In Utero Programming of Testicular Cancer
Abstract
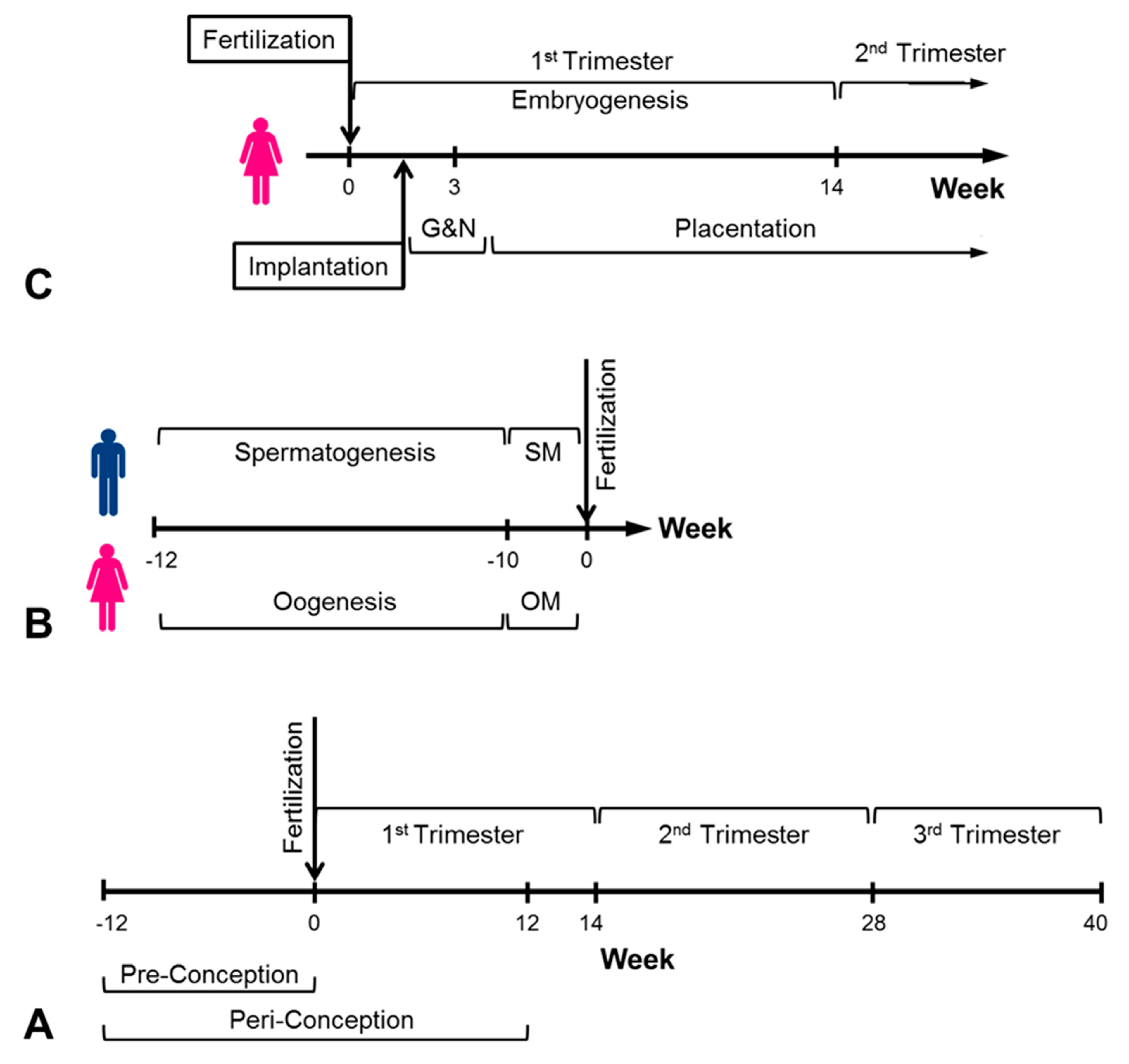
1. Introduction
2. Testicular Germ Cell Cancer
3. Testicular Cancer in Young Adults
4. In Utero Conditions and Cancer
5. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mahabir, S.; Aagaard, K.; Anderson, L.M.; Herceg, Z.; Hiatt, R.A.; Hoover, R.N.; Linet, M.S.; Medina, D.; Potischman, N.; Tretli, S.; et al. Challenges and opportunities in research on early-life events/exposures and cancer development later in life. Cancer Causes Control 2012, 23, 983–990. [Google Scholar] [CrossRef]
- Stephansson, O.; Wahnström, C.; Pettersson, A.; Sørensen, H.T.; Tretli, S.; Gissler, M.; Troisi, R.; Akre, O.; Grotmol, T. Perinatal risk factors for childhood testicular germ-cell cancer: A Nordic population-based study. Cancer Epidemiol. 2012, 35, e100–e104. [Google Scholar] [CrossRef]
- Faruqui, N.; Kummrow, A.; Fu, B.; Divieto, C.; Rojas, F.; Kisulu, F.; Cavalcante, J.; Wang, J.; Campbell, J.; Martins, J.L.; et al. Cellular Metrology: Scoping for a Value Proposition in Extra- and Intracellular Measurements. Front. Bioeng. Biotechnol. 2020, 7, 456. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.J.; Kim, Y.J. What is fetal programming? A lifetime health is under the control of in utero health. Obstet. Gynecol. Sci. 2017, 60, 506–519. [Google Scholar] [CrossRef] [PubMed]
- Duncan, J.M. On puerperal diabetes. Trans. Obstet. Soc. Lob. 1882, 24, 256–285. [Google Scholar]
- Lecture, F.N.B. Of pregnancy and progeny. Diabetes 1980, 29, 1023–1035. [Google Scholar]
- Strauss, R.S. Effects of the intrauterine environment on childhood growth. Br. Med Bull. 1997, 53, 81–95. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Hanson, M.; Cooper, C.; Thornburg, K. Effect of In Utero and Early-Life Conditions on Adult Health and Disease. N. Engl. J. Med. 2008, 359, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Tzahor, E.; Poss, K.D. Cardiac regeneration strategies: Staying young at heart. Science 2017, 356, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Kim, J.; Elghiaty, A.; Ham, W.S. Recent global trends in testicular cancer incidence and mortality. Medicine 2018, 97, e12390. [Google Scholar] [CrossRef]
- Gurney, J.K.; Florio, A.A.; Znaor, A.; Ferlay, J.; Laversanne, M.; Sarfati, D.; Bray, F.; McGlynn, K.A. International Trends in the Incidence of Testicular Cancer: Lessons from 35 Years and 41 Countries. Eur. Urol. 2019, 76, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Harper, A.; Ruan, Y.; Barr, R.; Frazier, A.L.; Ferlay, J.; Steliarova-Foucher, E.; Fidler-Benaoudia, M.M. International Trends in the Incidence of Cancer Among Adolescents and Young Adults. J. Natl. Cancer Inst. 2020, 112, 1105–1117. [Google Scholar] [CrossRef]
