1. Introduction
Adherens junctions establish the apical domain of epithelia through the connection of the Cadherin complex to filamentous actin (F-actin). Recent studies have shown that the connection between Cadherin-based apical junctions and F-actin is surprisingly dynamic. Our in vivo studies in
C. elegans have shown that WAVE-dependent branched F-actin contributes to the apical junctions, during their establishment, and during their maintenance [
1,
2]. To address precisely how branched actin promotes adhesion and polarity through Cadherin will require further development of in vivo models where endogenous Cadherin can be visualized and where temporal depletion of branched actin regulators is possible.
We have used the
C. elegans embryo as a model for understanding the polarized forces of morphogenesis. Genetic screens led us to focus on one major driver of embryonic morphogenesis, in
C. elegans and other organisms, the WAVE Complex. WAVE is a nucleation promoting factor (NPF) that creates branched F-actin by activating the Arp2/3 complex. WAVE is the major NPF that turns on branched actin through Arp2/3 during
C. elegans and Drosophila embryonic morphogenesis, despite that fact that other NPFs act in embryos, including WASP [
1,
3,
4]. WAVE is itself under tight regulation, and must be recruited to membranes to act. We have identified signals at the membrane that activate the GTPase Rac1/CED-10, which in turn binds and activates WAVE, so it can promote branched actin through Arp2/3 [
5].
The
C. elegans apical junction (CeAJ) is composed of at least two functionally distinct complexes, yet has a single electron dense junctional complex, compared to two in vertebrates and
Drosophila (reviewed in [
6]). The more apical Cadherin Catenin Complex (CCC) contains
C. elegans homologs of E-Cadherin/HMR-1, beta-catenin/HMP-2 and alpha-catenin/HMP-1 [
7]. The more basally localized DAC junction contains homologs of Discs Large/DLG-1 and AJM-1 [
8,
9,
10].
C. elegans has a homolog of the tight junction component ZO-1, ZOO-1, that is recruited to the Cadherin junction by E-Cadherin/HMR-1, but it does not appear to be required for paracellular gate function [
11,
12]. The apical-most complex in
C. elegans is the Cadherin complex, not a separate Tight Junction. Therefore, to understand the apical junction we focus here on
C. elegans E-Cadherin, which we will refer to hereafter as Cadherin/HMR-1.
We previously showed that in the embryonic intestine, WAVE complex components are enriched just apical to the CeAJ, suggesting WAVE is at the correct place to support junctions [
1,
13]. Paradoxically, in embryos, loss of WAVE leads to excess Cadherin accumulation, coupled with decreased apical F-actin [
2]. To understand these complex results, it helps to consider that branched actin may play multiple roles in regulating epithelial Cadherin. Actomyosin sets up the tension that maintains apical Cadherin [
14,
15,
16]. While actomyosin appears to mainly include linear actin, in human umbilical vein endothelial cells (HUVECs), platinum replica electron microscopy (PREM) illustrated that actomyosin linear fibers are connected to VE-Cadherin through branched actin [
17]. Thus, one function for branched actin at apical junctions may be to link the Cadherin complex to actomyosin in the apical actin ring that supports polarized epithelia.
A second proposed role for branched actin at junctions to push adult epithelial membranes together, for example when individual cells join an epithelium, and to repair breaks in adhesion [
18,
19,
20]. Branched actin may help maintain epithelial Cadherin in adult tissues that need to repair damage. For example, branched-actin dependent microspikes were reported in MDCK and Caco-2 cells, and proposed to permit repair of breaks in lateral adhesion [
21]. Our studies provide evidence that WAVE and Cadherin maintain apical junctions in an organismal setting. In an adult epithelium, the
C. elegans intestine, we used the F-actin dye phalloidin to show that regulators of branched actin (including WAVE), of linear actin (including the formin Diaphonous/CYK-1) and the Cadherin complex support the apically enriched F-actin of the adult intestine, while their loss reduced apical F-actin and resulted in an expanded apical lumen [
1]. However, effects at lateral membranes were not examined.
Cadherin is known to regulate adhesion, yet in
C. elegans, embryonic loss of Cadherin does not eliminate overall adhesion, likely due to redundancy with other adhesive molecules [
7]. However, embryonic Cadherin contributes to apicobasal polarity: it is needed for apical F-actin enrichment in the developing intestine [
1,
2] and PAR-dependent apicobasal polarity in blastomeres [
22]. We have found a shared role for Cadherin and the WAVE branched actin regulator in establishing embryonic apical junctions, and the proteins promote each other’s embryonic apical enrichment. In adults, we showed using RNAi depletion and electron microscopy (EM) that Cadherin and WAVE help maintain apical junctions [
2]. However, the subcellular distribution of adult Cadherin in a tissue that permits dissection of apicobasal distribution and regulation has not been reported.
