The Skull’s Girder: A Brief Review of the Cranial Base
Abstract
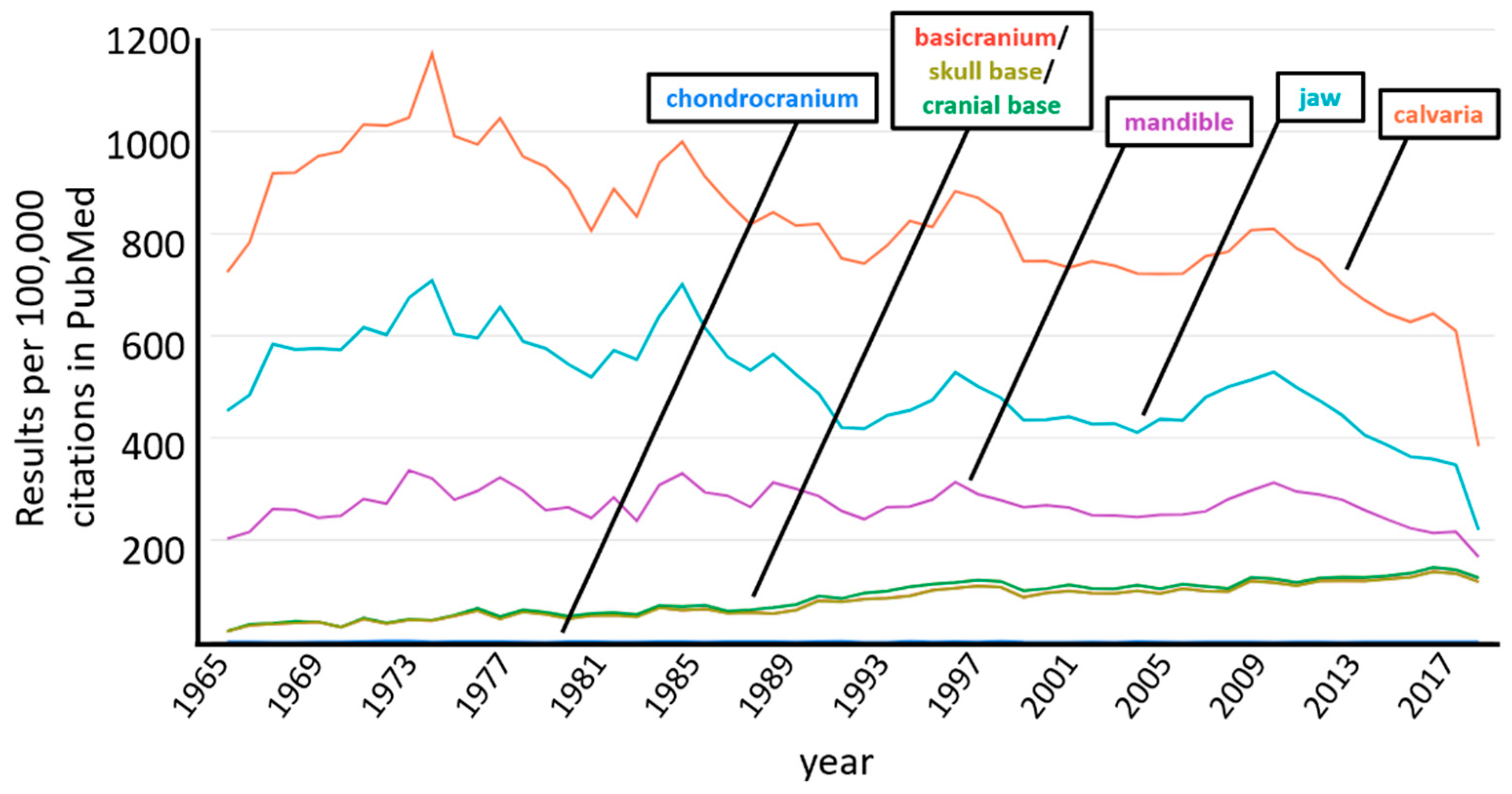
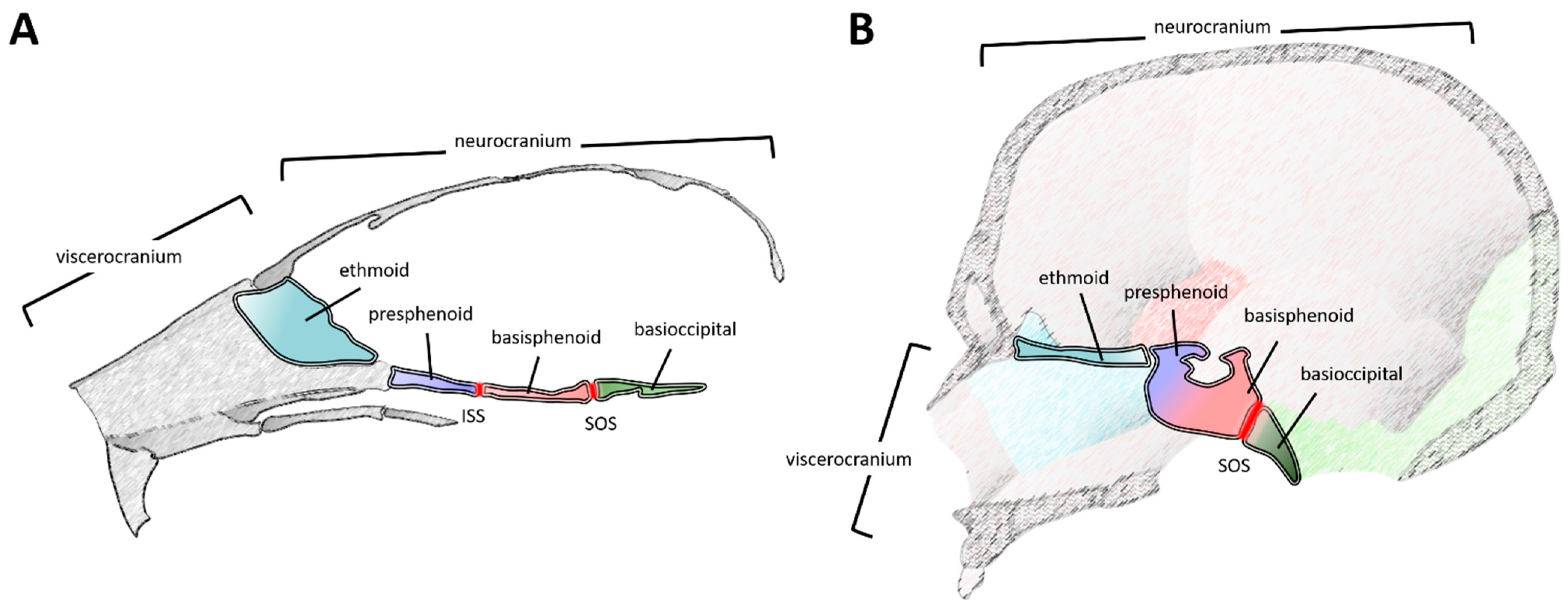
1. Introduction
2. Development and Developmental Origins of the Cranial Base—The Mouse and Chick as a Template
3. Pre- and Post-Natal Development of the Human Cranial Base
4. Genetic Control of Cranial Base Development
4.1. Foxc1, Six2, and Tbx1—Anterior to Posterior Control of Cranial Base Development
4.1.1. FOXC1
4.1.2. SIX2
4.1.3. TBX1
5. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lieberman, D.E.; Hallgrimsson, B.; Liu, W.; Parsons, T.E.; Jamniczky, H.A. Spatial packing, cranial base angulation, and craniofacial shape variation in the mammalian skull: Testing a new model using mice. J. Anat. 2008, 212, 720–735. [Google Scholar] [CrossRef] [PubMed]
- Nie, X. Cranial base in craniofacial development: Developmental features, influence on facial growth, anomaly, and molecular basis. Acta Odontol. Scand. 2005, 63, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Hu, M.; Mishina, Y.; Liu, F. Developmental Regulation of the Growth Plate and Cranial Synchondrosis. J. Dent. Res. 2016, 95, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Vora, S.R. Mouse models for the study of cranial base growth and anomalies. Orthod. Craniofac. Res. 2017, 20 (Suppl. 1), 18–25. [Google Scholar] [CrossRef]
- Di Ieva, A.; Bruner, E.; Haider, T.; Rodella, L.F.; Lee, J.M.; Cusimano, M.D.; Tschabitscher, M. Skull base embryology: A multidisciplinary review. Childs Nerv. Syst. 2014, 30, 991–1000. [Google Scholar] [CrossRef]
- Funato, N. New Insights Into Cranial Synchondrosis Development: A Mini Review. Front. Cell Dev. Biol. 2020, 8, 706. [Google Scholar] [CrossRef]
- McBratney-Owen, B.; Iseki, S.; Bamforth, S.D.; Olsen, B.R.; Morriss-Kay, G.M. Development and tissue origins of the mammalian cranial base. Dev. Biol. 2008, 322, 121–132. [Google Scholar] [CrossRef]
- Smith, T.D.; McMahon, M.J.; Millen, M.E.; Llera, C.; Engel, S.M.; Li, L.; Bhatnagar, K.P.; Burrows, A.M.; Zumpano, M.P.; DeLeon, V.B. Growth and Development at the Sphenoethmoidal Junction in Perinatal Primates. Anat. Rec. (Hoboken) 2017, 300, 2115–2137. [Google Scholar] [CrossRef]
- Wealthall, R.J.; Herring, S.W. Endochondral ossification of the mouse nasal septum. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2006, 288, 1163–1172. [Google Scholar] [CrossRef]
- Vora, S.R.; Camci, E.D.; Cox, T.C. Postnatal Ontogeny of the Cranial Base and Craniofacial Skeleton in Male C57BL/6J Mice: A Reference Standard for Quantitative Analysis. Front. Physiol. 2015, 6, 417. [Google Scholar] [CrossRef]
