Regeneration in Reptiles Generally and the New Zealand Tuatara in Particular as a Model to Analyse Organ Regrowth in Amniotes: A Review
Abstract
1. Introduction and Overview
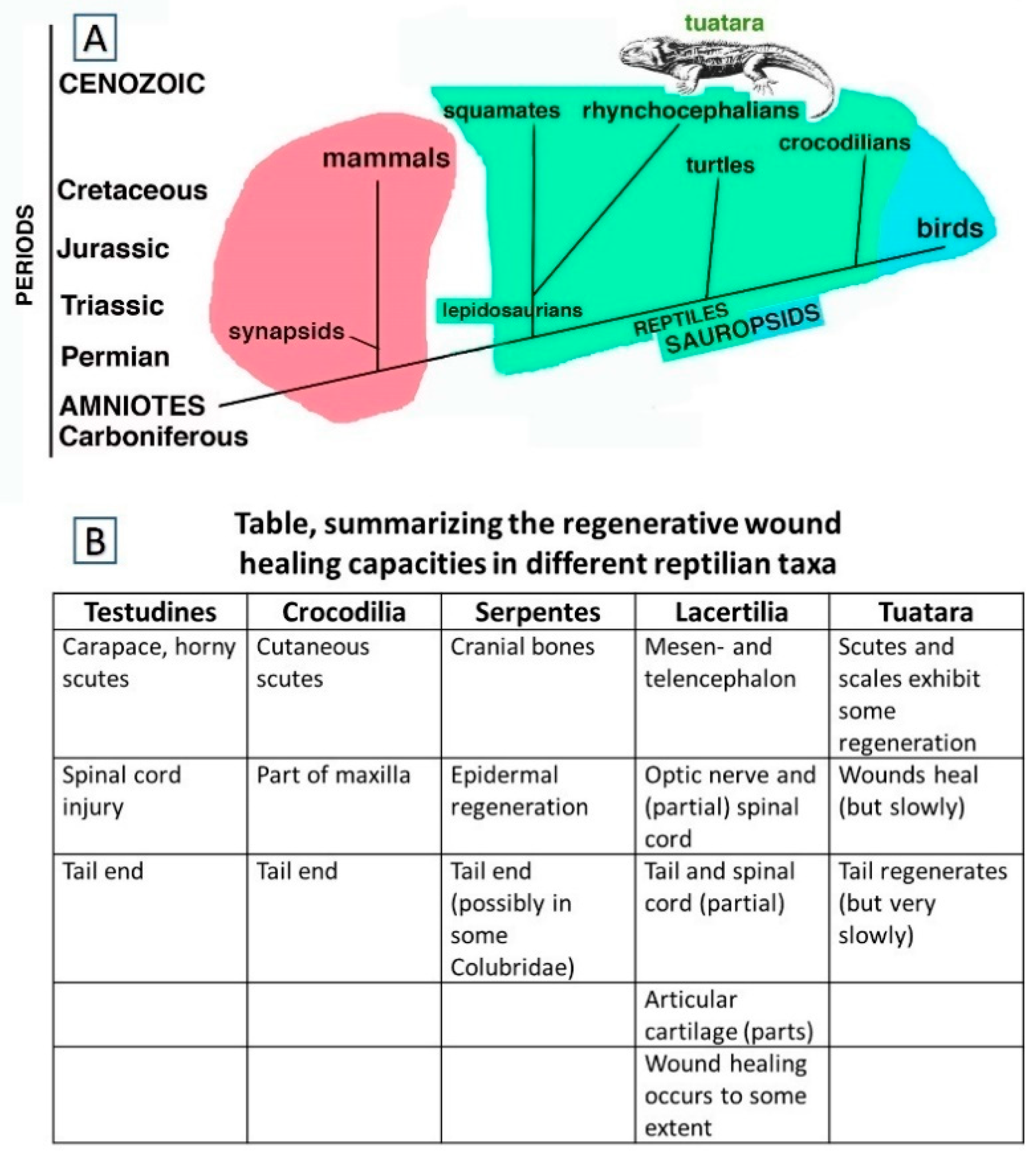
1.1. Wound Healing and Regeneration among Reptiles Generally
1.2. Focusing on Tail Autotomies in Reptiles
2. Caudal Autotomy in Tuatara
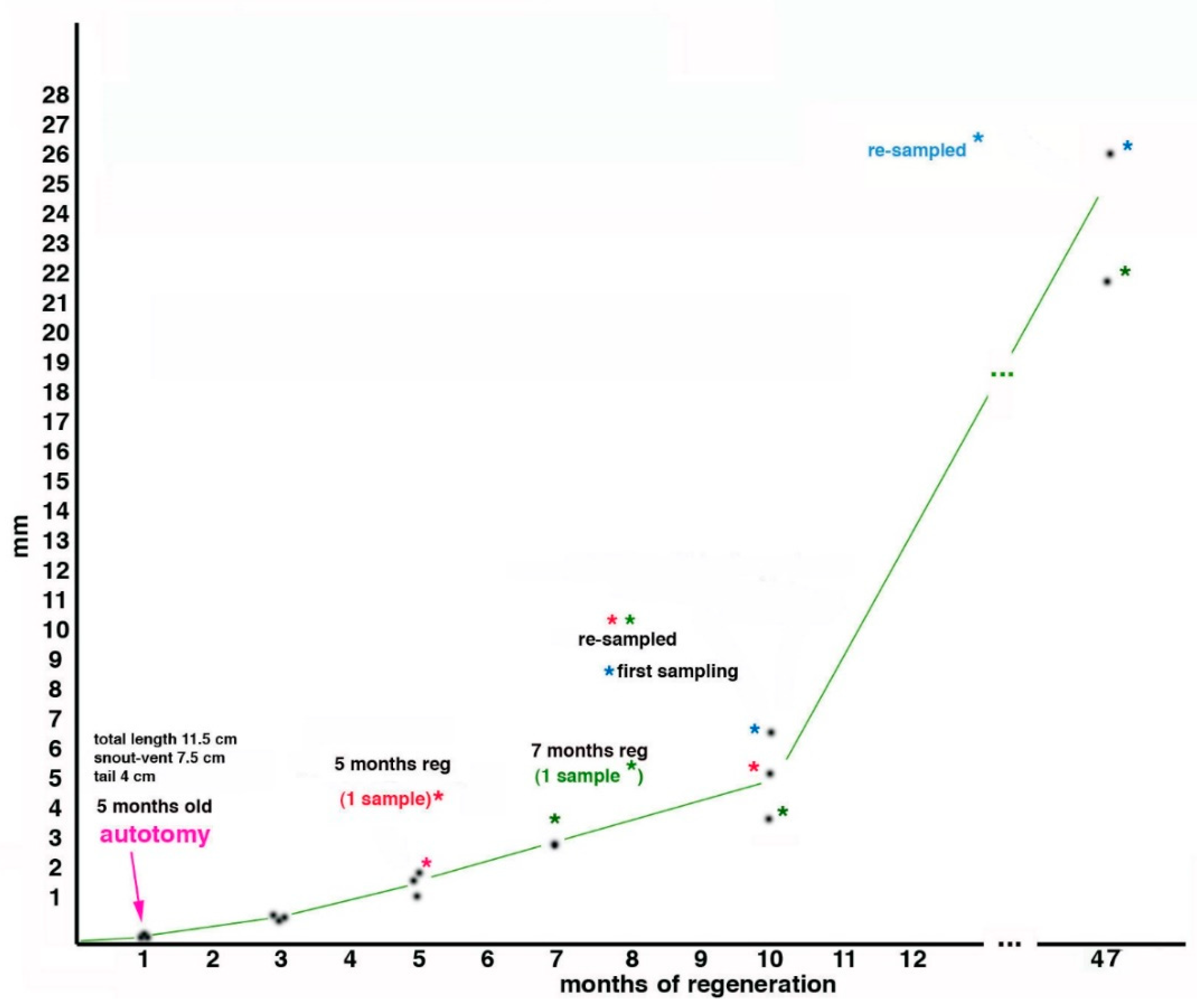
2.1. Sample Acquisition and Methodology
2.2. Tail Regeneration in the Tuatara Represents a Case of Regengrow
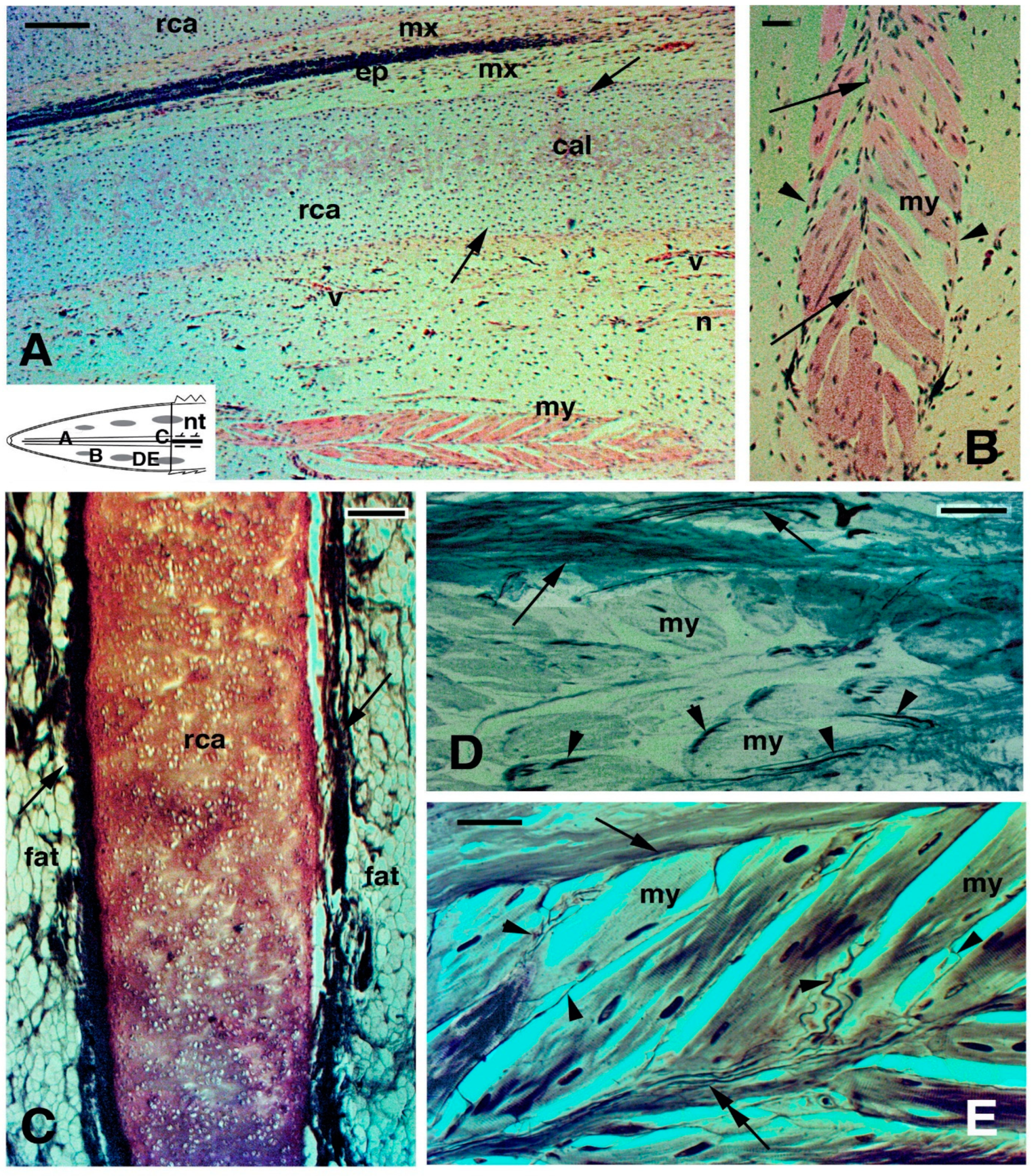
2.3. Histology of Regenerating and Regenerated Tails in the Tuatara
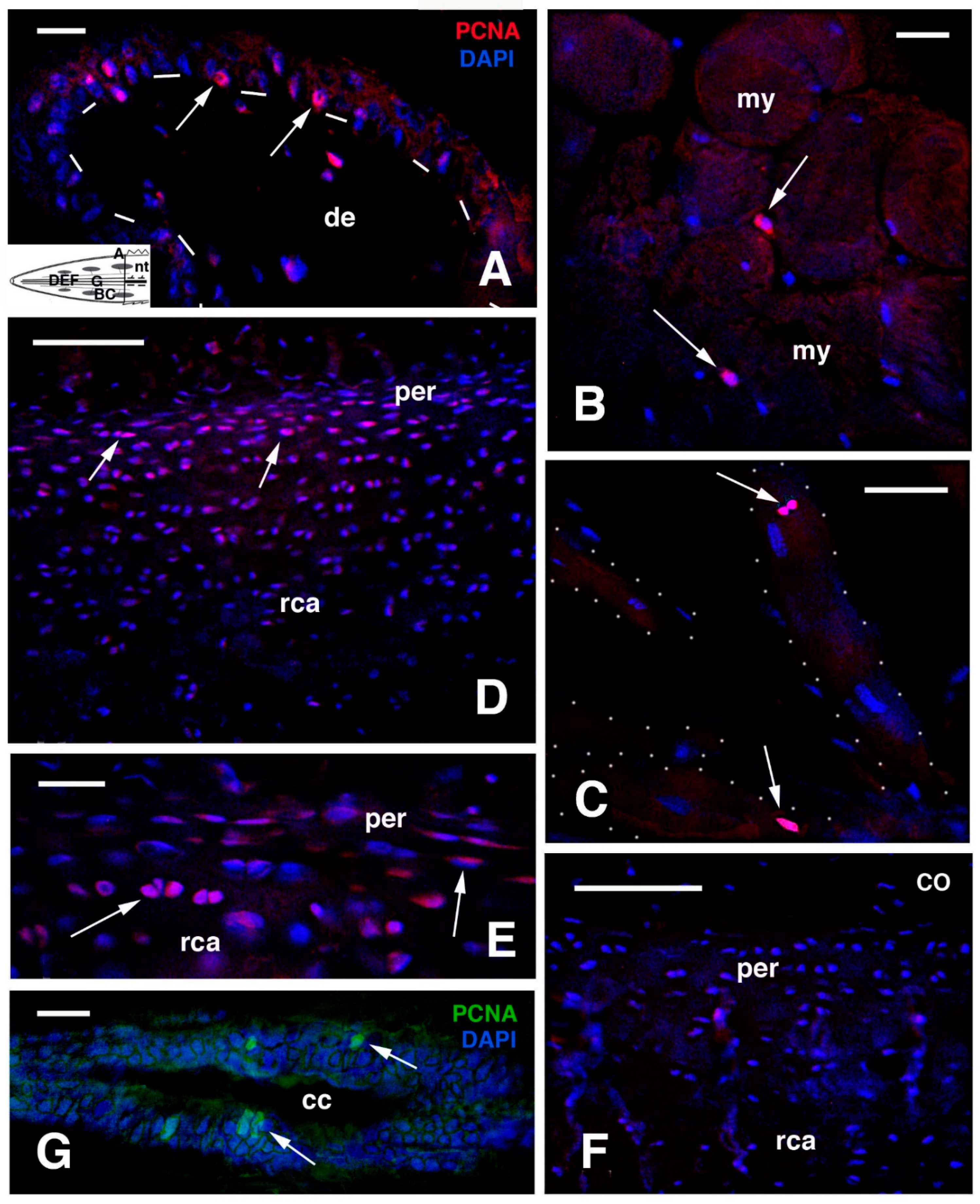
3. Immunohistochemical Considerations
Cell Proliferation in Old Regenerated Tails Supports the Concept of Regengrow
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Panini, G.P. The Prehistoric World; Treasure Press: London, UK, 1987. [Google Scholar]
- Pough, H.F.; Janis, C.M.; Heiser, J.B. Vertebrate Life; Pearson Benjamin Cummings: San Francisco, CA, USA, 2009. [Google Scholar]
- Alibardi, L. Review: Tail regeneration in lepidosauria as an exception to the generalized lack of organ regeneration in amniotes. J. Exp. Zool. B 2020, 336, 145–165. [Google Scholar] [CrossRef]
- Evans, S.E.; Jones, M.E.H. The origin, early history and diversification of lepidosauromorph reptiles. In New Aspects of Mesozoic Biodiversity Lecture Notes in Earth Sciences (132); Bandyopadhyay, S., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 27–44. [Google Scholar] [CrossRef]
- Ferguson, M.W.J.; O’Kane, S. Scar-free healing: From embryonic mechanisms to adult 235 therapeutic intervention. Philos. Trans. R. Soc. Lond. 2004, 359B, 839–850. [Google Scholar] [CrossRef]
- Gadow, H. On the reproduction of the carapax in tortoises. J. Anat. 1886, 20, 220–224. [Google Scholar]
- Howey, C.A.F.; Roosenburg, W.M. Effects of prescribed fire on the eastern box turtle (Terrapene carolina carolina). North East. Nat. 2013, 20, 493–497. [Google Scholar] [CrossRef]
- Negrini, J.; Ginel, P.J.; Novales, M.; Guerra, R.; Mozos, E. Clinical and histological findings of cutaneous wound healing in the red-eared slider turtle (Trachemys scripts elegans) housed in unheated outdoor enclosures. Vet. Dermatol. 2016, 27, 413-e106. [Google Scholar] [CrossRef]
