An Integrated Canonical and Non-Canonical Wnt Signaling Network Controls Early Anterior–Posterior Axis Formation in Sea Urchin Embryos
Abstract
1. Introduction
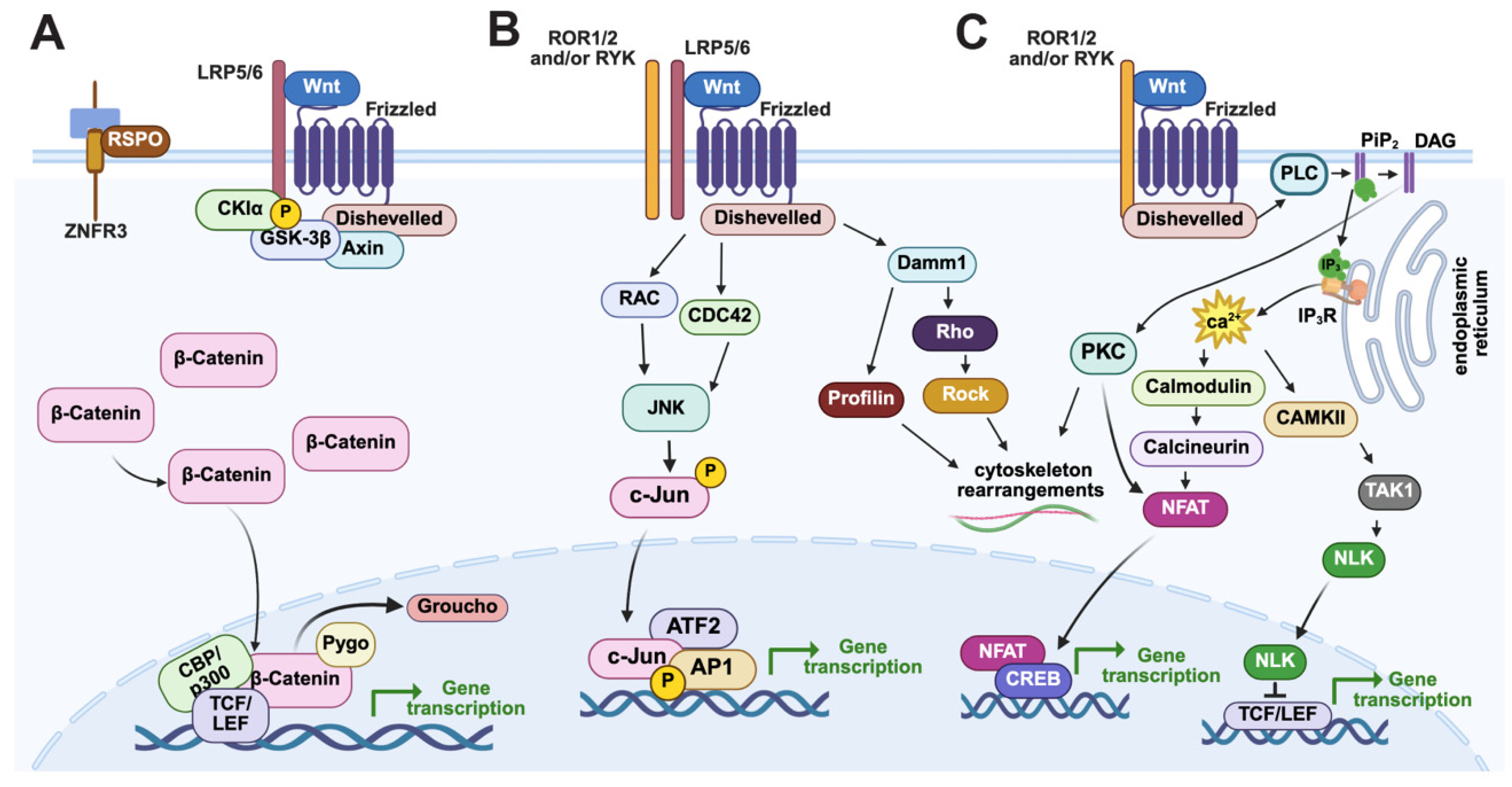
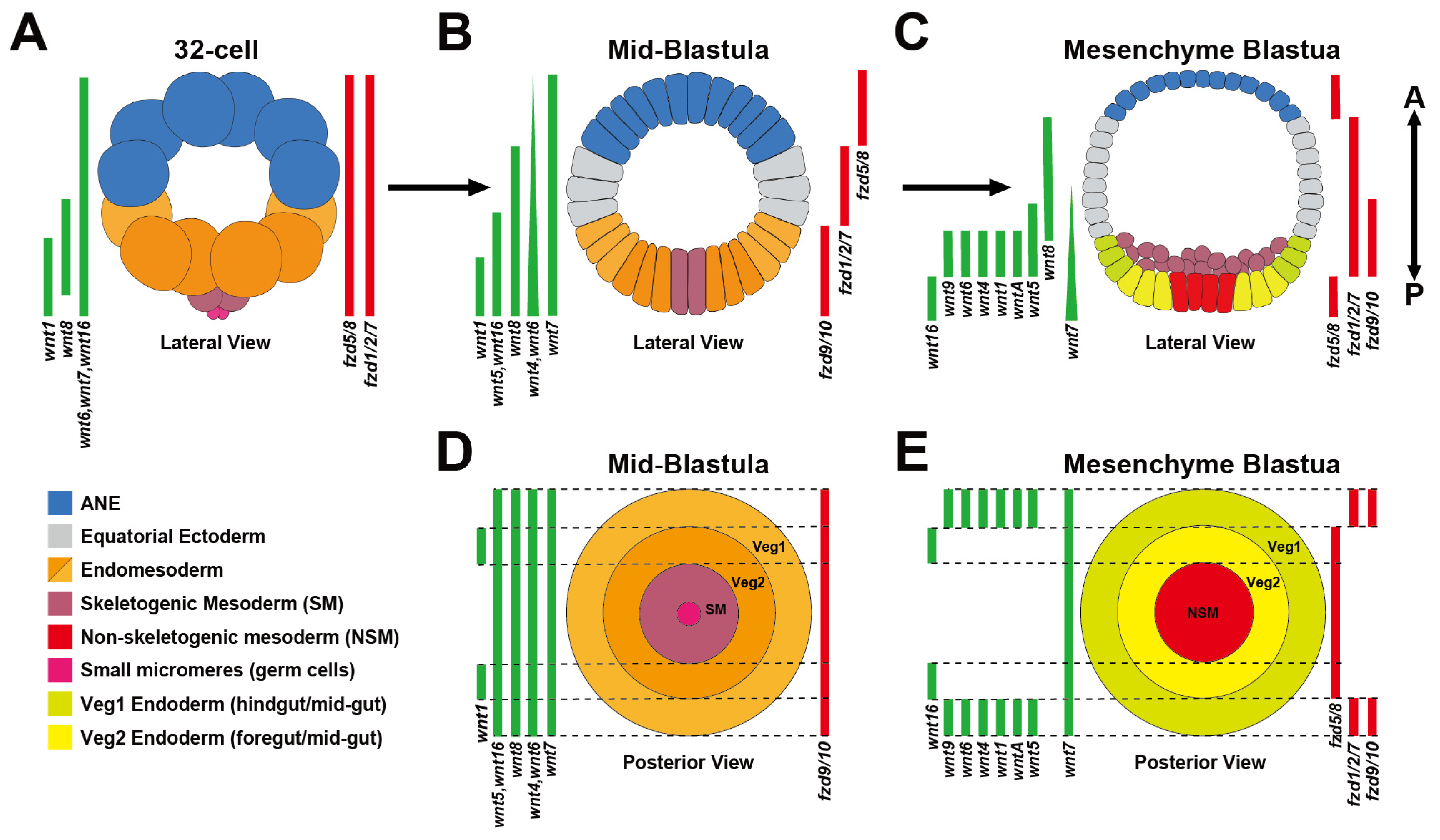
2. Dynamic Spatiotemporal Expression of the Early Sea Urchin AP Wnt Landscape
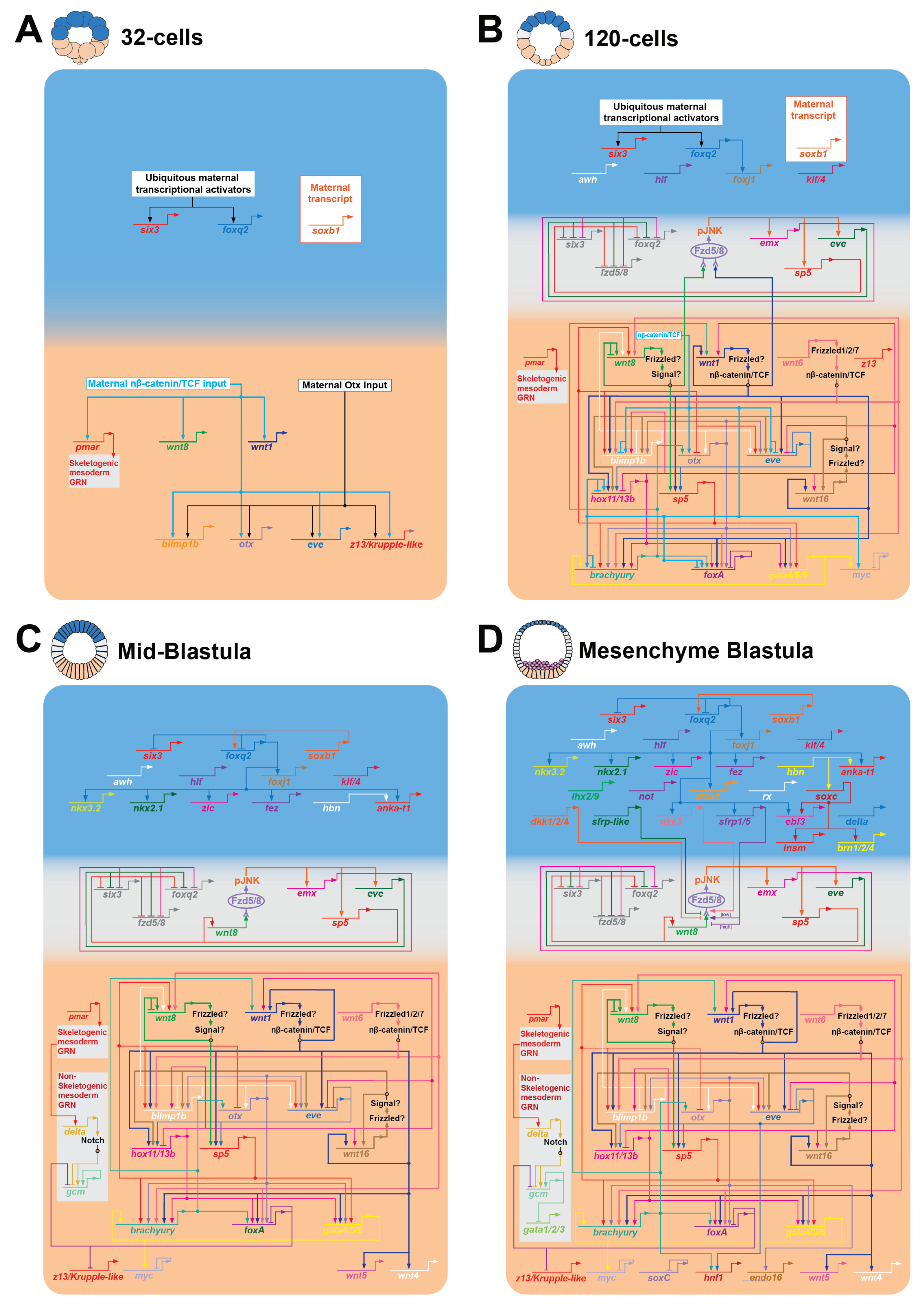
2.1. Wnt/β-Catenin Signaling Positions the First Two Gene Regulatory Networks Along the AP Axis by the 32-Cell Stage
2.2. A cWnt to Wnt/JNK Signaling Relay Mechanism Positions Ectodermal GRNs Along the AP Axis

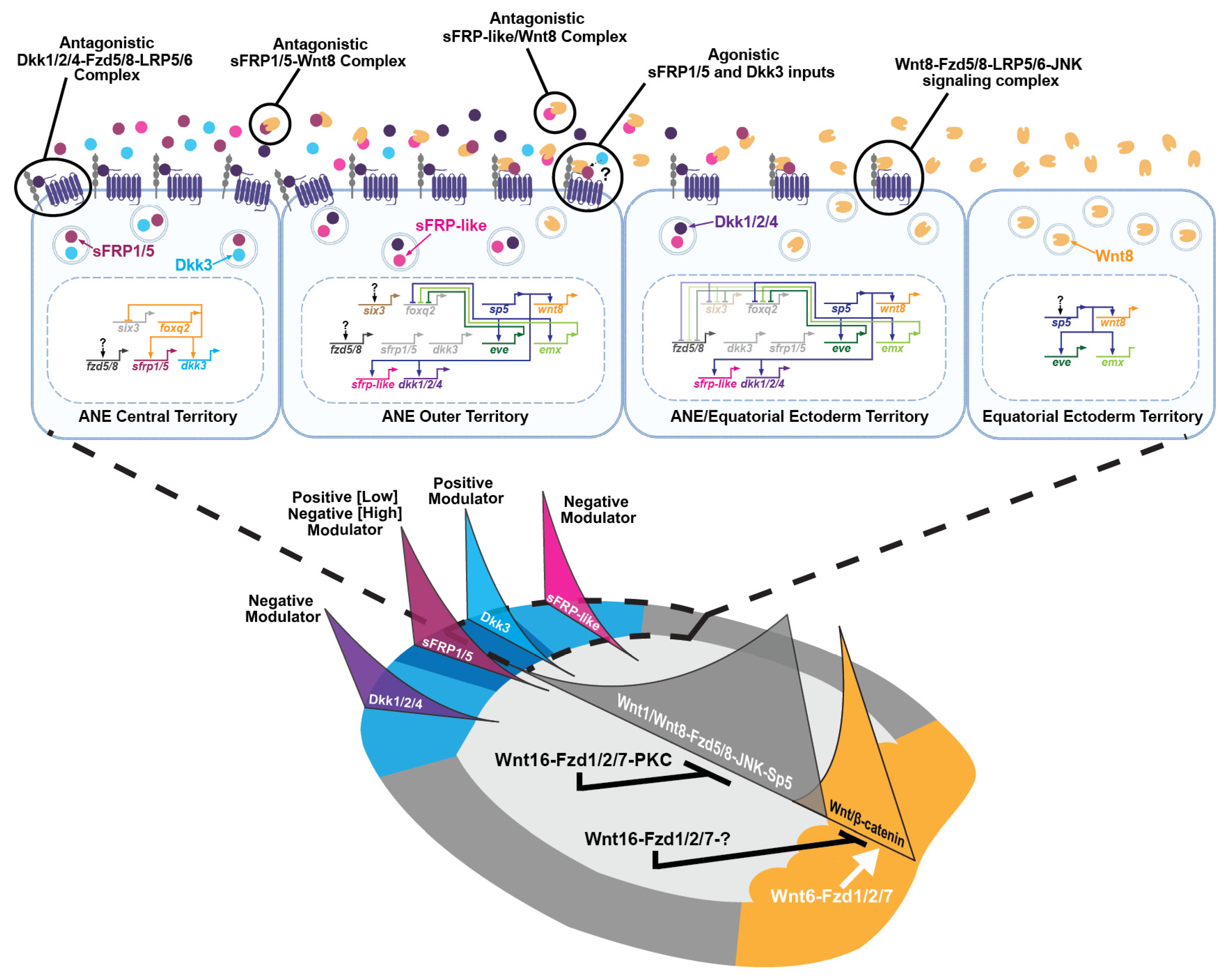
2.3. An Anterior Signaling Center Defines the Boundaries of the ANE Territory
2.4. Canonical and Non-Canonical Frizzled1/2/7 Signaling During AP Formation
2.5. Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Niehrs, C. On Growth and Form: A Cartesian Coordinate System of Wnt and BMP Signaling Specifies Bilaterian Body Axes. Development 2010, 137, 845–857. [Google Scholar] [CrossRef]
- Range, R. Specification and Positioning of the Anterior Neuroectoderm in Deuterostome Embryos. genesis 2014, 52, 222–234. [Google Scholar] [CrossRef]
- Holstein, T.W. The Evolution of the Wnt Pathway. Cold Spring Harb. Perspect. Biol. 2012, 4, a007922. [Google Scholar] [CrossRef]
- Kestler, H.A.; Kühl, M. From Individual Wnt Pathways towards a Wnt Signalling Network. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 1333–1347. [Google Scholar] [CrossRef] [PubMed]
- Loh, K.M.; van Amerongen, R.; Nusse, R. Generating Cellular Diversity and Spatial Form: Wnt Signaling and the Evolution of Multicellular Animals. Dev. Cell 2016, 38, 643–655. [Google Scholar] [CrossRef]
- Lee, P.N.; Pang, K.; Matus, D.Q.; Martindale, M.Q. A WNT of Things to Come: Evolution of Wnt Signaling and Polarity in Cnidarians. Semin. Cell Dev. Biol. 2006, 17, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sinha, T.; Wynshaw-Boris, A. Wnt Signaling in Mammalian Development: Lessons from Mouse Genetics. Cold Spring Harb. Perspect. Biol. 2012, 4, a007963. [Google Scholar] [CrossRef]
- Bejsovec, A. Wingless Signaling: A Genetic Journey from Morphogenesis to Metastasis. Genetics 2018, 208, 1311–1336. [Google Scholar] [CrossRef]
- Sawa, H.; Korswagen, H.C. Wnt Signaling in C. Elegans. WormBook Online Rev. C. Elegans Biol. 2013, 4, 1–30. [Google Scholar] [CrossRef]
- Lewis, E.B. A Gene Complex Controlling Segmentation in Drosophila. Nature 1978, 276, 565–570. [Google Scholar] [CrossRef]
- Nüsslein-Volhard, C.; Wieschaus, E. Mutations Affecting Segment Number and Polarity in Drosophila. Nature 1980, 287, 795–801. [Google Scholar] [CrossRef]
- Akam, M. Hox and HOM: Homologous Gene Clusters in Insects and Vertebrates. Cell 1989, 57, 347–349. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, T.; Wang, Y.; Xie, M.; Ji, X.; Luo, X.; Huang, W.; Xia, L. Homeobox Genes in Cancers: From Carcinogenesis to Recent Therapeutic Intervention. Front. Oncol. 2021, 11, 770428. [Google Scholar] [CrossRef]
- Ghosh, P.; Sagerström, C.G. Developing Roles for Hox Proteins in Hindbrain Gene Regulatory Networks. Int. J. Dev. Biol. 2018, 62, 767–774. [Google Scholar] [CrossRef]
- Mallo, M.; Alonso, C.R. The Regulation of Hox Gene Expression during Animal Development. Development 2013, 140, 3951–3963. [Google Scholar] [CrossRef]
- Olson, P.D. Hox Genes and the Parasitic Flatworms: New Opportunities, Challenges and Lessons from the Free-Living. Parasitol. Int. 2008, 57, 8–17. [Google Scholar] [CrossRef]
- Quinonez, S.C.; Innis, J.W. Human HOX Gene Disorders. Mol. Genet. Metab. 2014, 111, 4–15. [Google Scholar] [CrossRef]
- Petersen, C.P.; Reddien, P.W. Wnt Signaling and the Polarity of the Primary Body Axis. Cell 2009, 139, 1056–1068. [Google Scholar] [CrossRef]
- McMahon, A.P.; Moon, R.T. Ectopic Expression of the Proto-Oncogene Int-1 in Xenopus Embryos Leads to Duplication of the Embryonic Axis. Cell 1989, 58, 1075–1084. [Google Scholar] [CrossRef]
- Sokol, S.; Christian, J.L.; Moon, R.T.; Melton, D.A. Injected Wnt RNA Induces a Complete Body Axis in Xenopus Embryos. Cell 1991, 67, 741–752. [Google Scholar] [CrossRef]
- Wikramanayake, A.H.; Huang, L.; Klein, W.H. β-Catenin Is Essential for Patterning the Maternally Specified Animal-Vegetal Axis in the Sea Urchin Embryo. Proc. Natl. Acad. Sci. USA 1998, 95, 9343–9348. [Google Scholar] [CrossRef]
- Liu, P.; Wakamiya, M.; Shea, M.J.; Albrecht, U.; Behringer, R.R.; Bradley, A. Requirement for Wnt3 in Vertebrate Axis Formation. Nat. Genet. 1991, 22, 361–365. [Google Scholar] [CrossRef]
- Logan, C.Y.; Miller, J.R.; Ferkowicz, M.J.; McClay, D.R. Nuclear β-Catenin Is Required to Specify Vegetal Cell Fates in the Sea Urchin Embryo. Development 1999, 126, 345–357. [Google Scholar] [CrossRef]
- Vonica, A.; Weng, W.; Gumbiner, B.; Venuti, J. TCF Is the Nuclear Effector of the Beta-Catenin Signal That Patterns the Sea Urchin Animal-Vegetal Axis. Dev. Biol. 2000, 217, 230–243. [Google Scholar] [CrossRef]
- Emily-Fenouil, F.; Ghiglione, C.; Lhomond, G.; Lepage, T.; Gache, C. GSK3beta/Shaggy Mediates Patterning along the Animal-Vegetal Axis of the Sea Urchin Embryo. Development 1998, 125, 2489–2498. [Google Scholar] [CrossRef]
- Range, R.C.; Venuti, J.M.; McClay, D.R. LvGroucho and Nuclear H-Catenin Functionally Compete for Tcf Binding to Influence Activation of the Endomesoderm Gene Regulatory Network in the Sea Urchin Embryo. Dev. Biol. 2005, 279, 252–267. [Google Scholar] [CrossRef]
