Detecting Mild Water Stress in Olive with Multiple Plant-Based Continuous Sensors
Abstract
1. Introduction
2. Materials and Methods
2.1. Orchard Characteristics
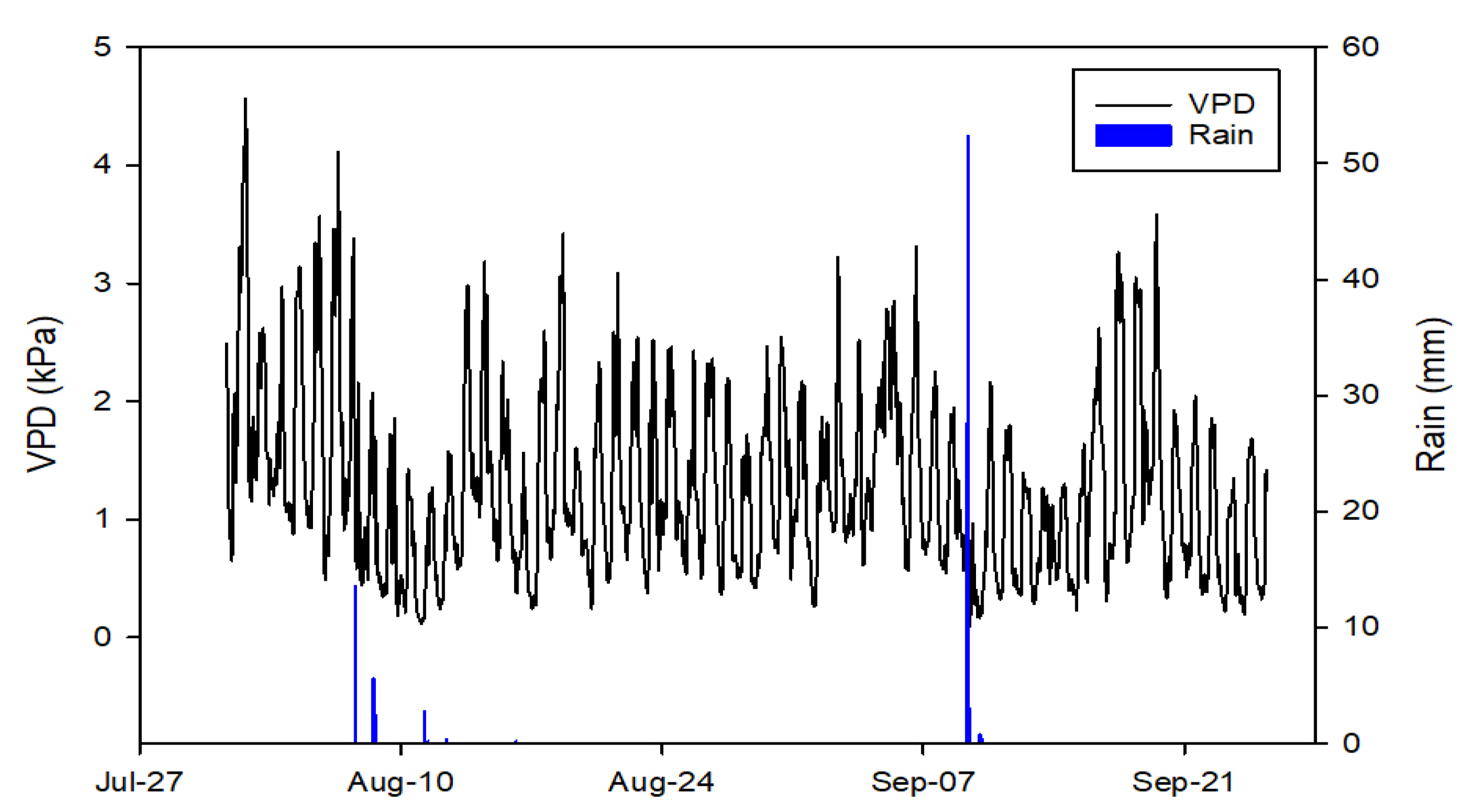
2.2. Environmental Conditions
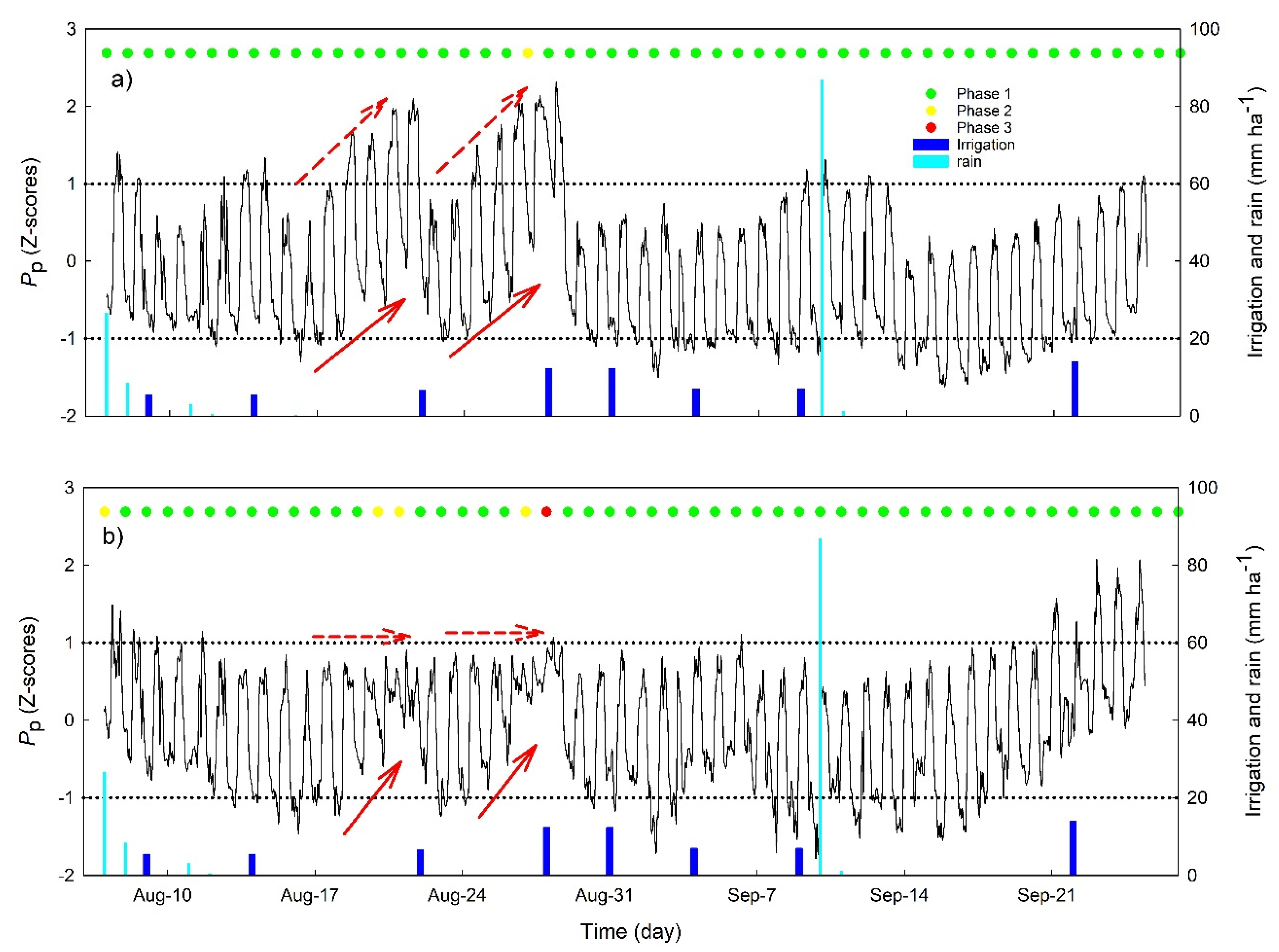
2.3. Irrigation Management and Experimental Design
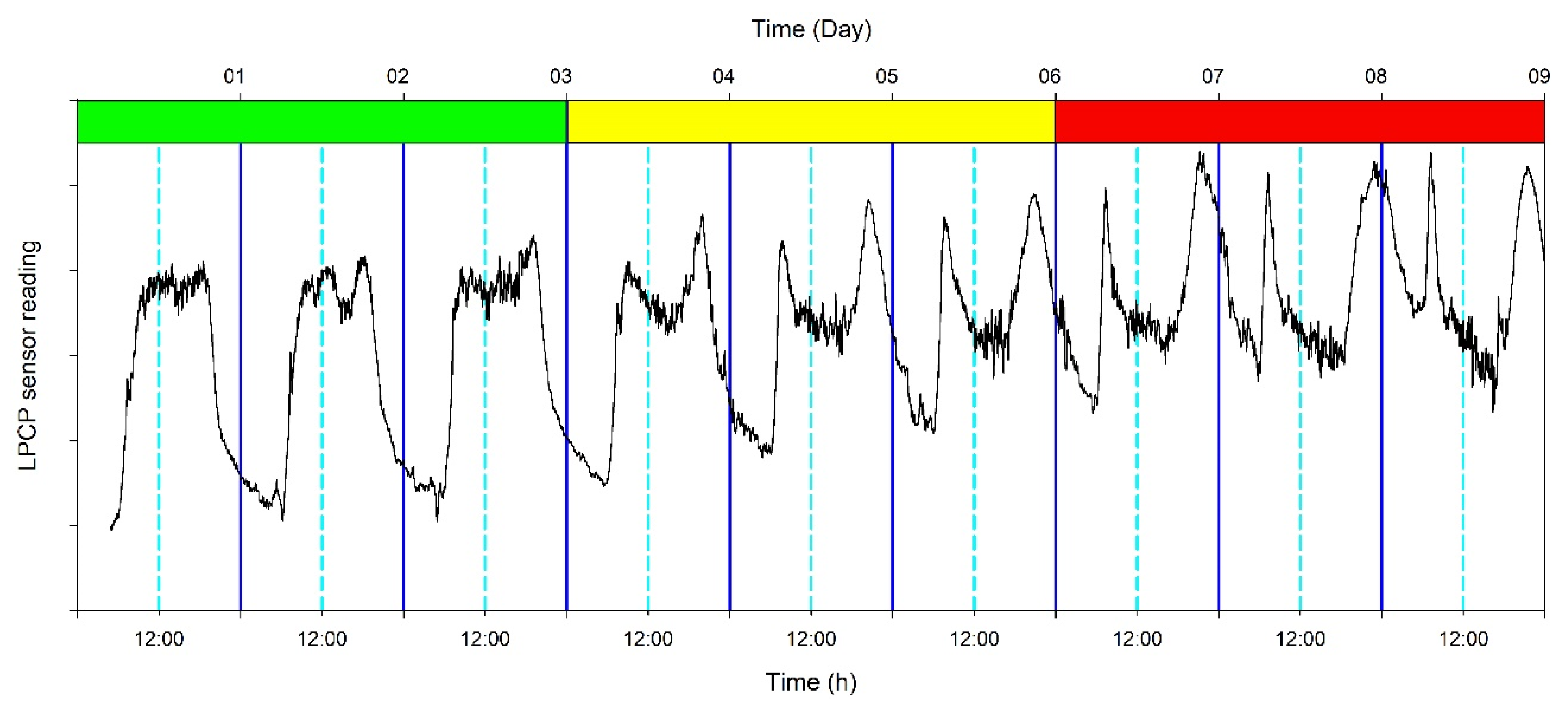
2.4. Measurements
2.5. Statistical Data Analysis
3. Results
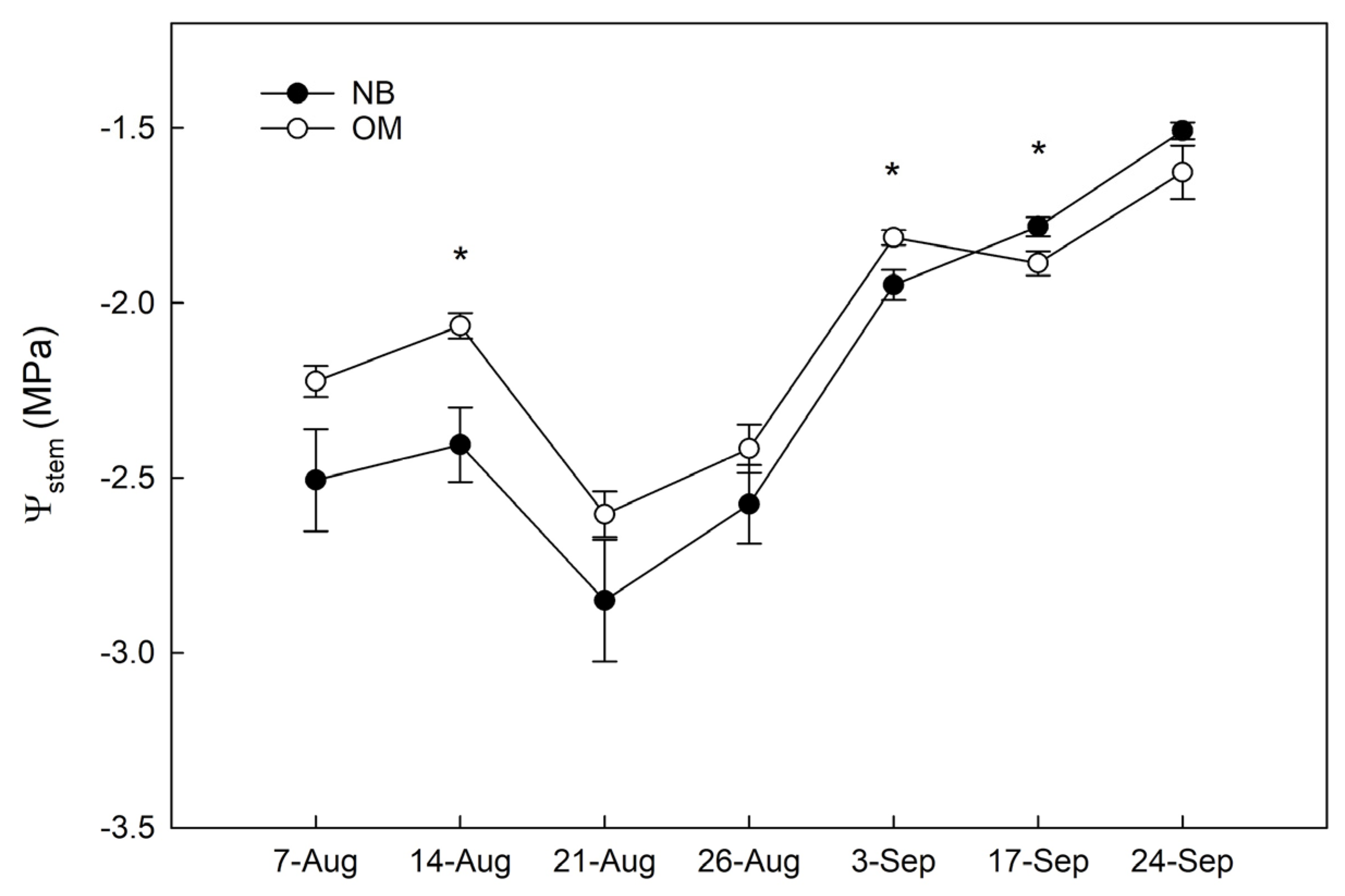
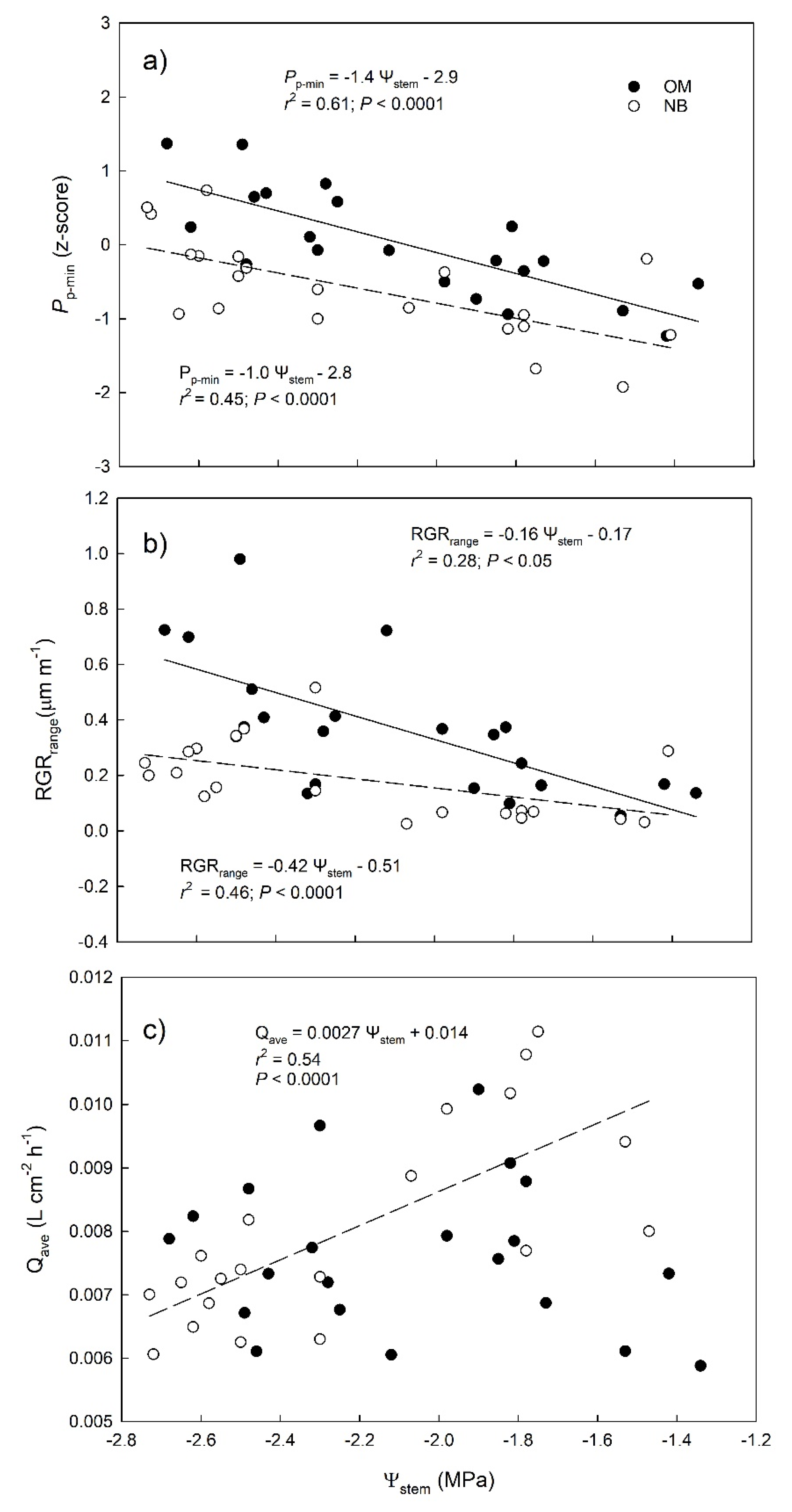
3.1. Stem Water Potential
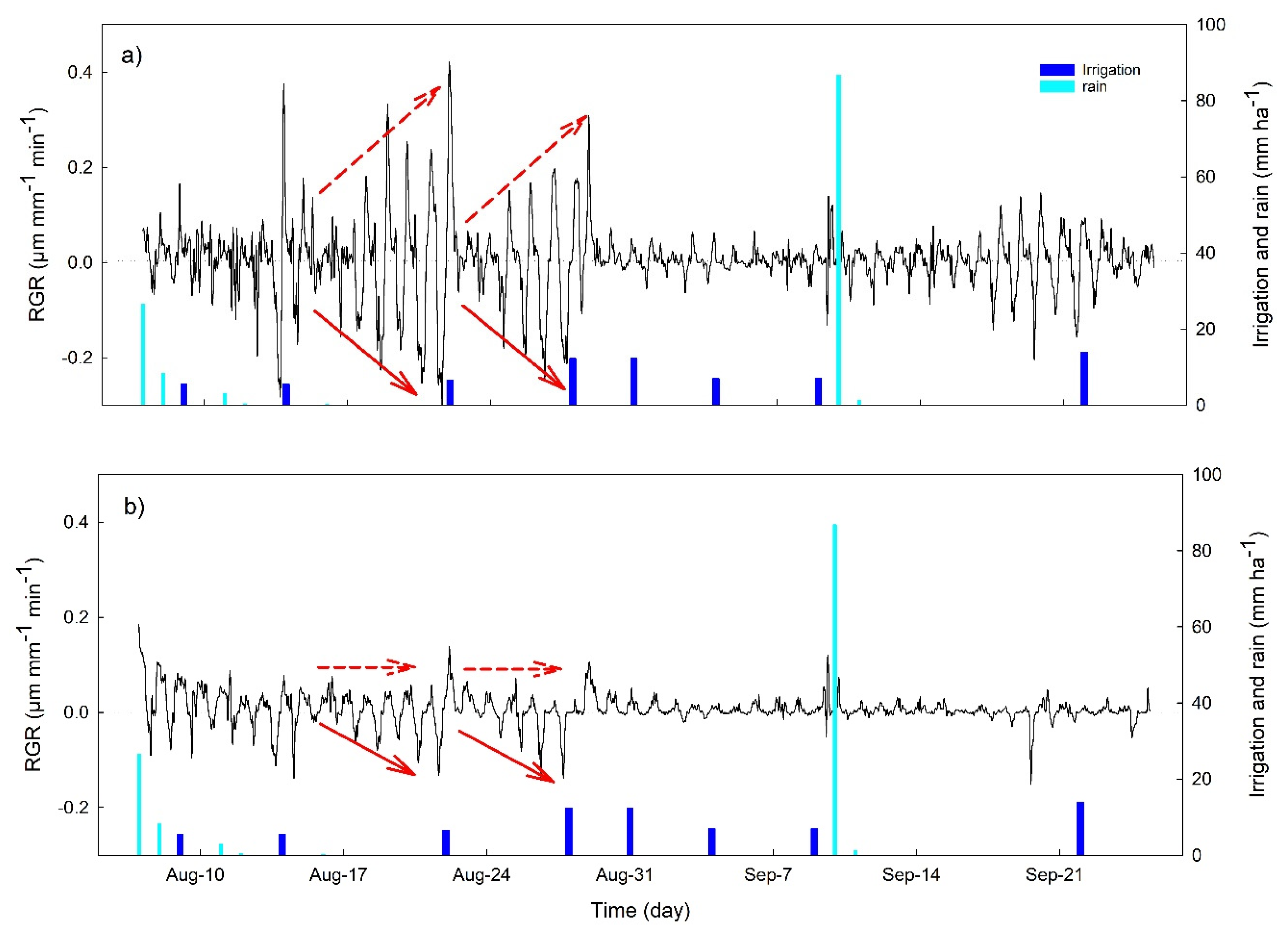
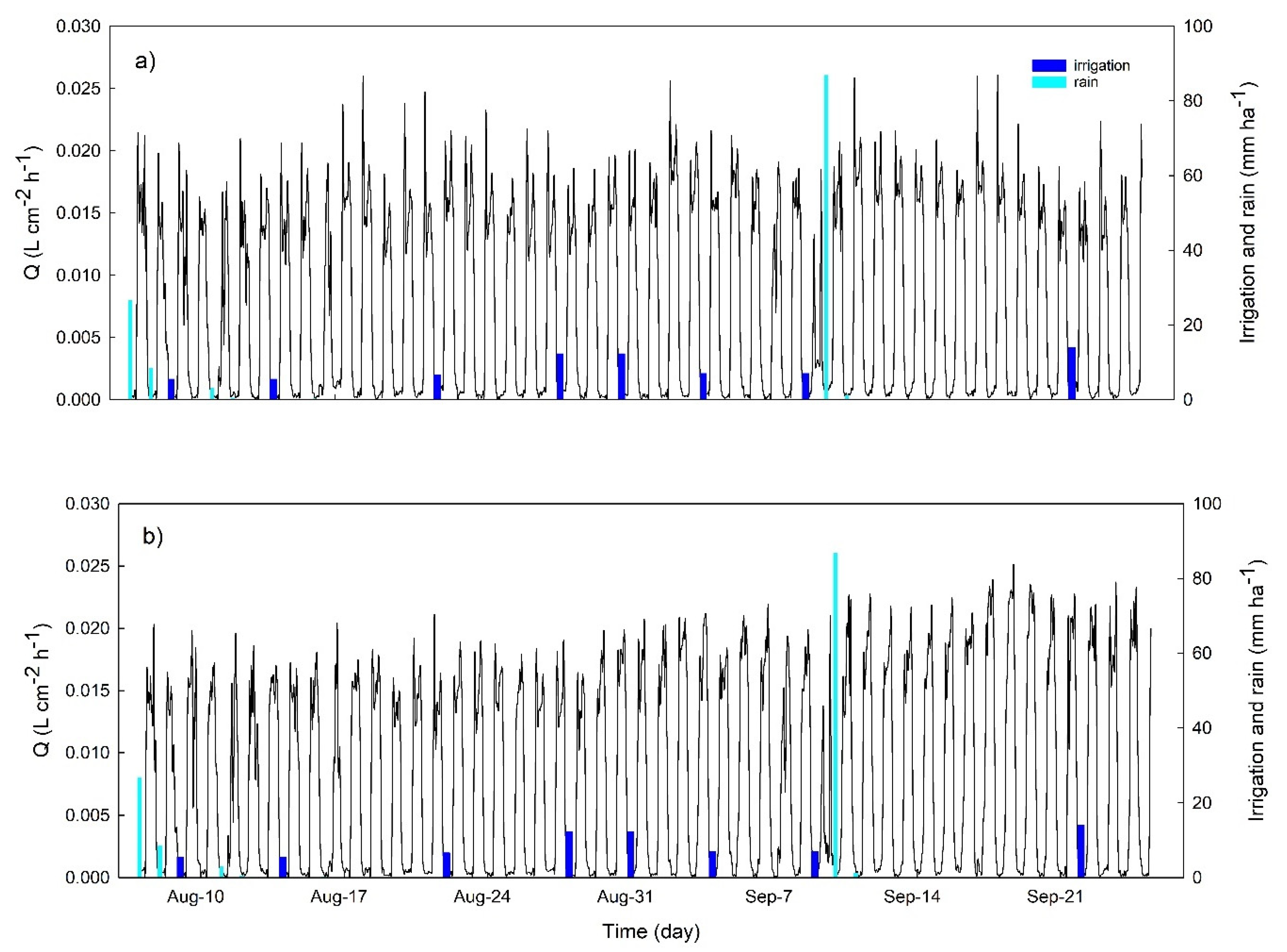
3.2. Continuous Trends of Leaf Turgor, Sap Flow and Fruit Relative Growth Rate
3.3. Relationships between Stem Water Potential and Sensor-Derived Physiological Parameters
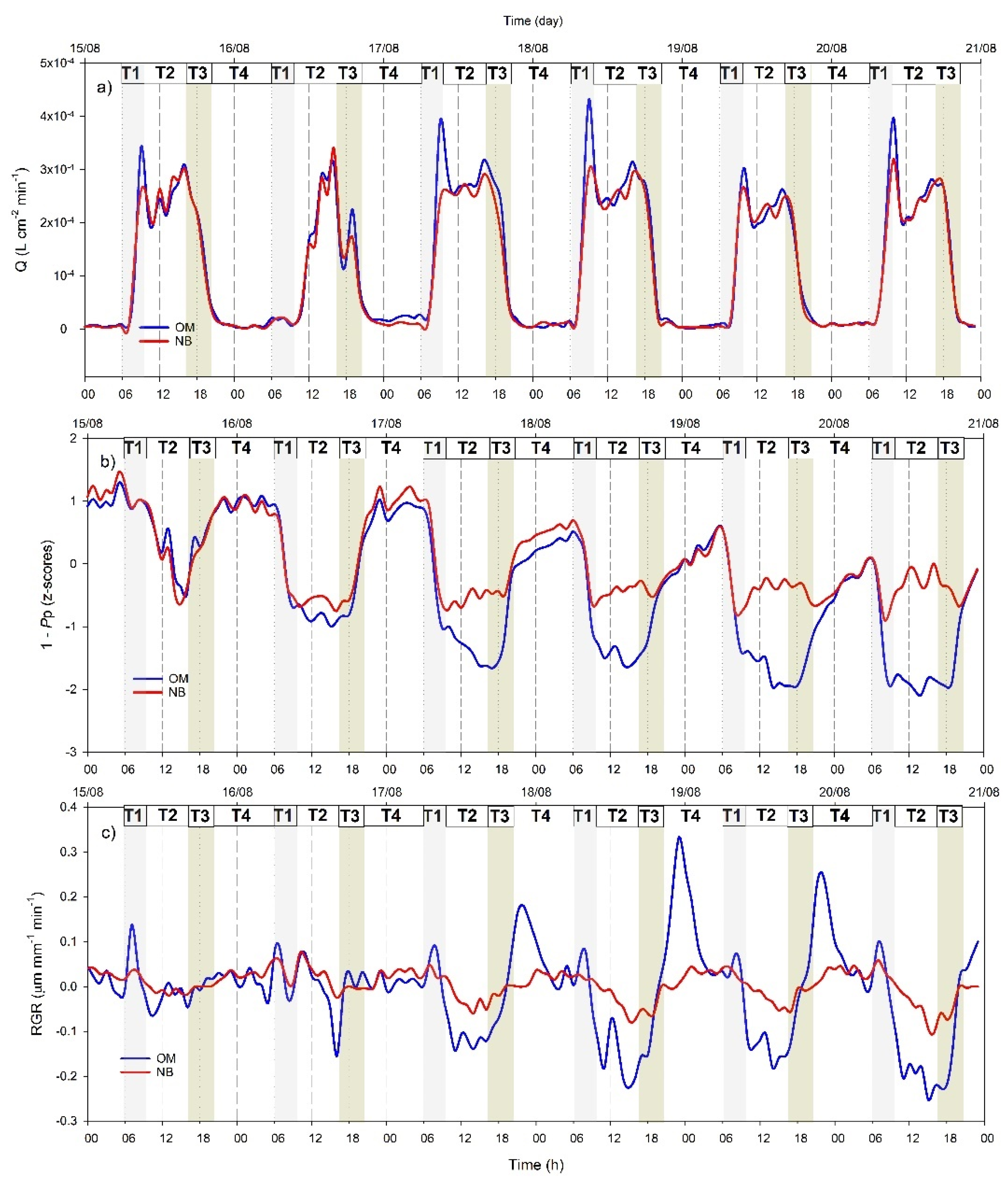
3.4. Hourly Dynamics of Leaf Turgor, Sap Flow, and Fruit Relative Grow Rate
4. Discussion
4.1. Use of Multiple Continuous Sensors Improves Mild Stress Detection
