranean clary sage: Phytotoxic activity. J. Essent. Oil Bear. Plants 2016, 19, 1192–1198. [Google Scholar] [CrossRef]
- Pinheiro, P.F.; Costa, A.V.; Tomaz, M.A.; Rodrigues, W.N.; Fialho Silva, W.P.; Moreira Valente, V.M. Characterization of the Essential Oil of Mastic Tree from Different Biomes and its Phytotoxic Potential on Cobbler’s Pegs. J. Essent. Oil Bear. Plants 2016, 19, 972–979. [Google Scholar] [CrossRef]
- Agnieszka, S.; Magdalena, R.; Jan, B.; Katarzyna, W.; Malgorzata, B.; Krzysztof, H.; Danuta, K. Phytotoxic effect of fiber hemp essential oil on germination of some weeds and crops. J. Essent. Oil Bear. Plants 2016, 19, 262–276. [Google Scholar] [CrossRef]
- Bali, A.S.; Batish, D.R.; Singh, H.P.; Kaur, S.; Kohli, R.K. Chemical characterization and phytotoxicity of foliar volatiles and essential oil of Callistemon viminalis. J. Essent. Oil Bear. Plants 2017, 20, 535–545. [Google Scholar] [CrossRef]
- Fouad, R.; Bousta, D.; Lalami, A.E.O.; Chahdi, F.O.; Amri, I.; Jamoussi, B.; Greche, H. Chemical composition and herbicidal effects of essential oils of Cymbopogon citratus (DC) Stapf, Eucalyptus cladocalyx, Origanum vulgare L and Artemisia absinthium L. cultivated in Morocco. J. Essent. Oil Bear. Plants 2015, 18, 112–123. [Google Scholar] [CrossRef]
- Poonpaiboonpipat, T.; Pangnakorn, U.; Suvunnamek, U.; Teerarak, M.; Charoenying, P.; Laosinwattana, C. Phytotoxic effects of essential oil from Cymbopogon citratus and its physiological mechanisms on barnyardgrass (Echinochloa crus-galli). Ind. Crop. Prod. 2013, 41, 403–407. [Google Scholar] [CrossRef]
- Kordali, S.; Cakir, A.; Ozer, H.; Cakmakci, R.; Kesdek, M.; Mete, E. Antifungal, phytotoxic and insecticidal properties of essential oil isolated from Turkish Origanum acutidens and its three components, carvacrol, thymol and p-cymene. Bioresour. Technol. 2008, 99, 8788–8795. [Google Scholar] [CrossRef]
- Verdeguer, M.; Blázquez, M.A.; Boira, H. Phytotoxic effects of Lantana camara, Eucalyptus camaldulensis and Eriocephalus africanus essential oils in weeds of Mediterranean summer crops. Biochem. Syst. Ecol. 2009, 37, 362–369. [Google Scholar] [CrossRef]
- Ulukanli, Z.; Çenet, M.; Öztürk, B.; Bozok, F.; Karabörklü, S.; Demirci, S.C. Chemical characterization, phytotoxic, antimicrobial and insecticidal activities of Vitex agnus-castus’ essential oil from East Mediterranean Region. J. Essent. Oil Bear. Plants 2015, 18, 1500–1507. [Google Scholar] [CrossRef]
- Kashkooli, A.B.; Saharkhiz, M.J. Essential oil compositions and natural herbicide activity of four Denaei Thyme (Thymus daenensis Celak.) ecotypes. J. Essent. Oil Bear. Plants 2014, 17, 859–874. [Google Scholar] [CrossRef]
- Ulukanli, Z.; Cenet, M.; Ince, H.; Yilmaztekin, M. Antimicrobial and herbicidal activities of the essential oil from the Mediterranean Thymus eigii. J. Essent. Oil Bear. Plants 2018, 21, 214–222. [Google Scholar] [CrossRef]
