Responses of Plant Bud Bank Characteristics to the Enclosure in Different Desertified Grasslands on the Tibetan Plateau
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Sampling Method
2.3. Experimental Method
2.4. Statistical Analyses
3. Results
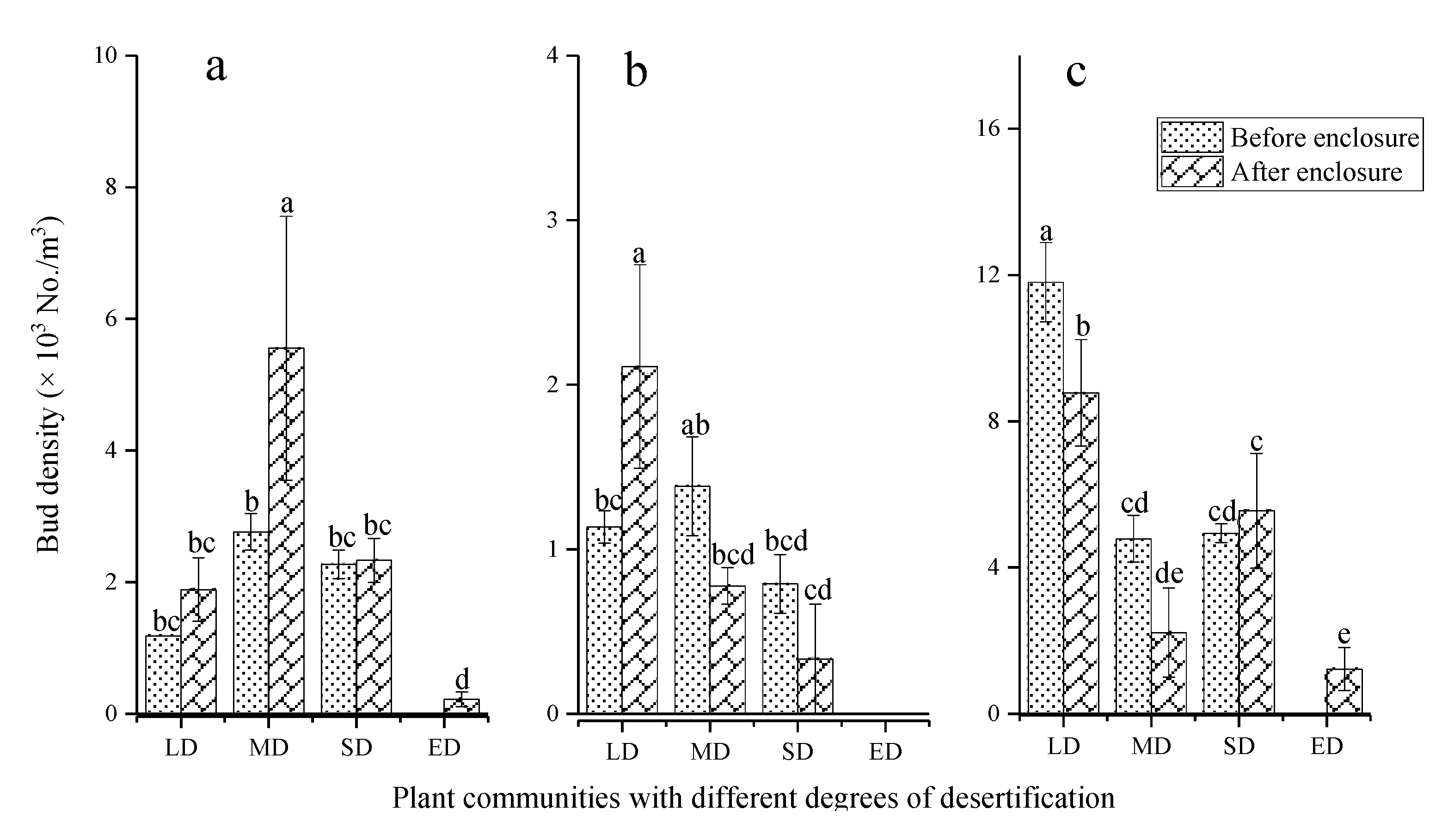
3.1. The Effect of Enclosure on the Bud Bank Distribution
3.2. The Effect of Enclosure on the Bud Bank Composition
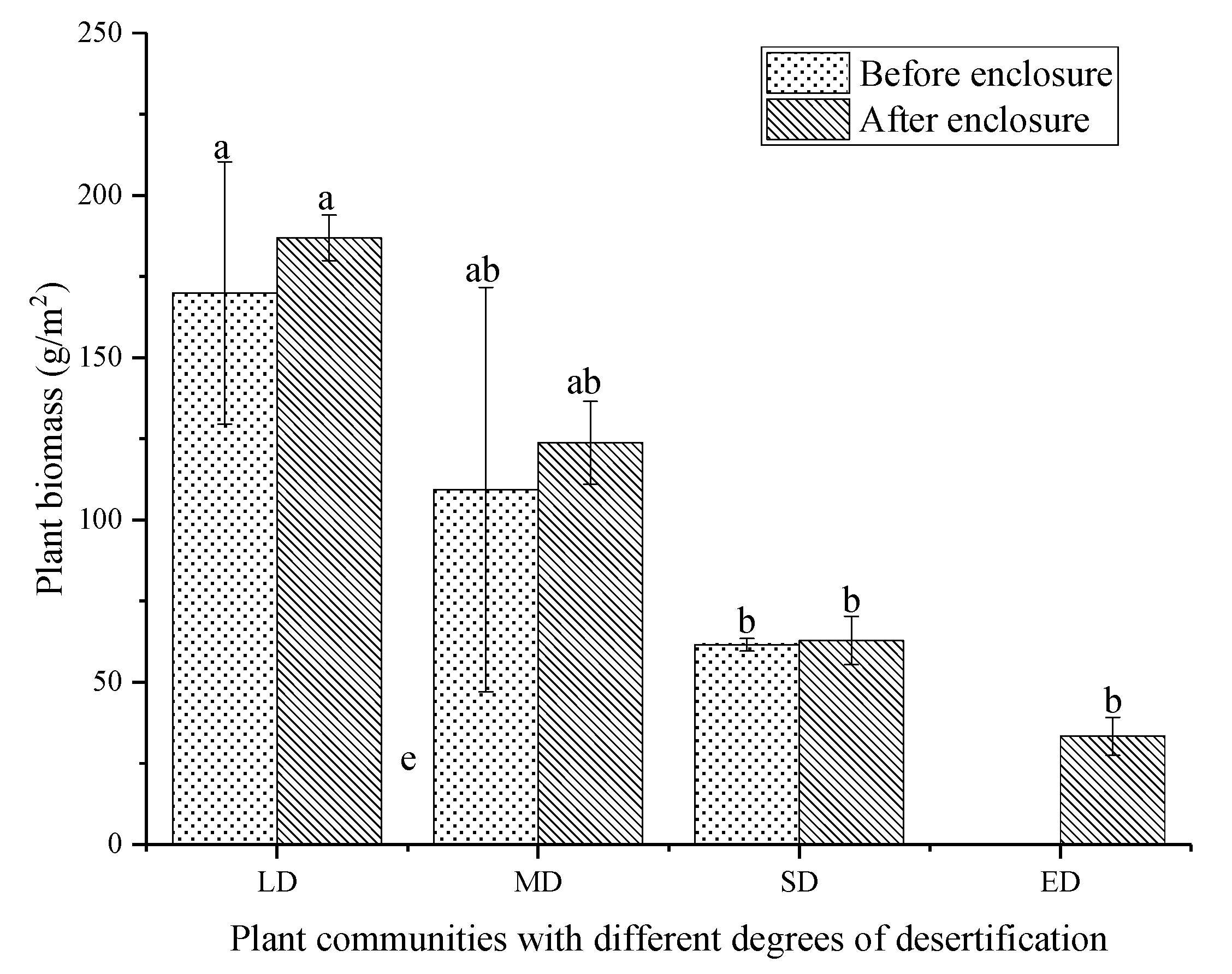
3.3. The Effect of Enclosure on Plant Species and Aboveground Biomass
3.4. Meristem Limitation Index along Desertification Gradient
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoekstra, J.M.; Boucher, T.M.; Ricketts, T.H.; Roberts, C.S. Confronting a biome crisis: Global disparities of habitat loss and protection. Ecol. Let. 2005, 8, 23–29. [Google Scholar] [CrossRef]
- Zhao, H.; He, Y.; Zhou, R.; Su, Y.; Li, Y.; Drake, S. Effects of desertification on soil organic C and N content in sandy farmland and grassland of Inner Mongolia. Catena 2009, 77, 187–191. [Google Scholar] [CrossRef]
- Zhang, D.M.; Zhao, W.Z.; Luo, W.C. Effect of the population density on belowground bud bank of a rhizomatous clonal plant leymus secalinus in mu us sandy land. J. Plant Res. 2019, 132, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gao, J.; Brierley, G.; Qiao, Y.M.; Zhang, J.; Yang, Y.W. Rangeland degradation on the Qinghai-Tibet plateau: Implications for rehabilitation. Land Degrad. Dev. 2013, 24, 72–80. [Google Scholar] [CrossRef]
- Hu, Z.; Li, S.; Guo, Q.; Niu, S.; He, N.; Li, L.; Yu, G. A synthesis of the effect of grazing exclusion on carbon dynamics in grasslands in China. Glob. Chang. Biol. 2016, 22, 1385–1393. [Google Scholar] [CrossRef]
- Akiyama, T.; Kawamura, K. Grassland degradation in china: Methods of monitoring, management and restoration. Grassl. Sci. 2010, 53, 1–17. [Google Scholar] [CrossRef]
- Bestelmeyer, B.T.; Okin, G.S.; Duniway, M.C.; Archer, S.R.; Sayre, N.F.; Williamson, J.C.; Herrick, J.E. Desertification, land use, and the transformation of global drylands. Front. Ecol. Environ. 2015, 13, 28–36. [Google Scholar] [CrossRef]
- Whalley, W. Grassland regeneration and reconstruction: The role of grazing animals. Ecol. Manag. Restor. 2010, 6, 3–4. [Google Scholar] [CrossRef]