- Moch, H.; Cubilla, A.L.; Humphrey, P.A.; Reuter, V.E.; Ulbright, T.M. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs—Part A: Renal, Penile, and Testicular Tumours. Eur. Urol. 2016, 70, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Dorssers, L.C.J.; Gillis, A.J.M.; Stoop, H.; Van Marion, R.; Nieboer, M.M.; van Riet, J.; Van De Werken, H.J.; Oosterhuis, J.W.; De Ridder, J.; Looijenga, L.H.J. Molecular heterogeneity and early metastatic clone selection in testicular germ cell cancer development. Br. J. Cancer 2019, 120, 444–452. [Google Scholar] [CrossRef]
- Oosterhuis, J.W.; Looijenga, L. Testicular germ-cell tumours in a broader perspective. Nat. Rev. Cancer 2005, 5, 210–222. [Google Scholar] [CrossRef]
- Ulbright, T.M. Recently Described and Clinically Important Entities in Testis Tumors: A Selective Review of Changes Incorporated Into the 2016 Classification of the World Health Organization. Arch. Pathol. Lab. Med. 2019, 143, 711–721. [Google Scholar] [CrossRef]
- Idrees, M.T.; Kao, C.S.; Epstein, J.I.; Ulbright, T.M. Nonchoriocarcinomatous Trophoblastic Tumors of the Testis: The Widening Spectrum of Trophoblastic Neoplasia. Am. J. Surg. Pathol. 2015, 39, 1468–1478. [Google Scholar] [CrossRef]
- Razafimahefa, J.; Gosset, C.; Culine, S.; Mongiat-Artus, P.; Verine, J. Placental Site Trophoblastic Tumor in Nonseminomatous Mixed Germ Cell Tumors of the Testis: A Case Report and Review of the Literature. Clin. Genitourin. Cancer 2018, 16, e349–e354. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Zheng, W.; Liang, Q.; Yin, T. Diagnosis and treatment of placental site trophoblastic tumor. Int. J. Clin. Exp. Pathol. 2013, 6, 1448–1451. [Google Scholar]
- Baird, D.C.; Meyers, G.J.; Hu, J.S. Testicular Cancer: Diagnosis and Treatment. Am. Fam. Physician 2018, 97, 261–268. [Google Scholar] [PubMed]
- Saltzman, A.F.; Cost, N.G. Adolescent and Young Adult Testicular Germ Cell Tumors: Special Considerations. Adv. Urol. 2018, 31, 2375176. [Google Scholar] [CrossRef] [PubMed]
- Ghazarian, A.A.; Kelly, S.P.; Altekruse, S.F.; Rosenberg, P.S.; McGlynn, K.A. Future of testicular germ cell tumor incidence in the United States: Forecast through 2026. Cancer 2017, 123, 2320–2328. [Google Scholar] [CrossRef] [PubMed]
- Ulytė, A.; Ulys, A.; Sužiedėlis, K.; Patašius, A.; Smailytė, G. Testicular cancer in two brothers of a quadruplet: A case report and a review of literature. Acta Med. Litu. 2017, 24, 12–17. [Google Scholar] [CrossRef]
- Grotmol, T.; Weiderpass, E.; Tretli, S. Conditions in utero and cancer risk. Eur. J. Epidemiol. 2006, 21, 561–570. [Google Scholar] [CrossRef][Green Version]
- Ekbom, A. Growing evidence that several human cancers may originate in utero. Semin. Cancer Biol. 1998, 8, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Timms, B.G.; Howdeshell, K.L.; Barton, L.; Bradley, S.; Richter, C.; Saal, F.S.V. Estrogenic chemicals in plastic and oral contraceptives disrupt development of the fetal mouse prostate and urethra. Proc. Natl. Acad. Sci. USA 2005, 102, 7014–7019. [Google Scholar] [CrossRef]