- Wikramanayake, A.H.; Peterson, R.; Chen, J.; Huang, L.; Bince, J.M.; McClay, D.R.; Klein, W.H. Nuclear β-Catenin-Dependent Wnt8 Signaling in Vegetal Cells of the Early Sea Urchin Embryo Regulates Gastrulation and Differentiation of Endoderm and Mesodermal Cell Lineages. Genesis 2003, 39, 194–205. [Google Scholar] [CrossRef]
- Galliot, B.; Wenger, Y. Organizer Formation, Organizer Maintenance and Epithelial Cell Plasticity in Hydra: Role of the Wnt3/β-Catenin/TCF/Sp5/Zic4 Gene Network. Cells Dev. 2025, 204002, in press. [Google Scholar] [CrossRef]
- Vogg, M.; Beccari, L.; Iglesias Ollé, L.; Rampon, C.; Vriz, S.; Perruchoud, C.; Wenger, Y.; Galliot, B. An Evolutionarily-Conserved Wnt3/β-Catenin/Sp5 Feedback Loop Restricts Head Organizer Activity in Hydra. Nat. Commun. 2019, 10, 312. [Google Scholar] [CrossRef]
- Guder, C.; Philipp, I.; Lengfeld, T.; Watanabe, H.; Hobmayer, B.; Holstein, T.W. The Wnt Code: Cnidarians Signal the Way. Oncogene 2006, 25, 7450–7460. [Google Scholar] [CrossRef]
- Kusserow, A.; Pang, K.; Sturm, C.; Hrouda, M.; Lentfer, J.; Schmidt, H.A.; Technau, U.; von Haeseler, A.; Hobmayer, B.; Martindale, M.Q.; et al. Unexpected Complexity of the Wnt Gene Family in a Sea Anemone. Nature 2005, 433, 156–160. [Google Scholar] [CrossRef]
- DuBuc, T.Q.; Stephenson, T.B.; Rock, A.Q.; Martindale, M.Q. Hox and Wnt Pattern the Primary Body Axis of an Anthozoan Cnidarian before Gastrulation. Nat. Commun. 2018, 9, 2007. [Google Scholar] [CrossRef]
- Larroux, C.; Fahey, B.; Degnan, S.M.; Adamski, M.; Rokhsar, D.S.; Degnan, B.M. The NK Homeobox Gene Cluster Predates the Origin of Hox Genes. Curr. Biol. 2007, 17, 706–710. [Google Scholar] [CrossRef]
- Fortunato, S.A.V.; Adamski, M.; Ramos, O.M.; Leininger, S.; Liu, J.; Ferrier, D.E.K.; Adamska, M. Calcisponges Have a ParaHox Gene and Dynamic Expression of Dispersed NK Homeobox Genes. Nature 2014, 514, 620–623. [Google Scholar] [CrossRef]
- Ramos, O.M.; Barker, D.; Ferrier, D.E.K. Ghost Loci Imply Hox and ParaHox Existence in the Last Common Ancestor of Animals. Curr. Biol. 2012, 22, 1951–1956. [Google Scholar] [CrossRef]
- Moroz, L.L.; Kocot, K.M.; Citarella, M.R.; Dosung, S.; Norekian, T.P.; Povolotskaya, I.S.; Grigorenko, A.P.; Dailey, C.; Berezikov, E.; Buckley, K.M.; et al. The Ctenophore Genome and the Evolutionary Origins of Neural Systems. Nature 2015, 510, 109–114. [Google Scholar] [CrossRef]
- Ryan, J.F.; Pang, K.; Program, N.C.S.; Mullikin, J.C.; Martindale, M.Q.; Baxevanis, A.D. The Homeodomain Complement of the Ctenophore Mnemiopsis Leidyi Suggests That Ctenophora and Porifera Diverged Prior to the ParaHoxozoa. EvoDevo 2010, 1, 9. [Google Scholar] [CrossRef]
- Pang, K.; Martindale, M.Q. Developmental Expression of Homeobox Genes in the Ctenophore Mnemiopsis Leidyi. Dev. Genes Evol. 2008, 218, 307–319. [Google Scholar] [CrossRef]
- Anlas, K.; Trivedi, V. Studying Evolution of the Primary Body Axis In Vivo and In Vitro. eLife 2021, 10, e69066. [Google Scholar] [CrossRef]
- Holzem, M.; Boutros, M.; Holstein, T.W. The Origin and Evolution of Wnt Signalling. Nat. Rev. Genet. 2024, 25, 500–512. [Google Scholar] [CrossRef]
- Wodarz, A.; Nusse, R. Mechanisms of Wnt Signaling in Development. Annu. Rev. Cell Dev. Biol. 1998, 14, 59–88. [Google Scholar] [CrossRef]
- Logan, C.Y.; Nusse, R. The Wnt Signaling Pathway in Development and Disease. Annu. Rev. CELL Dev. Biol. 2004, 20, 781–810. [Google Scholar] [CrossRef]
- Nusse, R.; Clevers, H. Wnt/β-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell 2017, 169, 985–999. [Google Scholar] [CrossRef]
- Van Amerongen, R.; Nusse, R. Towards an Integrated View of Wnt Signaling in Development. Development 2009, 136, 3205–3214. [Google Scholar] [CrossRef]
- Saneyoshi, T.; Kume, S.; Amasaki, Y.; Mikoshiba, K. The Wnt/Calcium Pathway Activates NF-AT and Promotes Ventral Cell Fate in Xenopus Embryos. Nature 2002, 417, 295–299. [Google Scholar] [CrossRef]
- Dejmek, J.; Säfholm, A.; Nielsen, C.K.; Andersson, T.; Leandersson, K. Wnt-5a/Ca2+-Induced NFAT Activity Is Counteracted by Wnt-5a/Yes-Cdc42-Casein Kinase 1α Signaling in Human Mammary Epithelial Cells. Mol. Cell. Biol. 2006, 26, 6024–6036. [Google Scholar] [CrossRef]
- Boutros, M.; Paricio, N.; Strutt, D.I.; Mlodzik, M. Dishevelled Activates JNK and Discriminates between JNK Pathways in Planar Polarity and Wingless Signaling. Cell 1998, 94, 109–118. [Google Scholar] [CrossRef]
- Ohkawara, B.; Niehrs, C. An ATF2-based Luciferase Reporter to Monitor Non-canonical Wnt Signaling in Xenopus Embryos. Dev. Dyn. 2011, 240, 188–194. [Google Scholar] [CrossRef]
- Cui, M.; Siriwon, N.; Li, E.; Davidson, E.H.; Peter, I.S. Specific Functions of the Wnt Signaling System in Gene Regulatory Networks throughout the Early Sea Urchin Embryo. Proc. Natl. Acad. Sci. USA 2014, 111, E5029–E5038. [Google Scholar] [CrossRef]
- Range, R.C.; Angerer, R.C.; Angerer, L.M. Integration of Canonical and Noncanonical Wnt Signaling Pathways Patterns the Neuroectoderm Along the Anterior–Posterior Axis of Sea Urchin Embryos. PLoS Biol. 2013, 11, e10011467. [Google Scholar] [CrossRef]
- Robert, N.; Lhomond, G.; Schubert, M.; Croce, J.C. A Comprehensive Survey of Wnt and Frizzled Expression in the Sea Urchin Paracentrotus Lividus. Genesis 2014, 52, 235–250. [Google Scholar] [CrossRef]