Apical–basal polarity in the epithelia is supported by polarized phospholipids, major components of biological membranes, which are themselves polarized by the distribution of polarity regulators. For example, some phosphoinositides (PI) are enriched at apical regions, where they help recruit membrane associated proteins, and help set up subcellular trafficking compartments (reviewed in Krahn 2020). Thus, if Cadherin and WAVE are mutually setting up polarity, changes in Cadherin or WAVE membrane enrichment may be reflected in changes in the distribution of phosphoinositides, like PI(4,5)P2, which is polarized to apical membranes, where it promotes polarized vesicle transport (Thapa and Anderson 2012).
To determine if WAVE-dependent membrane enrichment of Cadherin documented in embryos also occurs in post-embryonic larval and adult epithelia, and to explore possible mechanisms that contribute to this regulation, here we analyzed the membrane enrichment of Cadherin/HMR-1 that was fused to a fluorescent protein at its naturally occurring chromosomal location using CRISPR technology [
23]. We show that the distribution of Cadherin/HMR-1 in larval and adult intestines is highly polarized, and depends on WAVE. We generate a new transgenic strain to monitor apical F-actin in the post-embryonic intestine, to test for a shared role for WAVE and Cadherin in maintaining this epithelial apical F-actin. Analysis of the lateral junction suggests WAVE is particularly relevant to supporting the apicolateral pool of Cadherin. Live imaging and EM analysis of the larger postembryonic intestinal cells, relative to embryonic cells, reveals altered lateral membranes, and reduced recruitment of apical phosphoinositides in epithelia depleted of Cadherin or WAVE components. The data shown here support our hypothesis that maintenance of correct Cadherin localization is dependent on WAVE-dependent branched actin and suggest future directions to address the mechanism.
2. Materials and Methods
C. elegans strains built for this paper: To build intestinal F-actin strain OX966 Pglo-1::Lifeact::TAG::RFP; pRF4 rol-6(su1006), we cloned Lifeact::TAG::RFP from clone pDS355 (gift from Daniel Shaye) behind the intestinal glo-1 promoter (gift from Greg Hermann). The plasmid was verified by sequencing, and injected according to Mello et al., 1991. After UV-TMP integration we selected a strain with consistent rolling and Lifeact signal in the intestine.
The following strains which contain fluorescent proteins fused at naturally occurring chromosomal locations using CRISPR technology:
LP238 hmr-1::mKate2, and
LP431 gfp::3xFLAG-gex-3 [
23] as well as
RT1120 Pvha-6::PH::gfp (Barth Grant, Rutgers University) were combined to create doubly marked strains:
OX970 Pvha-6::PH::gfp; hmr-1::mKate2, OX974 hmr-1::mKate2; gfp::3xFLAG-gex-3 and
OX975 Pglo-1::Lifeact::TAG::RFP; Pvha-6::PH::gfp.
RNAi experiments: All RNAi bacterial strains used in this study were administered by the feeding protocol as in [
5]. RNAi feeding experiments were performed at 23 °C unless otherwise mentioned. Worms were synchronized and transferred onto seeded plates containing RNAi-expressing bacteria, or “Control” bacteria carrying the RNAi vector L4440 with no gene inserted. Effectiveness of the RNAi was monitored after 2 days using two methods, and worms were imaged on day 3 if both methods showed an effect. (1) We counted the percent dead embryos, which after two days is expected at >90% for
gex-3, gex-2 or arp-2, and at least 40% for
hmr-1, 50% for
hmp-2 and 20% for
hmp-1. (2) We monitored post-embryonic silencing of a gfp-tagged strain in the intestine, such as
gfp::gex-3 or
hmr-1::gfp by the corresponding RNAi. RNAi experiments with the expected embryonic lethality, and knock-down of the gfp signal of at least 75% for
gex-3 or
hmr-1, 24% for
hmp-2 and 5% for
hmp-1 were used. RNAi controls are included in
Supplemental Figure S1.
Live Imaging: Imaging was performed in a temperature-controlled room set to 23 °C on a Laser Spinning Disk Confocal Microscope with a Yokogawa scan head, on a Zeiss AxioImager Z1 Microscope using the Plan-Apo 63X/1.4NA or Plan-Apo 40X/1.3NA oil lenses. Images were captured on a Hamatsu CMOS Camera using MetaMorph software, and analyzed using ImageJ. Controls and mutants were imaged within 3 days of each other with the same imaging conditions. All measurements were performed on raw data using ImageJ. For fluorescent measurements, background intensity was subtracted by using a box or line of the same size and measuring average intensity in the same focal plane, near the animal.
Microscopy of adults: Young adult stage animals (one day after L4 stage) and L4 larvae were placed on agar pads in M9 solution and immobilized using 10 μL of Levamizole(10 μm) salts and covered with 1.5 um coverslips. Images were taken within 15 min of making the pads. Imaging was performed on the Zeiss AxioImager Z1 with a Yokogawa CSUX1-5000 spinning disc, using the Plan Apo 63X/1.4NA Oil lens. Images were analyzed and measured using ImageJ.