- Morimoto, L.M.; Zava, D.; McGlynn, K.A.; Stanczyk, F.Z.; Kang, A.Y.; Ma, X.; Wiemels, J.L.; Metayer, C. Neonatal Hormone Concentrations and Risk of Testicular Germ Cell Tumors (TGCT). Cancer Epidemiol. Biomark. Prev. 2018, 27, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.B.; Akre, O.; Forman, D.; Madigan, M.P.; Richiardi, L.; McGlynn, K.A. A systematic review and meta-analysis of perinatal variables in relation to the risk of testicular cancer—Experiences of the son. Int. J. Epidemiol. 2010, 39, 1605–1618. [Google Scholar] [CrossRef]
- Myatt, L. Placental adaptive responses and fetal programming. J. Physiol. 2006, 572, 25–30. [Google Scholar] [CrossRef]
- Desoye, G. The Human Placenta in Diabetes and Obesity: Friend or Foe? The 2017 Norbert Freinkel Award Lecture. Diabetes Care 2018, 41, 1362–1369. [Google Scholar] [CrossRef] [PubMed]
- Fall, C.H.D.; Kumaran, K. Metabolic programming in early life in humans. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20180123. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Tain, Y.L. The good, the bad, and the ugly of pregnancy nutrients and developmental programming of adult disease. Nutrients 2019, 11, 894. [Google Scholar] [CrossRef]
- Oosterhuis, J.W.; Looijenga, L.H.J. Human germ cell tumours from a developmental perspective. Nat. Rev. Cancer 2019, 19, 522–537. [Google Scholar] [CrossRef]
- Christoforou, E.R.; Sferruzzi-Perri, A.N. Molecular mechanisms governing offspring metabolic programming in rodent models of in utero stress. Cell Mol. Life Sci. 2020, 77, 4861–4898. [Google Scholar] [CrossRef]
- Stanner, S.A.; Yudkin, J.S. Fetal programming and the Leningrad Siege study. Twin Res. 2001, 4, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Painter, R.C.; Roseboom, T.J.; Bleker, O.P. Prenatal exposure to the Dutch famine and disease in later life: An overview. Reprod. Toxicol. 2005, 20, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Roseboom, T.J.; Painter, R.C.; van Abeelen, A.F.; Veenendaal, M.V.; de Rooij, S.R. Hungry in the womb: What are the consequences? Lessons from the Dutch famine. Maturitas 2011, 70, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Bianco-Miotto, T.; Craig, J.M.; Gasser, Y.P.; van Dijk, S.J.; Ozanne, S.E. Epigenetics and DOHaD: From basics to birth and beyond. J. Dev. Orig. Health Dis. 2017, 8, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Heijmans, B.T.; Tobi, E.; Stein, A.; Putter, H.; Blauw, G.J.; Susser, E.S.; Slagboom, P.; Lumey, L.H. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc. Natl. Acad. Sci. USA 2008, 105, 17046–17049. [Google Scholar] [CrossRef]
- Tobi, E.; Goeman, J.; Monajemi, R.; Gu, H.; Putter, H.; Zhang, Y.; Slieker, R.; Stok, A.P.; Thijssen, P.E.; Müller, F.; et al. DNA methylation signatures link prenatal famine exposure to growth and metabolism. Nat. Commun. 2014, 5, 5592. [Google Scholar] [CrossRef]
