Hoxa5: A Key Player in Development and Disease
Abstract
:1. Introduction
2. Hoxa5: An Imperative in Morphogenesis
2.1. Axial Skeleton
2.2. Appendicular Skeleton
2.3. Respiratory System
2.4. Digestive System
2.5. Thyroid Gland
2.6. Mammary Gland
2.7. Ovary
2.8. Hematopoiesis
3. Hoxa5: Deregulated Expression and Tumorigenesis
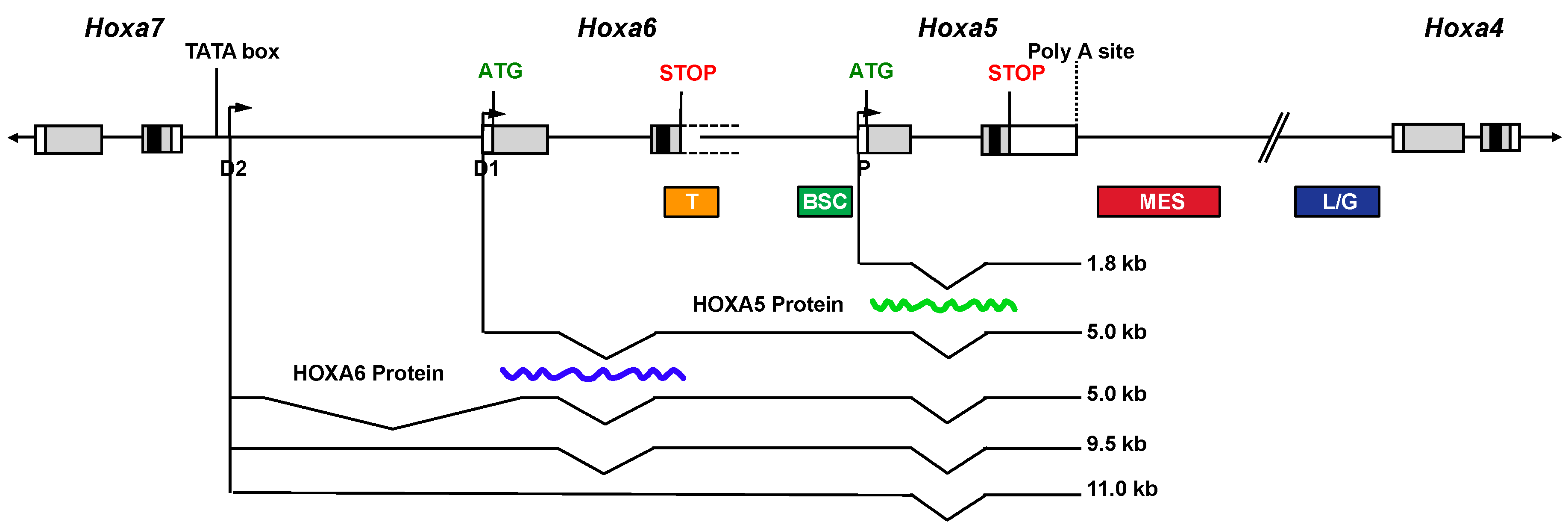
4. Hoxa5: Transcriptional Complexity and Mechanistic Integration
5. Conclusion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Akam, M. The molecular basis for metameric pattern in the Drosophila embryo. Development 1987, 101, 1–22. [Google Scholar] [PubMed]
- Lewis, E.B. A gene complex controlling segmentation in Drosophila. Nature 1978, 276, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Krumlauf, R. Hox genes in vertebrate development. Cell 1994, 78, 191–201. [Google Scholar] [CrossRef]
- Duboule, D. The rise and fall of Hox gene clusters. Development 2007, 134, 2549–2560. [Google Scholar] [CrossRef] [PubMed]
- Tschopp, P.; Tarchini, B.; Spitz, F.; Zakany, J.; Duboule, D. Uncoupling time and space in the collinear regulation of Hox genes. PLoS Genet. 2009, 5, e1000398. [Google Scholar] [CrossRef] [PubMed]
- Mallo, M.; Wellik, D.M.; Deschamps, J. Hox genes and regional patterning of the vertebrate body plan. Dev. Biol. 2010, 344, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Greer, J.M.; Puetz, J.; Thomas, K.R.; Capecchi, M.R. Maintenance of functional equivalence during paralogous Hox gene evolution. Nature 2000, 403, 661–665. [Google Scholar] [PubMed]
- Tvrdik, P.; Capecchi, M.R. Reversal of Hox1 gene subfunctionalization in the mouse. Dev. Cell 2006, 11, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Mann, R.S.; Lelli, K.M.; Joshi, R. Hox specificity: Unique roles for cofactors and collaborators. Curr. Top. Dev. Biol. 2009, 88, 63–101. [Google Scholar] [PubMed]
- Rezsohazy, R.; Saurin, A.J.; Maurel-Zaffran, C.; Graba, Y. Cellular and molecular insights into Hox protein action. Development 2015, 142, 1212–1227. [Google Scholar] [CrossRef] [PubMed]
- Pearson, J.C.; Lemons, D.; McGinnis, W. Modulating Hox gene functions during animal body patterning. Nat. Rev. Genet. 2005, 6, 893–904. [Google Scholar] [CrossRef] [PubMed]
- Quinonez, S.C.; Innis, J.W. Human HOX gene disorders. Mol. Genet. Metab. 2014, 111, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Bhatlekar, S.; Fields, J.Z.; Boman, B.M. HOX genes and their role in the development of human cancers. J. Mol. Med. 2014, 92, 811–823. [Google Scholar] [CrossRef] [PubMed]
- Odenwald, W.F.; Taylor, C.F.; Palmer-Hill, F.J.; Friedrich, V.; Tani, M.; Lazzarini, R.A. Expression of a homeo domain protein in noncontact-inhibited cultured cells and postmitotic neurons. Genes Dev. 1987, 1, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Jeannotte, L.; Lemieux, M.; Charron, J.; Poirier, F.; Robertson, E.J. Specification of axial identity in the mouse: Role of the Hoxa-5 (Hox1. 3) gene. Genes Dev. 1993, 7, 2085–2096. [Google Scholar] [CrossRef] [PubMed]
- Aubin, J.; Lemieux, M.; Tremblay, M.; Behringer, R.R.; Jeannotte, L. Transcriptional interferences at the Hoxa4/Hoxa5 locus: Importance of correct Hoxa5 expression for the proper specification of the axial skeleton. Dev. Dyn. 1998, 212, 141–156. [Google Scholar] [CrossRef]
- Horan, G.S.; Wu, K.; Wolgemuth, D.J.; Behringer, R.R. Homeotic transformation of cervical vertebrae in Hoxa-4 mutant mice. Proc. Natl. Acad. Sci. USA 1994, 91, 12644–12648. [Google Scholar] [CrossRef] [PubMed]
- Kostic, D.; Capecchi, M.R. Targeted disruptions of the murine Hoxa-4 and Hoxa-6 genes result in homeotic transformations of components of the vertebral column. Mech. Dev. 1994, 46, 231–247. [Google Scholar] [CrossRef]
- Coulombe, Y.; Lemieux, M.; Moreau, J.; Aubin, J.; Joksimovic, M.; Bérubé-Simard, F.A.; Tabariès, S.; Boucherat, O.; Guillou, F.; Larochelle, C.; et al. Multiple promoters and alternative splicing: Hoxa5 transcriptional complexity in the mouse embryo. PLoS ONE 2010, 12, e10600. [Google Scholar] [CrossRef] [PubMed]
