Wound-Induced Regeneration in Feather Follicles: A Stepwise Strategy to Regenerate Stem Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Plucking and Surgery of Feather Follicles
2.2. Hematoxylin and Eosin (H&E) and Immunostaining
2.3. Cell Proliferation Study
2.4. In Situ Hybridization
2.5. Statistical Analysis
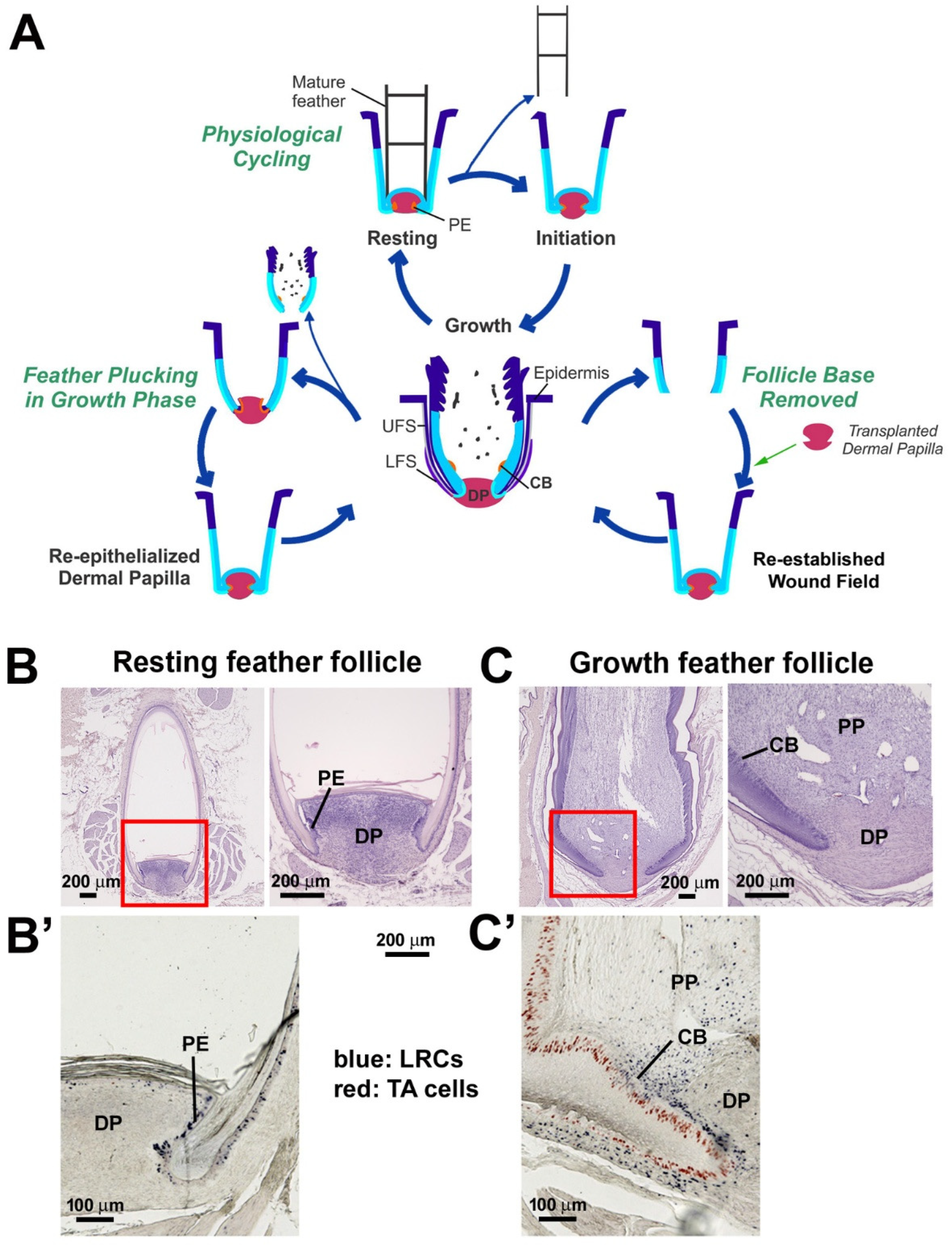
3. Results
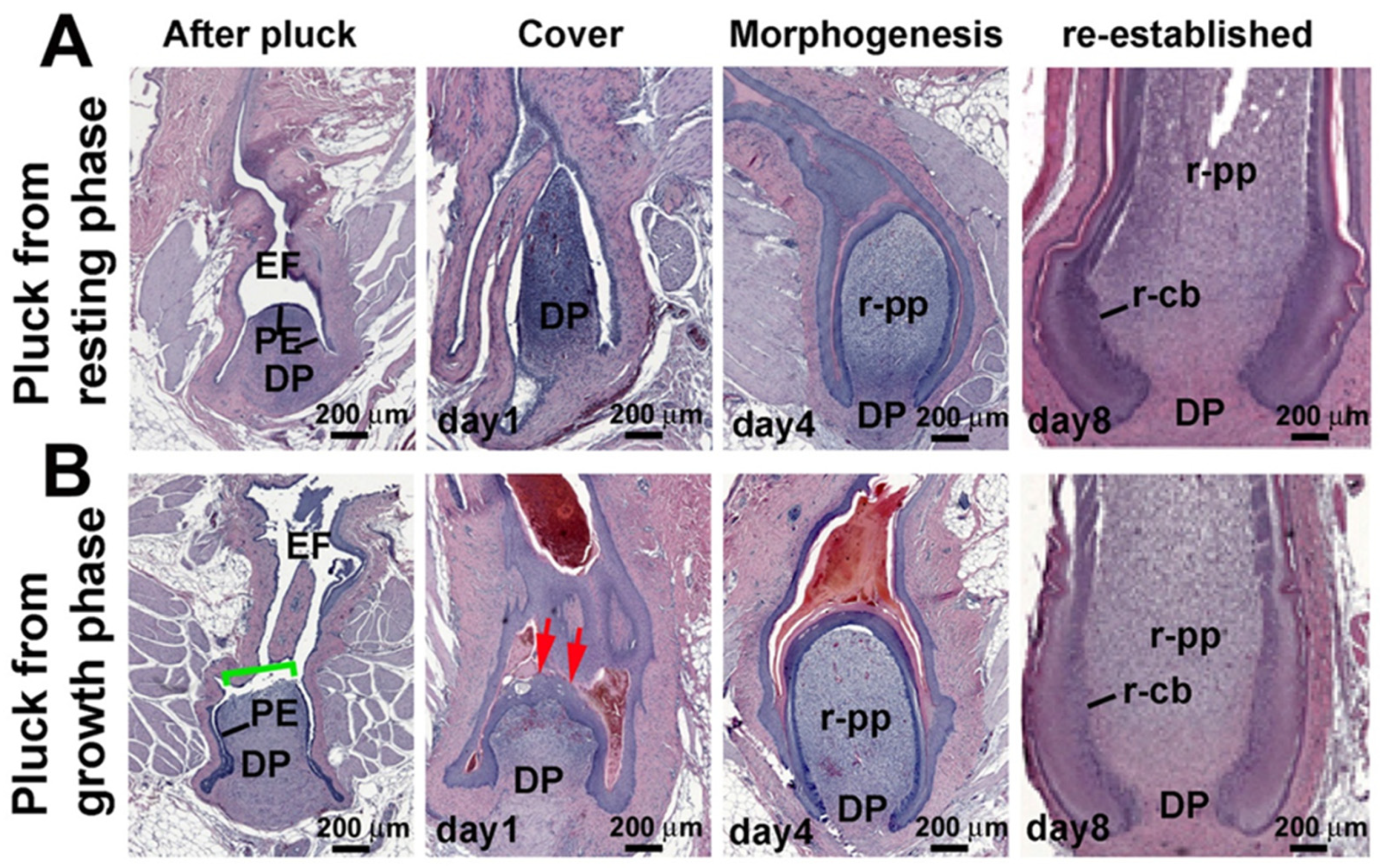
3.1. Comparison of Feather Regeneration Between Plucking During the Resting Phase or Growth Phase
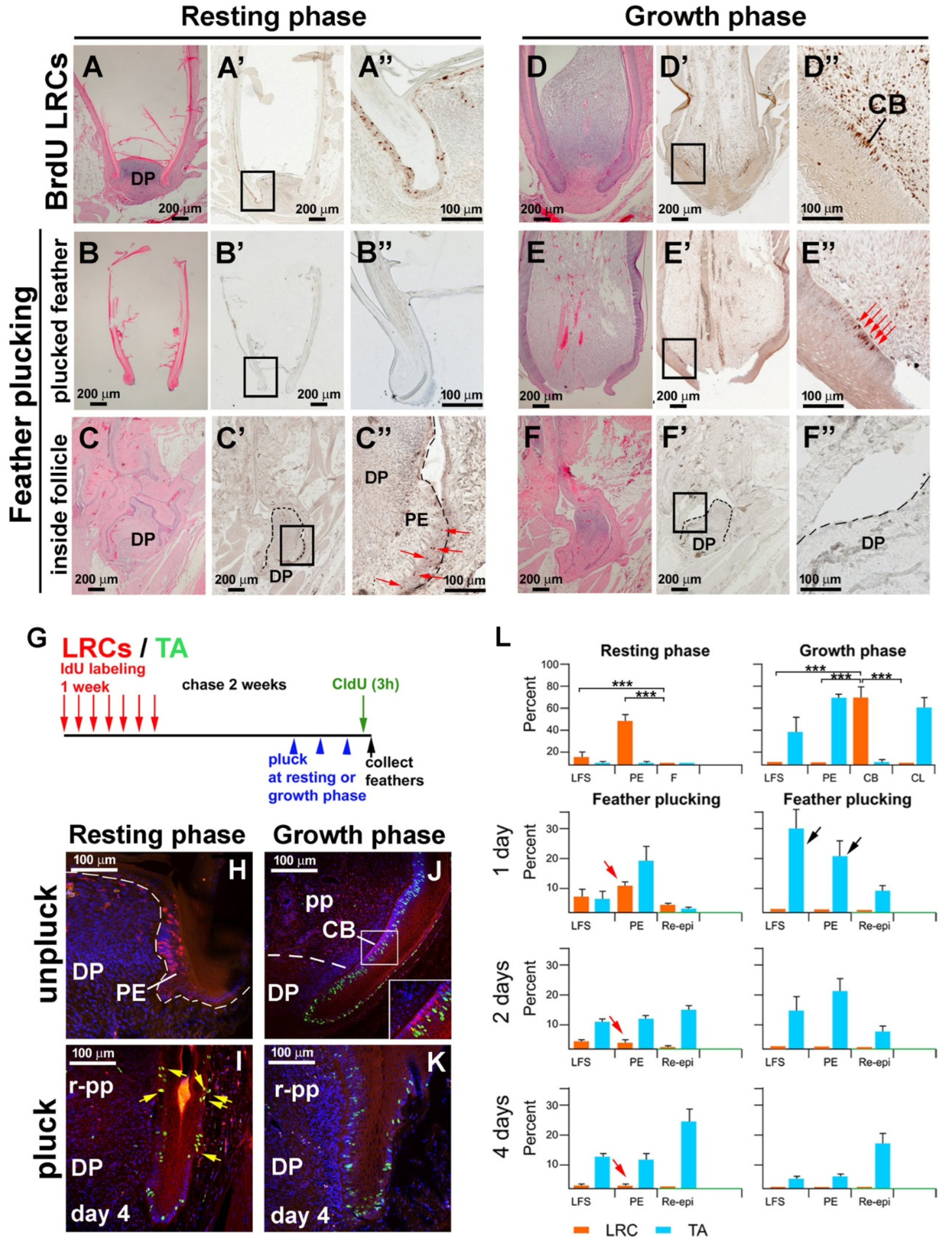
3.2. LRCs and TA Cell Activities During Feather Regeneration Following Feather Plucking in the Resting or Growth Phases
3.3. Altered Molecular Expression Following Feather Plucking in the Resting or Growth Phases
3.4. The Lost Regeneration Ability After Removing the Epithelial and Dermal Components of the Lower Follicle Can Be Restored Through DP Transplantation
4. Discussion
4.1. Collar Bulge and PE Stem Cells Represent Residential Stem Cells in the Growth and Resting Phases, Respectively, While Dermal Sheath Epithelium Are Induced by DP to De-Differentiate and Regenerate New Stem Cells
4.2. Regenerative Strategy Learned from Wounded Feather Follicles

5. Conclusions and Future Directions
6. Limitations of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CB | collar bulge |
| CL | collar |
| EF | empty follicle |
| DP | dermal papilla |
| DPC | dermal papilla complex |
| LRC | label-retaining cells |
| PE | papillary ectoderm |
| LFS | lower follicle sheath |
| PP | pulp |
| r-cb | regenerated collar bulge |
| r-PP | regenerated pulp |
| SC | stem cells |
| TA cells | transient amplifying cells |
| UFS | upper follicle sheath |
References
- Alibardi, L. Morphological and cellular aspects of tail and limb regeneration in lizards. A model system with implications for tissue regeneration in mammals. Adv. Anat. Embryol. Cell Biol. 2010, 207, 1–109. [Google Scholar]
