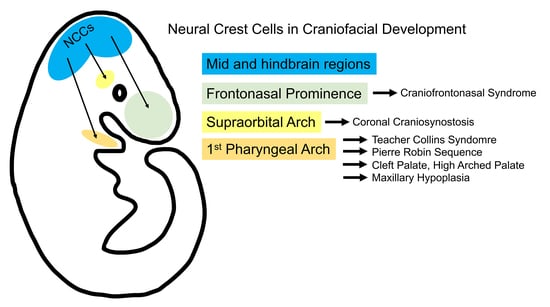
Cranial Neural Crest Cells and Their Role in the Pathogenesis of Craniofacial Anomalies and Coronal Craniosynostosis
Abstract
1. Introduction
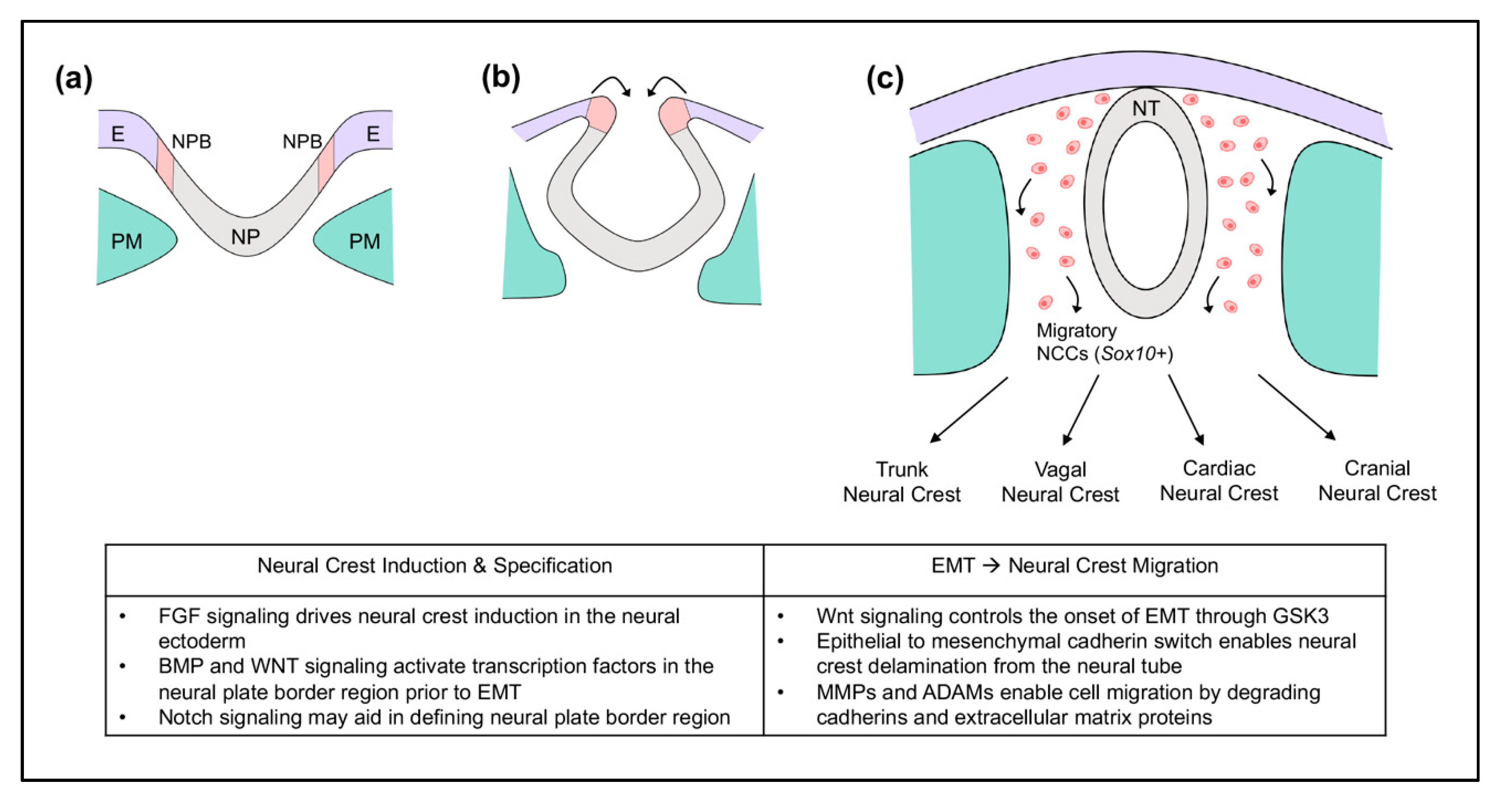
2. Neural Crest Cells
3. Cranial Neural Crest Cells in the Pathogenesis of Craniofacial Anomalies
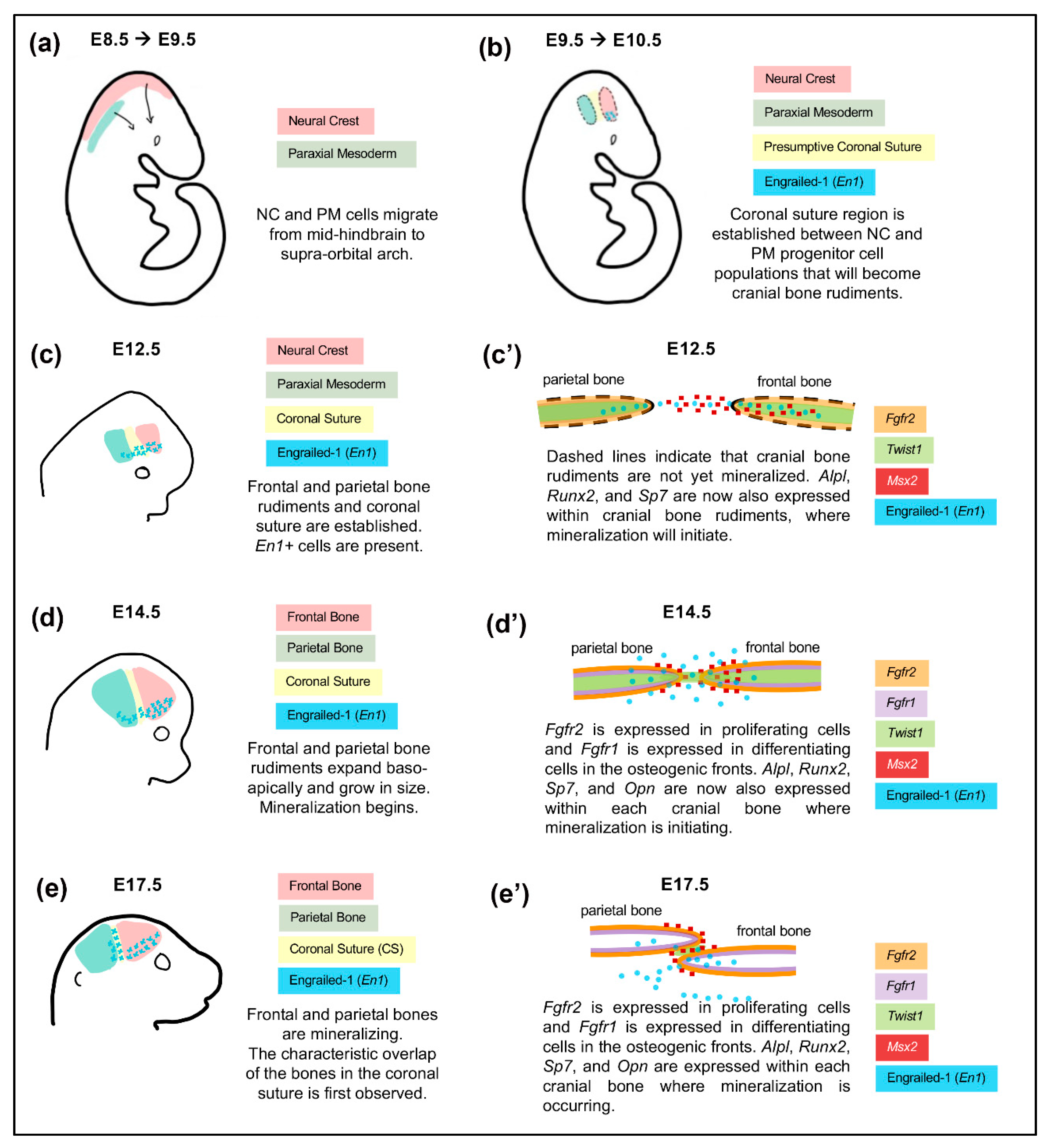
4. Cranial Neural Crest Cells in the Development of Cranial Bones and the Coronal Suture
5. Mechanisms Underlying Coronal Craniosynostosis
| Human Syndrome | Associated Mouse Model Genetic Mutation | Human Craniofacial Phenotype | Proposed Mechanism(s) of Anomaly |
|---|---|---|---|
| Craniofrontonasal Syndrome (OMIM #304110) | Efnb1−/− | anterior-posteriorly shortened skull, facial dysmorphologies, coronal suture fusion * | Neural crest-specific disruption of Efnb1 disrupts lineage-based boundary formation of coronal suture [137,142]. |
| Apert Syndrome (OMIM #101200) | Fgfr2S252W/+ | Coronal, sagittal, lambdoid suture fusion; proptosis, hypertelorism, midface hypoplasia | Enhanced osteogenic differentiation along osteogenic front of parietal bone enhanced by neural crest-derived frontal bone [91,93,126]. |
| Crouzon Syndrome (OMIM #123500) | Fgfr2C342Y/+ | Coronal suture fusion, proptosis, hypertelorism, midface hypoplasia | Embryonic dysregulation of Sox9 expression causing mesenchymal condensation defects, symptoms of neural tube defects, plus decreased craniofacial osteogenesis and increased chondrogenesis; postnatal enhanced osteogenic differentiation within osteogenic fronts; [84,87,130]. |
| Muenke Syndrome (OMIM #602849) | Fgfr3P250R/+ | Coronal suture fusion; pansynostosis; hearing loss; midface hypoplasia | Hearing loss due to embryonic fate switch of neural crest derived cochlear Deiters’ cells to pillar cells [143,144]. |
| Bent Bone Dysplasia (OMIM #614592) | FGFR2C1172TΦ | Coronal suture fusion; midface hypoplasia; prenatal teeth; low set ears; micrognathia; diminished bone mineralization; bent long bones | Mutations promote ribosomal transcription within the nucleus leading to enhanced osteoprogenitor cell proliferation with diminished differentiation [145,146]. |
| Saethre-Chotzen Syndrome (OMIM #101400) | Twist1+/− | Coronal suture fusion, low hairline, hypertelorism, ptosis, broad nasal bridge, digit fusions | Disruption of lineage-based boundary formation of coronal suture and cell lineage mixing. Enhanced osteogenic potential of parietal vs. frontal bones [10,90,135,147]. |
| TCF12 (OMIM # 600480) | Tcf12+/−/Twist+/− | Described as a milder form of Saethre-Chotzen syndrome. Coronal suture fusion, facial dysmorphologies, minor limb abnormalites | TCF12 is dimerization partner for TWIST1. Double mutant mice show accelerated parietal and/or frontal bone growth plus diminished pool of osteoprogenitors in coronal suture [147,148]. |
| Non-Syndromic Coronal Synostosis | EphA4−/− and Twist1+//EphA4+/− | Coronal suture fusion | Disruption of boundary formation and neural crest/mesoderm cell lineage mixing due to lack of Twist1 and its effector EphA4 [85]. |
| Infantile Hypophosphatasia (OMIM #241500) | Alpl−/− | Coronal or sagittal suture fusion #, hypomineralization, midface hypoplasia. | Hypomineralization and cell proliferation defects more severe in cells of neural crest derived craniofacial bones; enhanced FGFR2 signaling in osteoprogenitors; [149,150]. |
5.1. The Impact of Embryonic Origin on Cranial Bone and Coronal Suture Development
5.2. Defects in Neural Crest-Derived Progenitor Cell Proliferation, Differentiation, and Survival
5.3. Boundary Defects between Developing Cranial Bones
5.4. Premature Loss of Suture Stem Cells
5.5. Epigenetic Influences on Craniosynostosis
6. Development of Strategies for Treatment and Future Outlook
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lin, Y.; Lewallen, E.A.; Camilleri, E.T.; Bonin, C.A.; Jones, D.L.; Dudakovic, A.; Galeano-Garces, C.; Wang, W.; Karperien, M.J.; Larson, A.N.; et al. RNA-seq analysis of clinical-grade osteochondral allografts reveals activation of early response genes. J. Orthop. Res. 2016, 34, 1950–1959. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, F.; Calzolari, E.; Ciulli, L.; Cordier, S.; Gualandi, F.; Pierini, A.; Mossey, P.A. Environment and genetics in the etiology of cleft lip and cleft palate with reference to the role of folic acid. Epidemiol. Prev. 2000, 24, 21–27. [Google Scholar] [PubMed]
- Durham, E.; Howie, R.N.; Larson, N.; LaRue, A.; Cray, J. Pharmacological exposures may precipitate craniosynostosis through targeted stem cell depletion. Stem Cell Res. 2019, 40, 101528. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Rodriguez, G.; Han, X.; Janečková, E.; Kahng, S.; Song, B.; Chai, Y. Regulatory Mechanisms of Soft Palate Development and Malformations. J. Dent. Res. 2019, 98, 959–967. [Google Scholar] [CrossRef]
- Dixon, J.; Jones, N.C.; Sandell, L.L.; Jayasinghe, S.M.; Crane, J.; Rey, J.P.; Dixon, M.J.; Trainor, P.A. Tcof1/Treacle is required for neural crest cell formation and proliferation deficiencies that cause craniofacial abnormalities. Proc. Natl. Acad. Sci. USA 2006, 103, 13403–13408. [Google Scholar] [CrossRef]
- Parada, C.; Han, D.; Grimaldi, A.; Sarrion, P.; Park, S.S.; Pelikan, R.; Sanchez-Lara, P.A.; Chai, Y. Disruption of the ERK/MAPK pathway in neural crest cells as a potential cause of Pierre Robin sequence. Development 2015, 142, 3734–3745. [Google Scholar] [CrossRef]
- Lee, K.K.; Stanier, P.; Pauws, E. Mouse Models of Syndromic Craniosynostosis. Mol. Syndr. 2018, 10, 58–73. [Google Scholar] [CrossRef] [PubMed]
- Azoury, S.C.; Reddy, S.; Shukla, V.; Deng, C.-X. Fibroblast Growth Factor Receptor 2 (FGFR2) Mutation Related Syndromic Craniosynostosis. Int. J. Biol. Sci. 2017, 13, 1479–1488. [Google Scholar] [CrossRef]
- Twigg, S.R.; Wilkie, A. A Genetic-Pathophysiological Framework for Craniosynostosis. Am. J. Hum. Genet. 2015, 97, 359–377. [Google Scholar] [CrossRef] [PubMed]
- Deckelbaum, R.A.; Holmes, G.; Zhao, Z.; Tong, C.; Basilico, C.; Loomis, C.A. Regulation of cranial morphogenesis and cell fate at the neural crest-mesoderm boundary by engrailed 1. Development 2012, 139, 1346–1358. [Google Scholar] [CrossRef] [PubMed]
- Merrill, A.E.; Bochukova, E.G.; Brugger, S.M.; Ishii, M.; Pilz, D.T.; Wall, S.A.; Lyons, K.M.; Wilkie, A.; Maxson, R.E. Cell mixing at a neural crest-mesoderm boundary and deficient ephrin-Eph signaling in the pathogenesis of craniosynostosis. Hum. Mol. Genet. 2006, 15, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Simões-Costa, M.; Bronner, M.E. Establishing neural crest identity: A gene regulatory recipe. Development 2015, 142, 242–257. [Google Scholar] [CrossRef] [PubMed]
- Piacentino, M.L.; Li, Y.; Bronner, M.E. Epithelial-to-mesenchymal transition and different migration strategies as viewed from the neural crest. Curr. Opin. Cell Biol. 2020, 66, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Rogers, C.D.; Saxena, A.; Bronner, M.E. Sip1 mediates an E-cadherin-to-N-cadherin switch during cranial neural crest EMT. J. Cell Biol. 2013, 203, 835–847. [Google Scholar] [CrossRef] [PubMed]
- Malagon, S.G.G.; Muñoz, A.M.L.; Doro, D.; Bolger, T.G.; Poon, E.; Tucker, E.R.; Al-Lami, H.A.; Krause, M.; Phiel, C.J.; Chesler, L.; et al. Glycogen synthase kinase 3 controls migration of the neural crest lineage in mouse and Xenopus. Nat. Commun. 2018, 9, 1126. [Google Scholar] [CrossRef] [PubMed]
- Lander, R.; Nasr, T.; Ochoa, S.D.; Nordin, K.; Prasad, M.S.; LaBonne, C. Interactions between Twist and other core epithelial–mesenchymal transition factors are controlled by GSK3-mediated phosphorylation. Nat. Commun. 2013, 4, 1542. [Google Scholar] [CrossRef]