- Aubin, J.; Lemieux, M.; Moreau, J.; Lapointe, J.; Jeannotte, L. Cooperation of Hoxa5 and Pax1 genes during formation of the pectoral girdle. Dev. Biol. 2002, 244, 96–113. [Google Scholar] [CrossRef] [PubMed]
- Aubin, J.; Lemieux, M.; Tremblay, M.; Bérard, J.; Jeannotte, L. Early postnatal lethality in Hoxa-5 mutant mice is attributable to respiratory tract defects. Dev. Biol. 1997, 192, 432–445. [Google Scholar] [CrossRef] [PubMed]
- Boucherat, O.; Montaron, S.; Bérubé-Simard, F.A.; Aubin, J.; Philippidou, P.; Wellik, D.M.; Dasen, J.S.; Jeannotte, L. Partial functional redundancy between Hoxa5 and Hoxb5 paralog genes during lung morphogenesis. Am. J. Physiol. Lung Cell Mol. Physiol. 2013, 304, 817–830. [Google Scholar] [CrossRef] [PubMed]
- Mandeville, I.; Aubin, J.; LeBlanc, M.; Lalancette-Hébert, M.; Janelle, M.F.; Tremblay, G.M.; Jeannotte, L. Impact of the loss of Hoxa5 function on lung alveogenesis. Am. J. Pathol. 2006, 169, 1312–1327. [Google Scholar] [CrossRef] [PubMed]
- Boucherat, O.; Chakir, J.; Jeannotte, L. The loss of Hoxa5 function promotes Notch-dependent goblet cell metaplasia in lung airways. Biol. Open 2012, 1, 677–691. [Google Scholar] [CrossRef] [PubMed]
- Philippidou, P.; Walsh, C.M.; Aubin, J.; Jeannotte, L.; Dasen, J.S. Sustained Hox5 gene activity is required for respiratory motor neuron development. Nat. Neurosci. 2012, 15, 1636–1644. [Google Scholar] [CrossRef] [PubMed]
- Kinkead, R.; Leblanc, M.; Gulemetova, R.; Lalancette-Hébert, M.; Lemieux, M.; Mandeville, I.; Jeannotte, L. Respiratory adaptations to lung morphological defects in adult mice lacking Hoxa5 gene function. Pediatr. Res. 2004, 56, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Aubin, J.; Déry, U.; Lemieux, M.; Chailler, P.; Jeannotte, L. Stomach regional specification requires Hoxa5-driven mesenchymal-epithelial signaling. Development 2002, 129, 4075–4087. [Google Scholar] [PubMed]
- Aubin, J.; Chailler, P.; Ménard, D.; Jeannotte, L. Loss of Hoxa5 gene function in mice perturbs intestinal maturation. Am. J. Physiol. Cell Physiol. 1999, 277, 965–973. [Google Scholar]
- Meunier, D.; Aubin, J.; Jeannotte, L. Perturbed thyroid morphology and transient hypothyroidism symptoms in Hoxa5 mutant mice. Dev. Dyn. 2003, 227, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Garin, É.; Lemieux, M.; Coulombe, Y.; Robinson, G.W.; Jeannotte, L. Stromal Hoxa5 function controls the growth and differentiation of mammary alveolar epithelium. Dev. Dyn. 2006, 235, 1858–1871. [Google Scholar] [CrossRef] [PubMed]
- Gendronneau, G.; Boucherat, O.; Aubin, J.; Lemieux, M.; Jeannotte, L. The loss of Hoxa5 function causes estrous acyclicity and ovarian epithelial inclusion cysts. Endocrinology 2012, 153, 1484–1497. [Google Scholar] [CrossRef] [PubMed]
- Grüneberg, H. Genetical studies on the skeleton of the mouse. J. Genet. 1954, 52, 441–454. [Google Scholar] [CrossRef]
- Balling, R.; Deutsch, U.; Gruss, P. Undulated, a mutation affecting the development of the mouse skeleton, has a point mutation in the paired box of Pax 1. Cell 1988, 55, 531–535. [Google Scholar] [CrossRef]
- Wallin, J.; Wilting, J.; Koseki, H.; Fritsch, R.; Christ, B.; Balling, R. The role of Pax-1 in axial skeleton development. Development 1994, 120, 1109–1121. [Google Scholar] [PubMed]
- Bi, W.; Deng, J.M.; Zhang, Z.; Behringer, R.R.; de Crombrugghe, B. Sox9 is required for cartilage formation. Nat. Genet. 1999, 22, 85–89. [Google Scholar] [PubMed]
- Chen, J.W.; Zahid, S.; Shilts, M.H.; Weaver, S.J.; Leskowitz, R.M.; Habbsa, S.; Aronowitz, K.P.R.; Chang, Y.; Pinnella, Z.; Holloway, L.; et al. Hoxa-5 acts in segmented somites to regulate cervical vertebral morphology. Mech. Dev. 2013, 130, 226–240. [Google Scholar] [CrossRef] [PubMed]
- Rancourt, D.E.; Tsuzuki, T.; Capecchi, M.R. Genetic interaction between hoxb-5 and hoxb-6 is revealed by nonallelic noncomplementation. Genes Dev. 1995, 9, 108–122. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, D.C.; Rakshit, S.; Yallowitz, A.R.; Loken, L.; Jeannotte, L.; Capecchi, M.R.; Wellik, D.M. Hox patterning of the vertebrate rib cage. Development 2007, 134, 2981–2989. [Google Scholar] [CrossRef] [PubMed]