- Wei, X.; Thomas, N.; Hatch, N.E.; Hu, M.; Liu, F. Postnatal Craniofacial Skeletal Development of Female C57BL/6NCrl Mice. Front. Physiol. 2017, 8, 697. [Google Scholar] [CrossRef] [PubMed]
- Gross, J.B.; Hanken, J. Review of fate-mapping studies of osteogenic cranial neural crest in vertebrates. Dev. Biol. 2008, 317, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Noden, D.M. The control of avian cephalic neural crest cytodifferentiation. I. Skeletal and connective tissues. Dev. Biol. 1978, 67, 296–312. [Google Scholar] [CrossRef]
- Le Lievre, C.S. Participation of neural crest-derived cells in the genesis of the skull in birds. J. Embryol. Exp. Morphol. 1978, 47, 17–37. [Google Scholar]
- Couly, G.F.; Coltey, P.M.; Le Douarin, N.M. The developmental fate of the cephalic mesoderm in quail-chick chimeras. Development 1992, 114, 1–15. [Google Scholar] [PubMed]
- Couly, G.F.; Coltey, P.M.; Le Douarin, N.M. The triple origin of skull in higher vertebrates: A study in quail-chick chimeras. Development 1993, 117, 409–429. [Google Scholar] [PubMed]
- Le Douarin, N.M.; Ziller, C.; Couly, G.F. Patterning of neural crest derivatives in the avian embryo: In vivo and in vitro studies. Dev. Biol. 1993, 159, 24–49. [Google Scholar] [CrossRef] [PubMed]
- Gans, C.; Northcutt, R.G. Neural crest and the origin of vertebrates: A new head. Science 1983, 220, 268–273. [Google Scholar] [CrossRef]
- Muller, F.; O’Rahilly, R. The human chondrocranium at the end of the embryonic period, proper, with particular reference to the nervous system. Am. J. Anat. 1980, 159, 33–58. [Google Scholar] [CrossRef]
- Catala, M. Development and growth of the skull base. Neurochirurgie 2019, 65, 216–220. [Google Scholar] [CrossRef]
- Kjaer, I.; Fischer-Hansen, B. The adenohypophysis and the cranial base in early human development. J. Craniofac. Genet. Dev. Biol. 1995, 15, 157–161. [Google Scholar] [PubMed]
- Scott, J.H. The cranial base. Am. J. Phys. Anthropol. 1958, 16, 319–348. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, N. A high-resolution MRI study of linear growth of the human fetal skull base. Neuroradiology 2002, 44, 358–366. [Google Scholar] [CrossRef]
- Begnoni, G.; Serrao, G.; Musto, F.; Pellegrini, G.; Triulzi, F.M.; Dellavia, C. Craniofacial structures’ development in prenatal period: An MRI study. Orthod. Craniofac. Res. 2018, 21, 96–103. [Google Scholar] [CrossRef]
- Lieberman, D.E.; Ross, C.F.; Ravosa, M.J. The primate cranial base: Ontogeny, function, and integration. Am. J. Phys. Anthropol. 2000, 113 (Suppl. 31), 117–169. [Google Scholar] [CrossRef]