4.2. Different Physiological Responses of the Two Genotypes to Water Withholding
4.3. Cultivar Dependency of Continuous Indicator Sensitivity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fernández, J. Plant-Based Methods for Irrigation Scheduling of Woody Crops. HortTechnology 2017, 3, 35. [Google Scholar] [CrossRef]
- Shackel, K.A.; Ahmadi, H.; Biasi, W.; Buchner, R.; Goldhamer, D.; Gurusinghe, S.; Hasey, J.; Kester, D.; Krueger, B.; Lampinen, B.; et al. Plant Water Status as an Index of Irrigation Need in Deciduous Fruit Trees. HortTechnology 1997, 7, 23–29. [Google Scholar] [CrossRef]
- Shackel, K.A. A Plant-based Approach to Deficit Irrigation in Trees and Vines. HortScience 2011, 46, 173–177. [Google Scholar] [CrossRef]
- Marino, G.; Caruso, T.; Ferguson, L.; Marra, F.P. Gas Exchanges and Stem Water Potential Define Stress Thresholds for Efficient Irrigation Management in Olive (Olea europea L.). Water 2018, 10, 342. [Google Scholar] [CrossRef]
- Ahumada-Orellana, L.; Ortega-Farías, S.; Poblete-Echeverría, C.; Searles, P.S. Estimation of stomatal conductance and stem water potential threshold values for water stress in olive trees (cv. Arbequina). Irrig. Sci. 2019, 37, 461–467. [Google Scholar] [CrossRef]
- Ennajeh, M.; Tounekti, T.; Vadel, A.M.; Khemira, H.; Cochard, H. Water relations and drought-induced embolism in olive (Olea europaea) varieties ’Meski’ and ’Chemlali’ during severe drought. Tree Physiol. 2008, 28, 971–976. [Google Scholar] [CrossRef]
- Scalisi, A.; Marino, G.; Marra, F.P.; Caruso, T.; Bianco, R.L. A Cultivar-Sensitive Approach for the Continuous Monitoring of Olive (Olea europaea L.) Tree Water Status by Fruit and Leaf Sensing. Front. Plant Sci. 2020, 11. [Google Scholar] [CrossRef]
- Cocozza, C.; Marino, G.; Giovannelli, A.; Cantini, C.; Centritto, M.; Tognetti, R. Simultaneous measurements of stem radius variation and sap flux density reveal synchronisation of water storage and transpiration dynamics in olive trees. Ecohydrology 2015, 8, 33–45. [Google Scholar] [CrossRef]