- Desir, A.; Ghaye, B. Congenital abnormalities of intrathoracic airways. Radiol. Clin. North Am. 2009, 47, 203–225. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Dong, Y.; Yu, M.; Zhang, L.; Yan, X.; Sun, J.; Qiao, L.; Geng, H.; Nakajima, M.; Furuichi, T.; et al. Follistatin-like 1 (Fstl1) is a bone morphogenetic protein (BMP) 4 signaling antagonist in controlling mouse lung development. Proc. Natl. Acad. Sci. USA 2011, 108, 7058–7063. [Google Scholar] [CrossRef] [PubMed]
- Bohinski, R.J.; di Lauro, R.; Whitsett, J.A. The lung-specific surfactant protein B gene promoter is a target for thyroid transcription factor 1 and hepatocyte nuclear factor 3, indicating common factors for organ-specific gene expression along the foregut axis. Mol. Cell. Biol. 1994, 14, 5671–5681. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, W.V. Transcription factors and pattern formation in the developing lung. Am. J. Physiol. Lung Cell Mol. Physiol. 1995, 269, 429–442. [Google Scholar]
- Kim, N.; Vu, T.H. Parabronchial smooth muscle cells and alveolar myofibroblasts in lung development. Birth Defects Res. C. Embryo Today 2006, 78, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Korfhagen, T.R.; Bruno, M.D.; Kitzmiller, J.A.; Wan, H.; Wert, S.E.; Khurana Hershey, G.K.; Chen, G.; Whitsett, J.A. SPDEF regulates goblet cell hyperplasia in the airway epithelium. J. Clin. Invest. 2007, 117, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Verhaeghe, C.; Nguyenvu, L.T.; Barbeau, R.; Eisley, C.J.; Nakagami, Y.; Huang, X.; Woodruff, P.G.; Fahy, J.V.; Erle, D.J. Distinct roles of FOXA2 and FOXA3 in allergic airway disease and asthma. Am. J. Respir. Crit. Care Med. 2009, 180, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Kaestner, K.H.; Ang, S.L.; Ikegami, M.; Finkelman, F.D.; Stahlman, M.T.; Fulkerson, P.C.; Rothenberg, M.E.; Whitsett, J.A. Foxa2 regulates alveolarization and goblet cell hyperplasia. Development 2004, 131, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Korfhagen, T.R.; Xu, Y.; Kitzmiller, J.; Wert, S.E.; Maeda, Y.; Gregorieff, A.; Clevers, H.; Whitsett, J.A. SPDEF is required for mouse pulmonary goblet cell differentiation and regulates a network of genes associated with mucus production. J. Clin. Invest. 2009, 119, 2914–2924. [Google Scholar] [CrossRef] [PubMed]
- Guseh, J.S.; Bores, S.A.; Stanger, B.Z.; Zhou, Q.; Anderson, W.J.; Melton, D.A.; Rajagopal, J. Notch signaling promotes airway mucous metaplasia and inhibits alveolar development. Development 2009, 136, 1751–1759. [Google Scholar] [CrossRef] [PubMed]
- Hrycaj, S.M.; Dye, B.R.; Baker, N.C.; Larsen, B.M.; Burke, A.C.; Spence, J.R.; Wellik, D.M. Hox5 genes regulate the Wnt2/2b-Bmp4-signaling axis during lung development. Cell Rep. 2015, 12, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Gaunt, S.J.; Coletta, D.; Pravtcheva, D.; Sharpe, P.T. Mouse Hox-3.4: Homeobox sequence and embryonic expression patterns with other members of the Hox gene gen network. Development 1990, 109, 329–339. [Google Scholar] [PubMed]
- Liggins, G.C.; Vilos, G.A.; Campos, G.A.; Kitterman, J.A.; Lee, C.H. The effect of spinal cord transection on lung development in fetal sheep. J. Dev. Physiol. 1981, 3, 267–274. [Google Scholar] [PubMed]
- Inanlou, M.R.; Baguma-Nibasheka, M.; Kablar, B. The role of fetal breathing-like movements in lung organogenesis. Histol. Histopathol. 2005, 20, 1261–1266. [Google Scholar] [PubMed]
- Golpon, H.A.; Geraci, M.W.; Moore, M.D.; Miller, H.L.; Miller, G.J.; Tuder, R.M.; Voelkel, N.F. HOX genes in human lung: Altered expression in primary pulmonary hypertension and emphysema. Am. J. Pathol. 2001, 158, 955–966. [Google Scholar] [CrossRef]