- Yurkonis, K.A.; Mckenna, T.P. Aggregating Species at Seeding May Increase Initial Diversity during Grassland Reconstruction. Ecol. Restor. 2014, 32, 275–281. [Google Scholar] [CrossRef]
- Seahra, S.E.; Yurkonis, K.A.; Newman, J.A. Species patch size at seeding affects diversity and productivity responses in establishing grasslands. J. Ecol. 2016, 104, 479–486. [Google Scholar] [CrossRef]
- Zhang, F.; Nilsson, C.; Xu, Z.; Zhou, G. Evaluation of restoration approaches on the Inner Mongolian Steppe based on criteria of the Society for Ecological Restoration. Land Degrad. Dev. 2020, 31, 285–296. [Google Scholar] [CrossRef]
- Jiang, W.; Lv, J.; Wang, C.; Chen, Z.; Liu, Y. Marsh wetland degradation risk assessment and change analysis: A case study in the Zoige Plateau, China. Ecol. Indic. 2017, 82, 316–326. [Google Scholar] [CrossRef]
- Su, P.; Shi, R.; Zhou, Z.; Xie, T. Characteristics and relationships of foliar element content and specific leaf volume of alpine plant functional groups. Int. J. Agric. Biol. 2018, 20, 1663–1671. [Google Scholar]
- Na, R.; Du, H.; Na, L.; Shan, Y.; Huang, L. Spatiotemporal changes in the aeolian desertification of hulunbuir grassland and its driving factors in china during 1980–2015. Catena 2019, 182, 104123. [Google Scholar] [CrossRef]
- Wang, H.; Ren, J.; Yuan, H. A study on the changes of soil physical properties in the desertification process of source regions of the Yellow River using Maqu as an example. Acta Prataculturae Sin. 2007, 16, 30–33. [Google Scholar]
- Shen, G.; Yang, X.; Jin, Y.; Xu, B.; Zhou, Q. Remote sensing and evaluation of the wetland ecological degradation process of the Zoige Plateau Wetland in China. Ecol. Indic. 2019, 9, 48–58. [Google Scholar] [CrossRef]
- Harper, J.L. Population Biology of Plants; Academic Press: London, UK, 1977; pp. 33–60. [Google Scholar]
- Klimešová, J.; Klimeš, L. Bud banks and their role in vegetative regeneration—A literature review and proposal for simple classification and assessment. Perspect. Plant Ecol. Evol. Syst. 2007, 8, 115–129. [Google Scholar] [CrossRef]
- Willand, J.E.; Baer, S.G.; Gibson, D.J.; Klopf, R.P. Temporal dynamics of plant community regeneration sources during tallgrass prairie restoration. Plant Ecol. 2013, 214, 1169–1180. [Google Scholar] [CrossRef]
- Burke, N.W.; Bonduriansky, R. Sexual conflict, facultative asexuality, and the true paradox of sex. Trends Ecol. Evol. 2017, 32, 646–652. [Google Scholar] [CrossRef]