- Ulukanli, Z.; Bozok, F.; Cenet, M.; Ince, H.; Demirci, S.C.; Sezer, G. Secondary metabolites and bioactivities of Thymbra spicata var. spicata in Amanos mountains. J. Essent. Oil Bear. Plants 2016, 19, 1754–1761. [Google Scholar] [CrossRef]
- Bozok, F. Herbicidal Activity of Nepeta flavida Essential Oil. J. Essent. Oil Bear. Plants 2018, 21, 1687–1693. [Google Scholar] [CrossRef]
- Silva, E.R.; Overbeck, G.E.; Soares, G.L.G. Phytotoxicity of volatiles from fresh and dry leaves of two Asteraceae shrubs: Evaluation of seasonal effects. S. Afr. J. Bot. 2014, 93, 14–18. [Google Scholar] [CrossRef]
- Da Silva, E.R.; Lazarotto, D.C.; Pawlowski, A.; Schwambach, J.; Soares, G.L.G. Antioxidant Evaluation of Baccharis patens and Baccharis psiadioides Essential Oils. J. Essent. Oil Bear. Plants 2018, 21, 485–492. [Google Scholar] [CrossRef]
- Mancini, E.; Arnold, N.A.; De Martino, L.; De Feo, V.; Formisano, C.; Rigano, D.; Senatore, F. Chemical composition and phytotoxic effects of essential oils of Salvia hierosolymitana Boiss. and Salvia multicaulis Vahl. var. simplicifolia Boiss. growing wild in Lebanon. Molecules 2009, 14, 4725–4736. [Google Scholar] [CrossRef]
- Kaur, S.; Singh, H.P.; Mittal, S.; Batish, D.R.; Kohli, R.K. Phytotoxic effects of volatile oil from Artemisia scoparia against weeds and its possible use as a bioherbicide. Ind. Crop. Prod. 2010, 32, 54–61. [Google Scholar] [CrossRef]
- Aragão, F.; Palmieri, M.; Ferreira, A.; Costa, A.; Queiroz, V.; Pinheiro, P.; Andrade-Vieira, L. Phytotoxic and cytotoxic effects of Eucalyptus essential oil on lettuce (Lactuca sativa L.). Allelopath. J. 2015, 35, 259–272. [Google Scholar]
- Pinheiro, P.F.; Costa, A.V.; Alves, T.d.A.; Galter, I.N.; Pinheiro, C.A.; Pereira, A.F.; Oliveira, C.M.R.; Fontes, M.M.P. Phytotoxicity and cytotoxicity of essential oil from leaves of Plectranthus amboinicus, carvacrol, and thymol in plant bioassays. J. Agric. Food Chem. 2015, 63, 8981–8990. [Google Scholar] [CrossRef]
- Ulukanli, Z.; Karabörklü, S.; Bozok, F.; Burhan, A.; Erdogan, S.; Cenet, M.; KARAASLAN, M.G. Chemical composition, antimicrobial, insecticidal, phytotoxic and antioxidant activities of Mediterranean Pinus brutia and Pinus pinea resin essential oils. Chin. J. Nat. Med. 2014, 12, 901–910. [Google Scholar] [CrossRef]
- Amri, I.; Gargouri, S.; Hamrouni, L.; Hanana, M.; Fezzani, T.; Jamoussi, B. Chemical composition, phytotoxic and antifungal activities of Pinus pinea essential oil. J. Pest. Sci. 2012, 85, 199–207. [Google Scholar] [CrossRef]
- Ulukanli, Z.; Karabörklü, S.; Bozok, F.; Çenet, M.; Öztürk, B.; Balcilar, M. Antimicrobial, insecticidal and phytotoxic activities of Cotinus coggyria Scop. essential oil (Anacardiaceae). Nat. Prod. Res. 2014, 28, 2150–2157. [Google Scholar] [CrossRef]