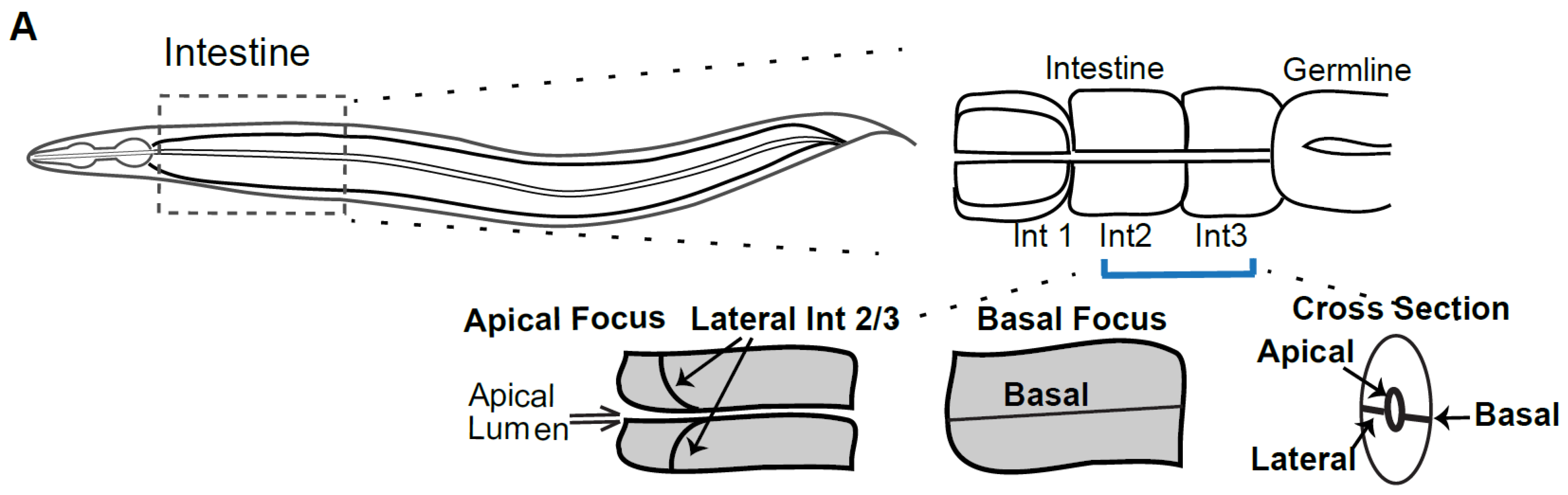
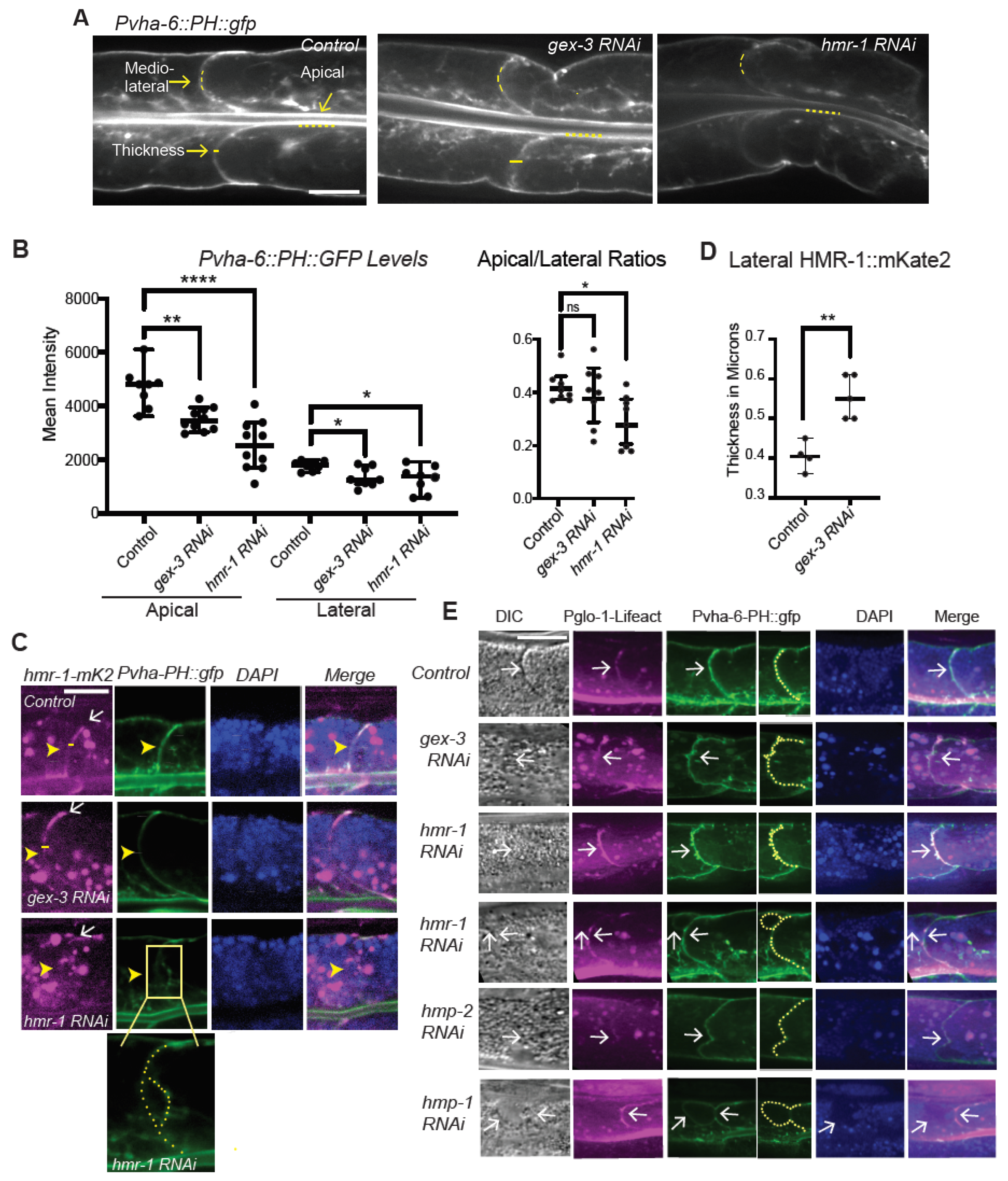
Quantitation of immunofluorescence: Quantitation of live fluorescence was performed using the line selection and the dynamic profile function of ImageJ to measure fluorescence along lines of equal length along, for example, the apical intestine or lateral regions of the intestinal cells, as indicated in
Figure 1,
Figure 2 and