- Knapp, D.; Tanaka, E.M. Regeneration and reprogramming. Curr. Opin. Genet. Dev. 2012, 22, 485–493. [Google Scholar] [CrossRef]
- Alibardi, L. Regeneration among animals: An evolutionary hypothesis related to aquatic versus terrestrial environment. Dev. Biol. 2023, 501, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Zuniga, A.; Zeller, R. Dynamic and self-regulatory interactions among gene regulatory networks control vertebrate limb bud morphogenesis. Curr. Top. Dev. Biol. 2020, 139, 61–88. [Google Scholar] [CrossRef] [PubMed]
- Murawala, P.; Tanaka, E.M.; Currie, J.D. Regeneration: The ultimate example of wound healing. Semin. Cell Dev. Biol. 2012, 23, 954–962. [Google Scholar] [CrossRef]
- Kawaguchi, A.; Wang, J.; Knapp, D.; Murawala, P.; Nowoshilow, S.; Masselink, W.; Taniguchi-Sugiura, Y.; Fei, J.-F.; Tanaka, E.M. A chromatin code for limb segment identity in axolotl limb regeneration. Dev. Cell 2024, 59, 2239–2253.e9. [Google Scholar] [CrossRef]
- Takeo, M.; Chou, W.C.; Sun, Q.; Lee, W.; Rabbani, P.; Loomis, C.; Taketo, M.M.; Ito, M. Wnt activation in nail epithelium couples nail growth to digit regeneration. Nature 2013, 499, 228–232. [Google Scholar] [CrossRef]
- Storer, M.A.; Miller, F.D. A finger on the pulse of regeneration: Insights into the cellular mechanisms of adult digit tip regeneration. Curr. Opin. Genet. Dev. 2021, 70, 1–6. [Google Scholar] [CrossRef]
- Leung, Y.; Kandyba, E.; Chen, Y.-B.; Ruffins, S.; Chuong, C.-M.; Kobielak, K. Bifunctional ectodermal stem cells around the nail display dual fate homeostasis and adaptive wounding response toward nail regeneration. Proc. Natl. Acad. Sci. USA 2014, 111, 15114–15119. [Google Scholar] [CrossRef]