- McIntyre, D.C.; Seay, N.W.; Croce, J.C.; McClay, D.R. Short-Range Wnt5 Signaling Initiates Specification of Sea Urchin Posterior Ectoderm. Development 2013, 140, 4881–4889. [Google Scholar] [CrossRef]
- Martínez-Bartolomé, M.; Range, R.C. A Biphasic Role of Non-Canonical Wnt16 Signaling during Early Anterior-Posterior Patterning and Morphogenesis of the Sea Urchin Embryo. Development 2019, 146, dev168799. [Google Scholar] [CrossRef]
- Swalla, B.J.; Smith, A.B. Deciphering Deuterostome Phylogeny: Molecular, Morphological and Palaeontological Perspectives. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 1557–1568. [Google Scholar] [CrossRef]
- Lowe, C.J. Chapter Three—Molecular Insights into Deuterostome Evolution from Hemichordate Developmental Biology. Curr. Top. Dev. Biol. 2021, 141, 75–117. [Google Scholar]
- Croce, J.C.; McClay, D.R. The Canonical Wnt Pathway in Embryonic Axis Polarity. Semin. Cell Dev. Biol. 2006, 17, 168–174. [Google Scholar] [CrossRef]
- Peng, C.J.; Wikramanayake, A.H. Differential Regulation of Disheveled in a Novel Vegetal Cortical Domain in Sea Urchin Eggs and Embryos: Implications for the Localized Activation of Canonical Wnt Signaling. PLoS ONE 2013, 8, e80693. [Google Scholar] [CrossRef]
- Horstadius, S. The Mechanisms of Sea Urchin Development, Studied by Operative Methods. Biol. Rev. 1939, 14, 105–242. [Google Scholar] [CrossRef]
- Horstadius, S. Experimental Biology of Echinoderms; Oxford University Press: Oxford, UK, 1973. [Google Scholar]
- McClay, D.R. Evolutionary Crossroads in Developmental Biology: Sea Urchins. Development 2011, 138, 2639–2648. [Google Scholar] [CrossRef]
- Weitzel, H.E.; Illies, M.R.; Byrum, C.A.; Xu, R.; Wikramanayake, A.H.; Ettensohn, C.A. Differential Stability of β-Catenin along the Animal-Vegetal Axis of the Sea Urchin Embryo Mediated by Dishevelled. Development 2004, 131, 2947–2956. [Google Scholar] [CrossRef]
- Leonard, J.D.; Ettensohn, C.A. Analysis of Dishevelled Localization and Function in the Early Sea Urchin Embryo. Dev. Biol. 2007, 306, 50–65. [Google Scholar] [CrossRef]
- Yaguchi, S.; Yaguchi, J.; Angerer, R.C.; Angerer, L.M. A Wnt-FoxQ2-Nodal Pathway Links Primary and Secondary Axis Specification in Sea Urchin Embryos. Dev. Cell 2008, 14, 97–107. [Google Scholar] [CrossRef]
- Wei, Z.; Yaguchi, J.; Yaguchi, S.; Angerer, R.C.; Angerer, L.M. The Sea Urchin Animal Pole Domain Is a Six3-Dependent Neurogenic Patterning Center. Development 2009, 136, 1179–1189. [Google Scholar] [CrossRef]
- Sethi, A.J.; Wikramanayake, R.M.; Angerer, R.C.; Range, R.C.; Angerer, L.M. Sequential Signaling Crosstalk Regulates Endomesoderm Segregation in Sea Urchin Embryos. Science 2012, 335, 590–593. [Google Scholar] [CrossRef]
- McClay, D.R.; Croce, J.C.; Warner, J.F. Conditional Specification of Endomesoderm. Cells Dev. 2021, 167, 203716. [Google Scholar] [CrossRef]
- Range, R.C. Canonical and Non-Canonical Wnt Signaling Pathways Define the Expression Domains of Frizzled 5/8 and Frizzled 1/2/7 along the Early Anterior-Posterior Axis in Sea Urchin Embryos. Dev. Biol. 2018, 444, 83–92. [Google Scholar] [CrossRef]
- Feuda, R.; Peter, I.S. Homologous Gene Regulatory Networks Control Development of Apical Organs and Brains in Bilateria. Sci. Adv. 2022, 8, eabo2416. [Google Scholar] [CrossRef]
- Gattoni, G.; Keitley, D.; Sawle, A.; Benito-Gutiérrez, E. An ancient apical patterning system sets the position of the forebrain in chordates. Sci. Adv. 2025, 11, eadq4731. [Google Scholar] [CrossRef]
- Lengfeld, T.; Watanabe, H.; Simakov, O.; Lindgens, D.; Gee, L.; Law, L.; Schmidt, H.A.; Özbek, S.; Bode, H.; Holstein, T.W. Multiple Wnts Are Involved in Hydra Organizer Formation and Regeneration. Dev. Biol. 2009, 330, 186–199. [Google Scholar] [CrossRef]
- Garriock, R.J.; Warkman, A.S.; Meadows, S.M.; D’Agostino, S.; Krieg, P.A. Census of Vertebrate Wnt Genes: Isolation and Developmental Expression of Xenopus Wnt2, Wnt3, Wnt9a, Wnt9b, Wnt10a, and Wnt16. Developmental Dynamics Off. Publ. Am. Assoc. Anat. 2007, 236, 1249–1258. [Google Scholar] [CrossRef]
- Khalturin, K.; Shinzato, C.; Khalturina, M.; Hamada, M.; Fujie, M.; Koyanagi, R.; Kanda, M.; Goto, H.; Anton-Erxleben, F.; Toyokawa, M.; et al. Medusozoan Genomes Inform the Evolution of the Jellyfish Body Plan. Nat. Ecol. Evol. 2019, 3, 811–822. [Google Scholar] [CrossRef]
- Robert, N.; Hammami, F.; Lhomond, G.; Dru, P.; Lepage, T.; Schubert, M.; Croce, J.C. A Wnt2 Ortholog in the Sea Urchin Paracentrotus Lividus. genesis 2019, 57, e23331. [Google Scholar] [CrossRef] [PubMed]
- Croce, J.C.; Wu, S.-Y.; Byrum, C.; Xu, R.; Duloquin, L.; Wikramanayake, A.H.; Gache, C.; McClay, D.R. A Genome-Wide Survey of the Evolutionarily Conserved Wnt Pathways in the Sea Urchin Strongylocentrotus Purpuratus. Dev. Biol. 2006, 300, 121–131. [Google Scholar] [CrossRef]
- Croce, J.; Range, R.C.; Wu, S.-Y.; Miranda, E.; Lhomond, G.; Peng, J.C.; Lepage, T.; McClay, D.R. Wnt6 Activates Endoderm in the Sea Urchin Gene Regulatory Network. Development 2011, 138, 3297–3306. [Google Scholar] [CrossRef]
- Gautam, S.; Fenner, J.L.; Wang, B.; Range, R.C. Evolutionarily Conserved Wnt/Sp5 Signaling Is Critical for Anterior-Posterior Axis Patterning in Sea Urchin Embryos. Iscience 2024, 27, 108616. [Google Scholar] [CrossRef]
- Yan, J.; Jia, H.; Ma, Z.; Ye, H.; Zhou, M.; Su, L.; Liu, J.; Guo, A.-Y. The Evolutionary Analysis Reveals Domain Fusion of Proteins with Frizzled-like CRD Domain. Gene 2014, 533, 229–239. [Google Scholar] [CrossRef]
