The Forgotten Skeletogenic Condensations: A Comparison of Early Skeletal Development Amongst Vertebrates
Abstract
:1. Introduction
2. Epithelial–Mesenchymal Induction: When, Where and How
3. Initiating and Maintaining Skeletogenic Condensations
4. Setting Condensation Boundaries
5. Condensation Growth
5.1. Condensation Adhesion and Cell Migration
5.2. Condensation Proliferation
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Atchley, W.R.; Hall, B.K. A model for development and evolution of complex morphological structures. Biol. Rev. 1991, 66, 101–157. [Google Scholar] [CrossRef] [PubMed]
- Hall, B.K.; Miyake, T. All for one and one for all: Condensations and the initiation of skeletal development. BioEssays 2000, 22, 138–147. [Google Scholar] [CrossRef]
- Dunlop, L.-L.T.; Hall, B.K. Relationships between cellular condensation, preosteoblast formation and epithelial-mesenchymal interactions in initiation of osteogenesis. Int. J. Dev. Biol. 1995, 39, 357–371. [Google Scholar] [PubMed]
- Hall, B.K. Bones and Cartilage; Academic Press: New York, NY, USA, 2015; pp. 299–348. [Google Scholar]
- Hall, B.K.; Miyake, T. Divide, accumulate, differentiate: Cell condensation in skeletal development revisited. Int. J. Dev. Biol. 1995, 39, 881–893. [Google Scholar] [PubMed]
- Hall, B.K.; Miyake, T. The membranous skeleton: The role of cell condensations in vertebrate skeletogenesis. Anat. Embryol. 1992, 186, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Eames, B.F.; Amores, A.; Yan, Y.-L.; Postlethwait, J.H. Evolution of the osteoblast: Skeletogenesis in gar and zebrafish. BMC Evol. Biol. 2012, 12, 27. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Picos, P.; Eames, B.F. On the evolutionary relationship between chondrocytes and osteoblasts. Front. Genet. 2015, 6, 297. [Google Scholar] [CrossRef] [PubMed]
- Eames, B.F.; Helms, J.A. Conserved molecular program regulating cranial and appendicular skeletogenesis. Dev. Dyn. 2004, 231, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Eames, B.F.; Sharpe, P.T.; Helms, J.A. Hierarchy revealed in the specification of three skeletal fates by Sox9 and Runx2. Dev. Biol. 2004, 274, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Åberg, T.; Rice, R.; Rice, D.; Thesleff, I.; Waltimo-Sirén, J. Chondrogenic potential of mouse calvarial mesenchyme. J. Histochem. Cytochem. 2005, 53, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Sauka-Spengler, T.; Bronner-Fraser, M. A gene regulatory network orchestrates neural crest formation. Nat. Rev. Mol. Cell Biol. 2008, 9, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Fisher, S.; Franz-Odendaal, T.A. Evolution of the bone gene regulatory network. Curr. Opin. Genet. Dev. 2012, 22, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Santoiemma, M. Epithelial-mesenchymal interactions: A fundamental developmental biology mechanism. Int. J. Dev. Biol. 2014, 58, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Hall, B.K. Tissue interactions and the initiation of osteogenesis and chondrogenesis in the neural crest-derived mandibular skeleton of the embryonic mouse as seen in isolated murine tissues and in recombinations of murine and avian tissues. J. Embryol. Exp. Morphol. 1980, 58, 251–264. [Google Scholar] [PubMed]