Figure 3. For all experiments shown, the images were captured at the same exposure settings for controls and mutants. All quantitation was performed on the raw images. The figure legends indicate when images were enhanced for contrast, and the same enhancement was applied to a mosaic of the related images for that experiment.
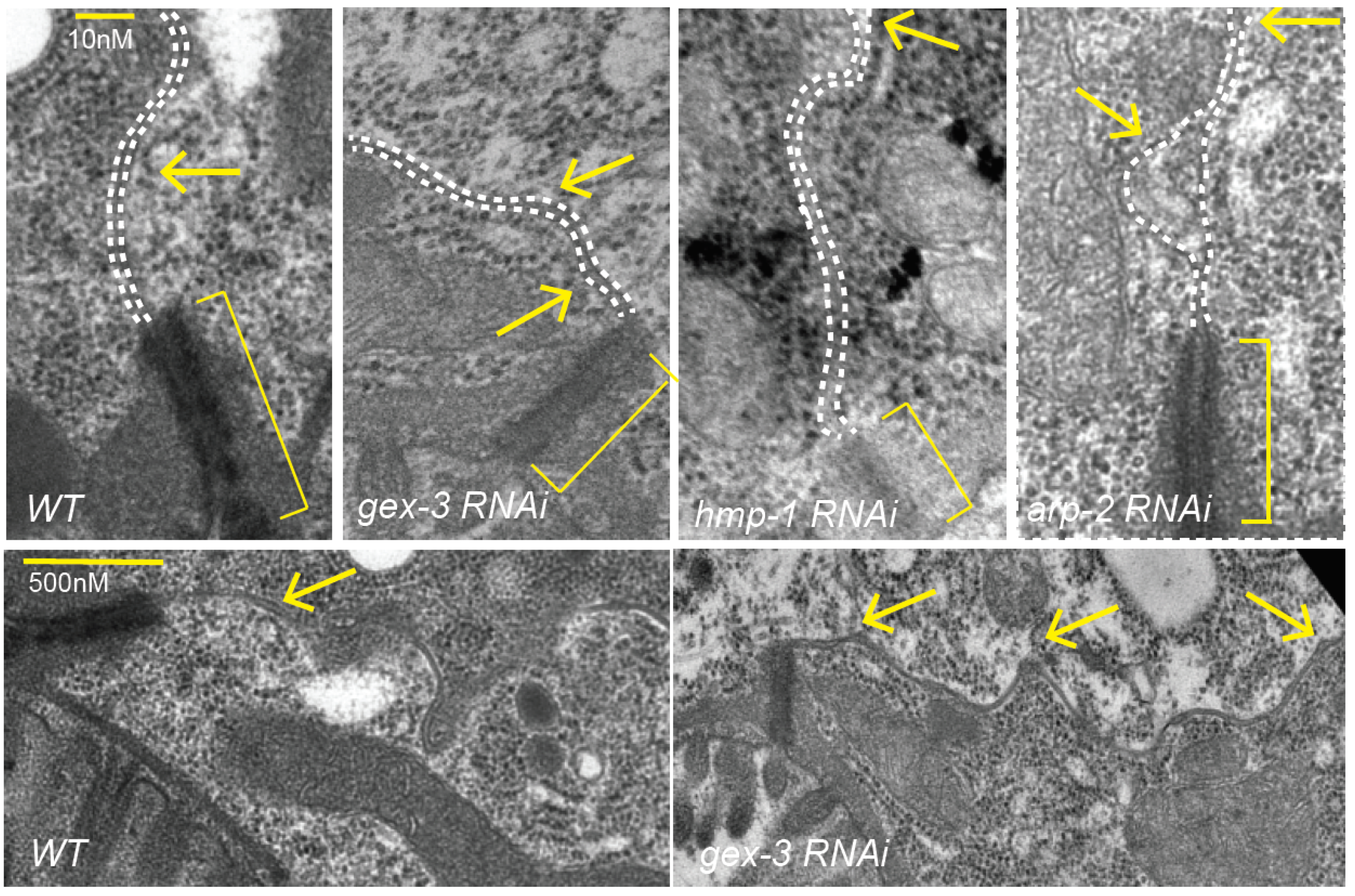
Electron Microscopy: was performed as described in [
1] but adapted for adults.