- Esenlik, E.; Sener, E.H.; Yilmaz, H.H.; Malas, M.A. Cephalometric investigation of craniomaxillofacial structures during the prenatal period: A cadaver study. Am. J. Orthod. Dentofac. Orthop. 2014, 145, 217–227. [Google Scholar] [CrossRef]
- Buschang, P.H.; Baume, R.M.; Nass, G.G. A craniofacial growth maturity gradient for males and females between 4 and 16 years of age. Am. J. Phys. Anthropol. 1983, 61, 373–381. [Google Scholar] [CrossRef]
- Wang, S.; Lai, X.; Deng, Y.; Song, Y. Correlation between mouse age and human age in anti-tumor research: Significance and method establishment. Life Sci. 2020, 242, 117242. [Google Scholar] [CrossRef]
- Nahhas, R.W.; Valiathan, M.; Sherwood, R.J. Variation in timing, duration, intensity, and direction of adolescent growth in the mandible, maxilla, and cranial base: The Fels longitudinal study. Anat. Rec. (Hoboken) 2014, 297, 1195–1207. [Google Scholar] [CrossRef]
- Rosenberg, P.; Arlis, H.R.; Haworth, R.D.; Heier, L.; Hoffman, L.; LaTrenta, G. The role of the cranial base in facial growth: Experimental craniofacial synostosis in the rabbit. Plast Reconstr. Surg. 1997, 99, 1396–1407. [Google Scholar] [CrossRef]
- Trenouth, M.J. Craniofacial shape in the anencephalic human fetus. J. Anat. 1989, 165, 215–224. [Google Scholar]
- Puschel, T.A.; Friess, M.; Manriquez, G. Morphological consequences of artificial cranial deformation: Modularity and integration. PLoS ONE 2020, 15, e0227362. [Google Scholar] [CrossRef]
- Ferros, I.; Mora, M.J.; Obeso, I.F.; Jimenez, P.; Martinez-Insua, A. The nasomaxillary complex and the cranial base in artificial cranial deformation: Relationships from a geometric morphometric study. Eur. J. Orthod. 2015, 37, 403–411. [Google Scholar] [CrossRef][Green Version]
- Gkantidis, N.; Halazonetis, D.J. Morphological integration between the cranial base and the face in children and adults. J. Anat. 2011, 218, 426–438. [Google Scholar] [CrossRef]
- Martinez-Abadias, N.; Mitteroecker, P.; Parsons, T.E.; Esparza, M.; Sjovold, T.; Rolian, C.; Richtsmeier, J.T.; Hallgrimsson, B. The Developmental Basis of Quantitative Craniofacial Variation in Humans and Mice. Evol. Biol. 2012, 39, 554–567. [Google Scholar] [CrossRef][Green Version]
- Weiss-Bilka, H.E.; Brill, J.A.; Ravosa, M.J. Non-sutural basicranium-derived cells undergo a unique mineralization pathway via a cartilage intermediate in vitro. PeerJ 2018, 6, e5757. [Google Scholar] [CrossRef]
- Carlsson, P.; Mahlapuu, M. Forkhead transcription factors: Key players in development and metabolism. Dev. Biol. 2002, 250, 1–23. [Google Scholar] [CrossRef]