- Hall, B.K.; Tremaine, R. Ability of neural crest cells from the embryonic chick to differentiate into cartilage before their migration away from the neural tube. Anat. Rec. 1979, 194, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Franz-Odendaal, T.A.; Vickaryous, M.K. Skeletal elements in the vertebrate eye and adnexa: Morphological and developmental perspectives. Dev. Dyn. 2006, 235, 1244–1255. [Google Scholar] [CrossRef] [PubMed]
- Hall, B.K. Specificity in the differentiation and morphogenesis of neural crest-derived scleral ossicles and of epithelial scleral papillae in the eye of the embryonic chick. J. Embryol. Exp. Morphol. 1981, 66, 175–190. [Google Scholar] [PubMed]
- Andrews, D.D.T.; Franz-Odendaal, T.A. Organotypic culture method to study the development of the embryonic chicken tissues. J. Vis. Exp. 2018, 138, e57619. [Google Scholar] [CrossRef] [PubMed]
- Coulombre, A.J.; Coulombre, J.L. The skeleton of the eye I. Conjunctival papillae and scleral ossicles. Dev. Biol. 1962, 5, 382–401. [Google Scholar] [CrossRef]
- Coulombre, A.J.; Coulombre, J.L. The skeleton of the eye II. Overlap of the scleral ossicles of the domestic fowl. Dev. Biol. 1973, 33, 257–267. [Google Scholar] [CrossRef]
- Epperlein, H.H. The ectomesenchymal-endodermal interaction-system (EEIS) of Triturus alpestris in tissue culture. 1. Observations on attachment, migration and differentiation of neural crest cells. Differentiation 1974, 2, 151–168. [Google Scholar] [CrossRef] [PubMed]
- Epperlein, H.H.; Lemann, R. The ectomesenchymal-endodermal interaction system (EEIS) of Triturus alpestris in tissue culture. 2. Observations on the differentiation of visceral cartilage. Differentiation 1975, 4, 159–174. [Google Scholar] [CrossRef]
- Piotrowski, T.; Nüsslein-Volhard, C. The endoderm plays an important role in patterning the segmented pharyngeal region in zebrafish (Danio rerio). Dev. Biol. 2000, 225, 339–356. [Google Scholar] [CrossRef] [PubMed]
- David, N.B.; Saint-Etienne, L.; Tsang, M.; Schilling, T.F.; Rosa, F.M. Requirement for endoderm and FGF3 in ventral head skeleton formation. Development 2002, 129, 4457–4468. [Google Scholar] [PubMed]
- Franz-Odendaal, T.A. Skeletons of the Eye: An Evolutionary and Developmental Perspective. Anat. Rec. 2018. [Google Scholar] [CrossRef] [PubMed]
- Pignatti, E.; Zeller, R.; Zuniga, A. To BMP or not to BMP during vertebrate limb bud development. Semin. Cell Dev. Biol. 2014, 32, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Knothe Tate, M.L.; Falls, T.D.; McBride, S.H.; Atit, R.; Knothe, U.R. Mechanical modulation of osteochondroprogenitor cell fate. Int. J. Biochem. Cell Biol. 2008, 40, 2720–2738. [Google Scholar] [CrossRef] [PubMed]