Synchronized L1s were plated on control food or on RNAi food for 2d for arp-2, 3d for others. Samples were washed in M9 Buffer, anesthetized with 10 mM levamisole, then fixed for 3 h–4 h in fresh 4% paraformaldehyde (15710; Electron Microscopy Sciences, Hatfield, PA, USA) and 2% glutaraldehyde (16220; Electron Microscopy Sciences) in 0.1 M HEPES pH 7.2, after cutting near the pharynx to preserve the intestine. After fixation worms were washed three times for 3 min in M9 or 0.2 M HEPES, aligned with an eye lash with heads in one direction, and gently covered in 2% agarose heated to 55 degrees. M9 was replaced with 1% OsO4 for a 1 h–2 h incubation. Samples were dehydrated on a nutator at room temperature for 10 min sequentially with 50% ethanol, 70% ethanol, 95% ethanol, and 100% ethanol and twice with 100% acetone. The samples were then incubated in a series of graded Epon/acetone solutions. Epon/acetone (1:1) solution was used at room temperature for 2 h, followed by (3:1) Epon/acetone incubation overnight at room temperature on a nutator. The solution was replaced with 100% resin and microwaved in a Pelco microwave (Ted Pella, Redding, CA, USA) at 250 W with the vacuum on for 3 min. The microwave cycle was repeated with new 100% resin, and the vacuum was left on for an additional 30 min. The agarose block was cut into sections, and resin was then replaced with clean 100% resin, spun, and baked at 70 °C overnight. Blocks were trimmed, and 80-nm to 90-nm thin sections were cut using a Diatome diamond knife. The sections were picked up on 200-mesh, thin-bar copper grids and stained with uranyl acetate and lead citrate. Samples were imaged using an Advanced Microscopy Techniques (Woburn, MA, USA) camera on a Philips (Briarcliff Manor, NY, USA) CM12 electron microscope.
Statistical Analysis: For grouped data statistical significance was established by performing a one-way Analysis of Variance (ANOVA) followed by a Sidak’s multiple comparisons test, for
Figure 1C, and Dunnett multiple comparison post-test for other figures. For ungrouped data an unpaired t-test, the unequal variance (Welch) t test was used. Error bars show 95% confidence intervals. Asterisks (*) denote
p values * =
p <
0.05, ** =
p < 0.001, *** =
p < 0.0001, *** =
p < 0.00001. All statistical analysis was performed using GraphPad Prism 8.
4. Discussion
In vivo studies and high-resolution imaging studies support that even after epithelia form and polarize, they continue to require support from branched actin. This study sets up a system in which to test models for the mechanisms that promote epithelial polarity through the force of branched actin.
We first addressed a puzzle in our field. Previous reports suggested Cadherin/HMR-1 was not polarized in the adult intestinal epithelium. This result seemed incongruous to us, since it was clear Cadherin was required for apical F-actin enrichment in this tissue, as shown by phalloidin staining that was significantly diminished when Cadherin was depleted [
1].