- Saharkhiz, M.J.; Smaeili, S.; Merikhi, M. Essential oil analysis and phytotoxic activity of two ecotypes of Zataria multiflora Boiss. growing in Iran. Nat. Prod. Res. 2010, 24, 1598–1609. [Google Scholar] [CrossRef]
- Mahdavikia, F.; Saharkhiz, M.J. Phytotoxic activity of essential oil and water extract of peppermint (Mentha× piperita L. CV. Mitcham). J. Appl. Res. Med. Aromat. Plants 2015, 2, 146–153. [Google Scholar] [CrossRef]
- De Almeida, L.F.R.; Frei, F.; Mancini, E.; De Martino, L.; De Feo, V. Phytotoxic activities of Mediterranean essential oils. Molecules 2010, 15, 4309–4323. [Google Scholar] [CrossRef]
- Zahed, N.; Hosni, K.; Ben Brahim, N.; Kallel, M.; Sebei, H. Allelopathic effect of Schinus molle essential oils on wheat germination. Acta Physiol. Plant. 2010, 32, 1221–1227. [Google Scholar] [CrossRef]
- De Oliveira, M.S.; da Costa, W.A.; Pereira, D.S.; Botelho, J.R.S.; de Alencar Menezes, T.O.; de Aguiar Andrade, E.H.; da Silva, S.H.M.; da Silva Sousa Filho, A.P.; de Carvalho Junior, R.N. Chemical composition and phytotoxic activity of clove (Syzygium aromaticum) essential oil obtained with supercritical CO2. J. Supercrit. Fluid. 2016, 118, 185–193. [Google Scholar] [CrossRef]
- Verdeguer, M.; García-Rellán, D.; Boira, H.; Pérez, E.; Gandolfo, S.; Blázquez, M.A. Herbicidal activity of Peumus boldus and Drimys winterii essential oils from Chile. Molecules 2011, 16, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Kim, J. Phytotoxic and antimicrobial activities and chemical analysis of leaf essential oil from Agastache rugosa. J. Plant. Biol. 2008, 51, 276–283. [Google Scholar] [CrossRef]
- Grichi, A.; Nasr, Z.; Khouja, M.L. Phytotoxic effects of essential oil from Eucalyptus lehmanii against weeds and its possible use as a bioherbicide. Bull. Environ. Pharmacol. Life Sci. 2016, 5, 17–23. [Google Scholar]
- Salamci, E.; Kordali, S.; Kotan, R.; Cakir, A.; Kaya, Y. Chemical compositions, antimicrobial and herbicidal effects of essential oils isolated from Turkish Tanacetum aucheranum and Tanacetum chiliophyllum var. chiliophyllum. Biochem. Syst. Ecol. 2007, 35, 569–581. [Google Scholar] [CrossRef]
- Singh, R.; Ahluwalia, V.; Singh, P.; Kumar, N.; Prakash Sati, O.; Sati, N. Antifungal and phytotoxic activity of essential oil from root of Senecio amplexicaulis Kunth.(Asteraceae) growing wild in high altitude-Himalayan region. Nat. Prod. Res. 2016, 30, 1875–1879. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, V.; Sisodia, R.; Walia, S.; Sati, O.P.; Kumar, J.; Kundu, A. Chemical analysis of essential oils of Eupatorium adenophorum and their antimicrobial, antioxidant and phytotoxic properties. J. Pest. Sci. 2014, 87, 341–349. [Google Scholar] [CrossRef]