- Cano, A.; Perez, F.P.; Rodrigo, I.; Locascio, A.; Blanco, M.J.; Del Barrio, M.G.; Portillo, F.; Nieto, M.A. The transcription factor snail controls epithelial–mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol. 2000, 2, 76–83. [Google Scholar] [CrossRef]
- Martik, M.L.; Bronner, M.E. Regulatory Logic Underlying Diversification of the Neural Crest. Trends Genet. 2017, 33, 715–727. [Google Scholar] [CrossRef]
- Chalpe, A.J.; Prasad, M.; Henke, A.J.; Paulson, A. Regulation of cadherin expression in the chicken neural crest by the Wnt/beta-catenin signaling pathway. Cell Adhes. Migr. 2010, 4, 431–438. [Google Scholar] [CrossRef]
- Monsonego-Ornan, E.; Kosonovsky, J.; Bar, A.; Roth, L.; Fraggi-Rankis, V.; Simsa, S.; Kohl, A.; Sela-Donenfeld, D. Matrix metalloproteinase 9/gelatinase B is required for neural crest cell migration. Dev. Biol. 2012, 364, 162–177. [Google Scholar] [CrossRef]
- Kalev-Altman, R.; Hanael, E.; Zelinger, E.; Blum, M.; Monsonego-Ornan, E.; Sela-Donenfeld, D. Conserved role of matrix metalloproteases 2 and 9 in promoting the migration of neural crest cells in avian and mammalian embryos. FASEB J. 2020, 34, 5240–5261. [Google Scholar] [CrossRef] [PubMed]
- Abbruzzese, G.; Cousin, H.; Salicioni, A.M.; Alfandari, M. GSK3 and Polo-like kinase regulate ADAM13 function during cranial neural crest cell migration. Mol. Biol. Cell 2014, 25, 4072–4082. [Google Scholar] [CrossRef]
- Liu, K.J.; Arron, J.R.; Stankunas, K.; Crabtree, G.R.; Longaker, M.T. Chemical rescue of cleft palate and midline defects in conditional GSK-3beta mice. Nature 2007, 446, 79–82. [Google Scholar] [CrossRef]
- Szabo-Rogers, H.; Yakob, W.; Liu, K.J. Frontal Bone Insufficiency in Gsk3beta Mutant Mice. PLoS ONE 2016, 11, e0149604. [Google Scholar] [CrossRef] [PubMed]
- Trainor, P.A. Specification of neural crest cell formation and migration in mouse embryos. Semin. Cell Dev. Biol. 2005, 16, 683–693. [Google Scholar] [CrossRef] [PubMed]
- McKinney, M.C.; McLennan, R.; Giniunaite, R.; Baker, R.E.; Maini, P.K.; Othmer, H.G.; Kulesa, P.M. Visualizing mesoderm and neural crest cell dynamics during chick head morphogenesis. Dev. Biol. 2020, 461, 184–196. [Google Scholar] [CrossRef]
- Schilling, T.F.; Le Pabic, P. Chapter 7—Neural Crest Cells in Craniofacial Skeletal Development. In Neural Crest Cells; Trainor, P.A., Ed.; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Graham, A.; Richardson, J. Developmental and evolutionary origins of the pharyngeal apparatus. EvoDevo 2012, 3, 24. [Google Scholar] [CrossRef]
- McLennan, R.; Dyson, L.; Prather, K.W.; Morrison, J.A.; Baker, R.E.; Maini, P.K.; Kulesa, P.M. Multiscale mechanisms of cell migration during development: Theory and experiment. Development 2012, 139, 2935–2944. [Google Scholar] [CrossRef]
- McLennan, R.; Teddy, J.M.; Kasemeier-Kulesa, J.C.; Romine, M.H.; Kulesa, P.M. Vascular endothelial growth factor (VEGF) regulates cranial neural crest migration in vivo. Dev. Biol. 2010, 339, 114–125. [Google Scholar] [CrossRef]
- Kubota, Y.; Ito, K. Chemotactic migration of mesencephalic neural crest cells in the mouse. Dev. Dyn. 2000, 217, 170–179. [Google Scholar] [CrossRef]
- Dunkel, H.; Chaverra, M.; Bradley, R.; Lefcort, F. FGF signaling is required for chemokinesis and ventral migration of trunk neural crest cells. Dev. Dyn. 2020. [Google Scholar] [CrossRef]
- Trumpp, A.; DePew, M.J.; Rubenstein, J.L.; Bishop, J.M.; Martin, G.R. Cre-mediated gene inactivation demonstrates that FGF8 is required for cell survival and patterning of the first branchial arch. Genes Dev. 1999, 13, 3136–3148. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, L.; Taiyab, A.; Melvin, V.S.; Jones, K.L.; Williams, T. Increased FGF8 signaling promotes chondrogenic rather than osteogenic development in the embryonic skull. Dis. Model. Mech. 2018, 11, dmm031526. [Google Scholar] [CrossRef] [PubMed]
- Tabler, J.M.; Barrell, W.B.; Szabo-Rogers, H.L.; Healy, C.; Yeung, Y.; Perdiguero, E.G.; Schulz, C.; Yannakoudakis, B.Z.; Mesbahi, A.; Wlodarczyk, B.J.; et al. Fuz Mutant Mice Reveal Shared Mechanisms between Ciliopathies and FGF-Related Syndromes. Dev. Cell 2013, 25, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Tabler, J.M.; Rice, C.P.; Liu, K.J.; Wallingford, J.B. A novel ciliopathic skull defect arising from excess neural crest. Dev. Biol. 2016, 417, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Ahlgren, S.C.; Bronner, M.E. Inhibition of Sonic hedgehog signaling in vivo results in craniofacial neural crest cell death. Curr. Biol. 1999, 9, 1304–1314. [Google Scholar] [CrossRef]
- Jeong, J.; Mao, J.; Tenzen, T.; Kottmann, A.H.; McMahon, A.P. Hedgehog signaling in the neural crest cells regulates the patterning and growth of facial primordia. Genes Dev. 2004, 18, 937–951. [Google Scholar] [CrossRef]
- Mead, T.J.; Yutzey, K.E. Notch pathway regulation of neural crest cell development in vivo. Dev. Dyn. 2012, 241, 376–389. [Google Scholar] [CrossRef]
- Dong, Y.; Jesse, A.M.; Kohn, A.; Gunnell, L.M.; Honjo, T.; Zuscik, M.J.; O’Keefe, R.J.; Hilton, M.J. RBPjkappa-dependent Notch signaling regulates mesenchymal progenitor cell proliferation and differentiation during skeletal development. Development 2010, 137, 1461–1471. [Google Scholar] [CrossRef]
- Wurdak, H.; Ittner, L.M.; Lang, K.S.; Leveen, P.; Suter, U.; Fischer, J.A.; Karlsson, S.; Born, W.; Sommer, L. Inactivation of TGFbeta signaling in neural crest stem cells leads to multiple defects reminiscent of DiGeorge syndrome. Genes Dev. 2005, 19, 530–535. [Google Scholar] [CrossRef]
- Conway, S.J.; Kaartinen, V. TGFbeta superfamily signaling in the neural crest lineage. Cell Adh. Migr. 2011, 5, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Ito, Y.; Bringas, P.; Chou, S.; Urata, M.M.; Slavkin, H.; Chai, Y. TGFbeta-mediated FGF signaling is crucial for regulating cranial neural crest cell proliferation during frontal bone development. Development 2006, 133, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Yeo, J.Y.; Chytil, A.; Han, J.; Bringas, P.; Nakajima, A.; Shuler, C.; Moses, H.L.; Chai, Y. Conditional inactivation of Tgfbr2 in cranial neural crest causes cleft palate and calvaria defects. Development 2003, 130, 5269–5280. [Google Scholar] [CrossRef] [PubMed]
- Yumoto, K.; Thomas, P.S.; Lane, J.; Matsuzaki, K.; Inagaki, M.; Ninomiya-Tsuji, J.; Scott, G.J.; Ray, M.K.; Ishii, M.; Maxson, R.; et al. TGF-beta-activated kinase 1 (Tak1) mediates agonist-induced Smad activation and linker region phosphorylation in embryonic craniofacial neural crest-derived cells. J. Biol. Chem. 2013, 288, 13467–13480. [Google Scholar] [CrossRef] [PubMed]
- Barriga, E.H.; Trainor, P.A.; Bronner, M.E.; Mayor, R. Animal models for studying neural crest development: Is the mouse different? Development 2015, 142, 1555–1560. [Google Scholar] [CrossRef]
- Acloque, H.; Adams, M.S.; Fishwick, K.; Bronner, M.E.; Nieto, M.A. Epithelial-mesenchymal transitions: The importance of changing cell state in development and disease. J. Clin. Investig. 2009, 119, 1438–1449. [Google Scholar] [CrossRef]
- Carmona-Fontaine, C.; Matthews, H.K.; Kuriyama, S.; Moreno, M.; Dunn, G.A.; Parsons, M.; Stern, C.D.; Mayor, R. Contact inhibition of locomotion in vivo controls neural crest directional migration. Nature 2008, 456, 957–961. [Google Scholar] [CrossRef]
- Matthews, H.K.; Broders-Bondon, F.; Thiery, J.P.; Mayor, R. Wnt11ris required for cranial neural crest migration. Dev. Dyn. 2008, 237, 3404–3409. [Google Scholar] [CrossRef]