- Darras, S.; Fritzenwanker, J.H.; Uhlinger, K.R.; Farrelly, E.; Pan, A.M.; Hurley, I.A.; Norris, R.P.; Osovitz, M.; Terasaki, M.; Wu, M.; et al. Anteroposterior Axis Patterning by Early Canonical Wnt Signaling during Hemichordate Development. PLoS Biol. 2018, 16, e2003698. [Google Scholar] [CrossRef] [PubMed]
- Yankura, K.A.; Koechlein, C.S.; Cryan, A.F.; Cheatle, A.; Hinman, V.F. Gene Regulatory Network for Neurogenesis Ina Sea Star Embryo Connects Broad Neuralspecification and Localized Patterning. Proc. Natl. Acad. Sci. USA 2013, 110, 8591–8596. [Google Scholar] [CrossRef]
- Croce, J.; Duloquin, L.; Lhomond, G.; McClay, D.R.; Gache, C. Frizzled5/8 Is Required in Secondary Mesenchyme Cells to Initiate Archenteron Invagination during Sea Urchin Development. Development 2006, 133, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Ka, C.; Gautam, S.; Marshall, S.; Tice, L.; Martinez-Bartolome, M.; Fenner, J.L.; Range, R.C. Receptor Tyrosine Kinases Ror1/2 and Ryk Are Co-Expressed with Multiple Wnt Signaling Components During Early Development of Sea Urchin Embryos. Biol. Bull. 2021, 241, 140–157. [Google Scholar] [CrossRef]
- Green, J.; Kuntz, S.G.; Sternberg, P.W. Ror Receptor Tyrosine Kinases: Orphans No More. Trends Cell Biol. 2008, 18, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Green, J.; Nusse, R.; Van Amerongen, R. The Role of Ryk and Ror Receptor Tyrosine Kinases in Wnt Signal Transduction. Cold Spring Harb. Perspect. Biol. 2024, 6, a009175. [Google Scholar] [CrossRef] [PubMed]
- Roy, J.P.; Halford, M.M.; Stacker, S.A. The Biochemistry, Signaling and Disease Relevance of RYK and Other WNT-Binding Receptor Tyrosine Kinases. Growth Factors 2018, 36, 15–40. [Google Scholar] [CrossRef]
- Bai, Y.; Tan, X.; Zhang, H.; Liu, B.; Zhao, Y.; Li, Y.; Lu, L.; Liu, Y.; Zhou, J. Ror2 Receptor Mediates Wnt11 Ligand Signaling and Affects Convergence and Extension Movements in Zebrafish. J. Biol. Chem. 2014, 289, 20664–20676. [Google Scholar] [CrossRef]
- Andre, P.; Wang, P.Q.; Wang, N.; Wang, B.; Gao, A.; Schilit, M.; Halford, S.; Stacker, S.A.; Zhang, X.; Yang, Y. The Wnt Coreceptor Ryk Regulates Wnt/Planar Cell Polarity by Modulating the Degradation of the Core Planar Cell Polarity Component Vangl2. J. Biol. Chem. 2012, 287, 4451844525. [Google Scholar] [CrossRef]
- Mikels, A.; Minami, Y.; Nusse, R. Ror2 Receptor Requires Tyrosine Kinase Activity to Mediate Wnt5A Signaling. J. Biol. Chem. 2009, 284, 30167–30176. [Google Scholar] [CrossRef]
- Mikels, A.J.; Nusse, R. Purified Wnt5a Protein Activates or Inhibits B-Catenin–TCF Signaling Depending on Receptor Context. PLoS Biol. 2006, 4, e115. [Google Scholar] [CrossRef]
- Cheyette, B.N.R. Ryk: Another Heretical Wnt Receptor Defies the Canon. Sci. STKE Signal Transduct. Knowl. Env. 2004, 2004, pe54. [Google Scholar] [CrossRef]
- Berndt, J.D.; Aoyagi, A.; Yang, P.; Anastas, J.N.; Tang, L.; Moon, R.T. Mindbomb 1, an E3 Ubiquitin Ligase, Forms a Complex with RYK to Activate Wnt/b-Catenin Signaling. J. Cell Biol. 2011, 194, 737750. [Google Scholar] [CrossRef]
- Janda, C.Y.; Waghray, D.; Levin, A.M.; Thomas, C.; Garcia, K.C. Structural Basis of Wnt Recognition by Frizzled. Science 2012, 337, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Niehrs, C. The Complex World of WNT Receptor Signaling. Nat. Rev. Mol. Cell Biol. 2012, 13, 767–779. [Google Scholar] [CrossRef]
- Lhomond, G.; Schubert, M.; Croce, J. Spatiotemporal Requirements of Nuclear β-Catenin Define Early Sea Urchin Embryogenesis. PLoS Biol. 2024, 22, e3002880. [Google Scholar] [CrossRef] [PubMed]
- Peter, I.S.; Davidson, E.H. A Gene Regulatory Network Controlling the Embryonic Specification of Endoderm. Nature 2011, 474, 635–639. [Google Scholar] [CrossRef]
- Sethi, A.J.; Angerer, R.C.; Angerer, L.M. Gene Regulatory Network Interactions in Sea Urchin Endomesoderm Induction. PLoS Biol. 2009, 7, e1000029. [Google Scholar] [CrossRef]
- Cary, G.A.; McCauley, B.S.; Zueva, O.; Pattinato, J.; Longabaugh, W.; Hinman, V.F. Systematic Comparison of Sea Urchin and Sea Star Developmental Gene Regulatory Networks Explains How Novelty Is Incorporated in Early Development. Nat. Commun. 2020, 11, 6235. [Google Scholar] [CrossRef]
- Hinman, V.F.; Nguyen, A.T.; Cameron, R.A.; Davidson, E.H. Developmental Gene Regulatory Network Architecture across 500 Million Years of Echinoderm Evolution. Proc. Natl. Acad. Sci. USA 2003, 100, 13356–13361. [Google Scholar] [CrossRef]
- Erkenbrack, E.M. Divergence of Ectodermal and Mesodermal Gene Regulatory Network Linkages in Early Development of Sea Urchins. Proc. Natl. Acad. Sci. USA 2016, 113, E7202–E7211. [Google Scholar] [CrossRef] [PubMed]
- Erkenbrack, E.M.; Ako-Asare, K.; Miller, E.; Tekelenburg, S.; Thompson, J.R.; Romano, L. Ancestral State Reconstruction by Comparative Analysis of a GRN Kernel Operating in Echinoderms. Dev. Genes Evol. 2016, 226, 37–45. [Google Scholar] [CrossRef]
- Hinman, V.F.; Davidson, E.H. Evolutionary Plasticity of Developmental Gene Regulatory Network Architecture. Proc. Natl. Acad. Sci. USA 2007, 104, 19404–19409. [Google Scholar] [CrossRef]
- Ben-Tabou De-Leon, S. The Conserved Role and Divergent Regulation of Foxa, a Pan-Eumetazoan Developmental Regulatory Gene. Dev. Biol. 2011, 357, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Maduro, M.F.; Rothman, J.H. Making Worm Guts: The Gene Regulatory Network of the Caenorhabditis Elegans Endoderm. Dev. Biol. 2002, 246, 68–85. [Google Scholar] [CrossRef]