- Boucherat, O.; Franco-Montoya, M.L.; Thibault, C.; Incitti, R.; Chailley-Heu, B.; Delacourt, C.; Bourbon, J.R. Gene expression profiling in lung fibroblasts reveals new players in alveolarization. Physiol. Genomics 2007, 32, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Lu, K.H.; Wang, K.M.; Sun, M.; Zhang, E.B.; Yang, J.S.; Yin, D.D.; Liu, X.L.; Zhou, J.; Liu, Z.J.; et al. MicroRNA-196a promotes non-small cell lung cancer cell proliferation and invasion through targeting HOXA5. BMC Cancer 2012, 12, 348. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Warburton, D. Is COPD in adulthood really so far removed from early development? Eur. Respir. J. 2010, 35, 12–13. [Google Scholar] [CrossRef] [PubMed]
- Grapin-Botton, A.; Melton, D.A. Endoderm development: From patterning to organogenesis. Trends Genet. 2000, 16, 124–130. [Google Scholar] [CrossRef]
- Roberts, D.J. Molecular mechanisms of development of the gastrointestinal tract. Dev. Dynam. 2000, 219, 109–120. [Google Scholar] [CrossRef]
- Dony, C.; Gruss, P. Specific expression of the Hox 1.3 homeo box gene in murine embryonic structures originating from or induced by the mesoderm. EMBO J. 1987, 6, 2965–2975. [Google Scholar] [PubMed]
- James, R.; Kazenwadel, J. Homeobox gene expression in the intestinal epithelium of adult mice. J. Biol. Chem. 1991, 266, 3246–3251. [Google Scholar] [PubMed]
- Hennighausen, L.; Robinson, G.W. Signaling pathways in mammary gland development. Dev. Cell 2001, 1, 467–475. [Google Scholar] [CrossRef]
- Ryuko, K.; Miura, H.; Abu-Musa, A.; Iwanari, O.; Kitao, M. Endosalpingiosis in association with ovarian surface papillary tumor of borderline malignancy. Gynecol. Oncol. 1992, 46, 107–110. [Google Scholar] [CrossRef]
- Auersperg, N.; Wong, A.S.; Choi, K.C.; Kang, S.K.; Leung, P.C. Ovarian surface epithelium: Biology, endocrinology, and pathology. Endocr. Rev. 2001, 22, 255–288. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, W.J.; McDonnel, A.C. Roles of the ovarian surface epithelium in ovulation and carcinogenesis. Reproduction 2002, 123, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Nicosia, S.V. The aging ovary. Med. Clin. North Am. 1987, 71, 1–9. [Google Scholar] [PubMed]
- Auersperg, N. The origin of ovarian carcinomas: A unifying hypothesis. Int. J. Gynecol. Pathol. 2011, 30, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Maines-Bandiera, S.; Quinn, M.A.; Unger, W.G.; Dedhar, S.; Auersperg, N. Molecular pathways regulating EGF-induced epithelio-mesenchymal transition in human ovarian surface epithelium. Am. J. Physiol. Cell Physiol. 2006, 290, 1532–1542. [Google Scholar] [CrossRef] [PubMed]
- Fuller, J.F.; McAdara, J.; Yaron, Y.; Sakaguchi, M.; Fraser, J.K.; Gasson, J.C. Characterization of HOX gene expression during myelopoiesis: Role of HOXA5 in lineage commitment and maturation. Blood 1999, 93, 3391–3400. [Google Scholar] [PubMed]
- Crooks, G.M.; Fuller, J.; Petersen, D.; Izadi, P.; Malik, P.; Pattengale, P.K.; Kohn, D.B.; Gasson, J.C. Constitutive HOXA5 expression inhibits erythropoiesis and increases myelopoiesis from human hematopoietic progenitors. Blood 1999, 94, 519–528. [Google Scholar] [PubMed]
- Abate-Shen, C. Deregulated homeobox gene expression in cancer: cause or consequence? Nat. Rev. Cancer 2002, 2, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Sukumar, S. The Hox genes and their roles in oncogenesis. Nat. Rev. Cancer 2010, 10, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Carrio, M.; Arderiu, G.; Myers, C.; Boudreau, N.J. Homeobox D10 induces phenotypic reversion of breast tumor cells in a three-dimensional culture model. Cancer Res. 2005, 65, 7177–7185. [Google Scholar] [CrossRef] [PubMed]
- Rhoads, K.; Arderiu, G.; Charboneau, A.; Hansen, S.L.; Hoffman, W.; Boudreau, N. A role for HoxA5 in regulating angiogenesis and vascular patterning. Lymphat. Res. Biol. 2005, 3, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Rubin, E.; Wu, X.; Zhu, T.; Cheung, J.C.; Chen, H.; Lorincz, A.; Pandita, R.K.; Sharma, G.G.; Ha, H.C.; Gasson, J.; et al. A role for the HOXB7 homeodomain protein in DNA repair. Cancer Res. 2007, 67, 1527–1535. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Lee, J.S.; Liang, X.; Zhang, H.; Zhu, T.; Zhang, Z.; Taylor, M.E.; Zahnow, C.; Feigenbaum, L.; Rein, A.; et al. Hoxb7 inhibits transgenic HER-2/neu–induced mouse mammary tumor onset but promotes progression and lung metastasis. Cancer Res. 2008, 68, 3637–3644. [Google Scholar] [CrossRef] [PubMed]