- Banerjee, S.; Gordon, L.; Donn, T.M.; Berti, C.; Moens, C.B.; Burden, S.J.; Granato, M. A novel role for MuSK and non-canonical Wnt signaling during segmental neural crest cell migration. Development 2011, 138, 3287–3296. [Google Scholar] [CrossRef]
- De Calisto, J.; Araya, C.; Marchant, L.; Riaz, C.F.; Mayor, R. Essential role of non-canonical Wnt signalling in neural crest migration. Development 2005, 132, 2587–2597. [Google Scholar] [CrossRef]
- Pryor, S.E.; Massa, V.; Savery, D.; Andre, P.; Yang, Y.; Greene, N.D.; Copp, A.J. Vangl-dependent planar cell polarity signalling is not required for neural crest migration in mammals. Development 2014, 141, 3153–3158. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M.S.; Charney, R.M.; García-Castro, M.I. Specification and formation of the neural crest: Perspectives on lineage segregation. Genesis 2019, 57, e23276. [Google Scholar] [CrossRef] [PubMed]
- Vega-Lopez, G.; Cerrizuela, S.; Tríbulo, C.; Aybar, M.J. Neurocristopathies: New insights 150 years after the neural crest discovery. Dev. Biol. 2018, 444 (Suppl. 1), S110–S143. [Google Scholar] [CrossRef]
- Rovin, S.; Dachi, S.F.; Borenstein, D.B.; Cotter, W.B. Mandibulofacial dysostosis, a familial study of five generations. J. Pediatr. 1964, 65, 215–221. [Google Scholar] [CrossRef]
- Collins, E.T. Cases with symmetrical congenital notches in the outer part of each lower lid and defective development of the malar bones. Trans. Ophthalmol. Soc. UK 1900, 20, 190–192. [Google Scholar]
- Franceschetti, A.; Klein, D. The mandibulofacial dysostosis; a new hereditary syndrome. Acta Ophthalmol. 1949, 27, 143–224. [Google Scholar]
- Trainor, P.A.; Dixon, J.; Dixon, M.J. Treacher Collins syndrome: Etiology, pathogenesis and prevention. Eur. J. Hum. Genet. 2009, 17, 275–283. [Google Scholar] [CrossRef]
- Werner, A.; Iwasaki, S.; McGourty, C.A.; Medina-Ruiz, S.; Teerikorpi, N.; Fedrigo, I.; Ingolia, N.T.; Rape, M. Cell-fate determination by ubiquitin-dependent regulation of translation. Nature 2015, 525, 523–527. [Google Scholar] [CrossRef]
- Abbott, B. The etiology of cleft palate: A 50-year search for mechanistic and molecular understanding. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2010, 89, 266–274. [Google Scholar] [CrossRef]
- Burg, M.L.; Chai, Y.; Yao, C.A.; Magee, W.; Figueiredo, J.C. Epidemiology, Etiology, and Treatment of Isolated Cleft Palate. Front. Physiol. 2016, 7, 67. [Google Scholar] [CrossRef]
- Sivertsen, A.; Wilcox, A.J.; Skjærven, R.; Vindenes, H.A.; Abyholm, F.; Harville, E.; Lie, R.T. Familial risk of oral clefts by morphological type and severity: Population based cohort study of first degree relatives. BMJ 2008, 336, 432–434. [Google Scholar] [CrossRef] [PubMed]
- Grosen, D.; Bille, C.; Petersen, I.; Skytthe, A.; Hjelmborg, J.B.; Pedersen, J.K.; Murray, J.C.; Christensen, K. Risk of Oral Clefts in Twins. Epidemiology 2011, 22, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Bush, J.O.; Jiang, R. Palatogenesis: Morphogenetic and molecular mechanisms of secondary palate development. Development 2012, 139, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Janečková, E.; Feng, J.; Li, J.; Rodriguez, G.; Chai, Y. Dynamic activation of Wnt, Fgf, and Hh signaling during soft palate development. PLoS ONE 2019, 14, e0223879. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Gao, Y.; Lan, Y.; Jia, S.; Jiang, R. Pax9 regulates a molecular network involving Bmp4, Fgf10, Shh signaling and the Osr2 transcription factor to control palate morphogenesis. Development 2013, 140, 4709–4718. [Google Scholar] [CrossRef]
- Zwahlen, R.A.; Bütow, K.-W.; Morkel, J.A.; Naidoo, S. Pierre Robin sequence: Subdivision, data, theories, and treatment—Part 2: Syndromic and nonsyndromic Pierre Robin sequence. Ann. Maxillofac. Surg. 2016, 6, 35–37. [Google Scholar] [CrossRef]
- Zwahlen, R.A.; Bütow, K.-W.; Morkel, J.A.; Naidoo, S. Pierre Robin sequence: Subdivision, data, theories, and treatment—Part 1: History, subdivisions, and data. Ann. Maxillofac. Surg. 2016, 6, 31–34. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Z.; Chen, Y.; Zhang, Y. Conditional deletion of Bmp2 in cranial neural crest cells recapitulates Pierre Robin sequence in mice. Cell Tissue Res. 2018, 376, 199–210. [Google Scholar] [CrossRef]
- Aljerian, A.; Gilardino, M.S. Gilardino, Treacher Collins Syndrome. Clin. Plast. Surg. 2019, 46, 197–205. [Google Scholar] [CrossRef]
- Zwahlen, R.A.; Bütow, K.-W.; Naidoo, S.; Morkel, J.A. Pierre Robin sequence: Subdivision, data, theories, and treatment—Part 4: Recommended management and treatment of Pierre Robin sequence and its application. Ann. Maxillofac. Surg. 2016, 6, 44–49. [Google Scholar] [CrossRef]
- Rasmussen, S.A.; Yazdy, M.M.; Frias, J.L.; Honein, M.A. Priorities for public health research on craniosynostosis: Summary and recommendations from a Centers for Disease Control and Prevention-sponsored meeting. Am. J. Med. Genet. Part A 2007, 146, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Flapper, W.J.; Anderson, P.J.; Roberts, R.M.; David, D.J. Intellectual Outcomes Following Protocol Management in Crouzon, Pfeiffer, and Muenke Syndromes. J. Craniofacial Surg. 2009, 20, 1252–1255. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, J.G.; Warren, S.M.; Bernstein, J.; Burnett, W.; Cunningham, M.L.; Edmond, J.C.; Figueroa, A.A.; Kapp-Simon, K.A.; Labow, B.I.; Peterson-Falzone, S.J.; et al. Parameters of care for craniosynostosis. Cleft Palate Craniofac. J. 2012, 49 (Suppl. 1), 1–24. [Google Scholar] [CrossRef]
- Okajima, K.; Robinson, L.K.; Hart, M.A.; Abuelo, D.N.; Cowan, L.S.; Hasegawa, T.; Maumenee, I.H.; Jabs, E.W. Ocular anterior chamber dysgenesis in craniosynostosis syndromes with a fibroblast growth factor receptor 2 mutation. Am. J. Med. Genet. 1999, 85, 160–170. [Google Scholar] [CrossRef]
- Seruya, M.; Oh, A.K.; Boyajian, M.J.; Posnick, J.C.; Keating, R.F. Treatment for delayed presentation of sagittal synostosis: Challenges pertaining to occult intracranial hypertension. J. Neurosurg. Pediatr. 2011, 8, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.M. Sutural biology and the correlates of craniosynostosis. Am. J. Med. Genet. 1993, 47, 581–616. [Google Scholar] [CrossRef] [PubMed]
- Abe, H.; Ikota, T.; Akino, M.; Kitami, K.; Tsuru, M. Functional prognosis of surgical treatment of craniosynostosis. Childs Nerv. Syst. 1985, 1, 53–61. [Google Scholar] [CrossRef]
- Baird, L.C.; Gonda, D.; Cohen, S.R.; Evers, L.H.; Lefloch, N.; Levy, M.L.; Meltzer, H.S. Craniofacial reconstruction as a treatment for elevated intracranial pressure. Childs Nerv. Syst. 2011, 28, 411–418. [Google Scholar] [CrossRef]
- Renier, D.; Lajeunie, E.; Arnaud, E.; Marchac, D. Management of craniosynostoses. Childs Nerv. Syst. 2000, 16, 645–658. [Google Scholar] [CrossRef]
- Torrance, J.S.; Wincenciak, J.; Hahn, A.C.; Debruine, L.M.; Jones, B.C. The Relative Contributions of Facial Shape and Surface Information to Perceptions of Attractiveness and Dominance. PLoS ONE 2014, 9, e104415. [Google Scholar] [CrossRef]
- Garza, R.M.; Khosla, R.K. Nonsyndromic Craniosynostosis. Semin. Plast. Surg. 2012, 26, 053–063. [Google Scholar] [CrossRef] [PubMed]
- Wilkie, A.; Byren, J.C.; Hurst, J.A.; Jayamohan, J.; Johnson, D.; Knight, S.J.L.; Lester, T.; Richards, P.G.; Twigg, S.R.; Wall, S.A. Prevalence and complications of single-gene and chromosomal disorders in craniosynostosis. Pediatrics 2010, 126, e391–e400. [Google Scholar] [CrossRef] [PubMed]