- Owraghi, M.; Broitman-Maduro, G.; Luu, T.; Roberson, H.; Maduro, M.F. Roles of the Wnt Effector POP-1/TCF in the C. Elegans Endomesoderm Specification Gene Network. Dev. Biol. 2010, 340, 209–221. [Google Scholar] [CrossRef]
- Ewe, C.K.; Sommermann, E.M.; Kenchel, J.; Flowers, S.E.; Maduro, M.F.; Josh, P.M.; Rothman, J.H. Feedforward Regulatory Logic Controls the Specification-to-Differentiation Transition and Terminal Cell Fate during Caenorhabditis Elegans Endoderm Development. Development 2022, 149, dev200337. [Google Scholar] [CrossRef]
- Weber, H.; Symes, C.E.; Walmsley, M.E.; Rodaway, A.R.F.; Patient, R.K. A Role for GATA5 in Xenopus Endoderm Specification. Development 2000, 127, 4345–4360. [Google Scholar] [CrossRef]
- Tseng, W.-F.; Jang, T.-H.; Huang, C.-B.; Yuh, C.-H. An Evolutionarily Conserved Kernel of Gata5, Gata6, Otx2 and Prdm1a Operates in the Formation of Endoderm in Zebrafish. Dev. Biol. 2011, 357, 541–557. [Google Scholar] [CrossRef][Green Version]
- Chan, T.-M.; Longabaugh, W.; Bolouri, H.; Chen, H.-L.; Tseng, W.-F.; Chao, C.-H.; Jang, T.-H.; Lin, Y.-I.; Hung, S.-C.; Wang, H.-D.; et al. Developmental Gene Regulatory Networks in the Zebrafish Embryo. Biochim. Et Biophys. Acta (BBA)-Gene Regul. Mech. 2009, 1789, 279–298. [Google Scholar] [CrossRef]
- Rojas, A.; Schachterle, W.; Xu, S.-M.; Black, B.L. An Endoderm-specific Transcriptional Enhancer from the Mouse Gata4 Gene Requires GATA and Homeodomain Protein–Binding Sites for Function in Vivo. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2009, 238, 2588–2598. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, L.A.; Lin, F.R.; Cuesta, I.; Friedman, D.; Jarnik, M.; Zaret, K.S. Opening of Compacted Chromatin by Early Developmental Transcription Factors HNF3 (FoxA) and GATA-4. Mol. Cell 2002, 9, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Bikoff, E.K.; Morgan, M.A.; Robertson, E.J. An Expanding Job Description for Blimp-1/PRDM1. Curr. Opin. Genet. Dev. 2009, 19, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Hohenauer, T.; Moore, A.W. The Prdm Family: Expanding Roles in Stem Cells and Development. Development 2012, 139, 2267–2282. [Google Scholar] [CrossRef]
- Khadka, A.; Martínez-Bartolomé, M.; Burr, S.D.; Range, R.C. A Novel Gene’s Role in an Ancient Mechanism: Secreted Frizzled-Related Protein 1 Is a Critical Component in the Anterior–Posterior Wnt Signaling Network That Governs the Establishment of the Anterior Neuroectoderm in Sea Urchin Embryos. EvoDevo 2018, 9, 1. [Google Scholar] [CrossRef]
- Yaguchi, J.; Takeda, N.; Inaba, K.; Yaguchi, S. Cooperative Wnt-Nodal Signals Regulate the Patterning of Anterior Neuroectoderm. PLOS Genet. 2016, 12, e1006001. [Google Scholar] [CrossRef]
- Range, R.C.; Wei, Z. An Anterior Signaling Center Patterns and Sizes the Anterior Neuroectoderm of the Sea Urchin Embryo. Development 2016, 143, 1523–1533. [Google Scholar] [CrossRef]
- Yaguchi, S.; Yaguchi, J.; Wei, Z.; Jin, Y.; Angerer, L.M.; Inaba, K. Fez Function Is Required to Maintain the Size of the Animal Plate in the Sea Urchin Embryo. Development 2011, 138, 4233–4243. [Google Scholar] [CrossRef]
- Yaguchi, J.; Angerer, L.M.; Inaba, K.; Yaguchi, S. Zinc Finger Homeobox Is Required for the Differentiation of Serotonergic Neurons in the Sea Urchin Embryo. Dev. Biol. 2012, 363, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Angerer, L.M.; Angerer, R.C. Neurogenic Gene Regulatory Pathways in the Sea Urchin Embryo. Development 2016, 143, 298–305. [Google Scholar] [CrossRef]
- Li, E.; Materna, S.C.; Davidson, E.H. New Regulatory Circuit Controlling Spatial and Temporal Gene Expression in the Sea Urchin Embryo Oral Ectoderm GRN. Dev. Biol. 2013, 382, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Yaguchi, S.; Yaguchi, J.; Wei, Z.; Shiba, K.; Angerer, L.M.; Inaba, K. ankAT-1 Is a Novel Gene Mediating the Apical Tuft Formation in the Sea Urchin Embryo. Dev. Biol. 2010, 348, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Vielmas, E.; Davidson, E.H.; Peter, I.S. Sequential Response to Multiple Developmental Network Circuits Encoded in an Intronic Cis-Regulatory Module of Sea Urchin Hox11/13b. Cell Rep. 2017, 19, 364–374. [Google Scholar] [CrossRef]
- Livi, C.B.; Davidson, E.H. Expression and Function of Blimp1/Krox, an Alternatively Transcribed Regulatory Gene of the Sea Urchin Endomesoderm Network. Dev. Biol. 2006, 293, 513–525. [Google Scholar] [CrossRef]
- Materna, S.C.; Ransick, A.; Li, E.; Davidson, E.H. Diversification of Oral and Aboral Mesodermal Regulatory States in Pregastrular Sea Urchin Embryos. Dev. Biol. 2013, 375, 92–104. [Google Scholar] [CrossRef]
- Minokawa, T.; Wikramanayake, A.H.; Davidson, E.H. Cis-Regulatory Inputs of the Wnt8 Gene in the Sea Urchin Endomesoderm Network. Dev. Biol. 2005, 288, 545–558. [Google Scholar] [CrossRef]
- Oliveri, P.; Walton, K.D.; Davidson, E.H.; McClay, D.R. Repression of Mesodermal Fate by Foxa, a Key Endoderm Regulator of the Sea Urchin Embryo. Development 2006, 133, 4173–4181. [Google Scholar] [CrossRef]
- Smith, J.; Davidson, E.H. Gene Regulatory Network Subcircuit Controlling a Dynamic Spatial Pattern of Signaling in the Sea Urchin Embryo. Proc. Natl. Acad. Sci. USA 2008, 105, 20089–20094. [Google Scholar] [CrossRef]