- Pawlowski, Â.; Kaltchuk-Santos, E.; Brasil, M.; Caramão, E.; Zini, C.; Soares, G. Chemical composition of Schinus lentiscifolius March. essential oil and its phytotoxic and cytotoxic effects on lettuce and onion. S. Afr. J. Bot. 2013, 88, 198–203. [Google Scholar] [CrossRef]
- Abd El-Gawad, A.M. Chemical constituents, antioxidant and potential allelopathic effect of the essential oil from the aerial parts of Cullen plicata. Ind. Crops Prod. 2016, 80, 36–41. [Google Scholar] [CrossRef]
- Zhu, X.; Han, C.; Gao, T.; Shao, H. Chemical composition, phytotoxic and antimicrobial activities of the essential oil of Scutellaria strigillosa Hemsley. J. Essent. Oil Bear. Plants 2016, 19, 664–670. [Google Scholar] [CrossRef]
- Razavi, S.M.; Narouei, M.; Majrohi, A.A.; Chamanabad, H.R.M. Chemical constituents and phytotoxic activity of the essential oil of Acroptilon repens (L.) Dc from Iran. J. Essent. Oil Bear. Plants 2012, 15, 943–948. [Google Scholar] [CrossRef]
- El Ayeb-Zakhama, A.; Ben Salem, S.; Sakka-Rouis, L.; Flamini, G.; Ben Jannet, H.; Harzallah-Skhiri, F. Chemical composition and phytotoxic effects of essential oils obtained from Ailanthus altissima (Mill.) swingle cultivated in Tunisia. Chem. Biodivers. 2014, 11, 1216–1227. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Gawad, A.; El Gendy, A.; El-Amier, Y.; Gaara, A.; Omer, S.; Al-Rowaily, S.; Assaeed, A.; Al-Rashed, S.; Elshamy, A. Essential oil of Bassia muricata: Chemical characterization, antioxidant activity, and allelopathic effect on the weed Chenopodium murale. Saudi J. Biol. Sci. 2020, 27, 1900–1906. [Google Scholar] [CrossRef] [PubMed]
- Martino, L.D.; Formisano, C.; Mancini, E.; Feo, V.D.; Piozzi, F.; Rigano, D.; Senatore, F. Chemical composition and phytotoxic effects of essential oils from four Teucrium species. Nat. Prod. Commun. 2010, 5, 1969–1976. [Google Scholar] [CrossRef] [PubMed]
- Razavi, S.M.; Nejad-Ebrahimi, S. Phytochemical analysis and allelopathic activity of essential oils of Ecballium elaterium A. Richard growing in Iran. Nat. Prod. Res. 2010, 24, 1704–1709. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, S.; Atici, Ö.; Esim, N.; Mete, E. Essential oils of catmint (Nepeta meyeri Benth.) induce oxidative stress in early seedlings of various weed species. Acta Physiol. Plant. 2011, 33, 943–951. [Google Scholar] [CrossRef]
- Kordali, S.; Tazegul, A.; Cakir, A. Phytotoxic effects of Nepeta meyeri Benth. extracts and essential oil on seed germinations and seedling growths of four weed species. Rec. Nat. Prod. 2015, 9, 404–418. [Google Scholar]
- Saharkhiz, M.J.; Zadnour, P.; Kakouei, F. Essential oil analysis and phytotoxic activity of catnip (Nepeta cataria L.). Am. J. Essent. Oils Nat. Prod. 2016, 4, 40–45. [Google Scholar]