- Peskett, E.; Kumar, S.; Baird, W.; Jaiswal, J.; Li, M.; Patel, P.; Britto, J.A.; Pauws, E. Analysis of the Fgfr2C342Y mouse model shows condensation defects due to misregulation of Sox9 expression in prechondrocytic mesenchyme. Biol. Open 2017, 6, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Ting, M.-C.; Wu, N.L.; Roybal, P.G.; Sun, J.; Liu, L.; Yen, Y.; Maxson, R.E. EphA4 as an effector of Twist1 in the guidance of osteogenic precursor cells during calvarial bone growth and in craniosynostosis. Development 2009, 136, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.K.L.; Peskett, E.; Quinn, C.M.; Aiello, R.; Adeeva, L.; Moulding, D.A.; Stanier, P.; Pauws, E. Overexpression of Fgfr2c causes craniofacial bone hypoplasia and ameliorates craniosynostosis in the Crouzon mouse. Dis. Model. Mech. 2018, 11, dmm035311. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Nam, H.K.; Wang, E.; Hatch, N.E. Further analysis of the Crouzon mouse: Effects of the FGFR2(C342Y) mutation are cranial bone-dependent. Calcif. Tissue Int. 2013, 92, 451–466. [Google Scholar] [CrossRef]
- Pfaff, M.J.; Xue, K.; Li, L.; Horowitz, M.C.; Steinbacher, D.M.; Eswarakumar, V.P. FGFR2c-mediated ERK-MAPK activity regulates coronal suture development. Dev. Biol. 2016, 415, 242–250. [Google Scholar] [CrossRef]
- Ratisoontorn, C.; Fan, G.-F.; McEntee, K.; Nah, H.-D. Activating (P253R, C278F) and dominant negative mutations of FGFR2: Differential effects on calvarial bone cell proliferation, differentiation, and mineralization. Connect. Tissue Res. 2003, 44 (Suppl. 1), 292–297. [Google Scholar] [CrossRef]
- Yen, H.-Y.; Ting, M.-C.; Maxson, R.E. Jagged1 functions downstream of Twist1 in the specification of the coronal suture and the formation of a boundary between osteogenic and non-osteogenic cells. Dev. Biol. 2010, 347, 258–270. [Google Scholar] [CrossRef]
- Holmes, G.; Rothschild, G.; Roy, U.B.; Deng, C.-X.; Mansukhani, A.; Basilico, C. Early onset of craniosynostosis in an Apert mouse model reveals critical features of this pathology. Dev. Biol. 2009, 328, 273–284. [Google Scholar] [CrossRef]
- Chen, L.; Li, D.; Li, C.; Engel, A.; Deng, C.-X. A Ser252Trp [corrected] substitution in mouse fibroblast growth factor receptor 2 (Fgfr2) results in craniosynostosis. Bone 2003, 33, 169–178. [Google Scholar] [CrossRef]
- Holmes, G.; Basilico, C. Mesodermal expression of Fgfr2S252W is necessary and sufficient to induce craniosynostosis in a mouse model of Apert syndrome. Dev. Biol. 2012, 368, 283–293. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Soriano, P. Dysregulated PDGFRalpha signaling alters coronal suture morphogenesis and leads to craniosynostosis through endochondral ossification. Development 2017, 144, 4026–4036. [Google Scholar] [CrossRef]
- Santagati, F.; Rijli, F.M. Cranial neural crest and the building of the vertebrate head. Nat. Rev. Neurosci. 2003, 4, 806–818. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Iseki, S.; Maxson, R.E.; Sucov, H.M.; Morriss-Kay, G.M. Tissue Origins and Interactions in the Mammalian Skull Vault. Dev. Biol. 2002, 241, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Vivatbutsiri, P.; Morriss-Kay, G.; Saga, Y.; Iseki, S. Cell lineage in mammalian craniofacial mesenchyme. Mech. Dev. 2008, 125, 797–808. [Google Scholar] [CrossRef]
- Ferguson, J.W.; Atit, R.P. A tale of two cities: The genetic mechanisms governing calvarial bone development. Genesis 2018, 57, e23248. [Google Scholar] [CrossRef]
- Mori-Akiyama, Y.; Akiyama, H.; Rowitch, D.H.; De Crombrugghe, B. Sox9 is required for determination of the chondrogenic cell lineage in the cranial neural crest. Proc. Natl. Acad. Sci. USA 2003, 100, 9360–9365. [Google Scholar] [CrossRef]
- John, N.; Cinelli, P.; Wegner, M.; Sommer, L. Transforming Growth Factor β-Mediated Sox10 Suppression Controls Mesenchymal Progenitor Generation in Neural Crest Stem Cells. Stem Cells 2011, 29, 689–699. [Google Scholar] [CrossRef]
- Schwarz, D.; Varum, S.; Zemke, M.; Scholer, A.; Baggiolini, A.; Draganova, K.; Koseki, H.; Schubeler, D.; Sommer, L. Ezh2 is required for neural crest-derived cartilage and bone formation. Development 2014, 141, 867–877. [Google Scholar] [CrossRef]
- Hall, B.K.; Miyake, T. All for one and one for all: Condensations and the initiation of skeletal development. Bioessays 2000, 22, 138–147. [Google Scholar] [CrossRef]
- Bi, W.; Huang, W.; Whitworth, D.J.; Deng, J.M.; Zhang, Z.; Behringer, R.R.; De Crombrugghe, B. Haploinsufficiency of Sox9 results in defective cartilage primordia and premature skeletal mineralization. Proc. Natl. Acad. Sci. USA 2001, 98, 6698–6703. [Google Scholar] [CrossRef] [PubMed]
- Roybal, P.G.; Wu, N.L.; Sun, J.; Ting, M.-C.; Schafer, C.A.; Maxson, R.E. Inactivation of Msx1 and Msx2 in neural crest reveals an unexpected role in suppressing heterotopic bone formation in the head. Dev. Biol. 2010, 343, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Lana-Elola, E.; Rice, R.; Grigoriadis, A.E.; Rice, D.P. Cell fate specification during calvarial bone and suture development. Dev. Biol. 2007, 311, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Ishii, M.; Bringas, P.; Maas, R.L.; Maxson, R.E.; Chai, Y. Concerted action of Msx1 and Msx2 in regulating cranial neural crest cell differentiation during frontal bone development. Mech. Dev. 2007, 124, 729–745. [Google Scholar] [CrossRef]
- Ishii, M.; Merrill, A.E.; Chan, Y.-S.; Gitelman, I.; Rice, D.P.; Sucov, H.M.; Maxson, R.E. Msx2 and Twist cooperatively control the development of the neural crest-derived skeletogenic mesenchyme of the murine skull vault. Development 2003, 130, 6131–6142. [Google Scholar] [CrossRef]
- Otto, F.; Thornell, A.P.; Crompton, T.; Denzel, A.; Gilmour, K.C.; Rosewell, I.R.; Stamp, G.W.; Beddington, R.S.; Mundlos, S.; Olsen, B.R.; et al. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell 1997, 89, 765–771. [Google Scholar] [CrossRef]
- Maeno, T.; Moriishi, T.; Yoshida, C.A.; Komori, H.; Kanatani, N.; Izumi, S.-I.; Takaoka, K.; Komori, T. Early onset of Runx2 expression caused craniosynostosis, ectopic bone formation, and limb defects. Bone 2011, 49, 673–682. [Google Scholar] [CrossRef]
- Komori, T.; Yagi, H.; Nomura, S.; Yamaguchi, A.; Sasaki, K.; Deguchi, K.; Shimizu, Y.; Bronson, R.T.; Gao, Y.-H.; Inada, M.; et al. Targeted Disruption of Cbfa1 Results in a Complete Lack of Bone Formation owing to Maturational Arrest of Osteoblasts. Cell 1997, 89, 755–764. [Google Scholar] [CrossRef]
- Nakashima, K.; Zhou, X.; Kunkel, G.R.; Zhang, Z.; Deng, J.M.; Behringer, R.R.; De Crombrugghe, B. The Novel Zinc Finger-Containing Transcription Factor Osterix Is Required for Osteoblast Differentiation and Bone Formation. Cell 2002, 108, 17–29. [Google Scholar] [CrossRef]
- Rice, D.P.; Aberg, T.; Chan, Y.; Tang, Z.; Kettunen, P.J.; Pakarinen, L.; Maxson, R.E.; Thesleff, I. Integration of FGF and TWIST in calvarial bone and suture development. Development 2000, 127, 1845–1855. [Google Scholar] [PubMed]
- Rice, D.P.; Kim, H.-J.; Thesleff, I. Apoptosis in murine calvarial bone and suture development. Eur. J. Oral Sci. 1999, 107, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Connerney, J.; Andreeva, V.; Leshem, Y.; Mercado, M.A.; Dowell, K.; Yang, X.; Lindner, V.; Friesel, R.E.; Spicer, D.B. Twist1 homodimers enhance FGF responsiveness of the cranial sutures and promote suture closure. Dev. Biol. 2008, 318, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.F.; Behringer, R.R. Twist is required in head mesenchyme for cranial neural tube morphogenesis. Genes Dev. 1995, 9, 686–699. [Google Scholar] [CrossRef] [PubMed]
- Soo, K.; O’Rourke, M.P.; Khoo, P.-L.; Steiner, K.A.; Wong, N.; Behringer, R.R.; Tam, P. Twist Function Is Required for the Morphogenesis of the Cephalic Neural Tube and the Differentiation of the Cranial Neural Crest Cells in the Mouse Embryo. Dev. Biol. 2002, 247, 251–270. [Google Scholar] [CrossRef]