- Smith, J.; Kraemer, E.; Liu, H.; Theodoris, C.; Davidson, E. A Spatially Dynamic Cohort of Regulatory Genes in the Endomesodermal Gene Network of the Sea Urchin Embryo. Dev. Biol. 2008, 313, 863–875. [Google Scholar] [CrossRef]
- Yamazaki, A.; Kawabata, R.; Shiomi, K.; Tsuchimoto, J.; Kiyomoto, M.; Amemiya, S.; Yamaguchi, M. Krüppel-like Is Required for Nonskeletogenic Mesoderm Specification in the Sea Urchin Embryo. Dev. Biol. 2008, 314, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Yuh, C.-H.; Dorman, E.R.; Howard, M.L.; Davidson, E.H. An Otx Cis-Regulatory Module: A Key Node in the Sea Urchin Endomesoderm Gene Regulatory Network. Dev. Biol. 2004, 269, 536–551. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Robertis, E.M.D.; Tejeda-Munoz, N. Evo-Devo of Urbilateria and Its Larval Forms. Dev. Biol. 2022, 487, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Marlow, H.; Tosches, M.A.; Tomer, R.; Steinmetz, P.R.; Lauri, A.; Larsson, T.; Arendt, D. Larval Body Patterning and Apical Organs Are Conserved in Animal Evolution. BMC Biol. 2014, 12, 7. [Google Scholar] [CrossRef]
- Steinmetz, P.R.; Urbach, R.; Posnien, N.; Eriksson, J.; Kostyuchenko, R.P.; Brena, C.; Guy, K.; Akam, M.; Bucher, G.; Arendt, D. Six3 Demarcates the Anterior-Most Developing Brain Region in Bilaterian Animals. EvoDevo 2010, 1, 1–9. [Google Scholar] [CrossRef]
- Lagutin, O.V.; Zhu, C.C.; Kobayashi, D.; Topczewski, J.; Shimamura, K.; Puelles, L.; Russell, H.R.C.; McKinnon, P.J.; Solnica-Krezel, L.; Oliver, G. Six3 Repression of Wnt Signaling in the Anterior Neuroectoderm Is Essential for Vertebrate Forebrain Development. Genes Dev. 2003, 17, 368–379. [Google Scholar] [CrossRef]
- Posnien, N.; Koniszewski, N.D.B.; Hein, H.J.; Bucher, G. Candidate Gene Screen in the Red Flour Beetle Tribolium Reveals Six3 as Ancient Regulator of Anterior Median Head and Central Complex Development. Genetics 2011, 7, e1002416. [Google Scholar] [CrossRef]
- He, B.; Buescher, M.; Farnworth, M.S.; Strobl, F.; Stelzer, E.H.; Koniszewski, N.D.; Muehlen, D.; Bucher, G. An Ancestral Apical Brain Region Contributes to the Central Complex under the Control of foxQ2 in the Beetle Tribolium. eLife 2019, 8, e49065. [Google Scholar] [CrossRef]
- Schacht, M.I.; Schomburg, C.; Bucher, G. Six3 Acts Upstream of foxQ2 in Labrum and Neural Development in the Spider Parasteatoda Tepidariorum. Dev. Genes Evol. 2020, 230, 95–104. [Google Scholar] [CrossRef]
- Kitzmann, P.; Weißkopf, M.; Schacht, M.I.; Bucher, G. A Key Role for foxQ2 in Anterior Head and Central Brain Patterning in Insects. Development 2017, 144, 2969–2981. [Google Scholar] [CrossRef] [PubMed]
- Sinigaglia, C.; Busengdal, H.; Leclere, L.; Technau, U.; Rentzsch, F. The Bilaterian Head Patterning Gene Six3/6 Controls Aboral Domain Development in a Cnidarian. PLoS Biol. 2013, 11, e1001488. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Toyama, R.; Takeda, H.; Dawid, I.B.; Kawakami, K. Overexpression of the Forebrain-Specific Homeobox Gene Six3 Induces Rostral Forebrain Enlargement in Zebrafish. Development 1998, 125, 2973–2982. [Google Scholar] [CrossRef] [PubMed]
- Angerer, L.M.; Yaguchi, S.; Angerer, R.C.; Burke, R.D. The Evolution of Nervous System Patterning: Insights from Sea Urchin Development. Development 2011, 138, 3613–3623. [Google Scholar] [CrossRef]
- Darras, S.; Gerhart, J.; Terasaki, M.; Kirschner, M.; Lowe, C.J. B-Catenin Specifies the Endomesoderm and Defines the Posterior Organizer of the Hemichordate Saccoglossus Kowalevskii. Development 2011, 138, 959–970. [Google Scholar] [CrossRef]
- Reversade, B.; Kuroda, H.; Lee, H.; Mays, A.; Robertis, E.M.D. Depletion of Bmp2, Bmp4, Bmp7 and Spemann Organizer Signals Induces Massive Brain Formation in Xenopus Embryos. Development 2005, 132, 3381–3392. [Google Scholar] [CrossRef]
- Varga, M.; Maegawa, S.; Weinberg, E.S. Correct Anteroposterior Patterning of the Zebrafish Neurectoderm in the Absence of the Early Dorsal Organizer. BMC Dev. Biol. 2011, 11, 26. [Google Scholar] [CrossRef]
- Eiraku, M.; Watanabe, K.; Matsuo-Takasaki, M.; Kawada, M.; Yonemura, S.; Matsumura, M.; Wataya, T.; Nishiyama, A.; Muguruma, K.; Sasai, Y. Self-Organized Formation of Polarized Cortical Tissues from ESCs and Its Active Manipulation by Extrinsic Signals. Cell Stem. Cell 2008, 3, 519–532. [Google Scholar] [CrossRef]
- Nakano, T.; Ando, S.; Takata, N.; Kawada, M.; Muguruma, K.; Sekiguchi, K.; Saito, K.; Yonemura, S.; Eiraku, M.; Sasai, Y. Self-Formation of Optic Cups and Storable Stratified Neural Retina from Human ESCs. Cell Stem. Cell 2012, 10, 771–785. [Google Scholar] [CrossRef]
- Wei, Z.; Range, R.C.; Angerer, R.C.; Angerer, L.M. Axial Patterning Interactions in the Sea Urchin Embryo: Suppression of Nodal by Wnt1 Signaling. Development 2012, 139, 1662–1669. [Google Scholar] [CrossRef][Green Version]
- Hudson, C.; Kawai, N.; Negishi, T.; Yasuo, H. B-Catenin-Driven Binary Fate Specification Segregates Germ Layers in Ascidian Embryos. Curr. Biol. 2013, 23, 491–495. [Google Scholar] [CrossRef]
- Imai, K.; Takada, N.; Satoh, N.; Satou, Y. β-Catenin Mediates the Specification of Endoderm Cells in Ascidian Embryos. Development 2000, 127, 3009–3020. [Google Scholar] [CrossRef] [PubMed]
- Kiecker, C.; Niehrs, C. A Morphogen Gradient of Wnt/β-Catenin Signalling Regulates Anteroposterior Neural Patterning in Xenopus. Development 2001, 128, 4189–4201. [Google Scholar] [CrossRef]
- Nordström, U.; Jessell, T.M.; Edlund, T. Progressive Induction of Caudal Neural Character by Graded Wnt Signaling. Nat. Neurosci. 2002, 5, 525–532. [Google Scholar] [CrossRef]