When CRISPR tagged Cadherin/HMR-1 became available [
23], we decided to revisit the question of how Cadherin is distributed in this mature epithelium. The images and measurements in
Figure 1 show that Cadherin/HMR-1 is indeed polarized from apical to basal regions. This polarized enrichment depends on WAVE (
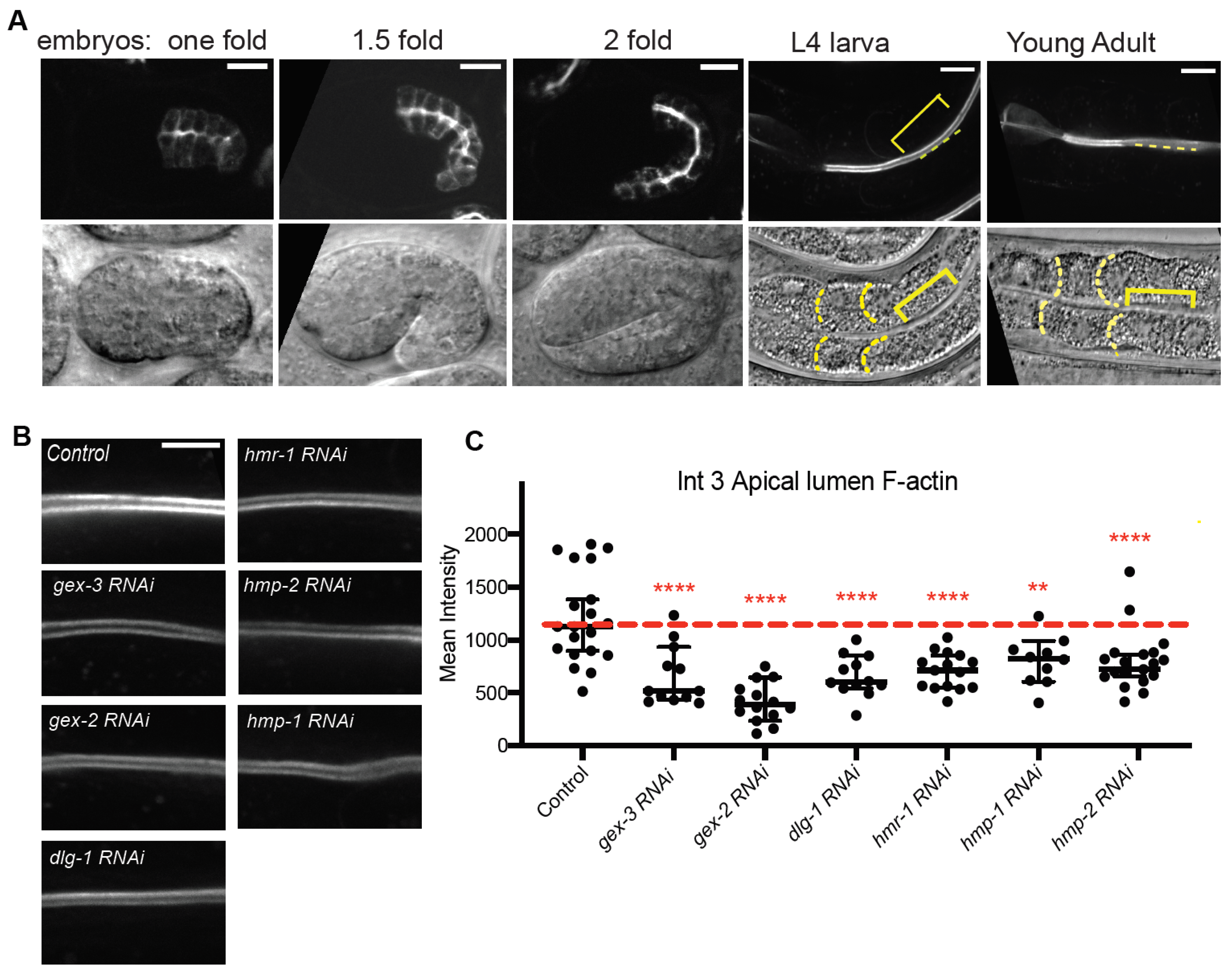
Figure 1). To determine if WAVE and Cadherin/HMR-1 mutually support apical F-actin, we built a new strain to image apical F-actin in the adult intestine. This strain,
Pglo-1::Lifeact::TAG-RFP, shows that apical F-actin enrichment depends on WAVE complex-dependent branched actin, and Cadherin.