- Mya, N.; Furutera, T.; Okuhara, S.; Kume, T.; Takechi, M.; Iseki, S. Transcription factor Foxc1 is involved in anterior part of cranial base formation. Congenit. Anom. (Kyoto) 2018, 58, 158–166. [Google Scholar] [CrossRef]
- Tumer, Z.; Bach-Holm, D. Axenfeld-Rieger syndrome and spectrum of PITX2 and FOXC1 mutations. Eur. J. Hum. Genet. 2009, 17, 1527–1539. [Google Scholar] [CrossRef]
- Seifi, M.; Footz, T.; Taylor, S.A.; Walter, M.A. Comparison of Bioinformatics Prediction, Molecular Modeling, and Functional Analyses of FOXC1 Mutations in Patients with Axenfeld-Rieger Syndrome. Hum. Mutat. 2017, 38, 169–179. [Google Scholar] [CrossRef]
- Seifi, M.; Walter, M.A. Axenfeld-Rieger syndrome. Clin. Genet. 2018, 93, 1123–1130. [Google Scholar] [CrossRef]
- Childers, N.K.; Wright, J.T. Dental and craniofacial anomalies of Axenfeld-Rieger syndrome. J. Oral Pathol. 1986, 15, 534–539. [Google Scholar] [CrossRef]
- Jena, A.K.; Kharbanda, O.P. Axenfeld-Rieger syndrome: Report on dental and craniofacial findings. J. Clin. Pediatr. Dent. 2005, 30, 83–88. [Google Scholar] [CrossRef]
- Waldron, J.M.; McNamara, C.; Hewson, A.R.; McNamara, C.M. Axenfeld-Rieger syndrome (ARS): A review and case report. Spec. Care Dent. 2010, 30, 218–222. [Google Scholar] [CrossRef]
- Bender, C.A.; Koudstaal, M.J.; van Elswijk, J.F.; Prahl, C.; Wolvius, E.B. Two cases of axenfeld-rieger syndrome, report of the complex pathology and treatment. Cleft Palate Craniofac. J. 2014, 51, 354–360. [Google Scholar] [CrossRef]
- Dunbar, A.C.; McIntyre, G.T.; Laverick, S.; Stevenson, B. Axenfeld-Rieger syndrome: A case report. J. Orthod. 2015, 42, 324–330. [Google Scholar] [CrossRef]
- Roomaney, I.A.; Chetty, M. Sella turcica morphology in patients with genetic syndromes: A systematic review. Orthod. Craniofac. Res. 2020. [Google Scholar] [CrossRef]
- Sasaki, H.; Hogan, B.L. Differential expression of multiple fork head related genes during gastrulation and axial pattern formation in the mouse embryo. Development 1993, 118, 47–59. [Google Scholar]
- Yoshida, M.; Hata, K.; Takashima, R.; Ono, K.; Nakamura, E.; Takahata, Y.; Murakami, T.; Iseki, S.; Takano-Yamamoto, T.; Nishimura, R.; et al. The transcription factor Foxc1 is necessary for Ihh-Gli2-regulated endochondral ossification. Nat. Commun. 2015, 6, 6653. [Google Scholar] [CrossRef]
- Young, B.; Minugh-Purvis, N.; Shimo, T.; St-Jacques, B.; Iwamoto, M.; Enomoto-Iwamoto, M.; Koyama, E.; Pacifici, M. Indian and sonic hedgehogs regulate synchondrosis growth plate and cranial base development and function. Dev. Biol. 2006, 299, 272–282. [Google Scholar] [CrossRef]