- Dalgleish, H.J.; Hartnett, D.C. The effects of fire frequency and grazing on tallgrass prairie productivity and plant composition are mediated through bud bank demography. Plant Ecol. 2009, 201, 411–420. [Google Scholar] [CrossRef]
- Eckert, C.G.; Dorken, M.E.; Mitchell, S.A. Loss of sex in clonal populations of a flowering plant, Decodon verticillatus (Lythraceae). Evolution 1999, 53, 1079–1092. [Google Scholar] [CrossRef] [PubMed]
- Ott, J.P.; Klimešová, J.; Hartnett, D.C. The ecology and significance of below-ground bud banks in plants. Ann. Bot. 2019, 123, 1099–1118. [Google Scholar] [CrossRef] [PubMed]
- Verburg, R.; Grava, D. Differences in allocation patterns in clonal and sexual offspring in woodland pseudo-annual. Oecologia 1998, 115, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Dalgleish, H.J.; Hartnett, D.C. Below-ground bud banks increase along a precipitation gradient of the North American Great Plains:a test of the meristem limitation hypothesis. New Phytol. 2006, 171, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Wellstein, C.; Kuss, P. Diversity and frequency of clonal traits along natural and land-use gradients in grasslands of the Swiss Alps. Folia Geobot. 2011, 46, 255–270. [Google Scholar] [CrossRef]
- Benson, E.J.; Hartnett, D.C.; Mann, K.H. Belowground bud banks and meristem limitation in tallgrass prairie plant populations. Am. J. Bot. 2004, 91, 416–421. [Google Scholar] [CrossRef]
- Klimešová, J.; Martínková, J.; Ottaviani, G. Belowground plant functional ecology: Towards an integrated perspective. Funct. Ecol. 2018, 32, 2115–2126. [Google Scholar] [CrossRef]
- Hartnett, D.C.; Setshogo, M.P.; Dalgleish, H.J. Bud banks of perennial savanna grasses in Botswana. Afr. J. Ecol. 2006, 44, 256–263. [Google Scholar] [CrossRef]
- Mudráket, O.; Fajmon, K.; Jongepierová, I.; Prach, K. Mass effects, clonality, and phenology but not seed traits predict species success in colonizing restored grasslands. Restor. Ecol. 2018, 26, 489–496. [Google Scholar] [CrossRef]
- Huhta, A.; Hellstrom, K.; Rautio, P.; Tuomi, J. Grazing tolerance of Gentianella amarella and other monocarpic herbs: Why is tolerance highest at low damage levels? Plant Ecol. 2003, 166, 49–61. [Google Scholar] [CrossRef]