- Richman, J.M.; Buchtová, M.; Boughner, J.C. Comparative ontogeny and phylogeny of the upper jaw skeleton in amniotes. Dev. Dyn. 2006, 235, 1230–1243. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, M.E.; Hall, B.K. Altered timing of the extracellular-matrix-mediated epithelial-mesenchymal interaction that initiates mandibular skeletogenesis in three inbred strains of mice: Development, heterochrony, and evolutionary change in morphology. J. Exp. Zool. 2001, 291, 258–273. [Google Scholar] [CrossRef] [PubMed]
- Pinto, C.B.; Hall, B.K. Toward an understanding of the epithelial requirement for osteogenesis in scleral mesenchyme of the embryonic chick. J. Exp. Zool. 1991, 259, 92–108. [Google Scholar] [CrossRef] [PubMed]
- Franz-Odendaal, T.A. Toward understanding the development of scleral ossicles in the chicken, Gallus gallus. Dev. Dyn. 2008, 237, 3240–3251. [Google Scholar] [CrossRef] [PubMed]
- Duench, K.; Franz-Odendaal, T.A. BMP and Hedgehog signaling during the development of scleral ossicles. Dev. Biol. 2012, 365, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Franz-Odendaal, T.A. Analysis of the FGFR spatiotemporal expression pattern within the chicken scleral ossicle system. Gene Expr. Patterns 2018, 30, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Roark, E.F.; Greer, K. Transforming growth factor-β and bone morphogenetic protein-2 act by distinct mechanisms to promote chick limb cartilage differentiation in vitro. Dev. Dyn. 1994, 200, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Gañan, Y.; Macias, D.; Duterque-Coquillaud, M.; Ros, M.A.; Hurle, J.M. Role of TGFβs and BMPs as signals controlling the position of the digits and the areas of interdigital cell death in the developing chick limb autopod. Development 1996, 122, 2349–2357. [Google Scholar] [PubMed]
- Chimal-Monroy, J.; Díaz de León, L. Expression of N-cadherin, N-CAM, fibronectin and tenascin is stimulated by TGF-β1, β2, β3 and β5 during the formation of precartilage condensations. Int. J. Dev. Biol. 1999, 43, 59–67. [Google Scholar] [PubMed]
- Mackie, E.J.; Thesleff, I.; Chiquet-Ehrismann, R. Tenascin is associated with chondrogenic and osteogenic differentiation in vivo and promotes chondrogenesis in vitro. J. Cell. Biol. 1987, 105, 2569–2579. [Google Scholar] [CrossRef] [PubMed]
- Billmyre, K.K.; Klingensmith, J. Sonic hedgehog from pharyngeal arch 1 epithelium is necessary for early mandibular arch cell survival and later cartilage condensation differentiation. Dev. Dyn. 2015, 244, 564–576. [Google Scholar] [CrossRef] [PubMed]