- Anbazhagan, R.; Raman, V. Homeobox genes: Molecular link between congenital anomalies and cancer. Eur. J. Cancer 1997, 33, 635–637. [Google Scholar] [CrossRef]
- Henderson, G.S.; van Diest, P.J.; Burger, H.; Russo, J.; Raman, V. Expression pattern of a homeotic gene, HOXA5, in normal breast and in breast tumors. Cell. Oncol. 2006, 28, 305–313. [Google Scholar] [PubMed]
- Raman, V.; Martensen, S.A.; Reisman, D.; Evron, E.; Odenwald, W.F.; Jaffee, E.; Marks, J.; Sukumar, S. Compromised HOXA5 function can limit p53 expression in human breast tumours. Nature 2000, 405, 974–978. [Google Scholar] [PubMed]
- Stasinopoulos, I.A.; Mironchik, Y.; Raman, A.; Wildes, F.; Winnard, P.; Raman, V. HOXA5-twist interaction alters p53 homeostasis in breast cancer cells. J. Biol. Chem. 2005, 280, 2294–2299. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chung, S.; Sukumar, S. HOXA5-induced apoptosis in breast cancer cells is mediated by caspases 2 and 8. Mol. Cell. Biol. 2004, 24, 924–935. [Google Scholar] [CrossRef] [PubMed]
- Duriseti, S.; Winnard, P.T.; Mironchik, Y.; Vesuna, F.; Raman, A.; Raman, V. HOXA5 regulates hMLH1 expression in breast cancer cells. Neoplasia 2006, 8, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Gendronneau, G.; Lemieux, M.; Morneau, M.; Paradis, J.; Têtu, B.; Frenette, N.; Aubin, J.; Jeannotte, L. Influence of Hoxa5 on p53 tumorigenic outcome in mice. Am. J. Pathol. 2010, 176, 995–1005. [Google Scholar] [CrossRef] [PubMed]
- Salazar, H.; Godwin, A.K.; Daly, M.B.; Laub, P.B.; Hogan, W.M.; Rosenblum, N.; Boente, M.P.; Lynch, H.T.; Hamilton, T.C. Microscopic benign and invasive malignant neoplasms and a cancer-prone phenotype in prophylactic oophorectomies. J. Natl. Cancer Inst. 1996, 88, 1810–1820. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.P.; Konishi, I.; Koshiyama, M.; Nanbu, Y.; Iwai, T.; Nonogaki, H.; Mori, T.; Fujii, S. Immunohistochemical localization of c-erbB-2 protein and epidermal growth factor receptor in normal surface epithelium, surface inclusion cysts, and common epithelial tumours of the ovary. Virchows Arch. A Pathol. Anat. Histopathol. 1992, 421, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Hutson, R.; Ramsdale, J.; Wells, M. p53 protein expression in putative precursor lesions of epithelial ovarian cancer. Histopathology 1995, 27, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Sundfeldt, K.; Piontkewitz, Y.; Ivarsson, K.; Nilsson, O.; Hellberg, P.; Brännström, M.; Janson, P.O.; Enerback, S.; Hedin, L. E-cadherin expression in human epithelial ovarian cancer and normal ovary. Int. J. Cancer 1997, 74, 275–280. [Google Scholar] [CrossRef]
- Tonary, A.M.; Macdonald, E.A.; Faught, W.; Senterman, M.K.; Vanderhyden, B.C. Lack of expression of c-KIT in ovarian cancers is associated with poor prognosis. Int. J. Cancer 2000, 89, 242–250. [Google Scholar] [CrossRef]
- Bowen, N.J.; Logani, S.; Dickerson, E.B.; Kapa, L.B.; Akhtar, M.; Benigno, B.B.; McDonald, J.F. Emerging roles for PAX8 in ovarian cancer and endosalpingeal development. Gynecol. Oncol. 2007, 104, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Madore, J.; Ren, F.; Filali-Mouhim, A.; Sanchez, L.; Köbel, M.; Tonin, P.N.; Huntsman, D.; Provencher, D.M.; Mes-Masson, A.M. Characterization of the molecular differences between ovarian endometrioid carcinoma and ovarian serous carcinoma. J. Pathol. 2010, 220, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.L.; Nie, F.Q.; Sun, M.; Xia, R.; Xie, M.; Lu, K.H.; Li, W. HOXA5 indicates poor prognosis and suppresses cell proliferation by regulating p21 expression in non-small cell lung cancer. Tumor Biol. 2015, 36, 3521–3531. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Kim, M.J.; Lee, J.Y.; Lee, S.M.; Choi, J.Y.; Yoon, G.S.; Na, Y.K.; Hong, H.S.; Kim, S.G.; Choi, J.E.; et al. Epigenetic inactivation of Homeobox A5 gene in non-small cell lung cancer and its relationship with clinicopathological features. Mol. Carcinog. 2009, 48, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Ordóñez-Morán, P.; Dafflon, C.; Imajo, M.; Nishida, E.; Huelsken, J. HOXA5 counteracts stem cell traits by inhibiting Wnt signaling in colorectal cancer. Cancer Cell 2015, 28, 815–829. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, I.; Layman, H.; Coussens, L.; Boudreau, N. Sustained endothelial expression of HoxA5 in vivo impairs pathological angiogenesis and tumor progression. PloS ONE 2015, 10, e0121720. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gorski, D.H. Regulation of angiogenesis through a microRNA (miR-130a) that down-regulates antiangiogenic homeobox genes GAX and HOXA5. Blood 2008, 111, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Dreyling, M.H.; Martinez-Climent, J.A.; Zheng, M.; Mao, J.; Rowley, J.D.; Bohlander, S.K. The t (10; 11)(p13; q14) in the U937 cell line results in the fusion of the AF10 gene and CALM, encoding a new member of the AP-3 clathrin assembly protein family. Proc. Natl. Acad. Sci. USA 1996, 93, 4804–4809. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Jiang, Q.; Lemieux, M.; Jeannotte, L.; Su, L.; Zhang, Y. Leukaemic transformation by CALM–AF10 involves upregulation of Hoxa5 by hDOT1L. Nat. Cell. Biol. 2006, 8, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Quentmeier, H.; Dirks, W.G.; Macleod, R.A.; Reinhardt, J.; Zaborski, M.; Drexler, H.G. Expression of HOX genes in acute leukemia cell lines with and without MLL translocations. Leuk. Lymphoma 2004, 45, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Raman, V.; Tamori, A.; Vali, M.; Zeller, K.; Korz, D.; Sukumar, S. HOXA5 regulates expression of the progesterone receptor. J. Biol. Chem. 2000, 275, 26551–26555. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Rubin, E.; Zhang, H.; Chung, S.; Jie, C.C.; Garrett, E.; Biswal, S.; Sukumar, S. Identification of transcriptional targets of HOXA5. J. Biol. Chem. 2005, 280, 19373–19380. [Google Scholar] [CrossRef] [PubMed]
- Gérard, M.; Chen, J.Y.; Gronemeyer, H.; Chambon, P.; Duboule, D.; Zakany, J. In vivo targeted mutagenesis of a regulatory element required for positioning the Hoxd-11 and Hoxd-10 expression boundaries. Genes Dev. 1996, 10, 2326–2334. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dupé, V.; Davenne, M.; Brocard, J.; Dollé, P.; Mark, M.; Dierich, A.; Chambon, P.; Rijli, F.M. In vivo functional analysis of the Hoxa-1 3′ retinoic acid response element (3′ RARE). Development 1997, 124, 399–410. [Google Scholar] [PubMed]
- Zákány, J.; Gérard, M.; Favier, B.; Duboule, D. Deletion of a HoxD enhancer induces transcriptional heterochrony leading to transposition of the sacrum. EMBO J. 1997, 16, 4393–4402. [Google Scholar] [CrossRef] [PubMed]
- Mallo, M.; Alonso, C.R. The regulation of Hox gene expression during animal development. Development 2013, 140, 3951–3963. [Google Scholar] [CrossRef] [PubMed]
- Montavon, T.; Soshnikova, N. Hox gene regulation and timing in embryogenesis. Semin. Cell. Dev. Biol. 2014, 34, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, J.; Nonchev, S.; Gould, A.; Whiting, J.; Krumlauf, R. Selectivity, sharing and competitive interactions in the regulation of Hoxb genes. EMBO J. 1998, 17, 1788–1798. [Google Scholar] [CrossRef] [PubMed]
- Spitz, F.; Duboule, D. Global control regions and regulatory landscapes in vertebrate development and evolution. Adv. Genet. 2008, 61, 175–205. [Google Scholar] [PubMed]
- Chambeyron, S.; Bickmore, W.A. Chromatin decondensation and nuclear reorganization of the HoxB locus upon induction of transcription. Genes Dev. 2004, 18, 1119–1130. [Google Scholar] [CrossRef] [PubMed]
- Ponting, C.P.; Oliver, P.L.; Reik, W. Evolution and functions of long noncoding RNAs. Cell 2009, 136, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Rinn, J.L.; Kertesz, M.; Wang, J.K.; Squazzo, S.L.; Xu, X.; Brugmann, S.A.; Goodnough, L.H.; Helms, J.A.; Farnham, P.J.; Segal, E.; et al. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell 2007, 129, 1311–1323. [Google Scholar] [CrossRef] [PubMed]