- Bozok, F.; Cenet, M.; Sezer, G.; Ulukanli, Z. Essential oil and bioherbicidal potential of the aerial parts of Nepeta nuda subsp. albiflora (Lamiaceae). J. Essent. Oil Bear. Plants 2017, 20, 148–154. [Google Scholar] [CrossRef]
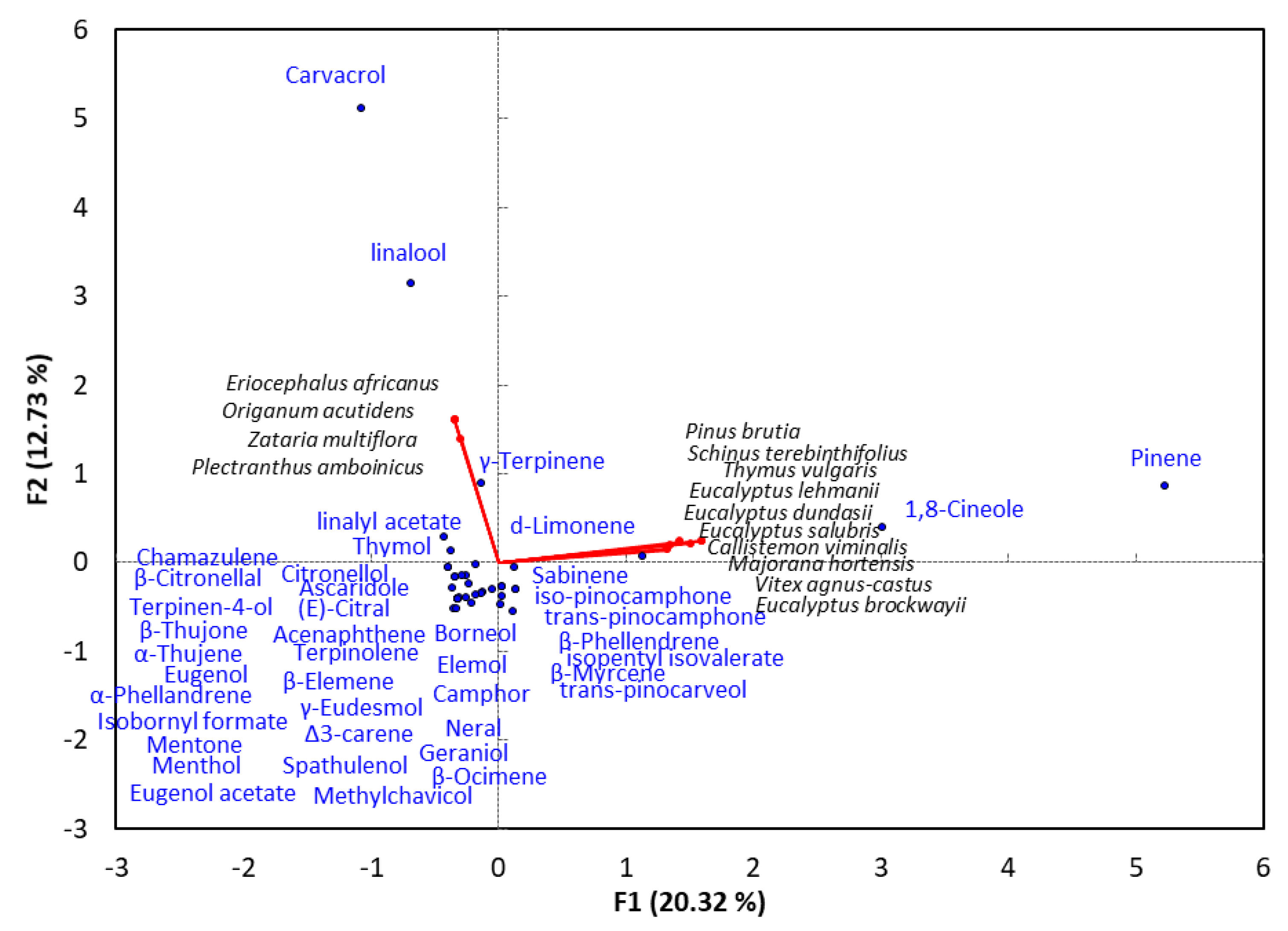
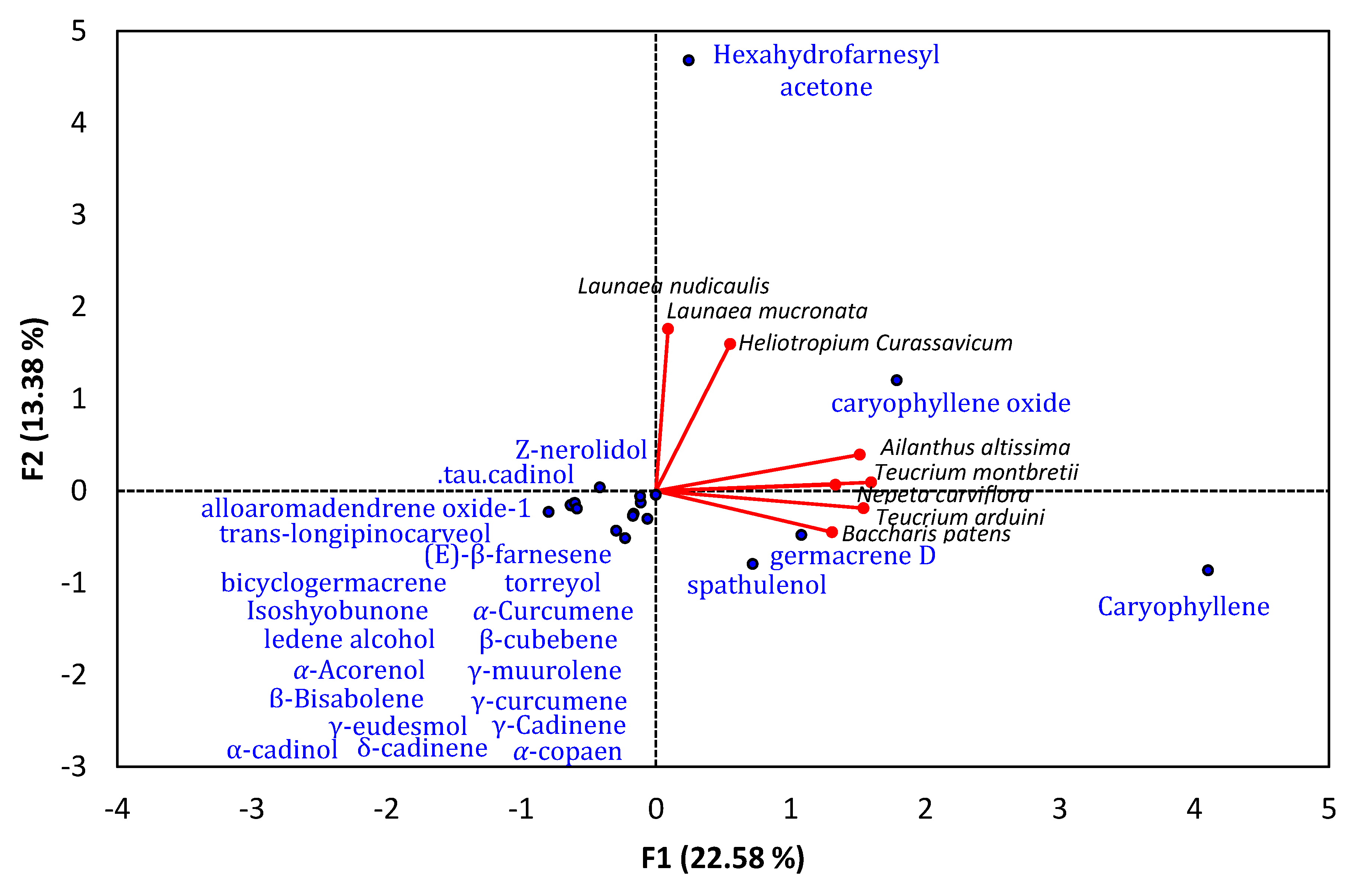
| Plant Name | Main Monoterpenoid Compounds | Phytotoxic against | Reference |
|---|---|---|---|
| Euphorbia heterophylla | 1,8-cineole, camphor, | Cenchrus echinatus * | [6] |
| Symphyotrichum squamatum | β-pinene | Bidens pilosa * | [15] |
| Salvia sclarea | l-linalool, linalyl acetate, α-terpineol, and geraniol | Lactuca sativa, Lepidium sativum, and Portulaca oleracea * | [16] |
| Schinus terebinthifolius | 3-carene, α-pinene, limonene, and β-pinene | Bidens pilosa * | [17] |
| Cannabis sativa | myrcene, terpinolene, and (E)-β-ocimene | Avena sativa, Zea mays, Brassica oleracea, Avena fatua *, Bromus secalinus *, Echinochloa crus-galli *, Amaranthus retroflexus *, Centaurea cyanus * | [18] |
| Callistemon viminalis | 1,8-cineole α-pinene, and d-limonene | Bidens pilosa *, Cassia occidentalis *, Echinochloa crus-galli *, and Phalaris minor * | [19] |
| Cymbopogon citratus | neral, geranial, and α-pinene | Sinapis arvensis * | [20] |
| Eucalyptus cladocalyx | 1.8-cineole, and p-cymene | ||
| Origanum vulgare | carvacrol, γ-terpinene, and p-cymene | ||
| Artemisia absinthium | β-thujone, and linalool | ||
| Cymbopogon citratus | neral, geranial, and β-myrcene | Echinochloa crus-galli * | [21] |
| Origanum acutidens | carvacrol, p-cymene, linalool acetate | Amaranthus retroflexus *, Chenopodium album *, and Rumex crispus * | [22] |
| Eriocephalus africanus | carvacrol, p-cymene, linalool acetate | Amaranthus hybridus * and Portulaca oleracea * | [23] |
| Vitex agnus-castus | 1,8-cineole, sabinene, and α-pinene | Lactuca sativa and Lepidium sativum | [24] |
| Thymus daenensis | thymol, carvacrol, and p-cymene | Amaranthus retroflexus *, Avena fatua *, Datura stramonium *, and Lepidium sativum | [25] |
| Thymus eigii | thymol, carvacrol, p-cymene, γ-terpinene, and borneol | Lactuca sativa, Lepidium sativum, and Portulaca oleracea * | [26] |
| Thymbra spicata | carvacrol, γ-terpinene, p-cymene | Triticum aestivum, Zea mays, Lactuca sativa, Lepidium sativum, and Portulaca oleracea * | [27] |
| Nepeta flavida | linalool, 1,8-cineole, and sabinene | Lepidium sativum, Raphanus sativus, and Eruca sativa | [28] |
| Heterothalamus psiadioides | β-pinene, δ3-carene, and limonene | Lactuca sativa and Allium cepa | [29,30] |
| Salvia hierosolymitana | α-pinene, myrtenol, and sabinyl acetate, | Raphanus sativus and Lepidium sativum | [31] |
| Artemisia scoparia | p-cymene, β-myrcene, and (+)-limonene | Achyranthes aspera, Cassia occidentalis *, Parthenium hysterophorus *, Echinochloa crus-galli *, and Ageratum conyzoides * | [32] |