- Smith, H.M. Total regeneration of the carapace in a box turtle. Turtox News 1958, 36, 234–238. [Google Scholar]
- Kuchling, G. Restoration of epidermal scute patterns during regeneration of the chelonian carapace. Chelonian Conserv. Biol. 1997, 2, 500–506. [Google Scholar]
- Flemming, G.J. Clinical technique: Chelonian shell repair. J. Exot. Pet. Med. 2008, 17, 246–258. [Google Scholar] [CrossRef]
- Sos, T. Two extreme cases of regeneration in Testudo graeca ibera Pallas 1814. Biharean Biologist 2012, 6, 128–131. [Google Scholar]
- Davenport, J. Regeneration of the tail spur in Testudo hermanni. Testudo 1995, 4, 79–80. [Google Scholar]
- Kuchling, G. Bifid tail regeneration in a turtle, Emydura sp. (Testudines:Chelidae). Chelonian Conserv. Biol. 2005, 4, 935–939. [Google Scholar]
- Martinez-Silvestre, A.; Soler-Massana, J. Regeneracion del caparazon en Testudo hermanni hermanni despues de un incendio forestal. Biol. Asoc. Herpetol. Esp. 2000, 11, 90–92. [Google Scholar]
- Mota-Rodrigues, J.E.; Feitosa-Silva, J.R. Phrynops tuberosus (Cotinga River toad-headed turtle); bifid tail. Herpetol. Rev. 2013, 44, 308–309. [Google Scholar]
- Reherman, M.I.; Marichal, N.; Russo, R.E.; Trujillo-Cenoz, O. Neural reconnection in the transected spinal cord of the freshwater turtle Trachemys dorbignyi. J. Comp. Neurol. 2009, 515, 197–214. [Google Scholar] [CrossRef] [PubMed]
- Reherman, M.I.; Santinaque, F.F.; Lopez-Carro, B.; Russo, R.E.; Trujillo-Cenoz, O. Cell proliferation and cytoarchitectural remodeling during spinal cord reconnection in the fresh-water turtle Trachemys dorbignyi. Cell Tissue Res. 2011, 344, 415–433. [Google Scholar] [CrossRef]
- Brazaitis, P. Maxillary regeneration in marsh crocodile, Crocodilus palustris. J. Herpetol. 1981, 15, 360–362. [Google Scholar] [CrossRef]
- Kälin, J.A. Über Skeletalanomalien bei Crocodiliden. Zeitschrift fuer Morphol. OekolgieTiere 1937, 32, 327–347. [Google Scholar]
- Rashid, D.J.; Chapman, S.C. The long and the short of tails. Dev. Dyn. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Dathe, H. Schwanz-Regeneration beim Brillenkaiman. Natur Volk. 1960, 90, 289–292. [Google Scholar]