- Weidinger, G.; Thorpe, C.J.; Wuennenberg-Stapleton, K.; Ngai, J.; Moon, R.T. The Sp1-Related Transcription Factors Sp5 and Sp5-like Act Downstream of Wnt/β-Catenin Signaling in Mesoderm and Neuroectoderm Patterning. Curr. Biol. 2005, 15, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Nikaido, M.; Law, E.W.P.; Kelsh, R.N. A Systematic Survey of Expression and Function of Zebrafish Frizzled Genes. PLoS ONE 2013, 8, e54833. [Google Scholar] [CrossRef] [PubMed]
- Lekven, A.C.; Thorpe, C.J.; Waxman, J.S.; Moon, R.T. Zebrafish Wnt8 Encodes Two Wnt8 Proteins on a Bicistronic Transcript and Is Required for Mesoderm and Neurectoderm Patterning. Dev. Cell 2001, 1, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Shin, J.; Park, H.-C.; Yeo, S.-Y.; Hong, S.-K.; Han, S.; Rhee, M.; Kim, C.-H.; Chitnis, A.B.; Huh, T.-L. Specification of an Anterior Neuroectoderm Patterning by Frizzled8a-Mediated Wnt8b Signalling during Late Gastrulation in Zebrafish. Development 2002, 129, 4443–4455. [Google Scholar] [CrossRef]
- Kim, S.-H.; Park, H.-C.; Yeo, S.-Y.; Hong, S.-K.; Choi, J.-W.; Kim, C.-H.; Weinstein, B.M.; Huh, T.-L. Characterization of Two Frizzled8 Homologues Expressed in the Embryonic Shield and Prechordal Plate of Zebrafish Embryos. Mech. Dev. 1998, 78, 193–198. [Google Scholar] [CrossRef]
- Dailey, S.C.; Kozmikova, I.; Somorjai, I.M.L. Amphioxus Sp5 Is a Member of a Conserved Specificity Protein Complement and Is Modulated by Wnt/β-Catenin Signalling. Int. J. Dev. Biol. 2017, 61, 723. [Google Scholar] [CrossRef]
- Thorpe, C.J.; Weidinger, G.; Moon, R.T. Wnt/β-Catenin Regulation of the Sp1-Related Transcription Factor Sp5l Promotes Tail Development in Zebrafish. Development 2005, 132, 1763–1772. [Google Scholar] [CrossRef]
- Zhao, J.; Cao, Y.; Zhao, C.; Postlethwait, J.; Meng, A. An SP1-like Transcription Factor Spr2 Acts Downstream of Fgf Signaling to Mediate Mesoderm Induction. EMBO J. 2003, 22, 6078–6088. [Google Scholar] [CrossRef]
- Zhao, C.; Meng, A. Sp1-like Transcription Factors Are Regulators of Embryonic Development in Vertebrates. Dev. Growth Differ. 2005, 47, 201–211. [Google Scholar] [CrossRef]
- Yu, J.-K.; Satou, Y.; Holland, N.D.; Shin-I, T.; Kohara, Y.; Satoh, N.; Bronner-Fraser, M.; Holland, L.Z. Axial Patterning in Cephalochordates and the Evolution of the Organizer. Nature 2007, 445, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.; Fritzenwanker, J.H.; Cunningham, D.D.; Lowe, C.J. Chapter Nineteen—Saccoglossus Kowalevskii: Evo-Devo Insights from the Mud. In Current Topics in Developmental Biology Emerging Model Systems in Developmental Biology; Academic Press: Cambridge, MA, USA, 2022; pp. 545–562. [Google Scholar]
- Bafico, A.; Gazit, A.; Pramila, T.; Finch, P.W.; Yaniv, A.; Aaronson, S.A. Interaction of Frizzled Related Protein (FRP) with Wnt Ligands and the Frizzled Receptor Suggests Alternative Mechanisms for FRP Inhibition of Wnt Signaling. J. Biol. Chem. 1999, 274, 16180–16187. [Google Scholar] [CrossRef] [PubMed]
- Mao, B.; Wu, W.; Li, Y.; Hoppe, D.; Stannek, P.; Glinka, A.; Niehrs, C. LDL-Receptor-Related Protein 6 Is a Receptor for Dickkopf Proteins. Nature 2001, 411, 321–325. [Google Scholar] [CrossRef]
- Semenov, M.V.; Tamai, K.; Brott, B.K.; Kuhl, M.; Sokol, S.; He, X. Head Inducer Dickkopf-1 Is a Ligand for Wnt Coreceptor LRP6. Curr. Biol. 2001, 11, 951–961. [Google Scholar] [CrossRef]
- Illies, M.R.; Peeler, M.T.; Dechtiaruk, A.; Ettensohn, C.A. Cloning and Developmental Expression of a Novel, Secreted Frizzledrelated Protein from the Sea Urchin, Strongylocentrotus Purpuratus. Mech. Dev. 2002, 13, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Bovolenta, P.; Esteve, P.; Ruiz, J.M.; Cisneros, E.; Lopez-Rios, J. Beyond Wnt Inhibition: New Functions of Secreted Frizzled-Related Proteins in Development and Disease. J. Cell Sci. 2008, 121, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Leyns, L.; Bouwmeester, T.; Kim, S.-H.; Piccolo, S.; Robertis, E.M.D. Frzb-1 Is a Secreted Antagonist of Wnt Signaling Expressed in the Spemann Organizer. Cell 1997, 88, 747–756. [Google Scholar] [CrossRef]
- Wang, S.; Krinks, M.; Lin, K.; Luyten, F.P.; Moos, M., Jr. Frzb, a Secreted Protein Expressed in the Spemann Organizer, Binds and Inhibits Wnt-8. Cell 1997, 88, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.-I.; Thisse, C.; Thisse, B. Identification and Mechanism of Regulation of the Zebrafish Dorsal Determinant. Proc. Natl. Acad. Sci. USA 2011, 108, 15876–15880. [Google Scholar] [CrossRef]
- Wen, B.; Hu, S.; Yin, J.; Wu, J.; Guo, W. Molecular Evolution and Protein Structure Variation of Dkk Family. Genes 2023, 14, 1863. [Google Scholar] [CrossRef]
- Veeck, J.; Dahl, E. Targeting the Wnt Pathway in Cancer: The Emerging Role of Dickkopf-3. Biochim. Et Biophys. Acta (BBA)-Rev. Cancer 2012, 1825, 18–28. [Google Scholar] [CrossRef]
- Mao, B.; Wu, W.; Davidson, G.; Marhold, J.; Li, M.; Mechler, B.M.; Delius, H.; Hoppe, D.; Stannek, P.; Walter, C.; et al. Kremen Proteins Are Dickkopf Receptors That Regulate Wnt/β-Catenin Signaling. Nature 2002, 417, 664–667. [Google Scholar] [CrossRef]
- Nakamura, R.E.I.; Hackam, A.S. Analysis of Dickkopf3 Interactions with Wnt Signaling Receptors. Growth Factors 2010, 28, 232–242. [Google Scholar] [CrossRef]