- Kumar, J.P. The sine oculis homeobox (SIX) family of transcription factors as regulators of development and disease. Cell. Mol. Life Sci. 2009, 66, 565–583. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Tavella, S.; Hanley, K.P.; Self, M.; Oliver, G.; Grifone, R.; Hanley, N.; Ward, C.; Bobola, N. Inactivation of Six2 in mouse identifies a novel genetic mechanism controlling development and growth of the cranial base. Dev. Biol. 2010, 344, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, H.; Hu, Y.C.; Lan, Y.; Jiang, R. Generation and characterization of Six2 conditional mice. Genesis 2020, 58, e23365. [Google Scholar] [CrossRef] [PubMed]
- Farlie, P.G.; Baker, N.L.; Yap, P.; Tan, T.Y. Frontonasal Dysplasia: Towards an Understanding of Molecular and Developmental Aetiology. Mol. Syndromol. 2016, 7, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Hufnagel, R.B.; Zimmerman, S.L.; Krueger, L.A.; Bender, P.L.; Ahmed, Z.M.; Saal, H.M. A new frontonasal dysplasia syndrome associated with deletion of the SIX2 gene. Am. J. Med. Genet. A 2016, 170a, 487–491. [Google Scholar] [CrossRef]
- Henn, A.; Weng, H.; Novak, S.; Rettenberger, G.; Gerhardinger, A.; Rossier, E.; Zirn, B. SIX2 gene haploinsufficiency leads to a recognizable phenotype with ptosis, frontonasal dysplasia, and conductive hearing loss. Clin. Dysmorphol. 2018, 27, 27–30. [Google Scholar] [CrossRef]
- Oliver, G.; Wehr, R.; Jenkins, N.A.; Copeland, N.G.; Cheyette, B.N.; Hartenstein, V.; Zipursky, S.L.; Gruss, P. Homeobox genes and connective tissue patterning. Development 1995, 121, 693–705. [Google Scholar]
- Guan, J.; Wang, D.; Cao, W.; Zhao, Y.; Du, R.; Yuan, H.; Liu, Q.; Lan, L.; Zong, L.; Yang, J.; et al. SIX2 haploinsufficiency causes conductive hearing loss with ptosis in humans. J. Hum. Genet. 2016, 61, 917–922. [Google Scholar] [CrossRef]
- Sweat, Y.Y.; Sweat, M.; Mansaray, M.; Cao, H.; Eliason, S.; Adeyemo, W.L.; Gowans, L.J.J.; Eshete, M.A.; Anand, D.; Chalkley, C.; et al. Six2 regulates Pax9 expression, palatogenesis and craniofacial bone formation. Dev. Biol. 2020, 458, 246–256. [Google Scholar] [CrossRef]
- Harel, I.; Maezawa, Y.; Avraham, R.; Rinon, A.; Ma, H.Y.; Cross, J.W.; Leviatan, N.; Hegesh, J.; Roy, A.; Jacob-Hirsch, J.; et al. Pharyngeal mesoderm regulatory network controls cardiac and head muscle morphogenesis. Proc. Natl. Acad. Sci. USA 2012, 109, 18839–18844. [Google Scholar] [CrossRef]
- Self, M.; Geng, X.; Oliver, G. Six2 activity is required for the formation of the mammalian pyloric sphincter. Dev. Biol. 2009, 334, 409–417. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhou, Z.; Wang, J.; Guo, C.; Chang, W.; Zhuang, J.; Zhu, P.; Li, X. Temporally Distinct Six2-Positive Second Heart Field Progenitors Regulate Mammalian Heart Development and Disease. Cell Rep. 2017, 18, 1019–1032. [Google Scholar] [CrossRef] [PubMed]