- Xu, C.; Palade, J.; Fisher, R.E.; Smith, C.I.; Clark, A.R.; Sampson, S.; Bourgeois, R.; Rawls, A.; Elsey, R.M.; Wilson-Rawls, J.; et al. Anatomical and histological analyses reveal that tail repair is coupled with regrowth in wild-caught, juvenile American alligators (Alligator mississippiensis). Sci. Rep. 2020, 10, 20122. [Google Scholar] [CrossRef]
- Alibardi, L. Regeneration in anamniotes was replaced by regengrow and scarring in amniotes after land colonization and the evolution of terrestrial biological cycles. Dev. Dyn. 2021. [Google Scholar] [CrossRef]
- Smith, D.A.; Barker, I.K. Healing of cutaneous wounds in the common garter snake (Thamnnophis sirtalis). Can. J. Vet. Res. 1988, 52, 111–119. [Google Scholar]
- Henle, K.; Grimm-Seyfarth, A. Exceptional occurrences of double, triple and quintuple tails in an Australian lizard community, with a review of supernumerary tails in natural populations of reptiles. Salamandra 2020, 56, 373–391. [Google Scholar]
- Redi, F. Osservazione intorno agli animali viventi che si trovano negli animali viventi; Lion d’Oro: Firenze, Italy, 1684; (cited in Henle & Grimm-Seyfahrt, 2020). [Google Scholar]
- Wallach, V. Axial bifurcation and duplication in snakes. Pt 1: A synopsis of authentic and anecdotal cases. Bull. Md. Herpetol. Soc. 2007, 43, 57–95, (cited in Henle & Grimm-Seyfahrt, 2020). [Google Scholar]
- Costa, H.C.; Moura, M.R.; Feio, R.N. A tale of lost tails: Pseudoautotomy in the neotropical snake genus Drymoliber (Serpentes; Colubridae). Can. J. Zool. 2014, 92, 811–816. [Google Scholar] [CrossRef]
- Gans, C. The characteristics and affinities of the Amphisbaenia. Trans. Zool. Soc. Lond. 1978, 34, 347–416. [Google Scholar] [CrossRef]
- Guedes, J.J.M.; Costa, H.C.; Moura, M.R. A new tale of lost tails: Correlates of tail breakage in the worm lizard Amphisbaena vermicularis. Ecol. Evol. 2020, 10, 14247–14255. [Google Scholar] [CrossRef]
- Bailleul, A.M.; Hall, B.K.; Horner, J.R. Secondary cartilage revealed in a non-avian dinosaur embryo. PLoS ONE 2013, 8, e56937. [Google Scholar] [CrossRef]
- Irwin, C.R.; Ferguson, M.W.J. Fracture repair of reptilian dermal bones: Can reptiles form secondary cartilage? J. Anat. 1986, 146, 53–64. [Google Scholar]