- Quint, E.; Smith, A.; Avaron, F.; Laforest, L.; Miles, J.; Gaffield, W.; Akimenko, M.-A. Bone patterning is altered in the regenerating zebrafish caudal fin after ectopic expression of sonic hedgehog and bmp2b or exposure to cyclopamine. Proc. Natl. Acad. Sci. USA 2002, 99, 8713–8718. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Avaron, F.; Guay, D.; Padhi, B.K.; Akimenko, M.A. Inhibition of BMP signaling during zebrafish fin regeneration disrupts fin growth and scleroblast differentiation and function. Dev. Biol. 2006, 299, 438–454. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.-X.; Yi, J.-R.; Ying, S.-Y.; Chuong, C.-M. Activin enhances chondrogenesis of limb bud cells: Stimulation of precartilagenous mesenchymal condensations and expression of NCAM. Dev. Biol. 1993, 155, 545–557. [Google Scholar] [CrossRef] [PubMed]
- DeLise, A.M.; Tuan, R.S. Alterations in the spatiotemporal expression pattern and function of N-Cadherin inhibit cellular condensation and chondrogenesis of limb mesenchymal cells in vitro. J. Cell. Biochem. 2002, 87, 342–359. [Google Scholar] [CrossRef] [PubMed]
- Rundus, V.R.; Marshall, G.B.; Parker, S.B.; Bales, E.S.; Hertzberg, E.L.; Minkoff, R. Association of cell and substrate adhesion molecules with connexin43 during intramembranous bone formation. Histochem. J. 1998, 30, 879–896. [Google Scholar] [CrossRef] [PubMed]
- Aulthouse, A.L.; Solursh, M. The detection of a precartilage, blastema-specific marker. Dev. Biol. 1987, 120, 377–384. [Google Scholar] [CrossRef]
- Zimmermann, B.; Thies, M. Alterations of lectin binding during chondrogenesis of mouse limb buds. Histochemistry 1984, 81, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Miyake, T.; Cameron, A.M.; Hall, B.K. Stage-specific onset of condensation and matrix deposition for Meckel’s and other first arch cartilages in inbred C57BL/6 mice. J. Craniofac. Genet. Dev. Biol. 1996, 16, 32–47. [Google Scholar] [PubMed]
- Maleski, M.P.; Knudson, C.B. Hyaluronan-mediated aggregation of limb bud mesenchyme and mesenchymal condensation during chondrogenesis. Exp. Cell Res. 1996, 225, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.; Lerea, K.M.; Peng, H.; Kaltner, H.; Gabius, H.-J.; Newman, S.A. A regulatory network of two galactins mediates the earliest steps of avian limb skeletal morphogenesis. BMC Dev. Biol. 2011, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Smith-Paredes, D.; Núñez-León, D.; Soto-Acuña, S.; O’Connor, J.; Botelho, J.F.; Vargas, A.O. Dinosaur ossification centres in embryonic birds uncover developmental evolution of the skull. Nat. Ecol. Evol. 2018, 2, 1966–1973. [Google Scholar] [CrossRef] [PubMed]
- Lemire, R.J. Embryology of the Skull. In Craniosynostosis: Diagnosis, Evaluation and Management; Cohen, M.M., Jr., Ed.; Raven Press: New York, NY, USA, 1986; Chapter 5. [Google Scholar]
- Gray, H.; Bannister, L.H.; Berry, M.M.; Williams, P.L. Gray’s Anatomy: The Anatomical Basis of Medicine and Surgery, 38th ed.; Churchill Livingstone: London, UK, 1995. [Google Scholar]
- Franz-Odendaal, T.A. Induction and patterning of intramembranous bone. Front. Biosci. 2011, 16, 2734–2746. [Google Scholar] [CrossRef]