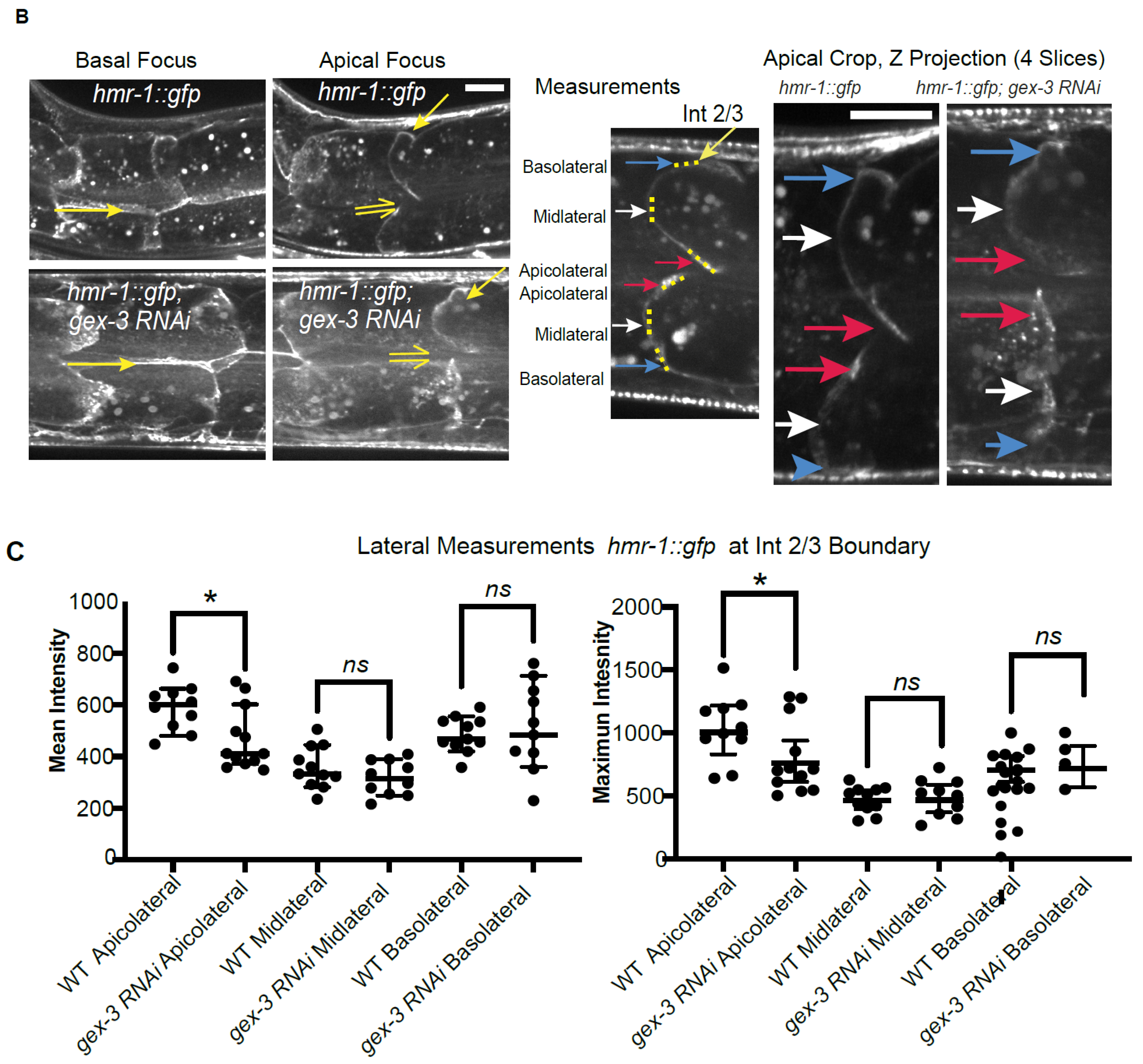
Imaging Cadherin/HMR-1 also revealed a substantial level of Cadherin/HMR-1 along basal membranes (
Figure 1). The basal intestine faces the pseudocoelomic space but is not thought to bind to other cells. It will be interesting to investigate if this pool of Cadherin is high due to the delivery of Cadherin to the basal regions for later transport to the apicolateral membranes, as is proposed in the Drosophila intestine [
31]. In this case, this pool of Cadherin might be an inactive reservoir formed by internal trafficking events. Alternatively, the basal intestine may experience shear forces or other mechanical stimuli that would require the establishment of a basal pool of Cadherin. In this latter case, the basal pool may be functional, and perhaps under external regulation. However, since the basal cells are not thought to adhere to other cells, we favor the idea that this is a reservoir of Cadherin that can be delivered to the apicolateral region as needed.
We further investigated if the effects of reducing branched actin created polarity defects, or adhesion defects for the Cadherin. At first glance, it did not seem that loss of branched actin by reducing WAVE components caused loss of adhesion, since, for example, we did not see large separations of the basolateral membrane. However, the changes in the Cadherin enrichment, including the diffuse signal at mid-lateral regions (for example,
Figure 3A,D) led us to look more carefully at the lateral regions. Using
Pglo-1::Lifeact-TAG-RFP to image intestinal F-actin, we saw F-actin accumulations in animals depleted of Cadherin or WAVE components that were much lower than is seen in controls (
Figure 2). Since Cadherin is expected to connect to the apical F-actin belt of this epithelium, the reduced lumenal F-actin may reflect a drop in the apical/basal polarity of the cells, and/or changes in the apical lumen membrane. Using a reporter for PIP2, PH:GFP, we saw more diffuse signal in the lateral region in animals depleted of WAVE component
gex-3. In addition, while PH::GFP is normally apically enriched, this apical enrichment and total membrane accumulation dropped in animals depleted of the WAVE complex (
Figure 3). Since these animals still showed adhesion at apicolateral and basolateral regions, the adhesive barrier appeared intact. However, these changes suggested a reduction in membrane polarity that normally enriches PIP2 apically. Loss WAVE or Cadherin reduced the PIP2 biosensor levels, especially at apical regions. Since PIP2 is known to reinforce apical/basal polarity [
28,
29], this result suggested an inter-dependent role for Cadherin, WAVE complex, and PIP2 in reinforcing apical/basal polarity. One mechanism for this integration may be through PIP2′s role promoting polarized transport of proteins [
28].