- LeBlanc, A.R.H.; MacDougall, M.J.; Haridy, Y.; Scott, D.; Reisz, R.R. Caudal autotomy as anti-predatory behavior in palaeozoic reptiles. Sci. Rep. 2018, 8, 3328. [Google Scholar] [CrossRef]
- Bellairs, A.A.; Bryant, S.V. Autotomy and regeneration in reptiles. In Biology of the Reptilia; Gans, C., Billet, F., Maderson, P.F.A., Eds.; John Wiley & Sons: New York, NY, USA, 1985; pp. 302–410. [Google Scholar]
- Arnold, E.N. Evolutionary aspects of tail shedding in lizards and their relatives. J. Nat. Hist. 1984, 18, 127–169. [Google Scholar] [CrossRef]
- Maginnis, T.L. The cost of autotomy and regeneration in animals: A review and framework for future research. Behav. Ecol. 2006, 17, 857–872. [Google Scholar] [CrossRef]
- Dunoyer, L.A.; Seifert, A.W.; Van Cleve, J. Evolutionary bedfellows: Reconstructing the ancestral state of autotomy and regeneration. J. Exp. Zool. Mol. Dev. Evol. 2020, 336, 94–115. [Google Scholar] [CrossRef]
- Gordeev, D.A.; Ananjeva, N.B.; Korost, D.V. Autotomy and regeneration in squamate reptiles (Squamata, Reptilia): Defensive behavior strategies and morphological characteristics (using computer microtomography methods). Biol. Bull. 2020, 47, 389–398. [Google Scholar] [CrossRef]
- Werber, I. Regeneration der Kiefer bei der Eidechse Lacerta agilis. Wilhelm Roux’s Arch. Entwickl. Mechnaik 1905, 19, 248–258. [Google Scholar]
- Peacock, H.M.; Gilbert, E.A.; Vickaryous, M.K. Scar-free cutaneous wound healing in the leopard gecko, Eublepharis macularius. J. Anat. 2015, 227, 596–610. [Google Scholar] [CrossRef]
- Alibardi, L.; Meyer-Rochow, V.B. Regeneration of adhesive tail pad scales in the New Zealand gecko (Hoplodactylus maculatus) (Reptilia; Squamata; Lacertilia) can serve as an experimental model to analyze setal formation in lizards generally. Zool. Res. 2017, 38, 191–197. [Google Scholar] [CrossRef][Green Version]
- Jacyniak, K.; Vickaryous, M.K. Constitutive cardiomyocyte proliferation in the leopard gecko (Eublepharis macularius). J. Morphol. 2018, 279, 1355–1367. [Google Scholar] [CrossRef] [PubMed]