- Yokoyama, H.; Kudo, N.; Todate, M.; Shimada, Y.; Suzuki, M.; Tamura, K. Skin regeneration of amphibians: A novel model for skin regeneration as adults. Dev. Growth Differ. 2018, 60, 316–325. [Google Scholar] [CrossRef]
- Sun, X.; Joost, S.; Kasper, M. Plasticity of Epithelial Cells during Skin Wound Healing. Cold Spring Harb. Perspect. Biol. 2023, 15, a041232. [Google Scholar] [CrossRef]
- Xue, Y.; Lim, C.H.; Plikus, M.V.; Ito, M.; Cotsarelis, G.; Garza, L.A. Wound-Induced Hair Neogenesis Model. J. Investig. Dermatol. 2022, 142, 2565–2569. [Google Scholar] [CrossRef]
- Wu, P.; Alibardi, L.; Chuong, C.-M. Regeneration of reptilian scales after wounding: Neogenesis, regional difference, and molecular modules. Regeneration 2014, 1, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Jahoda, C.A.; Horne, K.A.; Oliver, R.F. Induction of hair growth by implantation of cultured dermal papilla cells. Nature 1984, 311, 560–562. [Google Scholar] [CrossRef] [PubMed]
- Lillie, F.R.; Wang, H. Physiology of Development of the Feather V. Experimental Morphogenesis. 1941, 14, 103–135. [Google Scholar]
- Chuong, C.-M.; Yeh, C.-Y.; Jiang, T.-X.; Widelitz, R. Module-based complexity formation: Periodic patterning in feathers and hairs. Wiley Interdiscip. Rev. Dev. Biol. 2013, 2, 97–112. [Google Scholar] [CrossRef]
- Stenn, K.S.; Paus, R. Controls of hair follicle cycling. Physiol. Rev. 2001, 81, 449–494. [Google Scholar] [CrossRef] [PubMed]
- Panteleyev, A.A. Functional anatomy of the hair follicle: The Secondary Hair Germ. Exp. Dermatol. 2018, 27, 701–720. [Google Scholar] [CrossRef]
- Hsu, Y.-C.; Pasolli, H.A.; Fuchs, E. Dynamics between stem cells, niche, and progeny in the hair follicle. Cell 2011, 144, 92–105. [Google Scholar] [CrossRef]
- Greco, V.; Chen, T.; Rendl, M.; Schober, M.; Pasolli, H.A.; Stokes, N.; Dela Cruz-Racelis, J.; Fuchs, E. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell 2009, 4, 155–169. [Google Scholar] [CrossRef]