- Goodnough, L.H.; DiNuoscio, G.J.; Atit, R.P. Twist1contributes to cranial bone initiation and dermal condensation by maintaining wnt signaling responsiveness. Dev. Dyn. 2015, 245, 144–156. [Google Scholar] [CrossRef]
- Yoshida, T.; Phylactou, L.A.; Uney, J.B.; Ishikawa, I.; Eto, K.; Iseki, S. Twist is required for establishment of the mouse coronal suture. J. Anat. 2005, 206, 437–444. [Google Scholar] [CrossRef]
- Iseki, S.; Wilkie, A.O.; Heath, J.K.; Ishimaru, T.; Eto, K.; Morriss-Kay, G.M. Fgfr2 and osteopontin domains in the developing skull vault are mutually exclusive and can be altered by locally applied FGF2. Development 1997, 124, 3375–3384. [Google Scholar]
- Iseki, S.; Wilkie, A.O.; Morriss-Kay, G.M. Fgfr1 and Fgfr2 have distinct differentiation- and proliferation-related roles in the developing mouse skull vault. Development 1999, 126, 5611–5620. [Google Scholar]
- Quarto, N.; Behr, B.; Li, S.; Longaker, M.T. Differential FGF ligands and FGF receptors expression pattern in frontal and parietal calvarial bones. Cells Tissues Organs 2009, 190, 158–169. [Google Scholar] [CrossRef]
- Doro, D.; Liu, A.; Grigoriadis, A.E.; Liu, K.J. The Osteogenic Potential of the Neural Crest Lineage May Contribute to Craniosynostosis. Mol. Syndr. 2018, 10, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Muenke, M.; Schell, U.; Hehr, A.; Robin, N.H.; Losken, H.W.; Schinzel, A.; Pulleyn, L.J.; Rutland, P.; Reardon, W.; Malcolm, S.; et al. A common mutation in the fibroblast growth factor receptor 1 gene in Pfeiffer syndrome. Nat. Genet. 1994, 8, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-X.; Xu, X.; Chen, L.; Li, C.; Brodie, S.G.; Deng, C.-X. A Pro250Arg substitution in mouse Fgfr1 causes increased expression of Cbfa1 and premature fusion of calvarial sutures. Hum. Mol. Genet. 2000, 9, 2001–2008. [Google Scholar] [CrossRef] [PubMed]
- Roscioli, T.; Flanagan, S.; Kumar, P.; Masel, J.; Gattas, M.; Hyland, V.J.; Glass, I.A. Clinical findings in a patient with FGFR1 P252R mutation and comparison with the literature. Am. J. Med. Genet. 2000, 93, 22–28. [Google Scholar] [CrossRef]
- Wilkie, A.; Slaney, S.F.; Oldridge, M.; Poole, M.D.; Ashworth, G.J.; Hockley, A.; Hayward, R.D.; David, D.J.; Pulleyn, L.J.; Rutland, P.; et al. Apert syndrome results from localized mutations of FGFR2 and is allelic with Crouzon syndrome. Nat. Genet. 1995, 9, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Park, W.-J.; Theda, C.; Day, D.; Oriow, S.J.; Jones, M.C.; Jabs, E.W.; Meyers, G.A.; Li, X. Novel FGFR2 mutations in Crouzon and Jackson-Weiss syndromes show allelic heterogeneity and phenotypic variability. Hum. Mol. Genet. 1995, 4, 1229–1233. [Google Scholar] [CrossRef]
- Jabs, E.W.; Li, X.; Scott, A.F.; Meyers, G.; Chen, W.; Eccles, M.; Mao, J.-I.; Charnas, L.R.; Jackson, C.E.; Jaye, M. Jackson-Weiss and Crouzon syndromes are allelic with mutations in fibroblast growth factor receptor 2. Nat. Genet. 1994, 8, 275–279. [Google Scholar] [CrossRef]
- Holmes, G. Mouse models of Apert syndrome. Childs Nerv. Syst. 2012, 28, 1505–1510. [Google Scholar] [CrossRef]
- Eswarakumar, V.P.; Horowitz, M.C.; Locklin, R.; Morriss-Kay, G.M.; Lonai, P. A gain-of-function mutation of Fgfr2c demonstrates the roles of this receptor variant in osteogenesis. Proc. Natl. Acad. Sci. USA 2004, 101, 12555–12560. [Google Scholar] [CrossRef]
- Johnson, D.; Wilkie, A.O. Craniosynostosis. Eur. J. Hum. Genet. 2011, 19, 369–376. [Google Scholar] [CrossRef]
- Muenke, M.; Gripp, K.W.; McDonald-McGinn, D.M.; Gaudenz, K.; Whitaker, L.A.; Bartlett, S.P.; Markowitz, R.I.; Robin, N.H.; Nwokoro, N.; Mulvihill, J.J.; et al. A unique point mutation in the fibroblast growth factor receptor 3 gene (FGFR3) defines a new craniosynostosis syndrome. Am. J. Hum. Genet. 1997, 60, 555–564. [Google Scholar] [PubMed]
- Twigg, S.R.; Healy, C.; Babbs, C.; Sharpe, J.A.; Wood, W.G.; Sharpe, P.; Wilkie, A.; Morriss-Kay, G.M. Skeletal analysis of the Fgfr3P244R mouse, a genetic model for the Muenke craniosynostosis syndrome. Dev. Dyn. 2008, 238, 331–342. [Google Scholar] [CrossRef]
- El Ghouzzi, V.; Le Merrer, M.; Perrin-Schmitt, F.; Lajeunie, E.; Bénit, P.; Renier, D.; Bourgeois, P.; Bolcato-Bellemin, A.-L.; Munnich, A.; Bonaventure, J. Mutations of the TWIST gene in the Saethre-Chotzene syndrome. Nat. Genet. 1997, 15, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Carver, E.A.; Oram, K.F.; Gridley, T. Craniosynostosis inTwist heterozygous mice: A model for Saethre-Chotzen syndrome. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2002, 268, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Grutzner, E.; Gorlin, R.J. Craniofrontonasal dysplasia: Phenotypic expression in females and males and genetic considerations. Oral Surgery Oral Med. Oral Pathol. 1988, 65, 436–444. [Google Scholar] [CrossRef]
- Twigg, S.R.; Kan, R.; Babbs, C.; Bochukova, E.G.; Robertson, S.P.; Wall, S.A.; Morriss-Kay, G.M.; Wilkie, A. Mutations of ephrin-B1 (EFNB1), a marker of tissue boundary formation, cause craniofrontonasal syndrome. Proc. Natl. Acad. Sci. USA 2004, 101, 8652–8657. [Google Scholar] [CrossRef] [PubMed]
- Jabs, E.W.; Müller, U.; Li, X.; Ma, L.; Luo, W.; Haworth, I.S.; Klisak, I.; Sparkes, R.; Warman, M.L.; Mulliken, J.B.; et al. A mutation in the homeodomain of the human MSX2 gene in a family affected with autosomal dominant craniosynostosis. Cell 1993, 75, 443–450. [Google Scholar] [CrossRef]
- Warman, M.L.; Mulliken, J.B.; Hayward, P.G.; Müller, U. Newly recognized autosomal dominant disorder with craniosynostosis. Am. J. Med. Genet. 1993, 46, 444–449. [Google Scholar] [CrossRef]
- Ma, L.; Golden, S.; Wu, L.; Maxson, R. The molecular basis of Boston-type craniosynostosis: The Pro148-->His mutation in the N-terminal arm of the MSX2 homeodomain stabilizes DNA binding without altering nucleotide sequence preferences. Hum. Mol. Genet. 1996, 5, 1915–1920. [Google Scholar] [CrossRef]
- Bildsoe, H.K.; Loebel, D.A.; Jones, V.J.; Hor, A.C.; Braithwaite, A.W.; Chen, Y.-T.; Behringer, R.R.; Tam, P.P.L. The mesenchymal architecture of the cranial mesoderm of mouse embryos is disrupted by the loss of Twist1 function. Dev. Biol. 2012, 374, 295–307. [Google Scholar] [CrossRef]
- Compagni, A.; Logan, M.; Klein, R.; Adams, R.H. Control of Skeletal Patterning by EphrinB1-EphB Interactions. Dev. Cell 2003, 5, 217–230. [Google Scholar] [CrossRef]
- Mansour, S.L.; Li, C.; Urness, L.D. Genetic rescue of Muenke syndrome model hearing loss reveals prolonged FGF-dependent plasticity in cochlear supporting cell fates. Genes Dev. 2013, 27, 2320–2331. [Google Scholar] [CrossRef] [PubMed]
- Nah, H.-D.; Koyama, E.; Agochukwu, N.B.; Bartlett, S.P.; Muenke, M. Phenotype profile of a genetic mouse model for Muenke syndrome. Childs Nerv. Syst. 2012, 28, 1483–1493. [Google Scholar] [CrossRef] [PubMed]
- Merrill, A.E.; Sarukhanov, A.; Krejci, P.; Idoni, B.; Camacho, N.; Estrada, K.D.; Lyons, K.M.; Deixler, H.; Robinson, H.; Chitayat, D.; et al. Bent Bone Dysplasia-FGFR2 type, a Distinct Skeletal Disorder, Has Deficient Canonical FGF Signaling. Am. J. Hum. Genet. 2012, 90, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Neben, C.L.; Idoni, B.; Salva, J.E.; Tuzon, C.T.; Rice, J.C.; Krakow, D.; Merrill, A.E. Bent bone dysplasia syndrome reveals nucleolar activity for FGFR2 in ribosomal DNA transcription. Hum. Mol. Genet. 2014, 23, 5659–5671. [Google Scholar] [CrossRef]
- Teng, C.S.; Ting, M.-C.; Farmer, D.T.; Brockop, M.; Maxson, R.E.; Crump, J.G. Altered bone growth dynamics prefigure craniosynostosis in a zebrafish model of Saethre-Chotzen syndrome. eLife 2018, 7, 7. [Google Scholar] [CrossRef]