- Little, M.H.; McMahon, A.P. Mammalian kidney development: Principles, progress, and projections. Cold Spring Harb. Perspect. Biol. 2012, 4. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto-Shiraishi, Y.; Kuroiwa, A. Wnt and BMP signaling cooperate with Hox in the control of Six2 expression in limb tendon precursor. Dev. Biol. 2013, 377, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, M.; Iwamoto, M.; Hargett, A.; Kamiya, N.; Tamamura, Y.; Young, B.; Morrison, T.; Takeuchi, H.; Pacifici, M.; Enomoto-Iwamoto, M.; et al. Wnt/beta-catenin signaling regulates cranial base development and growth. J. Dent. Res. 2008, 87, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Shum, L.; Wang, X.; Kane, A.A.; Nuckolls, G.H. BMP4 promotes chondrocyte proliferation and hypertrophy in the endochondral cranial base. Int. J. Dev. Biol. 2003, 47, 423–431. [Google Scholar]
- Papaioannou, V.E. The T-box gene family: Emerging roles in development, stem cells and cancer. Development 2014, 141, 3819–3833. [Google Scholar] [CrossRef]
- Funato, N.; Srivastava, D.; Shibata, S.; Yanagisawa, H. TBX1 Regulates Chondrocyte Maturation in the Spheno-occipital Synchondrosis. J. Dent. Res. 2020, 99, 1182–1191. [Google Scholar] [CrossRef]
- McDonald-McGinn, D.M.; Sullivan, K.E.; Marino, B.; Philip, N.; Swillen, A.; Vorstman, J.A.; Zackai, E.H.; Emanuel, B.S.; Vermeesch, J.R.; Morrow, B.E.; et al. 22q11.2 deletion syndrome. Nat. Rev. Dis. Primers 2015, 1, 15071. [Google Scholar] [CrossRef]
- Morrow, B.E.; McDonald-McGinn, D.M.; Emanuel, B.S.; Vermeesch, J.R.; Scambler, P.J. Molecular genetics of 22q11.2 deletion syndrome. Am. J. Med. Genet. A 2018, 176, 2070–2081. [Google Scholar] [CrossRef]
- Dalben Gda, S.; Richieri-Costa, A.; Taveira, L.A. Craniofacial morphology in patients with velocardiofacial syndrome. Cleft Palate Craniofac. J. 2010, 47, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Lewyllie, A.; Roosenboom, J.; Indencleef, K.; Claes, P.; Swillen, A.; Devriendt, K.; Carels, C.; Cadenas De Llano-Perula, M.; Willems, G.; Hens, G.; et al. A Comprehensive Craniofacial Study of 22q11.2 Deletion Syndrome. J. Dent. Res. 2017, 96, 1386–1391. [Google Scholar] [CrossRef] [PubMed]