- Ito, M.; Kizawa, K.; Toyoda, M.; Morohashi, M. Label-retaining cells in the bulge region are directed to cell death after plucking, followed by healing from the surviving hair germ. J. Investig. Dermatol. 2002, 119, 1310–1316. [Google Scholar] [CrossRef]
- Alibardi, L.; Wu, P.; Chuong, C.-M. Ultrastructural characteristics of 5BrdU labeling retention cells including stem cells of regenerating feathers in chicken. J. Morphol. 2014, 275, 768–774. [Google Scholar] [CrossRef]
- Morris, R.J.; Liu, Y.; Marles, L.; Yang, Z.; Trempus, C.; Li, S.; Lin, J.S.; Sawicki, J.A.; Cotsarelis, G. Capturing and profiling adult hair follicle stem cells. Nat. Biotechnol. 2004, 22, 411–417. [Google Scholar] [CrossRef]
- Tumbar, T.; Guasch, G.; Greco, V.; Blanpain, C.; Lowry, W.E.; Rendl, M.; Fuchs, E. Defining the epithelial stem cell niche in skin. Science 2004, 303, 359–363. [Google Scholar] [CrossRef]
- Yue, Z.; Jiang, T.-X.; Widelitz, R.B.; Chuong, C.-M. Mapping stem cell activities in the feather follicle. Nature 2005, 438, 1026–1029. [Google Scholar] [CrossRef]
- Wu, P.; Jiang, T.-X.; Lei, M.; Chen, C.-K.; Hsieh Li, S.-M.; Widelitz, R.B.; Chuong, C.-M. Cyclic growth of dermal papilla and regeneration of follicular mesenchymal components during feather cycling. Development 2021, 148. [Google Scholar] [CrossRef]
- Xu, X.; Zheng, X.; You, H. Exceptional dinosaur fossils show ontogenetic development of early feathers. Nature 2010, 464, 1338–1341. [Google Scholar] [CrossRef]
- Chang, W.-L.; Wu, H.; Chiu, Y.-K.; Wang, S.; Jiang, T.-X.; Luo, Z.-L.; Lin, Y.-C.; Li, A.; Hsu, J.-T.; Huang, H.-L.; et al. The Making of a Flight Feather: Bio-architectural Principles and Adaptation. Cell 2019, 179, 1409–1423.e17. [Google Scholar] [CrossRef]