- Sharma, V.P.; 500 Whole-Genome Sequences (WGS500) Consortium; Fenwick, A.L.; Brockop, M.S.; McGowan, S.J.; Goos, J.A.C.; Hoogeboom, A.J.M.; Brady, A.F.; Jeelani, N.U.O.; Lynch, S.A.; et al. Mutations in TCF12, encoding a basic helix-loop-helix partner of TWIST1, are a frequent cause of coronal craniosynostosis. Nat. Genet. 2013, 45, 304–307. [Google Scholar] [CrossRef]
- Liu, J.; Nam, H.K.; Campbell, C.; da Silva Gasque, K.C.; Millán, J.L.; Hatch, N.E. Tissue-nonspecific alkaline phosphatase deficiency causes abnormal craniofacial bone development in the Alpl(−/−) mouse model of infantile hypophosphatasia. Bone 2014, 67, 81–94. [Google Scholar] [CrossRef]
- Nam, H.K.; Vesela, I.; Siismets, E.; Hatch, N.E. Tissue nonspecific alkaline phosphatase promotes calvarial progenitor cell cycle progression and cytokinesis via Erk1,2. Bone 2019, 120, 125–136. [Google Scholar] [CrossRef]
- Li, S.; Quarto, N.; Longaker, M.T. Activation of FGF Signaling Mediates Proliferative and Osteogenic Differences between Neural Crest Derived Frontal and Mesoderm Parietal Derived Bone. PLoS ONE 2010, 5, e14033. [Google Scholar] [CrossRef]
- Wu, T.; Chen, G.; Tian, F.; Liu, H.-X. Contribution of cranial neural crest cells to mouse skull development. Int. J. Dev. Biol. 2017, 61, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Quarto, N.; Wan, D.C.; Kwan, M.D.; Panetta, N.J.; Li, S.; Longaker, M.T. Origin Matters: Differences in Embryonic Tissue Origin and Wnt Signaling Determine the Osteogenic Potential and Healing Capacity of Frontal and Parietal Calvarial Bones. J. Bone Miner. Res. 2009, 25, 1680–1694. [Google Scholar] [CrossRef] [PubMed]
- Teng, C.S.; Cavin, L.; Maxson, R.E.; Sánchez-Villagra, M.R.; Crump, J.G. Resolving homology in the face of shifting germ layer origins: Lessons from a major skull vault boundary. eLife 2019, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.M.; Kreiborg, S.; Krelborg, S. Birth prevalence studies of the Crouzon syndrome: Comparison of direct and indirect methods. Clin. Genet. 2008, 41, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Neilson, K.M.; Friesel, R.E. Constitutive Activation of Fibroblast Growth Factor Receptor-2 by a Point Mutation Associated with Crouzon Syndrome. J. Biol. Chem. 1995, 270, 26037–26040. [Google Scholar] [CrossRef] [PubMed]
- Galvin, B.D.; Hart, K.C.; Meyer, A.N.; Webster, M.K.; Donoghue, D.J. Constitutive receptor activation by Crouzon syndrome mutations in fibroblast growth factor receptor (FGFR)2 and FGFR2/Neu chimeras. Proc. Natl. Acad. Sci. USA 1996, 93, 7894–7899. [Google Scholar] [CrossRef]
- Robertson, S.C.; Meyer, A.N.; Hart, K.C.; Galvin, B.D.; Webster, M.K.; Donoghue, D.J. Activating mutations in the extracellular domain of the fibroblast growth factor receptor 2 function by disruption of the disulfide bond in the third immunoglobulin-like domain. Proc. Natl. Acad. Sci. USA 1998, 95, 4567–4572. [Google Scholar] [CrossRef]
- Reardon, W.; Winter, R.M.; Rutland, P.; Pulleyn, L.J.; Jones, B.M.; Malcolm, S. Mutations in the fibroblast growth factor receptor 2 gene cause Crouzon syndrome. Nat. Genet. 1994, 8, 98–103. [Google Scholar] [CrossRef]
- Mansukhani, A.; Bellosta, P.; Sahni, M.; Basilico, C. Signaling by Fibroblast Growth Factors (Fgf) and Fibroblast Growth Factor Receptor 2 (Fgfr2)–Activating Mutations Blocks Mineralization and Induces Apoptosis in Osteoblasts. J. Cell Biol. 2000, 149, 1297–1308. [Google Scholar] [CrossRef]
- Cohen, M.M.; Kreiborg, S. Visceral anomalies in the Apert syndrome. Am. J. Med. Genet. 1993, 45, 758–760. [Google Scholar] [CrossRef]
- Cohen, M.M.; Kreiborg, S. Skeletal abnormalities in the Apert syndrome. Am. J. Med. Genet. 1993, 47, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zhang, L.; Weng, T.; Zhang, S.; Sun, S.; Chang, M.; Li, Y.; Zhang, B.; Zhang, L. A Ser252Trp Mutation in Fibroblast Growth Factor Receptor 2 (FGFR2) Mimicking Human Apert Syndrome Reveals an Essential Role for FGF Signaling in the Regulation of Endochondral Bone Formation. PLoS ONE 2014, 9, e87311. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.; Burns, H.D.; Enriquez-Harris, P.; Wilkie, A.; Heath, J.K. Apert syndrome mutations in fibroblast growth factor receptor 2 exhibit increased affinity for FGF ligand. Hum. Mol. Genet. 1998, 7, 1475–1483. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Herr, A.B.; Waksman, G.; Ornitz, D.M. Loss of fibroblast growth factor receptor 2 ligand-binding specificity in Apert syndrome. Proc. Natl. Acad. Sci. USA 2000, 97, 14536–14541. [Google Scholar] [CrossRef] [PubMed]
- Lemonnier, J.; Hay, E.; Delannoy, P.; Fromigué, O.; Lomri, A.; Modrowski, D.; Marie, P.J. Increased Osteoblast Apoptosis in Apert Craniosynostosis. Am. J. Pathol. 2001, 158, 1833–1842. [Google Scholar] [CrossRef]
- Dufour, C.; Guenou, H.; Kaabeche, K.; Bouvard, D.; Sanjay, A.; Marie, P.J. FGFR2-Cbl interaction in lipid rafts triggers attenuation of PI3K/Akt signaling and osteoblast survival. Bone 2008, 42, 1032–1039. [Google Scholar] [CrossRef]
- Lomri, A.; Lemonnier, J.; Hott, M.; De Parseval, N.; Lajeunie, E.; Munnich, A.; Renier, D.; Marie, P.J. Increased calvaria cell differentiation and bone matrix formation induced by fibroblast growth factor receptor 2 mutations in Apert syndrome. J. Clin. Investig. 1998, 101, 1310–1317. [Google Scholar]
- Deckelbaum, R.A.; Majithia, A.; Booker, T.; Henderson, J.E.; Loomis, C.A. The homeoprotein engrailed 1 has pleiotropic functions in calvarial intramembranous bone formation and remodeling. Development 2006, 133, 63–74. [Google Scholar] [CrossRef]
- Johnson, D.; Iseki, S.; Wilkie, A.; Morriss-Kay, G.M. Expression patterns of Twist and Fgfr1, -2 and -3 in the developing mouse coronal suture suggest a key role for twist in suture initiation and biogenesis. Mech. Dev. 2000, 91, 341–345. [Google Scholar] [CrossRef]
- Kindberg, A.A.; Bush, J.O. Cellular organization and boundary formation in craniofacial development. Genesis 2019, 57, e23271. [Google Scholar] [CrossRef]
- Zhao, H.; Feng, J.; Ho, T.-V.; Grimes, W.; Urata, M.; Chai, Y. The suture provides a niche for mesenchymal stem cells of craniofacial bones. Nat. Cell Biol. 2015, 17, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, T.; Jeong, J.; Sheu, T.-J.; Hsu, W. Stem cells of the suture mesenchyme in craniofacial bone development, repair and regeneration. Nat. Commun. 2016, 7, 10526. [Google Scholar] [CrossRef] [PubMed]
- Harr, J.C.; Gonzalez-Sandoval, A.; Gasser, S.M. Histones and histone modifications in perinuclear chromatin anchoring: From yeast to man. EMBO Rep. 2016, 17, 139–155. [Google Scholar] [CrossRef] [PubMed]
- Prado, F.; Jimeno-González, S.; Reyes, J.C. Histone availability as a strategy to control gene expression. RNA Biol. 2016, 14, 281–286. [Google Scholar] [CrossRef]
- Kuzmichev, A.; Nishioka, K.; Erdjument-Bromage, H.; Tempst, P.; Reinberg, D. Histone methyltransferase activity associated with a human multiprotein complex containing the Enhancer of Zeste protein. Genes Dev. 2002, 16, 2893–2905. [Google Scholar] [CrossRef]
- Cao, R.; Wang, L.; Wang, H.; Xia, L.; Erdjument-Bromage, H.; Tempst, P.; Jones, R.S.; Zhang, Y. Role of Histone H3 Lysine 27 Methylation in Polycomb-Group Silencing. Science 2002, 298, 1039–1043. [Google Scholar] [CrossRef]
- Dudakovic, A.; Camilleri, E.T.; Xu, F.; Riester, S.M.; McGee-Lawrence, M.E.; Bradley, E.W.; Paradise, C.R.; Lewallen, E.A.; Thaler, R.; Deyle, D.R.; et al. Epigenetic Control of Skeletal Development by the Histone Methyltransferase Ezh2*. J. Biol. Chem. 2015, 290, 27604–27617. [Google Scholar] [CrossRef]