- Crnobrnja-Isailoic, J.; Corovic, J.; Halpern, B. Deliberate tail loss in Dolichophis caspius and Natrix tesselata (Serpentes: Colubridae) with a brief review of pseudoautotomy in contemporary snake families. North West. J. Zool. 2016, 12, 367–372. [Google Scholar]
- Ananjeva, N.B.; Orlov, N.I. Caudal autotomy in colubrid snake Xenochrophis piscator from Vietnam. Russ. J. Herpetol. 1994, 1, 169–171. [Google Scholar]
- Broadley, D.G. Caudal autotomy in African snakes of the genera Natriciteres Loveridge and Psammophis Boie. J. Herpetol. Assoc. Afr. 1987, 33, 18–19. [Google Scholar]
- Cooper, W.E., Jr.; Alfieri, K.J. Caudal autotomy in the eastern garter snake, Thamnophis s. sirtalis. Amphib. Reptil. 1993, 14, 86–89. [Google Scholar]
- Loveridge, A. Revision of the African snakes of the genera Dromophis and Psammophis. Bull. Mus. Comp. Zool. Harv. Coll. 1940, 87, 1–69. [Google Scholar]
- Sharma, B.D. A rare case of autotomy seen in Amphiesma stolata (Linn., Serpentes, Colubridae). Snake 1980, 12, 60. [Google Scholar]
- Mello, R.S.R.; Besson, A.A.; Hare, K.M.; Fay, V.; Smith, E.; Cree, A. Adjustment of juvenile tuatara to a cooler, southern climate: Operative temperatures, emergence behaviour, and growth rate. N. Z. J. Zool. 2013, 40, 290–303. [Google Scholar] [CrossRef]
- Cree, A. Tuatara: Biology and conservation of a venerable survivor; Canterbury University Press: Christchurch, New Zealand, 2014. [Google Scholar]
- Alibardi, L.; Meyer-Rochow, V.B. Microscopical observations on the regenerating tail in the tuatara Sphenodon punctatus indicate a tendency to scarring, but also influence from somatic growth. J. Morphol. 2019, 280, 411–422. [Google Scholar] [CrossRef]
- Batemen, P.W.; Fleming, P.A. To cut a long tail short: A review of lizard caudal autotomy studies carried out over the last 20 years. J. Zool. 2009, 277, 1–14. [Google Scholar] [CrossRef]
- Woodland, W.N.F. Some observations on caudal autotomy and regeneration in the gecko (Hemidactylus flaviviridis, Ruppel), with notes on the tails of Sphenodon and Pygopus. Q. J. Microsc. Sci. 1920, 65, 63–100. [Google Scholar]
- Ali, S.M. Studies on the comparative anatomy of the tail in sauria and rhynchocephalian Sphenodon punctatus Gray. Proc. Indian Acad. Sci. 1941, B13, 171–192. [Google Scholar]