- Haenssler, A.E.; Baylis, A.; Perry, J.L.; Kollara, L.; Fang, X.; Kirschner, R. Impact of Cranial Base Abnormalities on Cerebellar Volume and the Velopharynx in 22q11.2 Deletion Syndrome. Cleft Palate Craniofac. J. 2020, 57, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Mølsted, K.; Boers, M.; Kjaer, I. The morphology of the sella turcica in velocardiofacial syndrome suggests involvement of a neural crest developmental field. Am. J. Med. Genet. A 2010, 152a, 1450–1457. [Google Scholar] [CrossRef] [PubMed]
- Chapman, D.L.; Garvey, N.; Hancock, S.; Alexiou, M.; Agulnik, S.I.; Gibson-Brown, J.J.; Cebra-Thomas, J.; Bollag, R.J.; Silver, L.M.; Papaioannou, V.E. Expression of the T-box family genes, Tbx1-Tbx5, during early mouse development. Dev. Dyn. 1996, 206, 379–390. [Google Scholar] [CrossRef]
- Garg, V.; Yamagishi, C.; Hu, T.; Kathiriya, I.S.; Yamagishi, H.; Srivastava, D. Tbx1, a DiGeorge syndrome candidate gene, is regulated by sonic hedgehog during pharyngeal arch development. Dev. Biol. 2001, 235, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Funato, N.; Nakamura, M.; Richardson, J.A.; Srivastava, D.; Yanagisawa, H. Loss of Tbx1 induces bone phenotypes similar to cleidocranial dysplasia. Hum. Mol. Genet. 2015, 24, 424–435. [Google Scholar] [CrossRef]
- Yamagishi, H.; Maeda, J.; Hu, T.; McAnally, J.; Conway, S.J.; Kume, T.; Meyers, E.N.; Yamagishi, C.; Srivastava, D. Tbx1 is regulated by tissue-specific forkhead proteins through a common Sonic hedgehog-responsive enhancer. Genes Dev. 2003, 17, 269–281. [Google Scholar] [CrossRef]
- Wei, C.J.; Zhang, K. RETrace: Simultaneous retrospective lineage tracing and methylation profiling of single cells. Genome Res. 2020, 30, 602–610. [Google Scholar] [CrossRef]
- Shaffer, J.R.; Orlova, E.; Lee, M.K.; Leslie, E.J.; Raffensperger, Z.D.; Heike, C.L.; Cunningham, M.L.; Hecht, J.T.; Kau, C.H.; Nidey, N.L.; et al. Genome-Wide Association Study Reveals Multiple Loci Influencing Normal Human Facial Morphology. PLoS Genet. 2016, 12, e1006149. [Google Scholar] [CrossRef]
- Eames, B.F.; Helms, J.A. Conserved molecular program regulating cranial and appendicular skeletogenesis. Dev. Dyn. 2004, 231, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Abzhanov, A.; Rodda, S.J.; McMahon, A.P.; Tabin, C.J. Regulation of skeletogenic differentiation in cranial dermal bone. Development 2007, 134, 3133–3144. [Google Scholar] [CrossRef] [PubMed]
- Meulemans, D.; Bronner-Fraser, M. Central role of gene cooption in neural crest evolution. J. Exp. Zool. B Mol. Dev. Evol. 2005, 304, 298–303. [Google Scholar] [CrossRef]
- Kaucka, M.; Adameyko, I. Evolution and development of the cartilaginous skull: From a lancelet towards a human face. Semin. Cell Dev. Biol. 2019, 91, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Cattell, M.; Lai, S.; Cerny, R.; Medeiros, D.M. A new mechanistic scenario for the origin and evolution of vertebrate cartilage. PLoS ONE 2011, 6, e22474. [Google Scholar] [CrossRef]