- Zimmermann, D.; Reuss, R.; Westhoff, M.; Geßner, P.; Bauer, W.; Bamberg, E.; Bentrup, F.-W.; Zimmermann, U. A novel, non-invasive, online-monitoring, versatile and easy plant-based probe for measuring leaf water status. J. Exp. Bot. 2008, 59, 3157–3167. [Google Scholar] [CrossRef]
- Fernández, J.; Rodriguez-Dominguez, C.M.; Perez-Martin, A.; Zimmermann, U.; Rüger, S.; Martín-Palomo, M.; Torres-Ruiz, J.M.; Cuevas, M.V.; Sann, C.; Ehrenberger, W.; et al. Online-monitoring of tree water stress in a hedgerow olive orchard using the leaf patch clamp pressure probe. Agric. Water Manag. 2011, 100, 25–35. [Google Scholar] [CrossRef]
- Padilla-Díaz, C.M.; Rodriguez-Dominguez, C.; Hernandez-Santana, V.; Perez-Martin, A.; Fernández, J. Scheduling regulated deficit irrigation in a hedgerow olive orchard from leaf turgor pressure related measurements. Agric. Water Manag. 2016, 164, 28–37. [Google Scholar] [CrossRef]
- Ehrenberger, W.; Rüger, S.; Domínguez, C.M.R.; Díaz-Espejo, A.; Fernández, J.; Moreno, J.; Zimmermann, D.; Sukhorukov, V.L. Leaf patch clamp pressure probe measurements on olive leaves in a nearly turgorless state. Plant. Biol. 2012, 14, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Marino, G.; Pernice, F.; Marra, F.P.; Caruso, T. Validation of an online system for the continuous monitoring of tree water status for sustainable irrigation managements in olive (Olea europaea L.). Agric. Water Manag. 2016, 177, 298–307. [Google Scholar] [CrossRef]
- Bacelar, E.; Moutinho-Pereira, J.M.; Gonçalves, B.C.; Lopes, J.I.; Correia, C.M. Physiological responses of different olive genotypes to drought conditions. Acta Physiol. Plant. 2009, 31, 611–621. [Google Scholar] [CrossRef]
- Girón, I.; Corell, M.; Galindo, A.; Torrecillas, A.S.; Morales, D.; Dell’Amico, J.; Moreno, I.F.G.; Moriana, A. Changes in the physiological response between leaves and fruits during a moderate water stress in table olive trees. Agric. Water Manag. 2015, 148, 280–286. [Google Scholar] [CrossRef]