- Dudakovic, A.; Camilleri, E.T.; Paradise, C.R.; Samsonraj, R.M.; Gluscevic, M.; Paggi, C.A.; Begun, D.L.; Khani, F.; Pichurin, O.; Ahmed, F.S.; et al. Enhancer of zeste homolog 2 (Ezh2) controls bone formation and cell cycle progression during osteogenesis in mice. J. Biol. Chem. 2018, 293, 12894–12907. [Google Scholar] [CrossRef]
- Camilleri, E.T.; Dudakovic, A.; Riester, S.M.; Galeano-Garces, C.; Paradise, C.R.; Bradley, E.W.; McGee-Lawrence, M.E.; Im, H.-J.; Karperien, H.; Krych, A.J.; et al. Loss of histone methyltransferase Ezh2 stimulates an osteogenic transcriptional program in chondrocytes but does not affect cartilage development. J. Biol. Chem. 2018, 293, 19001–19011. [Google Scholar] [CrossRef]
- Dudakovic, A.; Camilleri, E.T.; Lewallen, E.A.; McGee-Lawrence, M.E.; Riester, S.M.; Kakar, S.; Montecino, M.; Stein, G.S.; Ryoo, H.-M.; Dietz, A.B.; et al. Histone Deacetylase Inhibition Destabilizes the Multi-Potent State of Uncommitted Adipose-Derived Mesenchymal Stromal Cells. J. Cell. Physiol. 2015, 230, 52–62. [Google Scholar] [CrossRef]
- De Laat, W.L.; Duboule, D. Topology of mammalian developmental enhancers and their regulatory landscapes. Nature 2013, 502, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.; Ferrai, C.; Chiariello, A.M.; Schueler, M.; Rito, T.; Laudanno, G.; Barbieri, M.; Moore, B.L.; Kraemer, D.C.; Aitken, S.; et al. Hierarchical folding and reorganization of chromosomes are linked to transcriptional changes in cellular differentiation. Mol. Syst. Biol. 2015, 11, 852. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, M.C.; Sungalee, S.; Zufferey, M.; Tavernari, D.; Katanayeva, N.; Battistello, E.; Mina, M.; Douglass, K.M.; Rey, T.; Raynaud, F.; et al. EZH2 oncogenic mutations drive epigenetic, transcriptional, and structural changes within chromatin domains. Nat. Genet. 2019, 51, 517–528. [Google Scholar] [CrossRef]
- Tatton-Brown, K.; Murray, A.; Hanks, S.; Douglas, J.; Armstrong, R.; Banka, S.; Bird, L.M.; Clericuzio, C.L.; Cormier, V.; Cushing, T.; et al. Weaver syndrome and EZH2 mutations: Clarifying the clinical phenotype. Am. J. Med. Genet. Part A 2013, 161, 2972–2980. [Google Scholar] [CrossRef] [PubMed]
- Lui, J.C.; Barnes, K.M.; Dong, L.; Yue, S.; Graber, E.; Rapaport, R.; Dauber, A.; Nilsson, O.; Baron, J. Ezh2 Mutations Found in the Weaver Overgrowth Syndrome Cause a Partial Loss of H3K27 Histone Methyltransferase Activity. J. Clin. Endocrinol. Metab. 2018, 103, 1470–1478. [Google Scholar] [CrossRef]
- Wilderman, A.; VanOudenhove, J.; Kron, J.; Noonan, J.P.; Cotney, J. High-Resolution Epigenomic Atlas of Human Embryonic Craniofacial Development. Cell Rep. 2018, 23, 1581–1597. [Google Scholar] [CrossRef]
- Brinkley, J.; Borromeo, C.; Clarkson, M.D.; Cox, T.C.; Cunningham, M.J.; Detwiler, L.; Heike, C.; Hochheiser, H.; Mejino, J.; Travillian, R.; et al. The ontology of craniofacial development and malformation for translational craniofacial research. Am. J. Med. Genet. Part C Semin. Med. Genet. 2013, 163, 232–245. [Google Scholar] [CrossRef][Green Version]
- Holmes, G.; Gonzalez-Reiche, A.S.; Lu, N.; Zhou, X.; Rivera, J.; Kriti, D.; Sebra, R.; Williams, A.A.; Donovan, M.J.; Potter, S.S.; et al. Integrated Transcriptome and Network Analysis Reveals Spatiotemporal Dynamics of Calvarial Suturogenesis. Cell Rep. 2020, 32, 107871. [Google Scholar] [CrossRef]
- Poot, M. Structural Genome Variations Related to Craniosynostosis. Mol. Syndr. 2018, 10, 24–39. [Google Scholar] [CrossRef]
- Online Mendelian Inheritance in Man, OMIM®. Available online: https://www.omim.org/about (accessed on 31 July 2020).
- Woo, K.M.; Chen, V.J.; Jung, H.-M.; Kim, T.-I.; Shin, H.-I.; Baek, J.-H.; Ryoo, H.-M.; Ma, P.X. Comparative Evaluation of Nanofibrous Scaffolding for Bone Regeneration in Critical-Size Calvarial Defects. Tissue Eng. Part A 2009, 15, 2155–2162. [Google Scholar] [CrossRef]
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Levi, B.; Hyun, J.S.; Montoro, D.T.; Lo, D.D.; Chan, C.K.F.; Hu, S.; Sun, N.; Lee, M.; Grova, M.; Connolly, A.J.; et al. In vivo directed differentiation of pluripotent stem cells for skeletal regeneration. Proc. Natl. Acad. Sci. USA 2012, 109, 20379–20384. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Y.; Chen, Y.E.; Chen, J.; Ma, P.X. Cell-free 3D scaffold with two-stage delivery of miRNA-26a to regenerate critical-sized bone defects. Nat. Commun. 2016, 7, 10376. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; Irizarry, D.; Lopez, M.; Moore, A.L.; Ransom, R.; Longaker, M.T.; Wan, D.C.; Chan, C.K. The Role of Skeletal Stem Cells in the Reconstruction of Bone Defects. J. Craniofacial Surg. 2017, 28, 1136–1141. [Google Scholar] [CrossRef] [PubMed]
- Hixon, K.R.; Melvin, A.M.; Lin, A.Y.; Hall, A.F.; Sell, S.A. Cryogel scaffolds from patient-specific 3D-printed molds for personalized tissue-engineered bone regeneration in pediatric cleft-craniofacial defects. J. Biomater. Appl. 2017, 32, 598–611. [Google Scholar] [CrossRef] [PubMed]
- Gupte, M.J.; Swanson, W.B.; Hu, J.; Jin, X.; Ma, H.; Zhang, Z.; Liu, Z.; Feng, K.; Feng, G.; Xiao, G.; et al. Pore size directs bone marrow stromal cell fate and tissue regeneration in nanofibrous macroporous scaffolds by mediating vascularization. Acta Biomater. 2018, 82, 1–11. [Google Scholar] [CrossRef]
- Ransom, R.; Carter, A.C.; Salhotra, A.; Leavitt, T.; Marecic, O.; Murphy, M.P.; Lopez, M.L.; Wei, Y.; Marshall, C.D.; Shen, E.Z.; et al. Mechanoresponsive stem cells acquire neural crest fate in jaw regeneration. Nature 2018, 563, 514–521. [Google Scholar] [CrossRef]
- Nam, H.K.; Sharma, M.; Liu, J.; Hatch, N.E. Tissue Nonspecific Alkaline Phosphatase (TNAP) Regulates Cranial Base Growth and Synchondrosis Maturation. Front. Physiol. 2017, 8, 161. [Google Scholar] [CrossRef]
- Hatch, N.E.; Li, Y.; Franceschi, R.T. FGF2 Stimulation of the Pyrophosphate-Generating Enzyme, PC-1, in Pre-Osteoblast Cells Is Mediated by RUNX2. J. Bone Miner. Res. 2008, 24, 652–662. [Google Scholar] [CrossRef]
- Liu, J.; Kwon, T.G.; Nam, H.K.; Hatch, N.E. Craniosynostosis-associated Fgfr2(C342Y) mutant bone marrow stromal cells exhibit cell autonomous abnormalities in osteoblast differentiation and bone formation. BioMed. Res. Int. 2013, 2013, 292506. [Google Scholar]
- Nam, H.K.; Veselá, I.; Schutte, S.D.; Hatch, N.E. Viral delivery of tissue nonspecific alkaline phosphatase diminishes craniosynostosis in one of two FGFR2C342Y/+ mouse models of Crouzon syndrome. PLoS ONE 2020, 15, e0234073. [Google Scholar] [CrossRef] [PubMed]
- MacGregor, G.R.; Zambrowicz, B.P.; Soriano, P. Tissue non-specific alkaline phosphatase is expressed in both embryonic and extraembryonic lineages during mouse embryogenesis but is not required for migration of primordial germ cells. Development 1995, 121, 1487–1496. [Google Scholar] [PubMed]
- Lomelí, H.; Ramos-Mejía, V.; Gertsenstein, M.; Lobe, C.G.; Nagy, A. Targeted insertion of Cre recombinase into the TNAP gene: Excision in primordial germ cells. Genesis 2000, 26, 116–117. [Google Scholar] [CrossRef]
- De Felici, M. Origin, Migration, and Proliferation of Human Primordial Germ Cells. In Oogenesis; Coticchio, G.A.D., De Santis, L., Eds.; Springer: London, UK, 2013; pp. 19–37. [Google Scholar]
- Langer, D.; Ikehara, Y.; Takebayashi, H.; Hawkes, R.; Zimmermann, H. The ectonucleotidases alkaline phosphatase and nucleoside triphosphate diphosphohydrolase 2 are associated with subsets of progenitor cell populations in the mouse embryonic, postnatal and adult neurogenic zones. Neuroscience 2007, 150, 863–879. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, M.H. The Atlas of Mouse Development; Academic Press: Cambridge, MA, USA, 1992. [Google Scholar]