- Amatruda, J.F.; Ross, J.A.; Christensen, B.; Fustino, N.J.; Chen, K.S.; Hooten, A.J.; Nelson, H.; Kuriger, J.K.; Rakheja, D.; Frazier, A.L.; et al. DNA methylation analysis reveals distinct methylation signatures in pediatric germ cell tumors. BMC Cancer 2013, 13, 313. [Google Scholar] [CrossRef]
- Moreno-Fernandez, J.; Ochoa, J.J.; Lopez-Frias, M.; Diaz-Castro, J. Impact of Early Nutrition, Physical Activity and Sleep on the Fetal Programming of Disease in the Pregnancy: A Narrative Review. Nutrients 2020, 12, 3900. [Google Scholar] [CrossRef] [PubMed]
- Elad, D.; Jaffa, A.J.; Grisaru, D. Biomechanics of Early Life in the Female Reproductive Tract. Physiology 2020, 35, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Takakuwa, T. 3D Analysis of Human Embryos and Fetuses Using Digitized Datasets From the Kyoto Collection. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2018, 301, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Padhee, M.; Zhang, S.; Lie, S.; Wang, K.C.; Botting, K.J.; McMillen, I.C.; MacLaughlin, S.M.; Morrison, J.L. The Periconceptional Environment and Cardiovascular Disease: Does In Vitro Embryo Culture and Transfer Influence Cardiovascular Development and Health? Nutrients 2015, 7, 1378–1425. [Google Scholar] [CrossRef] [PubMed]
- van Uitert, E.M.; Van Der Elst-Otte, N.; Wilbers, J.J.; Exalto, N.; Willemsen, S.P.; Eilers, P.H.C.; Koning, A.H.J.; Steegers, E.A.P.; Steegers-Theunissen, R.P.M. Periconception maternal characteristics and embryonic growth trajectories: The Rotterdam Predict study. Hum. Reprod. 2013, 28, 3188–3196. [Google Scholar] [CrossRef]
- Louis, G.M.; Cooney, M.A.; Lynch, C.D.; Handal, A. Periconception window: Advising the pregnancy-planning couple. Fertil. Steril. 2008, 89, e119–e121. [Google Scholar] [CrossRef] [PubMed]
- Hafizi, L.; Asgarieh, E.R.; Taheri, N.; Ghomian, N. Successful Management of Spontaneous Quadruplet Pregnancy: A Case Report. J. Fam. Reprod. Health 2018, 12, 173–176. [Google Scholar]
- Schlueter, R.; Arnett, C.; Huang, C.; Burlingame, J. Successful quintuplet pregnancy of monochorionic male quadruplets and single female after double embryo transfer: Case report and review of the literature. Fertil. Steril. 2018, 109, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Baroni, T.; Arato, I.; Mancuso, F.; Calafiore, R.; Luca, G. On the Origin of Testicular Germ Cell Tumors: From Gonocytes to Testicular Cancer. Front. Endocrinol. 2019, 10, 343. [Google Scholar] [CrossRef]
- Das, M.K.; Kleppa, L.; Haugen, T.B. Functions of genes related to testicular germ cell tumour development. Andrology 2019, 7, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Bo, H.; Cao, K.; Tang, R.; Zhang, H.; Gong, Z.; Liu, Z.; Liu, J.; Li, J.; Fan, L. A network-based approach to identify DNA methylation and its involved molecular pathways in testicular germ cell tumors. J. Cancer 2019, 10, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Myers, K.M.; Elad, D. Biomechanics of the human uterus. Wiley Interdiscip. Rev. Syst. Biol. Med. 2017, 9, e1388. [Google Scholar] [CrossRef]