- Hammer, C.L.; Franz-Odendaal, T.A. Towards understanding the dose and timing effect of hydrocortisone treatment on the scleral ossicle system within the chicken eye. J. Anat. 2018, 232, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Drake, P.M.; Franz-Odendaal, T.A. A potential role for MMPs during the formation of non-neurogenic placodes. J. Dev. Biol. 2018, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Kanzler, B.; Kuschert, S.J.; Liu, Y.H.; Mallo, M. Hoxa-2 restricts the chondrogenic domain and inhibits bone formation during development of the branchial area. Development 1998, 125, 2587–2597. [Google Scholar] [PubMed]
- Iyyanar, P.P.R.; Nazarali, A.J. Hoxa2 inhibits bone morphogenetic protein signaling during osteogenic differentiation of the palatal mesenchyme. Front. Physiol. 2017, 8, 929. [Google Scholar] [CrossRef] [PubMed]
- Glimm, T.; Headon, D.; Kiskowski, M.A. Computational and mathematical models of chondrogenesis in vertebrate limbs. Birth Defects Res. C 2012, 96, 176–192. [Google Scholar] [CrossRef] [PubMed]
- Karsenty, G. The complexities of skeletal biology. Nature 2003, 423, 316–318. [Google Scholar] [CrossRef] [PubMed]
- Widelitz, R.B.; Jiang, T.-X.; Murray, B.A.; Chuong, C.-M. Adhesion molecules in skeletogenesis: II. Neural cell adhesion molecules mediate precartilagenous mesenchymal condensations and enhance chondrogenesis. J. Cell. Physiol. 1993, 156, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Kostetskii, I.; Radice, G.L. N-cadherin is not essential for limb mesenchymal chondrogenesis. Dev. Dyn. 2005, 232, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Yokouchi, Y.; Nakazato, S.; Yamamoto, M.; Goto, Y.; Kameda, T.; Iba, H.; Kuroiwa, A. Misexpression of Hoxa-13 induces cartilage homeotic transformation and changes cell adhesiveness in chick limb buds. Genes Dev. 1995, 9, 2509–2522. [Google Scholar] [CrossRef] [PubMed]
- Stadler, H.S.; Higgins, K.M.; Capecchi, M.R. Loss of Eph-receptor expression correlates with loss of cell adhesion and chondrogenic capacity in Hoxa13 mutant limbs. Development 2001, 128, 4177–4188. [Google Scholar] [PubMed]
- Horakova, D.; Cela, P.; Krejci, P.; Balek, L.; Moravcova Balkova, S.; Matalova, E.; Buchtova, M. Effect of FGFR inhibitors on chicken limb development. Dev. Growth Differ. 2014, 56, 555–572. [Google Scholar] [CrossRef] [PubMed]
- Downie, S.A.; Newman, S.A. Different roles for fibronectin in the generation of fore and hind limb precartilage condensations. Dev. Biol. 1995, 172, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Stewart, T.A.; Bhat, R.; Newman, S.A. The evolutionary origin of digit patterning. EvoDevo 2017, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.; Chapman, S.C. Cytoskeletal reorganization drives mesenchymal condensation and regulates downstream molecular signaling. PLoS ONE 2015, 10, e0134702. [Google Scholar] [CrossRef] [PubMed]