- Parsons, T.E.; Downey, C.M.; Jirik, F.R.; Hallgrimsson, B.; Jamniczky, H.A. Mind the gap: Genetic manipulation of basicranial growth within synchondroses modulates calvarial and facial shape in mice through epigenetic interactions. PLoS ONE 2015, 10, e0118355. [Google Scholar] [CrossRef]
- Pitirri, M.K.; Kawasaki, K.; Richtsmeier, J.T. It takes two: Building the vertebrate skull from chondrocranium and dermatocranium. Vertebr. Zool. 2020, 70, 587–600. [Google Scholar]
- Marulanda, J.; Murshed, M. Role of Matrix Gla protein in midface development: Recent advances. Oral Dis. 2018, 24, 78–83. [Google Scholar] [CrossRef]
- Hallgrimsson, B.; Brown, J.J.; Ford-Hutchinson, A.F.; Sheets, H.D.; Zelditch, M.L.; Jirik, F.R. The brachymorph mouse and the developmental-genetic basis for canalization and morphological integration. Evol. Dev. 2006, 8, 61–73. [Google Scholar] [CrossRef]
- Downey, C.M.; Horton, C.R.; Carlson, B.A.; Parsons, T.E.; Hatfield, D.L.; Hallgrimsson, B.; Jirik, F.R. Osteo-chondroprogenitor-specific deletion of the selenocysteine tRNA gene, Trsp, leads to chondronecrosis and abnormal skeletal development: A putative model for Kashin-Beck disease. PLoS Genet. 2009, 5, e1000616. [Google Scholar] [CrossRef]
- Sakagami, N.; Ono, W.; Ono, N. Diverse contribution of Col2a1-expressing cells to the craniofacial skeletal cell lineages. Orthod. Craniofac. Res. 2017, 20 (Suppl. 1), 44–49. [Google Scholar] [CrossRef] [PubMed]
- Szabova, L.; Yamada, S.S.; Wimer, H.; Chrysovergis, K.; Ingvarsen, S.; Behrendt, N.; Engelholm, L.H.; Holmbeck, K. MT1-MMP and type II collagen specify skeletal stem cells and their bone and cartilage progeny. J. Bone Miner. Res. 2009, 24, 1905–1916. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rengasamy Venugopalan, S.; Van Otterloo, E. The Skull’s Girder: A Brief Review of the Cranial Base. J. Dev. Biol. 2021, 9, 3. https://doi.org/10.3390/jdb9010003
Rengasamy Venugopalan S, Van Otterloo E. The Skull’s Girder: A Brief Review of the Cranial Base. Journal of Developmental Biology. 2021; 9(1):3. https://doi.org/10.3390/jdb9010003
Chicago/Turabian StyleRengasamy Venugopalan, Shankar, and Eric Van Otterloo. 2021. "The Skull’s Girder: A Brief Review of the Cranial Base" Journal of Developmental Biology 9, no. 1: 3. https://doi.org/10.3390/jdb9010003
APA StyleRengasamy Venugopalan, S., & Van Otterloo, E. (2021). The Skull’s Girder: A Brief Review of the Cranial Base. Journal of Developmental Biology, 9(1), 3. https://doi.org/10.3390/jdb9010003