- Zhao, L.P.; Wang, D.; Liang, F.H.; Liu, Y.; Wu, G.L. Grazing exclusion promotes grasses functional group dominance via increasing of bud banks in steppe community. J. Environ. Manag. 2019, 251, 109589. [Google Scholar] [CrossRef] [PubMed]
- Clarke, P.J.; Lawes, M.J.; Midgley, J.J.; Lamont, B.B.; Ojeda, F.; Burrows, G.E.; Enright, N.J.; Knox, K.J.E. Resprouting as a key functional trait: How buds, protection and resources drive persistence after fire. New Phytol. 2013, 197, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Wang, Z.; Liu, Z.; Busso, V.A. Belowground Bud Bank Responses to Grazing Intensity in the Inner-Mongolia Steppe, China. Land Degrad. Dev. 2017, 28, 822–832. [Google Scholar] [CrossRef]
- Dalgleish, H.J.; Kula, A.R.; Hartnett, D.C.; Sandercock, B.K. Responses of two bunchgrasses to nitrogen addition in tallgrass prairie: The role of bud bank demography. Am. J. Bot. 2008, 95, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Klimešová, J.; De Bello, F. CLO-PLA: The database of clonal and bud bank traits of Central European flora §. J. Veg. Sci. 2009, 20, 511–516. [Google Scholar] [CrossRef]
- Ma, Q.; Qian, J.; Tian, L.; Liu, Z. Responses of belowground bud bank to disturbance and stress in the sand dune ecosystem. Ecol. Indic. 2019, 106, 105521. [Google Scholar] [CrossRef]
- Ding, X.; Su, P.; Zhou, Z.; Shi, R. Belowground Bud Bank Distribution and Aboveground Community Characteristics along Different Moisture Gradients of Alpine Meadow in the Zoige Plateau, China. Sustainability 2019, 11, 2602. [Google Scholar] [CrossRef]
- Niu, B.; Zeng, C.; Zhang, X.; He, Y.; Shi, P.; Tian, Y.; Feng, Y.; Li, M.; Wang, Z.; Wang, X.; et al. High Below-Ground Productivity Allocation of Alpine Grasslands on the Northern Tibet. Plants 2019, 8, 535. [Google Scholar] [CrossRef]
- Jin, X.; Jin, H.; Wu, X.; Luo, D.; Yu, S.; Li, X.; He, R.; Wang, Q.; Knops, J.M.H. Permafrost Degradation Leads to Biomass and Species Richness Decreases on the Northeastern Qinghai-Tibet Plateau. Plants 2020, 9, 1453. [Google Scholar] [CrossRef]
- Wu, X.; Wang, Y.; Sun, S. Long-term fencing decreases plant diversity and soil organic carbon concentration of the Zoige alpine meadows on the eastern Tibetan plateau. Plant Soil 2019, 1–10. [Google Scholar] [CrossRef]