- Cruciat, C.-M.; Niehrs, C. Secreted and Transmembrane Wnt Inhibitors and Activators. Cold Spring Harb. Perspect. Biol. 2013, 5, a015081. [Google Scholar] [CrossRef]
- Üren, A.; Reichsman, F.; Anest, V.; Taylor, W.G.; Muraiso, K.; Bottaro, D.P.; Cumberledge, S.; Rubin, J.S. Secreted Frizzled-Related Protein-1 Binds Directly to Wingless and Is a Biphasic Modulator of Wnt Signaling. J. Biol. Chem. 2000, 275, 4372–4382. [Google Scholar] [CrossRef]
- Mii, Y.; Taira, M. Secreted Frizzled-Related Proteins Enhance the Diffusion of Wnt Ligands and Expand Their Signalling Range. Development 2009, 136, 4083–4088. [Google Scholar] [CrossRef]
- Mii, Y.; Taira, M. Secreted Wnt “Inhibitors” Are Not Just Inhibitors: Regulation of Extracellular Wnt by Secreted Frizzled-related Proteins. Dev. Growth Differ. 2011, 53, 911–923. [Google Scholar] [CrossRef]
- de Almeida Magalhaes, T.; Liu, J.; Chan, C.; Borges, K.S.; Zhang, J.; Kane, A.J.; Wierbowski, B.M.; Salic, A. Extracellular Carriers Control Lipid-Dependent Secretion, Delivery, and Activity of WNT Morphogens. Dev. Cell 2024, 59, 244–261. [Google Scholar] [CrossRef]
- Sato, A.; Yamamoto, H.; Sakane, H.; Koyama, H.; Kikuchi, A. Wnt5a Regulates Distinct Signaling Pathways by Binding to Frizzled2. EMBO J. 2010, 29, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Ring, L.; Neth, P.; Weber, C.; Steffens, S.; Faussner, A. β-Catenin-Dependent Pathway Activation by Both Promiscuous “Canonical” WNT3a–, and Specific “Noncanonical” WNT4– and WNT5a–FZD Receptor Combinations with Strong Differences in LRP5 and LRP6 Dependency. Cell Signal. 2014, 26, 260–267. [Google Scholar] [CrossRef]
- Cha, S.-W.; Tadjuidje, E.; Tao, Q.; Wylie, C.; Heasman, J. Wnt5a and Wnt11 Interact in a Maternal Dkk1-Regulated Fashion to Activate Both Canonical and Non-Canonical Signaling in Xenopus Axis Formation. Development 2008, 135, 3719–3729. [Google Scholar] [CrossRef] [PubMed]
- Slusarski, D.C.; Corces, V.G.; Moon, R.T. Interaction of Wnt and a Frizzled Homologue Triggers G-Protein-Linked Phosphatidylinositol Signaling. Nature 1997, 390, 410–413. [Google Scholar] [CrossRef]
- Westfall, T.A.; Brimeyer, R.; Twedt, J.; Gladon, J.; Olberding, A.; Furutani-Seiki, M.; Slusarski, D.C. Wnt-5/Pipetail Functions in Vertebrate Axis Formation as a Negative Regulator of Wnt/β-Catenin Activity. J. Cell Biol. 2003, 162, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, M.; Sheldahl, L.C.; Malbon, C.C.; Moon, R.T. Ca21/Calmodulin-Dependent Protein Kinase II Is Stimulated by Wnt and Frizzled Homologs and Promotes Ventral Cell Fates in Xenopus. J. Biol. Chem. 2000, 275, 12701–12711. [Google Scholar] [CrossRef]
- Tao, Q.; Yokota, C.; Puck, H.; Kofron, M.; Birsoy, B.; Yan, D.; Asashima, M.; Wylie, C.C.; Lin, X.; Heasman, J. Maternal Wnt11 Activates the Canonical Wnt Signaling Pathway Required for Axis Formation in Xenopus Embryos. Cell 2005, 120, 857–871. [Google Scholar] [CrossRef] [PubMed]
- Heisenberg, C.-P.; Tada, M.; Rauch, G.-J.; Sauade, L.; Concha, M.L.; Geisler, R.; Stemple, D.L.; Smith, J.C.; Wilson, S.W. Silberblick/Wnt11mediates Convergent Extension Movements during Zebrafish Gastrulation. Nature 2000, 405, 76–81. [Google Scholar] [CrossRef]
- McLeod, J.J.; Rothschild, S.C.; Francescatto, L.; Kim, H.; Tombes, R.M. Specific CaMKIIs Mediate Convergent Extension Cell Movements in Early Zebrafish Development. Dev. Dyn. 2024, 253, 390–403. [Google Scholar] [CrossRef] [PubMed]
- Medina, A.; Reintsch, W.; Steinbeisser, H. Xenopus Frizzled 7 Can Act in Canonical and Non-Canonical Wnt Signaling Pathways: Implications on Early Patterning and Morphogenesis. Mech. Dev. 2000, 92, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.D.; Hallagan, S.E.; McGrew, L.L.; Miller, J.R.; Moon, R.T. The Maternal Xenopus B-Catenin Signaling Pathway, Activated by Frizzled Homologs, Induces Goosecoid in a Cell Non-Autonomous Manner. Dev. Growth Differ. 2000, 42, 347–357. [Google Scholar] [CrossRef]
- Carron, C.; Shi, D.-L. Specification of Anteroposterior Axis by Combinatorial Signaling during Xenopus Development. Wiley Interdiscip. Rev. Dev. Biol. 2015, 5, 150–168. [Google Scholar] [CrossRef]
- Huang, Y.-L.; Niehrs, C. Polarized Wnt Signaling Regulates Ectodermal Cell Fate in Xenopus. Dev. Cell 2014, 29, 250–257. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fenner, J.L.; Wang, B.; Ka, C.; Gautam, S.; Range, R.C. An Integrated Canonical and Non-Canonical Wnt Signaling Network Controls Early Anterior–Posterior Axis Formation in Sea Urchin Embryos. J. Dev. Biol. 2025, 13, 36. https://doi.org/10.3390/jdb13040036
Fenner JL, Wang B, Ka C, Gautam S, Range RC. An Integrated Canonical and Non-Canonical Wnt Signaling Network Controls Early Anterior–Posterior Axis Formation in Sea Urchin Embryos. Journal of Developmental Biology. 2025; 13(4):36. https://doi.org/10.3390/jdb13040036
Chicago/Turabian StyleFenner, Jennifer L., Boyuan Wang, Cheikhouna Ka, Sujan Gautam, and Ryan C. Range. 2025. "An Integrated Canonical and Non-Canonical Wnt Signaling Network Controls Early Anterior–Posterior Axis Formation in Sea Urchin Embryos" Journal of Developmental Biology 13, no. 4: 36. https://doi.org/10.3390/jdb13040036
APA StyleFenner, J. L., Wang, B., Ka, C., Gautam, S., & Range, R. C. (2025). An Integrated Canonical and Non-Canonical Wnt Signaling Network Controls Early Anterior–Posterior Axis Formation in Sea Urchin Embryos. Journal of Developmental Biology, 13(4), 36. https://doi.org/10.3390/jdb13040036