- Seligmann, H.; Moravec, J.; Werner, Y.L. Morphological, functional and evolutionary aspects of tail autotomy and regeneration in the ‘living fossil’ Sphenodon (Reptilia, Rhynchocephalia). Biol. J. Linn. Soc. 2008, 93, 721–743. [Google Scholar] [CrossRef]
- Quattrini, D. Piano di autotomia e rigenerazione della coda nei sauri. Arch. Ital. Anat. Embriol. 1954, 59, 225–282. [Google Scholar]
- Alibardi, L.; Meyer-Rochow, V.B. Ultrastructural survey of the spinal cord of young tuataras (Sphenodon punctatus) with emphasis on the glia. N. Z. J. Zool. 1990, 17, 73–85. [Google Scholar] [CrossRef]
- Alibardi, L.; Meyer-Rochow, V.B. Comparative fine structure of the axial skeleton inside the regenerated tail of lizards and the tuatara (Sphenodon punctatus). Gegenbaurs Morphol. Jahrb. (Leipzig) 1989, 135, 705–716. [Google Scholar]
- Barr, J.I.; Boisvert, C.A.; Somaweera, R.; Trinajstic, K.; Bateman, P.W. Re-regeneration to reduce negative effects associated with tail loss in lizards. Sci. Rep. 2019, 9, 18717. [Google Scholar] [CrossRef] [PubMed]
- Nelson, N.J.; Thompson, M.B.; Pledger, S.; Keall, S.N.; Daugherty, C.H. Egg mass determines hatchling size, and incubation temperature influences post-hatching growth, of tuatara Sphenodon punctatus. J. Zool. 2004, 263, 77–87. [Google Scholar] [CrossRef]
- Alibardi, L.; Meyer-Rochow, V.B. Fine structure of regenerating caudal spinal cord in the tuatara (Sphenodon punctatus). J. Hirnforsch. 1990, 31, 613–621. [Google Scholar] [PubMed]
- Worthy, T.H.; Holdaway, R.N. The Lost World of the Moa: Prehistoric Life of New Zealand; Indiana University Press: Bloomington, IN, USA, 2002; p. 718. ISBN 13-978-0253340344. [Google Scholar]
- Meyer-Rochow, V.B.; Wohlfahrt, S.; Ahnelt, P.K. Photoreceptor cell types in the retina of the tuatara (Sphenodon punctatus) have cone characteristics. Micron 2005, 36, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Rochow, V.B. Observations on the behaviour of young tuatara Sphenodon punctatus in conditions of total darkness. Tuatara 1988, 30, 36–38. [Google Scholar]
- Meyer-Rochow, V.B.; The, K.L. Visual predation by tuatara (Sphenodon punctatus) on the beach beetle Chaerodestrachyscelides as a selective force in the production of distinct colour morphs. Tuatara 1991, 31, 1–8. [Google Scholar]