| Eucalyptus grandis | α-pinene, γ-terpinene, and p-cymene | Lactuca sativa | [33] |
| Eucalyptus citriodora | β-citronellal, geraniol, and citronellol | ||
| Plectranthus amboinicus | carvacrol | Lactuca sativa and Sorghum bicolor | [34] |
| Pinus brutia | α-pinene, and β-pinene | Lactuca sativa, Lepidium sativum, and Portulaca oleracea * | [35] |
| Pinus pinea | limonene, α-pinene, and β-pinene | Sinapis arvensis *, Lolium rigidum *, and Raphanus raphanistrum * | [36] |
| Cotinus coggyria Scop. | α-pinene, limonene, and β-myrcene | Silybum marianum *, and Portulaca oleracea * | [37] |
| Zataria multiflora | carvacrol, linalool and p-cymene | Hordeum spontaneum *, Secale cereale *, and Amaranthus retroflexus *, and Cynodon dactylon * | [38] |
| Mentha × piperita | menthol, mentone, and menthofuran | Lycopersicon esculentum, Raphanus sativus, Convolvulus arvensis *, Portulaca oleracea *, and Echinochloa colonum * | [39] |
| Hyssopus officinalis | β-pinene, iso-pinocamphone, and, trans-pinocamphone | Raphanus sativus, Lactuca sativa, and Lepidium sativum | [40] |
| Lavandula angustifolia | β-pinene, iso-pinocamphone, and trans-pinocamphone | ||
| Majorana hortensis | 1,8-cineole, β-phellandrene, and α-pinene | ||
| Melissa officinalis | (-)-citronellal, carvacrol, and citronellol | ||
| Ocimum basilicum | linalol, and borneol | ||
| Origanum vulgare | o-cymene, carvacrol, and linalyl acetate | ||
| Salvia officinalis | trans-thujone, camphor, and borneol | ||
| Thymus vulgaris | o-cymene, and α-pinene | ||
| Verbena officinalis | isobornyl formate , and (E)-citral | ||
| Shinus molle | β-phellendrene, α-phellendrene, and myrcene | Triticum aestivum | [41] |
| Syzygium aromaticum | eugenol, and eugenol acetate | Mimosa pudic *a and Senna obtusifolia * | [42] |
| Peumus boldus | ascaridole, p-cymene and 1,8-cineole | Amaranthus hybridus * and Portulaca oleracea * | [43] |
| Drimys winterii | terpinen-4-ol, γ-terpinene, and sabinene | ||
| Agastache rugosa | d-limonene, and linalool | Majorana hortensis *, Trifolium repens *, Rudbeckia hirta, Chrysanthemum zawadskii, Melissa officinalis *, Taraxacum platycarpum *, and Tagetes patula | [44] |
| Eucalyptus lehmanii | 1,8-cineole, α-thujene, and α-pinene | Sinapis arvensis *, Diplotaxis harra *, Trifolium campestre *, Desmazeria rigida *, and Phalaris canariensis * | [45] |
| Tanacetum aucheranum | 1,8-cineole, camphor, and terpinen-4-ol | Amaranthus retroflexus *, Chenopodium album *, and Rumex crispus * | [46] |
| Tanacetum chiliophyllum | camphor, 1,8-cineole and borneol | ||
| Heterothalamus psiadioides | β-pinene, δ3-carene, and limonene | Lactuca sativa and Allium cepa | [29] |
| Baccharis patens | linalool | ||
| Senecio amplexicaulis | α-phellandrene, o-cymene and β-ocimene | Phalaris minor * and Triticum aestivum | [47] |