- Klimeš, L.; Klimešová, J.; Osbornová, J. Regeneration capacity and carbohydrate reserves in a clonal plant rumex alpinus: Effect of burial. Vegetatio 1993, 109, 153–160. [Google Scholar] [CrossRef]
- Palacio, S.; Escudero, A.; Montserrat-Martí, G.; Maestro, M.; Milla, R.; Albert, M.J. Plants living on gypsum: Beyond the specialist model. Ann. Bot. 2007, 99, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Vesk, P.A.; Westoby, M. Sprouting by plants: The effects of modular organization. Funct. Ecol. 2004, 18, 939–945. [Google Scholar] [CrossRef]
- Herben, T.; Nováková, Z.; Klimešová, J.; Hrouda, L. Species traits and plant performance: Functional trade-offs in a large set of species in a botanical garden. J. Ecol. 2012, 100, 1522–1533. [Google Scholar] [CrossRef]
- Hendrickson, J.R.; Briske, D.D. Axillary bud banks of two semiarid perennial grasses: Occurrence, longevity, and contribution to population persistence. Oecology 1997, 110, 584–591. [Google Scholar] [CrossRef]
- Herben, T.; Klimešová, J. Evolution of clonal growth forms in angiosperms. New Phytol. 2019, 225, 999–1010. [Google Scholar] [CrossRef]
- Macdonald, S.E.; Lieffers, V.J. Rhizome plasticity and clonal foraging of Calamagrostis canadensis in response to habitat heterogeneity. J. Ecol. 1993, 81, 769–776. [Google Scholar] [CrossRef]
- Russell, M.L.; Vermeire, L.T.; Ganguli, A.C.; Hendrickson, K.R. Season of fire manipulates bud bank dynamics in northern mixed-grass prairie. Plant Ecol. 2015, 216, 835–846. [Google Scholar] [CrossRef]
- Chen, X.S.; Deng, Z.M.; Xie, Y.H.; Li, F.; Hou, Z.Y.; Li, X. Belowground bud banks of four dominant macrophytes along a small-scale elevational gradient in dongting lake wetlands, China. Aquat. Bot. 2015, 122, 9–14. [Google Scholar] [CrossRef]
- Knapp, A.K.; Smith, M.D. Variation among biomes in temporal dynamics of aboveground primary production. Science 2001, 291, 481–484. [Google Scholar] [CrossRef]
- Reichmann, L.G.; Sala, O.E. Differential sensitivities of grassland structural components to changes in precipitation mediate productivity response in a desert ecosystem. Funct. Ecol. 2014, 28, 1292–1298. [Google Scholar] [CrossRef]




| Community Type | Vegetation Coverage/% | Quicksand Area/% |
|---|---|---|
| LD | 50 ≤ C < 60 | 5 ≤ S < 10 |
| MD | 20 ≤ C < 50 | 10 ≤ S < 30 |
| SD | 10 ≤ C < 20 | 30 ≤ S < 50 |
| ED | <10 | ≥50 |