- Alibardi, L.; Meyer-Rochow, V.B. General and specific microscopic characteristics of the dorsal tail scales and the spines of the crest in the tuatara Sphenodon pucntatus (Reptilia; Rhynchocephalia; Sphenodontidae). Micron 2020, 137, 102909. [Google Scholar] [CrossRef]
- Alibardi, L. Electron microscopic observations on the myelination of the long-term regenerated caudal spinal cord in lizards and Sphenodon. Biol. Struct. Morphog. 1990, 3, 147–158. [Google Scholar]
- Alibardi, L.; Maderson, P.F.A. Observations on the histochemistry and ultrastructure of the epidermis of the tuatara, Sphenodon punctatus (Sphenodontida, Lepidosauri, Reptiles): A contribution to an understanding of the lepidosaurian epidermal generation and the evolutionary origin of the squamate shedding complex. J. Morphol. 2003, 256, 111–133. [Google Scholar]
- Alibardi, L.; Maderson, P.F.A. Observations on the histochemistry and ultrastructure of regenerating caudal epidermis of the tuatara Sphenodon punctatus (Sphenodontida, Lepidosauria, Reptilia). J. Morphol. 2003, 256, 134–145. [Google Scholar] [CrossRef]
- Alibardi, L. The regenerating tail blastema of lizards as a model to study organ regeneration and tumor growth regulation in amniotes. Anat. Rec. 2019, 302, 1469–1490. [Google Scholar] [CrossRef]
- Dawbin, W.H. The tuatara Sphenodon punctatus: Aspects of life history, growth and longevity. In New Zealand Herpetology; Newman, D.G., Ed.; New Zealand Wildlife Service Occasional Publication Nr 2: Wellington, New Zeeland, 1982; pp. 237–250. [Google Scholar]
- Elias, P.M.; Wood, L.C.; Feingold, K.R. Epidermal pathogenesis of inflammatory dermatoses. Am. J. Contact Dermat. 1999, 10, 119–126. [Google Scholar]
- Alibardi, L. Ultrastructural immunolocalization of antimicrobial peptides targeting bacteria in the corneous layer supports the presence of an anti-microbial barrier in reptilian epidermis. J. Tissue Biol. Cytol. 2016, 1, 1–7. [Google Scholar]
- Maderson, P.F.A. Observations on the epidermis of the tuatara (Sphenodon punctatus). J. Anat. 1968, 103, 311–320. [Google Scholar] [PubMed]