| Eucalyptus salubris | 1,8-cineole, α-pinene and p-cymene | Solanum elaeagnifolium * | [14] |
| Eucalyptus dundasii | 1,8-cineole, α-pinene and trans-pinocarveol | ||
| Eucalyptus spathulata | 1,8-cineole and α-pinene | ||
| Eucalyptus brockwayii | α-pinene, 1,8-cineole and isopentyl isovalerate | ||
| Carum carvi | estragole, limonene, and β-pinene | Raphanus sativus, Lactuca sativa, and Lepidium sativum | [40] |
| Plant Name | Major Sesquiterpenes Compounds | Phytotoxic Against | Reference |
|---|---|---|---|
| Lactuca serriola | isoshyobunone, and alloaromadendrene oxide-1 | Bidens pilosa * | [7] |
| Launaea mucronata | hexahydrofarnesyl acetone and (-)-spathulenol | Portulaca oleracea * | [6] |
| Launaea nudicaulis | hexahydrofarnesyl acetone and γ-gurjunen epoxide (2) | ||
| Launaea spinosa | α-acorenol, trans-longipinocarveol, and γ-eudesmol | ||
| Heliotropium curassavicum | Hexahydrofarnesyl acetone, (-)-caryophyllene oxide, farnesyl acetone | Chenopodium murale * | [6] |
| Xanthium strumarium | α-eudesmol, (-)-spathulenol, and ledene alcohol | Bidens pilosa * | [3] |
| Cullen plicata | (−)-caryophyllene oxide, z-nerolidol, tau.cadinol and α-cadinol | Bidens pilosa * and Urospermum picroides * | [50] |
| Scutellaria strigillosa | germacrene D, bicyclogermacrene, and β-caryophyllene | Amaranthus retroflexus * and Poa annua * | [51] |
| Acroptilon repens | caryophyllene oxide, β-cubebene, β-caeyophyllen, and α-copaen | Amaranthus retroflexus * and Cardaria draba * | [52] |
| Lantana camara | α-curcumene, β-caryophyllene, and γ-curcumene | Amaranthus hybridus * and Portulaca oleracea * | [23] |
| Eucalyptus camaldulensis | spathulenol, and isobicyclogermacrenal | ||
| Eupatorium adenophorum | γ-cadinene, γ-muurolene, and 3-acetoxyamorpha-4,7(11)-diene-8-one | Phalaris minor * and Triticum aestivum * | |
| Baccharis patens | β-caryophyllene, and spathulenol | Lactuca sativa and Allium cepa | [29] |
| Salvia multicaulis | α-Copaene, β-caryophyllene, and aromadendrene | Raphanus sativus and Lepidium sativum | [40] |
| Teucrium arduini | caryophyllene, caryophyllene oxide, germacrene D, and spathulenol | ||
| Teucrium maghrebinum | germacrene d, δ-cadinene, γ-cadinene, and caryophyllene | ||
| Teucrium polium | caryophyllene, torreyol, and α-cadinol | ||
| Teucrium montbretii | carvacrol, caryophyllene, and caryophyllene oxide | ||
| Nepeta curviflora | β-caryophyllene, caryophyllene oxide, and (E)-β-farnesene | [31] | |
| Nepeta nuda | β-bisabolene | ||
| Ailanthus altissima | β-caryophyllene, (Z)-caryophyllene, and germacrene D, | Lactuca sativa | [53] |
| Schinus lentiscifolius | δ-cadinene, α-cadinol, and β-caryophyllene | [49] | |
| Pulicaria somalensis | Juniper camphor (24.7%), α-sinensal (7.7%), 6-epi-shyobunol (6.6%), and α-zingiberene (5.8%) | Dactyloctenium aegyptium * and Bidens pilosa * | [4] |