- Sessa, L.; Breiling, A.; Lavorgna, G.; Silvestri, L.; Casari, G.; Orlando, V. Noncoding RNA synthesis and loss of Polycomb group repression accompanies the colinear activation of the human HOXA cluster. RNA 2007, 13, 223–239. [Google Scholar] [CrossRef] [PubMed]
- Püschel, A.W.; Balling, R.; Gruss, P. Position-specific activity of the Hox1.1 promoter in transgenic mice. Development 1990, 108, 435–442. [Google Scholar] [PubMed]
- Wang, K.C.; Yang, Y.W.; Liu, B.; Sanyal, A.; Corces-Zimmerman, R.; Chen, Y.; Lajoie, B.R.; Protacio, A.; Flynn, R.A.; Gupta, R.A.; et al. A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature 2011, 472, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, B.; Wapinski, O.L.; Tsai, M.-C.; Qu, K.; Zhang, J.; Carlson, J.C.; Lin, M.; fang, F.; Gupta, R.A.; et al. Targeted disruption of Hotair leads to homeotic transformation and gene derepression. Cell Rep. 2013, 5, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Bérubé-Simard, F.A.; Prudhomme, C.; Jeannotte, L. YY1 Acts as a Transcriptional Activator of Hoxa5. PLoS ONE 2014, 9, e93989. [Google Scholar]
- Zakany, J.; Tuggle, C.K.; Patel, M.D.; Nguyen-Huu, M.C. Spatial regulation of homeobox gene fusions in the embryonic central nervous system of transgenic mice. Neuron 1988, 1, 679–691. [Google Scholar] [CrossRef]
- Tuggle, C.K.; Zakany, J.; Cianetti, L.; Peschle, C.; Nguyen-Huu, M.C. Region-specific enhancers near two mammalian homeo box genes define adjacent rostrocaudal domains in the central nervous system. Genes Dev. 1990, 4, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Nowling, T.; Zhou, W.; Krieger, K.E.; Larochelle, C.; Nguyen-Huu, M.C.; Jeannotte, L.; Tuggle, C.K. Hoxa5 gene regulation: A gradient of binding activity to a brachial spinal cord element. Dev. Biol. 1999, 208, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Larochelle, C.; Tremblay, M.; Bernier, D.; Aubin, J.; Jeannotte, L. Multiple cis-acting regulatory regions are required for restricted spatio-temporal Hoxa5 gene expression. Dev. Dyn. 1999, 214, 127–140. [Google Scholar] [CrossRef]
- Tabariès, S.; Lapointe, J.; Besch, T.; Carter, M.; Woollard, J.; Tuggle, C.K.; Jeannotte, L. Cdx protein interaction with Hoxa5 regulatory sequences contributes to Hoxa5 regional expression along the axial skeleton. Mol. Cell. Biol. 2005, 25, 1389–1401. [Google Scholar] [CrossRef] [PubMed]
- Moreau, J.; Jeannotte, L. Sequence analysis of a Hoxa4-Hoxa5 intergenic region including shared regulatory elements. DNA Seq. 2002, 13, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Oosterveen, T.; van Vliet, P.; Deschamps, J.; Meijlink, F. The direct context of a Hox retinoic acid response element is crucial for its activity. J. Biol. Chem. 2003, 278, 24103–24107. [Google Scholar] [CrossRef] [PubMed]
- Packer, A.I.; Crotty, D.A.; Elwell, V.A.; Wolgemuth, D.J. Expression of the murine Hoxa4 gene requires both autoregulation and a conserved retinoic acid response element. Development 1998, 125, 1991–1998. [Google Scholar] [PubMed]
- Chen, H.; Zhang, H.; Lee, J.; Liang, X.; Wu, X.; Zhu, T.; Lo, P.K.; Zhang, X.; Sukumar, S. HOXA5 acts directly downstream of retinoic acid receptor β and contributes to retinoic acid–induced apoptosis and growth inhibition. Cancer Res. 2007, 67, 8007–8013. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Cao, P.; Wan, M.M.; Sui, G. Yin Yang 1: A multifaceted protein beyond a transcription factor. Transcription 2010, 1, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Boucherat, O.; Landry-Truchon, K.; Bérubé-Simard, F.A.; Houde, N.; Beuret, L.; Lezmi, G.; Foulkes, W.D.; Delacourt, C.; Charron, J.; Jeannotte, L. Epithelial inactivation of Yy1 abrogates lung branching morphogenesis. Development 2015, 142, 2981–2995. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Paylor, S.W.; Magnuson, T.; Schumacher, A. Juxtaposed Polycomb complexes co-regulate vertebral identity. Development 2006, 133, 4957–4968. [Google Scholar] [CrossRef] [PubMed]