- Wang, S.; Chang, W.-L.; Zhang, Q.; Ma, M.; Yang, F.; Zhuo, D.; Hans, H.I.-C.; Yang, R.; Wu, P.; Habib, M.; et al. Variations of Mesozoic feathers: Insights from the morphogenesis of extant feather rachises. Evolution 2020, 74, 2121–2133. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, Z.; Dudley, R.; Mackem, S.; Chuong, C.-M.; Erickson, G.M.; Varricchio, D.J. An integrative approach to understanding bird origins. Science 2014, 346, 1253293. [Google Scholar] [CrossRef]
- Cohen, J.; Espinasse, P.G. On the normal and abnormal development of the feather. Journal of embryology and experimental morphology 1961, 9, 223–251. [Google Scholar]
- Lucas, A.M.; Stettenheim, P.R. Avian Anatomy Integument; US Department of Agriculture: Washington, DC, USA, 1972; Volume 362 PT. [Google Scholar]
- Yu, M.; Yue, Z.; Wu, P.; Wu, D.-Y.; Mayer, J.-A.; Medina, M.; Widelitz, R.B.; Jiang, T.-X.; Chuong, C.-M. The biology of feather follicles. Int. J. Dev. Biol. 2004, 48, 181–191. [Google Scholar] [CrossRef]
- Chuong, C.M.; Chen, H.M.; Jiang, T.X.; Chia, J. Adhesion molecules in skin development: Morphogenesis of feather and hair. Ann. N. Y. Acad. Sci. 1991, 642, 263–280. [Google Scholar] [CrossRef]
- Wu, P.; Lai, Y.-C.; Widelitz, R.; Chuong, C.-M. Comprehensive molecular and cellular studies suggest avian scutate scales are secondarily derived from feathers, and more distant from reptilian scales. Sci. Rep. 2018, 8, 16766. [Google Scholar] [CrossRef]
- Jones, P.H.; Harper, S.; Watt, F.M. Stem cell patterning and fate in human epidermis. Cell 1995, 80, 83–93. [Google Scholar] [CrossRef]
- Vidal, V.P.I.; Chaboissier, M.-C.; Lützkendorf, S.; Cotsarelis, G.; Mill, P.; Hui, C.-C.; Ortonne, N.; Ortonne, J.-P.; Schedl, A. Sox9 is essential for outer root sheath differentiation and the formation of the hair stem cell compartment. Curr. Biol. 2005, 15, 1340–1351. [Google Scholar] [CrossRef]