| Community Type | Plant Species | Coverage % |
|---|---|---|
| Light desertified grassland | Elymus nutans | 23.3 |
| Leymus secalinus | 18 | |
| Anaphalis lactea | 15 | |
| Poa pratensis | 6.6 | |
| Ligularia virgaurea | 3 | |
| Artemisia hedinii | 2.8 | |
| Agropyron cristatum | 2.7 | |
| Lancea tibetica | 2.7 | |
| Kobresia setchwanensis | 1 | |
| Potentilla anserina | 0.2 | |
| Moderate desertified grassland | Kobresia setchwanensis | 21.3 |
| Anaphalis lactea | 12.3 | |
| Potentilla anserina | 2.3 | |
| Stellera chamaejasme | 1.1 | |
| Leymus secalinus | 0.9 | |
| Ajuga lupulina | 0.4 | |
| Severe desertified grassland | Carex moorcroftii | 5.3 |
| Leymus secalinus | 4.9 | |
| Elymus nutans | 1.9 | |
| Dracocephalum heterophyllum | 1.2 | |
| Gentiana straminea | 0.8 | |
| Extreme desertified grassland | Agropyron cristatum | 4.8 |
| Chenopodium aristatum | 0.3 |
| Community Type | Plant Species | |
|---|---|---|
| Before Enclosure | After Enclosure | |
| LD | 3 ± 1 a | 4 ± 0.6 a |
| MD | 2.7 ± 0.3 a | 3 ± 0 ab |
| SD | 2 ± 0 a | 2 ± 0 b |
| ED | - | 1.3 ± 0.3 c |
| Plant Community | Shoot Density Number/m2 | Meristem Limitation Index |
|---|---|---|
| LD | 3589 ± 3030 a | 1.07 ± 0.24 c |
| MD | 6891 ± 2589 a | 0.37 ± 0.06 d |
| SD | 528 ± 25 a | 4.67 ± 0.67 b |
| ED | 32 ± 16 a | 13.54 ± 1.36 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, X.; Su, P.; Zhou, Z.; Shi, R.; Yang, J. Responses of Plant Bud Bank Characteristics to the Enclosure in Different Desertified Grasslands on the Tibetan Plateau. Plants 2021, 10, 141. https://doi.org/10.3390/plants10010141
Ding X, Su P, Zhou Z, Shi R, Yang J. Responses of Plant Bud Bank Characteristics to the Enclosure in Different Desertified Grasslands on the Tibetan Plateau. Plants. 2021; 10(1):141. https://doi.org/10.3390/plants10010141
Chicago/Turabian StyleDing, Xinjing, Peixi Su, Zijuan Zhou, Rui Shi, and Jianping Yang. 2021. "Responses of Plant Bud Bank Characteristics to the Enclosure in Different Desertified Grasslands on the Tibetan Plateau" Plants 10, no. 1: 141. https://doi.org/10.3390/plants10010141
APA StyleDing, X., Su, P., Zhou, Z., Shi, R., & Yang, J. (2021). Responses of Plant Bud Bank Characteristics to the Enclosure in Different Desertified Grasslands on the Tibetan Plateau. Plants, 10(1), 141. https://doi.org/10.3390/plants10010141