- Hershko, A.Y.; Kafri, T.; Fainsod, A.; Razin, A. Methylation of HoxA5 and HoxB5 and its relevance to expression during mouse development. Gene 2003, 302, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Mujahid, S.; Nielsen, H.C.; Volpe, M.V. MiR-221 and miR-130a regulate lung airway and vascular development. PLoS ONE 2013, 8, e55911. [Google Scholar]
- Yang, F.; Miao, L.; Mei, Y.; Wu, M. Retinoic acid-induced HOXA5 expression is co-regulated by HuR and miR-130a. Cell. Signal. 2013, 25, 1476–1485. [Google Scholar] [PubMed]
- Lee, D.H.; Forscher, C.; di Vizio, D.; Koeffler, H.P. Induction of p53-independent apoptosis by ectopic expression of HOXA5 in human liposarcomas. Sci. Rep. 2015, 5, 12580. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, L.; Jiang, L. miR-1271 promotes non-small-cell lung cancer cell proliferation and invasion via targeting HOXA5. Biochem. Biophys. Res. Commun. 2015, 458, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Watson, R.E.; Curtin, G.M.; Hellmann, G.M.; Doolittle, D.J.; Goodman, J.I. Increased DNA methylation in the HoxA5 promoter region correlates with decreased expression of the gene during tumor promotion. Mol. Carcinog. 2004, 41, 54–66. [Google Scholar] [PubMed]
- Strathdee, G.; Sim, A.; Soutar, R.; Holyoake, T.L.; Brown, R. HOXA5 is targeted by cell-type-specific CpG island methylation in normal cells and during the development of acute myeloid leukaemia. Carcinogenesis 2006, 28, 299–309. [Google Scholar] [PubMed]
| Organs/Structures | Mutant Phenotypes | References | |
|---|---|---|---|
| Axial skeleton | Homeotic transformations:
Sternum malformations:
| [15,16] | |
| Appendicular skeleton | Reduced/interrupted/missing acromion | [16,20] | |
| Respiratory system | Tracheal mesenchyme | Reduced luminal surface to complete occlusion Thickening of the lamina propria Abnormal patterning of cartilage rings | [21,22] |
| Tracheal epithelium | Epithelium disorganization:
| [21] | |
| Lung mesenchyme | Lung hypoplasia:
Disorganization of the lung mesenchyme:
Impaired motility of alveolar myofibroblast precursors:
| [21,22,23] | |
| Lung epithelium | Decreased surfactant protein expression Reduced number of secretory club cells Reduced number of alveolar type I pneumocytes Goblet cell metaplasia and mucus hypersecretion | [21,22,23,24] | |
| Lung endothelium | Undeveloped lung microvasculature:
| [22] | |
| Phrenic motor column | Impaired diaphragm innervation | [22,25] | |
| Respiratory function | Impaired breathing:
| [26] | |
| Digestive system | Stomach | Perturbed cell specification of the gastric epithelium Goblet cell hyperplasia Reduced number of zymogenic and enteroendocrine cells:
| [27] |
| Intestine | No obvious morphological defects Delay in the functional enzymatic activity of intestinal epithelial cells | [28] | |
| Colon | Abnormal distribution of goblet cells | [27] | |
| Thyroid gland | Embryo:
Adult:
| [29] | |
| Mammary gland | Abnormal development:
| [30] | |
| Ovary | Precocious puberty Early onset of estrous acyclicity that worsens with age Ovarian epithelial cyst formation in older females | [31] | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeannotte, L.; Gotti, F.; Landry-Truchon, K. Hoxa5: A Key Player in Development and Disease. J. Dev. Biol. 2016, 4, 13. https://doi.org/10.3390/jdb4020013
Jeannotte L, Gotti F, Landry-Truchon K. Hoxa5: A Key Player in Development and Disease. Journal of Developmental Biology. 2016; 4(2):13. https://doi.org/10.3390/jdb4020013
Chicago/Turabian StyleJeannotte, Lucie, Florian Gotti, and Kim Landry-Truchon. 2016. "Hoxa5: A Key Player in Development and Disease" Journal of Developmental Biology 4, no. 2: 13. https://doi.org/10.3390/jdb4020013
APA StyleJeannotte, L., Gotti, F., & Landry-Truchon, K. (2016). Hoxa5: A Key Player in Development and Disease. Journal of Developmental Biology, 4(2), 13. https://doi.org/10.3390/jdb4020013