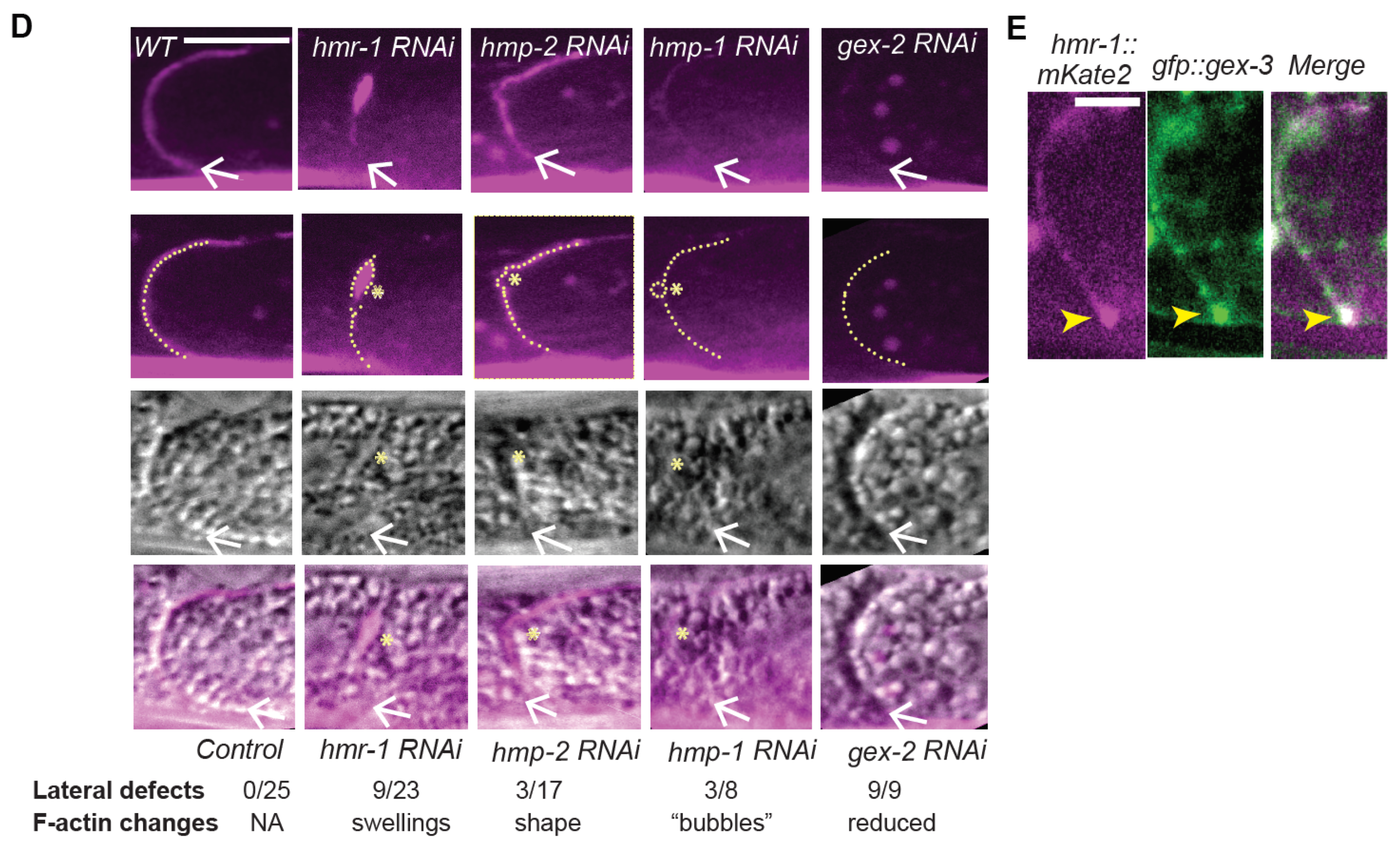
Previous studies suggested a second role for branched actin at apical junctions: to continually push lateral membranes together in adult tissues to repair small breaks. In our system, we did not see evidence that animals under laboratory conditions experience lateral breaks in their intestinal cells. However, C. elegans intestinal cells are not replaced if they are injured. Therefore, animals may need to repair altered or damaged membranes when they are challenged by pathogens, or when they ingest harmful chemical products. Our studies suggest that there is a dynamic and polarized pool of Cadherin present throughout the life of the worm, and that branched actin is active in stabilizing and polarizing this dynamic pool. This suggests that even in cells that are not migrating, or obviously damaged, in an animal that is constantly moving, and therefore putting these cells under some strain, branched actin supports and stabilizes a pool of dynamic Cadherin at the apicolateral junction. In the future, it will be interesting to investigate if different environmental conditions affect the requirements for Cadherin and branched actin in the mature intestine, and to explore mechanisms that promote branched-actin dependent polarized enrichment of Cadherin/HMR-1.