- Mammoto, T.; Mammoto, A.; Ingber, D.E. Mechanobiology and Developmental Control. Annu. Rev. Cell Dev. Biol. 2013, 29, 27–61. [Google Scholar] [CrossRef] [PubMed]
- Le Noble, F.; Moyon, D.; Pardanaud, L.; Yuan, L.; Djonov, V.; Matthijsen, R.; Bréant, C.; Fleury, V.; Eichmann, A. Flow regulates arterial-venous differentiation in the chick embryo yolk sac. Development 2004, 131, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Hove, J.R.; Köster, R.W.; Forouhar, A.S.; Acevedo-Bolton, G.; Fraser, S.E.; Gharib, M. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature 2003, 421, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Hove, J.R. Quantifying cardiovascular flow dynamics during early development. Ped. Res. 2006, 60, 6–13. [Google Scholar] [CrossRef]
- Critchley, H.O.D.; Maybin, J.A.; Armstrong, G.M.; Williams, A.R.W. Physiology of the Endometrium and Regulation of Menstruation. Physiol. Rev. 2020, 100, 1149–1179. [Google Scholar] [CrossRef]
- Kamm, R.D.; Bashir, R.; Arora, N.; Dar, R.D.; Gillette, M.U.; Griffith, L.G.; Kemp, M.L.; Kinlaw, K.; Levin, M.; Martin, A.C.; et al. Perspective: The promise of multicellular engineered living systems. APL Bioeng. 2018, 2, 040901. [Google Scholar] [CrossRef]
- Biechonski, S.; Gourevich, D.; Rall, M.; Aqaqe, N.; Yassin, M.; Zipin-Roitman, A.; Trakhtenbrot, L.; Olender, L.; Raz, Y.; Jaffa, A.J.; et al. Quercetin alters the DNA damage response in human hematopoietic cells via TopoII- and PI3K- dependent mechanisms synergizing in leukemogenic rearrangements. Int. J. Cancer 2017, 140, 864–876. [Google Scholar] [CrossRef] [PubMed]
- Makoolati, Z.; Movahedin, M.; Forouzandeh-Moghadam, M. Proliferation in culture of primordial germ cells derived from embryonic stem cell: Induction by retinoic acid. Biosci. Rep. 2016, 36. [Google Scholar] [CrossRef] [PubMed]
- Gell, J.; Liu, W.; Sosa, E.; Chialastri, A.; Hancock, G.; Tao, Y.; Wamaitha, S.; Bower, G.; Dey, S.S.; Clark, A.T. An Extended Culture System that Supports Human Primordial Germ Cell-like Cell Survival and Initiation of DNA Methylation Erasure. Stem Cell Rep. 2020, 14, 433–446. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elad, D.; Jaffa, A.J.; Grisaru, D.; Leibovitch, I. In Utero Programming of Testicular Cancer. J. Dev. Biol. 2021, 9, 35. https://doi.org/10.3390/jdb9030035
Elad D, Jaffa AJ, Grisaru D, Leibovitch I. In Utero Programming of Testicular Cancer. Journal of Developmental Biology. 2021; 9(3):35. https://doi.org/10.3390/jdb9030035
Chicago/Turabian StyleElad, David, Ariel J. Jaffa, Dan Grisaru, and Ilan Leibovitch. 2021. "In Utero Programming of Testicular Cancer" Journal of Developmental Biology 9, no. 3: 35. https://doi.org/10.3390/jdb9030035
APA StyleElad, D., Jaffa, A. J., Grisaru, D., & Leibovitch, I. (2021). In Utero Programming of Testicular Cancer. Journal of Developmental Biology, 9(3), 35. https://doi.org/10.3390/jdb9030035