- Maderson, P.F.A.; Flaxman, B.A.; Roth, S.I.; Szabo, G. Ultrastructural contributions to the identification of cell types in the lizard epidermal generation. J. Morphol. 1972, 136, 191–209. [Google Scholar] [CrossRef] [PubMed]
- Alibardi, L. Original and regenerating lizard tail cartilage contain putative resident stem/progenitor cells. Micron 2015, 78, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Maderson, P.F.A.; Rabinowitz, T.; Tandler, B.; Alibardi, L. Ultrastructural contributions to an understanding of the cellular mechanisms involved in lizard skin shedding with comments on the function and evolution of a unique lepidosaurian phenomenon. J. Morphol. 1998, 236, 1–24. [Google Scholar] [CrossRef]
- Wu, P.; Lai, Y.-C.; Widelitz, R.; Chuong, C.-M. Comprehensive molecular and cellular studies suggest avian scutate scales are secondarily derived from feathers, and more distant from reptilian scales. Sci. Rep. 2018, 8, 16766. [Google Scholar] [CrossRef]
- Lozito, T.P.; Tuan, R.S. Lizard tail skeletal regeneration combines aspects of fracture healing and blastema-based regeneration. Development 2016, 143, 2946–2957. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alibardi, L.; Meyer-Rochow, V.B. Regeneration in Reptiles Generally and the New Zealand Tuatara in Particular as a Model to Analyse Organ Regrowth in Amniotes: A Review. J. Dev. Biol. 2021, 9, 36. https://doi.org/10.3390/jdb9030036
Alibardi L, Meyer-Rochow VB. Regeneration in Reptiles Generally and the New Zealand Tuatara in Particular as a Model to Analyse Organ Regrowth in Amniotes: A Review. Journal of Developmental Biology. 2021; 9(3):36. https://doi.org/10.3390/jdb9030036
Chicago/Turabian StyleAlibardi, Lorenzo, and Victor Benno Meyer-Rochow. 2021. "Regeneration in Reptiles Generally and the New Zealand Tuatara in Particular as a Model to Analyse Organ Regrowth in Amniotes: A Review" Journal of Developmental Biology 9, no. 3: 36. https://doi.org/10.3390/jdb9030036
APA StyleAlibardi, L., & Meyer-Rochow, V. B. (2021). Regeneration in Reptiles Generally and the New Zealand Tuatara in Particular as a Model to Analyse Organ Regrowth in Amniotes: A Review. Journal of Developmental Biology, 9(3), 36. https://doi.org/10.3390/jdb9030036