- Harada, M.; Murakami, H.; Okawa, A.; Okimoto, N.; Hiraoka, S.; Nakahara, T.; Akasaka, R.; Shiraishi, Y.; Futatsugi, N.; Mizutani-Koseki, Y.; et al. FGF9 monomer-dimer equilibrium regulates extracellular matrix affinity and tissue diffusion. Nat. Genet. 2009, 41, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Janners, M.Y.; Searls, R.L. Changes in rate of cellular proliferation during the differentiation of cartilage and muscle in the mesenchyme of the embryonic chick wing. Dev. Biol. 1970, 23, 136–165. [Google Scholar] [CrossRef]
- Thorogood, P.V.; Hinchliffe, J.R. An analysis of the condensation process during chondrogenesis in the embryonic chick hind limb. J. Embryol. Exp. Morphol. 1975, 33, 581–606. [Google Scholar] [PubMed]
- Jabalee, J.; Hillier, S.; Franz-Odendaal, T.A. An investigation of cellular dynamics during the development of intramembranous bones: The scleral ossicles. J. Anat. 2013, 223, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.; Jheon, A.H.; Ealba, E.L.; Eames, B.F.; Butcher, K.D.; Mak, S.-S.; Ladher, R.; Alliston, T.; Schneider, R.A. Evolution of a developmental mechanism: Species-specific regulation of the cell cycle and the timing of events during craniofacial osteogenesis. Dev. Biol. 2014, 385, 380–395. [Google Scholar] [CrossRef] [PubMed]
- Boulet, A.M.; Capecchi, M.R. Multiple roles of Hoxa11 and Hoxd11 in the formation of the mammalian forelimb zeugopod. Development 2004, 131, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Ornitz, D.M. FGF signaling regulates mesenchymal differentiation and skeletal patterning along the limb bud proximodistal axis. Development 2008, 135, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; Chaboissier, M.-C.; Martin, J.F.; Schedl, A.; de Crombrugghe, B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Gene. Dev. 2002, 16, 2813–2828. [Google Scholar] [CrossRef] [PubMed]
- Rice, R.; Rice, D.P.C.; Olsen, B.R.; Thesleff, I. Progression of calvarial bone development requires Foxc1 regulation of Msx2 and Alx4. Dev. Biol. 2003, 262, 75–87. [Google Scholar] [CrossRef]
- Sun, J.; Ishii, M.; Ting, M.-C.; Maxson, R. Foxc1 controls the growth of the murine frontal bone rudiment by direct regulation of a Bmp response threshold of Msx2. Development 2013, 140, 1034–1044. [Google Scholar] [CrossRef] [PubMed]
- Abzhanov, A.; Protas, M.; Grant, B.R.; Grant, P.R.; Tabin, C.J. Bmp4 and morphological variation of beaks in Darwin’s finches. Science 2004, 305, 1462–1465. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Colnot, C.; Marcucio, R.S. Effect of bone morphogenetic protein signaling on development of the jaw skeleton. Dev. Dyn. 2008, 237, 3727–3737. [Google Scholar] [CrossRef] [PubMed]
- Abzhanov, A.; Rodda, S.J.; McMahon, A.P.; Tabin, C.J. Regulation of skeletogenic differentiation in cranial dermal bone. Development 2007, 134, 3133–3144. [Google Scholar] [CrossRef] [PubMed]