- Sun, J.; Ishii, M.; Ting, M.-C.; Maxson, R.E. Foxc1 controls the growth of the murine frontal bone rudiment by direct regulation of a Bmp response threshold of Msx2. Development 2013, 140, 1034–1044. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.; Grose, R. Fibroblast growth factor signalling: From development to cancer. Nat. Rev. Cancer 2010, 10, 116–129. [Google Scholar] [CrossRef]
- Pollock, P.M.; Project, C.G.; Gartside, M.G.; Dejeza, L.C.; Powell, M.A.; Mallon, M.A.; Davies, H.; Mohammadi, M.; Futreal, P.A.; Stratton, M.R.; et al. Frequent activating FGFR2 mutations in endometrial carcinomas parallel germline mutations associated with craniosynostosis and skeletal dysplasia syndromes. Oncogene 2007, 26, 7158–7162. [Google Scholar] [CrossRef]
- Chaffer, C.L.; Dopheide, B.; Savagner, P.; Thompson, E.W.; Williams, E.D. Aberrant fibroblast growth factor receptor signaling in bladder and other cancers. Differentiation 2007, 75, 831–842. [Google Scholar] [CrossRef]
- Hoelder, S.; Clarke, P.A.; Workman, P. Discovery of small molecule cancer drugs: Successes, challenges and opportunities. Mol. Oncol. 2012, 6, 155–176. [Google Scholar] [CrossRef]
- Shukla, V.; Coumoul, X.; Wang, R.-H.; Kim, H.-S.; Deng, C.-X. RNA interference and inhibition of MEK-ERK signaling prevent abnormal skeletal phenotypes in a mouse model of craniosynostosis. Nat. Genet. 2007, 39, 1145–1150. [Google Scholar] [CrossRef]
- Eswarakumar, V.P.; Özcan, F.; Lew, E.D.; Bae, J.H.; Tomé, F.; Booth, C.J.; Adams, D.J.; Lax, I.; Schlessinger, J. Attenuation of signaling pathways stimulated by pathologically activated FGF-receptor 2 mutants prevents craniosynostosis. Proc. Natl. Acad. Sci. USA 2006, 103, 18603–18608. [Google Scholar] [CrossRef] [PubMed]
- Perlyn, C.A.; Morriss-Kay, G.; Darvann, T.; Tenenbaum, M.; Ornitz, D.M. A model for the pharmacological treatment of crouzon syndrome. Neurosurgery 2006, 59, 215. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chilà, R.; Hall, T.; Abbadessa, G.; Broggini, M.; Damia, G. Multi-Chemotherapeutic Schedules Containing the pan-FGFR Inhibitor ARQ 087 are Safe and Show Antitumor Activity in Different Xenograft Models. Transl. Oncol. 2017, 10, 153–157. [Google Scholar] [CrossRef]
- Mazzaferro, V.; El-Rayes, B.F.; Busset, M.D.D.; Cotsoglou, C.; Harris, W.P.; Damjanov, N.; Masi, G.; Rimassa, L.; Personeni, N.; Braiteh, F.; et al. Derazantinib (ARQ 087) in advanced or inoperable FGFR2 gene fusion-positive intrahepatic cholangiocarcinoma. Br. J. Cancer 2019, 120, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Balek, L.; Gudernova, I.; Veselá, I.; Hampl, M.; Oralova, V.; Bosakova, M.K.; Varecha, M.; Nemec, P.; Hall, T.; Abbadessa, G.; et al. ARQ 087 inhibits FGFR signaling and rescues aberrant cell proliferation and differentiation in experimental models of craniosynostoses and chondrodysplasias caused by activating mutations in FGFR1, FGFR2 and FGFR3. Bone 2017, 105, 57–66. [Google Scholar] [CrossRef]
- Evans, C.G.; Chang, L.; Gestwicki, J.E. Heat Shock Protein 70 (Hsp70) as an Emerging Drug Target. J. Med. Chem. 2010, 53, 4585–4602. [Google Scholar] [CrossRef]
- Lamberti, D.; Cristinziano, G.; Porru, M.; Leonetti, C.; Egan, J.B.; Shi, C.-X.; Buglioni, S.; Amoreo, C.A.; Castellani, L.; Borad, M.J.; et al. HSP90 Inhibition Drives Degradation of FGFR2 Fusion Proteins: Implications for Treatment of Cholangiocarcinoma. Hepatology 2019, 69, 131–142. [Google Scholar] [CrossRef]
- Chung, C.; Yoo, G.; Kim, T.; Lee, D.; Lee, C.-S.; Cha, H.R.; Park, Y.H.; Moon, J.Y.; Jung, S.S.; Kim, J.O.; et al. The E3 ubiquitin ligase CHIP selectively regulates mutant epidermal growth factor receptor by ubiquitination and degradation. Biochem. Biophys. Res. Commun. 2016, 479, 152–158. [Google Scholar] [CrossRef]
- Díaz-Villanueva, J.F.; Díaz-Molina, R.; García-González, V. Protein Folding and Mechanisms of Proteostasis. Int. J. Mol. Sci. 2015, 16, 17193–17230. [Google Scholar] [CrossRef]
- Trepel, J.; Mollapour, M.; Giaccone, G.; Neckers, L. Targeting the dynamic HSP90 complex in cancer. Nat. Rev. Cancer 2010, 10, 537–549. [Google Scholar] [CrossRef]
- Kaabeche, K.; Lemonnier, J.; Le Mée, S.; Caverzasio, J.; Marie, P.J. Cbl-mediated Degradation of Lyn and Fyn Induced by Constitutive Fibroblast Growth Factor Receptor-2 Activation Supports Osteoblast Differentiation. J. Biol. Chem. 2004, 279, 36259–36267. [Google Scholar] [CrossRef]
- Kaabeche, K.; Guenou, H.; Bouvard, D.; Didelot, N.; Listrat, A.; Marie, P.J. Cbl-mediated ubiquitination of 5 integrin subunit mediates fibronectin-dependent osteoblast detachment and apoptosis induced by FGFR2 activation. J. Cell Sci. 2005, 118 Pt 6, 1223–1232. [Google Scholar] [CrossRef]
- Hatch, N.E.; Hudson, M.; Seto, M.L.; Cunningham, M.L.; Bothwell, M.A. Intracellular Retention, Degradation, and Signaling of Glycosylation-deficient FGFR2 and Craniosynostosis Syndrome-associated FGFR2C278F. J. Biol. Chem. 2006, 281, 27292–27305. [Google Scholar] [CrossRef] [PubMed]
- Yoon, W.-J.; Cho, Y.-D.; Cho, K.-H.; Woo, K.-M.; Baek, J.-H.; Cho, J.-Y.; Kim, G.-S.; Ryoo, H.-M. The Boston-type Craniosynostosis Mutation MSX2 (P148H) Results in Enhanced Susceptibility of MSX2 to Ubiquitin-dependent Degradation. J. Biol. Chem. 2008, 283, 32751–32761. [Google Scholar] [CrossRef] [PubMed]
- Lin-Shiao, E.; Lan, Y.; Welzenbach, J.; Alexander, K.A.; Zhang, Z.; Knapp, M.; Mangold, E.; Sammons, M.A.; Ludwig, K.U.; Berger, S.L. p63 establishes epithelial enhancers at critical craniofacial development genes. Sci. Adv. 2019, 5, eaaw0946. [Google Scholar] [CrossRef] [PubMed]
- Soldatov, R.; Kaucka, M.; Kastriti, M.E.; Peters, J.A.; Chontorotzea, T.; Englmaier, L.; Akkuratova, N.; Yang, Y.; Häring, M.; Dyachuk, V.; et al. Spatiotemporal structure of cell fate decisions in murine neural crest. Science 2019, 364, eaas9536. [Google Scholar] [CrossRef]
- Miller, K.; Twigg, S.R.; McGowan, S.J.; Phipps, J.M.; Fenwick, A.L.; Johnson, D.; Wall, S.A.; Noons, P.; Rees, K.E.M.; Tidey, E.A.; et al. Diagnostic value of exome and whole genome sequencing in craniosynostosis. J. Med. Genet. 2016, 54, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Malagon, S.G.G.; Dobson, L.; Muñoz, A.M.L.; Dawson, M.; Barrell, W.; Marangos, P.; Krause, M.; Liu, K.J. Dissection, Culture and Analysis of Primary Cranial Neural Crest Cells from Mouse for the Study of Neural Crest Cell Delamination and Migration. J. Vis. Exp. 2019, 2019, e60051. [Google Scholar] [CrossRef]
- Ishii, M.; Arias, A.C.; Liu, L.; Chen, Y.-B.; Bronner, M.E.; Maxson, R.E. A Stable Cranial Neural Crest Cell Line from Mouse. Stem Cells Dev. 2012, 21, 3069–3080. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siismets, E.M.; Hatch, N.E. Cranial Neural Crest Cells and Their Role in the Pathogenesis of Craniofacial Anomalies and Coronal Craniosynostosis. J. Dev. Biol. 2020, 8, 18. https://doi.org/10.3390/jdb8030018
Siismets EM, Hatch NE. Cranial Neural Crest Cells and Their Role in the Pathogenesis of Craniofacial Anomalies and Coronal Craniosynostosis. Journal of Developmental Biology. 2020; 8(3):18. https://doi.org/10.3390/jdb8030018
Chicago/Turabian StyleSiismets, Erica M., and Nan E. Hatch. 2020. "Cranial Neural Crest Cells and Their Role in the Pathogenesis of Craniofacial Anomalies and Coronal Craniosynostosis" Journal of Developmental Biology 8, no. 3: 18. https://doi.org/10.3390/jdb8030018
APA StyleSiismets, E. M., & Hatch, N. E. (2020). Cranial Neural Crest Cells and Their Role in the Pathogenesis of Craniofacial Anomalies and Coronal Craniosynostosis. Journal of Developmental Biology, 8(3), 18. https://doi.org/10.3390/jdb8030018