| Bassia muricata | hexahydrofarnesyl acetone, and α-gurjunene | Chenopodium murale * | [54] |
| Plant Name | Main Components | Major Compounds | Phytotoxic Against | Reference |
|---|---|---|---|---|
| Nepeta nuda | Iridoids | 4a-α,7-α,7a-β-nepetalactone, 2(1h)-naphthalenone, and octahydro-8a-methyl-trans- | Triticum aestivum, Raphanus sativus, Lactuca sativa, Lepidium sativum, and Portulaca oleracea * | [60] |
| Nepeta cataria | Iridoids | 4a-α,7-α,7a-β-nepetalactone and 4a-α,7-β,7a-α-nepetalactone | Hordeum spontaneum *, Taraxacum officinale *, Avena fatua *, and Lipidium sativum | [59] |
| Nepeta meyeri | Iridoids | 4a-α,7-α,7a-β-nepetalactone and 4a-α,7-β,7a-α-nepetalactone | Amaranthus retroflexus *, Bromus danthoniae *, Bromus intermedius *, Chenopodium album *, Cynodon dactylon *, Lactuca serriola *, Portulaca oleracea *, Cirsium arvense *, and Sinapsis arvensis * | [57,58] |
| Ecballium elaterium | Phenolics and hydrocarbons | e-anethol, octyl octanoate, 3-(6,6-dimethyl-5-oxohept-2-enyl)-cyclohexanone, and tetracosane | Lactuca sativa | [56] |
| Pimpinella anisum | Non-terpenoidial phenols | cis-anethole | Raphanus sativus, Lactuca sativa, and Lepidium sativum | [40] |
| Foeniculum vulgare | Non-terpenoidial phenols | cis-anethole |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd-ElGawad, A.M.; El Gendy, A.E.-N.G.; Assaeed, A.M.; Al-Rowaily, S.L.; Alharthi, A.S.; Mohamed, T.A.; Nassar, M.I.; Dewir, Y.H.; Elshamy, A.I. Phytotoxic Effects of Plant Essential Oils: A Systematic Review and Structure-Activity Relationship Based on Chemometric Analyses. Plants 2021, 10, 36. https://doi.org/10.3390/plants10010036
Abd-ElGawad AM, El Gendy AE-NG, Assaeed AM, Al-Rowaily SL, Alharthi AS, Mohamed TA, Nassar MI, Dewir YH, Elshamy AI. Phytotoxic Effects of Plant Essential Oils: A Systematic Review and Structure-Activity Relationship Based on Chemometric Analyses. Plants. 2021; 10(1):36. https://doi.org/10.3390/plants10010036
Chicago/Turabian StyleAbd-ElGawad, Ahmed M., Abd El-Nasser G. El Gendy, Abdulaziz M. Assaeed, Saud L. Al-Rowaily, Abdullah S. Alharthi, Tarik A. Mohamed, Mahmoud I. Nassar, Yaser H. Dewir, and Abdelsamed I. Elshamy. 2021. "Phytotoxic Effects of Plant Essential Oils: A Systematic Review and Structure-Activity Relationship Based on Chemometric Analyses" Plants 10, no. 1: 36. https://doi.org/10.3390/plants10010036
APA StyleAbd-ElGawad, A. M., El Gendy, A. E.-N. G., Assaeed, A. M., Al-Rowaily, S. L., Alharthi, A. S., Mohamed, T. A., Nassar, M. I., Dewir, Y. H., & Elshamy, A. I. (2021). Phytotoxic Effects of Plant Essential Oils: A Systematic Review and Structure-Activity Relationship Based on Chemometric Analyses. Plants, 10(1), 36. https://doi.org/10.3390/plants10010036