- Pomper, K.W.; Breen, P.J. Expansion and Osmotic Adjustment of Strawberry Fruit during Water Stress. J. Am. Soc. Hortic. Sci. 1997, 122, 183–189. [Google Scholar] [CrossRef]
- Greenspan, M.D.; Schultz, H.R.; Matthews, M.A. Field evaluation of water transport in grape berries during water deficits. Physiol. Plant. 1996, 97, 55–62. [Google Scholar] [CrossRef]
- Rodriguez-Dominguez, C.M.; Ehrenberger, W.; Sann, C.; Rüger, S.; Sukhorukov, V.; Martín-Palomo, M.; Diaz-Espejo, A.; Cuevas, M.V.; Torres-Ruiz, J.M.; Perez-Martin, A.; et al. Concomitant measurements of stem sap flow and leaf turgor pressure in olive trees using the leaf patch clamp pressure probe. Agric. Water Manag. 2012, 114, 50–58. [Google Scholar] [CrossRef]
- Scalisi, A.; Marra, F.; Caruso, T.; Illuminati, C.; Costa, F.; Bianco, R.L. Transpiration rates and hydraulic conductance of two olive genotypes with different sensitivity to drought. Acta Hortic. 2019, 421–428. [Google Scholar] [CrossRef]
- Begg, J.E.; Turner, N.C. Water Potential Gradients in Field Tobacco. Plant. Physiol. 1970, 46, 343–346. [Google Scholar] [CrossRef]
- Westhoff, M.; Reuss, R.; Zimmermann, D.; Netzer, Y.; Gessner, A.; Geßner, P.; Zimmermann, G.; Wegner, L.H.; Bamberg, E.; Schwartz, A.; et al. A non-invasive probe for online-monitoring of turgor pressure changes under field conditions. Plant Biol. 2009, 11, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Granier, A. Une nouvelle méthode pour la mesure du flux de sève brute dans le tronc des arbres. Ann. Sci. Forestières 1985, 42, 193–200. [Google Scholar] [CrossRef]
- Savitzky, A.; Golay, M.J.E. Smoothing and Differentiation of Data by Simplified Least Squares Procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014; Available online: http//www.R-project.org/ (accessed on 9 March 2020).