- Celá, P.; Buchtová, M.; Veselá, I.; Fu, K.; Bogardi, J.-P.; Song, Y.; Barlow, A.; Buxton, P.; Medalová, J.; Francis-West, P.; et al. BMP signaling regulates the fate of chondro-osteoprogenitor cells in facial mesenchyme in a stage-specific manner. Dev. Dyn. 2016, 245, 947–962. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Tu, X.; Choi, K.; Akiyama, H.; Mishina, Y.; Long, F. BMP-Smad4 signaling is required for precartilaginous mesenchymal condensation independent of Sox9 in the mouse. Dev. Biol. 2015, 400, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Haas, A.R.; Tuan, R.S. Chondrogenic differentiation of murine C3H10T1/2 multipotential mesenchymal cells: II. Stimulation by bone morphogenetic protein-2 requires modulation of N-cadherin expression and function. Differentiation 1999, 64, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zheng, Y.; Chen, D.; Chen, Y. Enhanced BMP signaling prevents degradation and leads to endochondral ossification of Meckel’s cartilage in mice. Dev. Biol. 2013, 381, 301–311. [Google Scholar] [CrossRef] [PubMed]
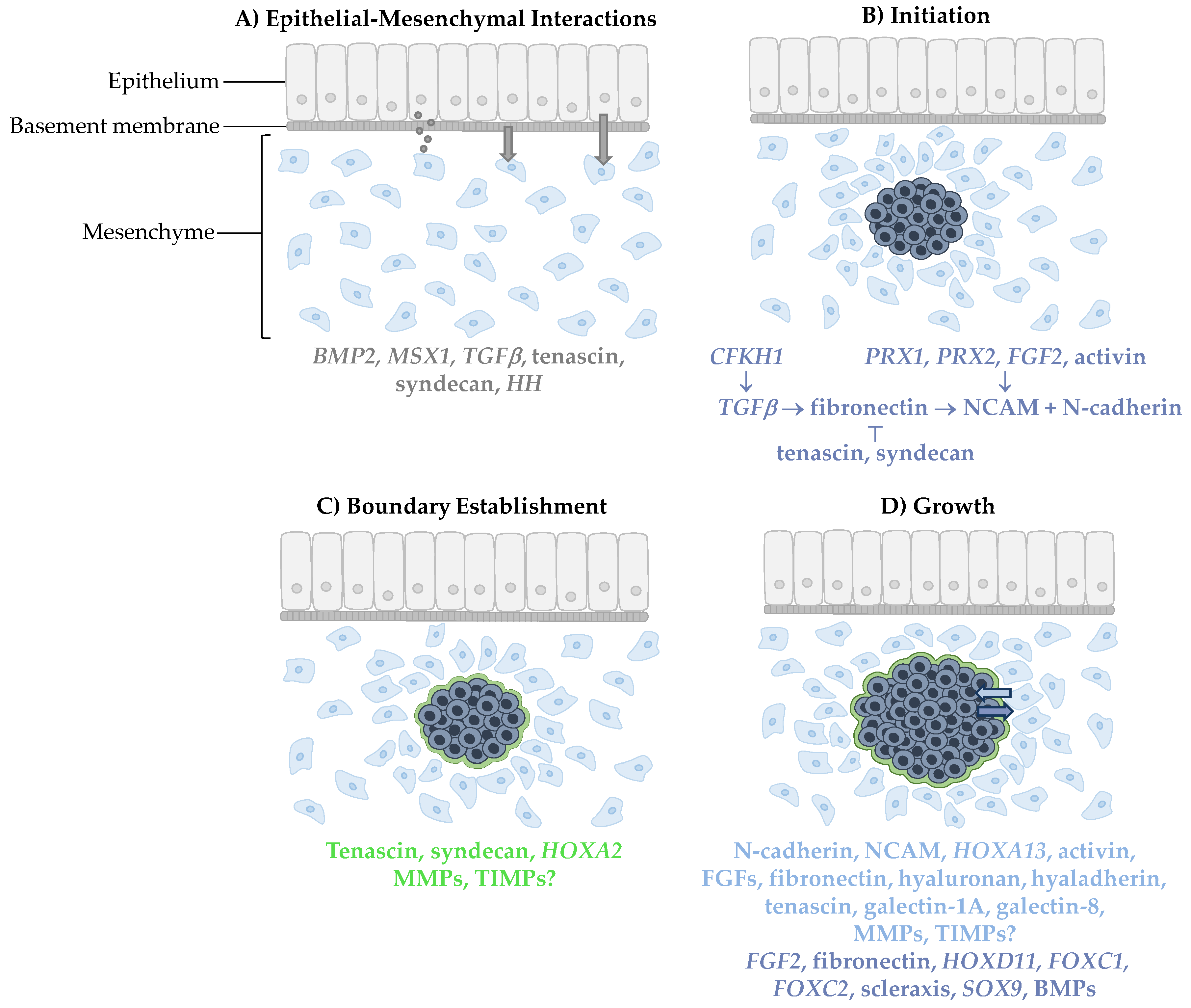
| Stage | Gene/Gene Product | Function |
|---|---|---|
| Epithelial–Mesenchymal Interactions | BMP2 | Inductive epithelial signaling molecules |
| MSX1 | ||
| TGFβ | ||
| Tenascin | ||
| Syndecan | ||
| HH | ||
| Initiation | CFKH1 | Regulates TGFβ |
| TGFβ | Regulates fibronectin | |
| Fibronectin | Regulates NCAM | |
| PRX1, PRX2 | ||
| FGF2 | ||
| Activin | ||
| Tenascin | Adhesion; inhibits fibronectin | |
| Syndecan | ||
| NCAM | Adhesion; stabilization and maintenance of condensation | |
| N-cadherin | Adhesion | |
| Boundary Establishment | Tenascin | Boundary formation |
| Syndecan | ||
| MMPs, TIMPs | Potential role in boundary degradation | |
| HOXA2 | Establishment of osteogenic and chondrogenic domains | |
| Growth | N-cadherin | Cell adhesion |
| NCAM | ||
| Activin | ||
| FGFs | ||
| Fibronectin | ||
| Hyaluronan, hyaladherin | ||
| Tenascin | ||
| HOXA13 | Cell adhesion and condensation patterning | |
| Galectin-1A, galectin-8 | ||
| FGF2 | Cell proliferation and survival | |
| HOXD11 | ||
| FOXC1 | ||
| FOXC2 | ||
| Scleraxis | ||
| SOX9 | ||
| BMPs | ||
| MMPs, TIMPs | Potential role in boundary degradation to allow growth |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giffin, J.L.; Gaitor, D.; Franz-Odendaal, T.A. The Forgotten Skeletogenic Condensations: A Comparison of Early Skeletal Development Amongst Vertebrates. J. Dev. Biol. 2019, 7, 4. https://doi.org/10.3390/jdb7010004
Giffin JL, Gaitor D, Franz-Odendaal TA. The Forgotten Skeletogenic Condensations: A Comparison of Early Skeletal Development Amongst Vertebrates. Journal of Developmental Biology. 2019; 7(1):4. https://doi.org/10.3390/jdb7010004
Chicago/Turabian StyleGiffin, Jennifer L., Danielle Gaitor, and Tamara A. Franz-Odendaal. 2019. "The Forgotten Skeletogenic Condensations: A Comparison of Early Skeletal Development Amongst Vertebrates" Journal of Developmental Biology 7, no. 1: 4. https://doi.org/10.3390/jdb7010004
APA StyleGiffin, J. L., Gaitor, D., & Franz-Odendaal, T. A. (2019). The Forgotten Skeletogenic Condensations: A Comparison of Early Skeletal Development Amongst Vertebrates. Journal of Developmental Biology, 7(1), 4. https://doi.org/10.3390/jdb7010004