- Zimmermann, U.; Bitter, R.; Marchiori, P.E.R.; Rüger, S.; Ehrenberger, W.; Sukhorukov, V.L.; Schüttler, A.; Ribeiro, R.V. A non-invasive plant-based probe for continuous monitoring of water stress in real time: A new tool for irrigation scheduling and deeper insight into drought and salinity stress physiology. Theor. Exp. Plant. Physiol. 2013, 25, 2–11. [Google Scholar] [CrossRef]
- Morandi, B.; Manfrini, L.; Zibordi, M.; Noferini, M.; Fiori, G.; Grappadelli, L.C. A Low-cost Device for Accurate and Continuous Measurements of Fruit Diameter. HortScience 2007, 42, 1380–1382. [Google Scholar] [CrossRef]
- Lang, A. Xylem, Phloem and Transpiration Flows in Developing Apple Fruits. J. Exp. Bot. 1990, 41, 645–651. [Google Scholar] [CrossRef]
- Morandi, B.; Manfrini, L.; Losciale, P.; Zibordi, M.; Grappadelli, L.C. The positive effect of skin transpiration in peach fruit growth. J. Plant Physiol. 2010, 167, 1033–1037. [Google Scholar] [CrossRef]
- Morandi, B.; Manfrini, L.; Zibordi, M.; Losciale, P.; Grappadelli, L.C. Effects of drought stress on the growth, water relations and vascular flows of young ’summerkiwi’ fruit. Acta Hortic. 2011, 355–359. [Google Scholar] [CrossRef]
- Morandi, B.; Losciale, P.; Manfrini, L.; Zibordi, M.; Anconelli, S.; Galli, F.; Pierpaoli, E.; Grappadelli, L.C. Increasing water stress negatively affects pear fruit growth by reducing first its xylem and then its phloem inflow. J. Plant Physiol. 2014, 171, 1500–1509. [Google Scholar] [CrossRef]
- Fernandes, R.D.M.; Cuevas, M.V.; Diaz-Espejo, A.; Hernández-Santana, V. Effects of water stress on fruit growth and water relations between fruits and leaves in a hedgerow olive orchard. Agric. Water Manag. 2018, 210, 32–40. [Google Scholar] [CrossRef]
- Clothier, B.; Green, S. Transpiration and root water uptake by olive trees. Plant Soil 1996, 184, 85–96. [Google Scholar] [CrossRef]
- Tognetti, R.; D’Andria, R.; Morelli, G.; Calandrelli, D.; Fragnito, F. Irrigation effects on daily and seasonal variations of trunk sap flow and leaf water relations in olive trees. Plant Soil 2004, 263, 249–264. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Huang, G.; Feng, F.; Liu, X.; Guo, R.; Gu, F.; Hu, X.; Yang, Z.; Zhong, X.; et al. Atmospheric humidity and genotype are key determinants of the diurnal stomatal conductance pattern. J. Agron. Crop. Sci. 2019, 206, 161–168. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Wei, Z.; Liu, F. ABA-mediated modulation of elevated CO2 on stomatal response to drought. Curr. Opin. Plant Biol. 2020, 56, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Gucci, R.; Grimelli, A.; Costagli, G.; Tognetti, R.; Minnocci, A.; Vitagliano, C. Stomatal characteristics of two olive cultivars “frantoio” and “leccino”. Acta Hortic. 2002, 541–544. [Google Scholar] [CrossRef]
- Gullo, M.A.; Salleo, S. Different strategies of drought resistance in three Mediterranean sclerophyllous trees growing in the same environmental conditions. New Phytol. 1988, 108, 267–276. [Google Scholar] [CrossRef]
- Fan, S.; Blake, T.J.; Blumwald, E. The relative contribution of elastic and osmotic adjustments to turgor maintenance of woody species. Physiol. Plant. 1994, 90, 408–413. [Google Scholar] [CrossRef]
- Scalisi, A.; O’Connell, M.; Stefanelli, D.; Bianco, R.L. Fruit and Leaf Sensing for Continuous Detection of Nectarine Water Status. Front. Plant Sci. 2019, 10, 805. [Google Scholar] [CrossRef]
- Berger, A.; Selles, G. Diurnal fruit shrinkage: A model. In Water Transport in Plants under Climatic Stress; Borghetti, M., Grace, J., Raschi, A., Eds.; Cambridge University Press (CUP): Cambridge, UK, 1993; pp. 261–269. [Google Scholar]
- Johnson, R.W.; Dixon, M.A.; Lee, D.R. Water relations of the tomato during fruit growth. Plant Cell Environ. 1992, 15, 947–953. [Google Scholar] [CrossRef]
- Tromp, J. Diurnal fruit shrinkage in apple as affected by leaf water potential and vapour pressure deficit of the air. Sci. Hortic. 1984, 22, 81–87. [Google Scholar] [CrossRef]
- Matthews, M.A.; Shackel, K.A. Growth and water transport in fleshy fruit. In Vascular Transport in Plants; Holbrook, N.M., Zwieniecki, M.A., Eds.; Elsevier Academic Press: Burlington, VT, USA, 2005; pp. 181–197. [Google Scholar]
- Dell’Amico, J.; Moriana, A.; Corell, M.; Girón, I.; Morales, D.; Torrecillas, A.S.; Moreno, I.F.G. Low water stress conditions in table olive trees (Olea europaea L.) during pit hardening produced a different response of fruit and leaf water relations. Agric. Water Manag. 2012, 114, 11–17. [Google Scholar] [CrossRef]
- Fernández, J. Plant-based sensing to monitor water stress: Applicability to commercial orchards. Agric. Water Manag. 2014, 142, 99–109. [Google Scholar] [CrossRef]
| Parameter | Slopes | SE | d.f. | t Ratio | p Value |
|---|---|---|---|---|---|
| Pp-min | −0.389 | 0.371 | 38 | −1.049 | 0.301 |
| RGRrange | −0.259 | 0.118 | 38 | −2.195 | 0.034 |
| Qave | −0.003 | 0.0009 | 37 | −3.444 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marino, G.; Scalisi, A.; Guzmán-Delgado, P.; Caruso, T.; Marra, F.P.; Lo Bianco, R. Detecting Mild Water Stress in Olive with Multiple Plant-Based Continuous Sensors. Plants 2021, 10, 131. https://doi.org/10.3390/plants10010131
Marino G, Scalisi A, Guzmán-Delgado P, Caruso T, Marra FP, Lo Bianco R. Detecting Mild Water Stress in Olive with Multiple Plant-Based Continuous Sensors. Plants. 2021; 10(1):131. https://doi.org/10.3390/plants10010131
Chicago/Turabian StyleMarino, Giulia, Alessio Scalisi, Paula Guzmán-Delgado, Tiziano Caruso, Francesco Paolo Marra, and Riccardo Lo Bianco. 2021. "Detecting Mild Water Stress in Olive with Multiple Plant-Based Continuous Sensors" Plants 10, no. 1: 131. https://doi.org/10.3390/plants10010131
APA StyleMarino, G., Scalisi, A., Guzmán-Delgado, P., Caruso, T., Marra, F. P., & Lo Bianco, R. (2021). Detecting Mild Water Stress in Olive with Multiple Plant-Based Continuous Sensors. Plants, 10(1), 131. https://doi.org/10.3390/plants10010131