- Cotsarelis, G. Epithelial stem cells: A folliculocentric view. J. Investig. Dermatol. 2006, 126, 1459–1468. [Google Scholar] [CrossRef]
- Snippert, H.J.; Haegebarth, A.; Kasper, M.; Jaks, V.; van Es, J.H.; Barker, N.; van de Wetering, M.; van den Born, M.; Begthel, H.; Vries, R.G.; et al. Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science 2010, 327, 1385–1389. [Google Scholar] [CrossRef]
- Wu, P.; Wu, X.; Jiang, T.-X.; Elsey, R.M.; Temple, B.L.; Divers, S.J.; Glenn, T.C.; Yuan, K.; Chen, M.-H.; Widelitz, R.B.; et al. Specialized stem cell niche enables repetitive renewal of alligator teeth. Proc. Natl. Acad. Sci. USA 2013, 110, E2009-18. [Google Scholar] [CrossRef]
- Moore, G.P.; Jackson, N.; Isaacs, K.; Brown, G. Pattern and morphogenesis in skin. J. Theor. Biol. 1998, 191, 87–94. [Google Scholar] [CrossRef]
- Chuong, C.-M.; Widelitz, R.B. The river of stem cells. Cell Stem Cell 2009, 4, 100–102. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rompolas, P.; Mesa, K.R.; Kawaguchi, K.; Park, S.; Gonzalez, D.; Brown, S.; Boucher, J.; Klein, A.M.; Greco, V. Spatiotemporal coordination of stem cell commitment during epidermal homeostasis. Science 2016, 352, 1471–1474. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.; Jiang, T.-X.; Widelitz, R.B.; Chuong, C.-M. Wnt3a gradient converts radial to bilateral feather symmetry via topological arrangement of epithelia. Proc. Natl. Acad. Sci. USA 2006, 103, 951–955. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, T.-X.; Wu, P.; Li, A.; Widelitz, R.B.; Chuong, C.-M. Wound-Induced Regeneration in Feather Follicles: A Stepwise Strategy to Regenerate Stem Cells. J. Dev. Biol. 2025, 13, 10. https://doi.org/10.3390/jdb13020010
Jiang T-X, Wu P, Li A, Widelitz RB, Chuong C-M. Wound-Induced Regeneration in Feather Follicles: A Stepwise Strategy to Regenerate Stem Cells. Journal of Developmental Biology. 2025; 13(2):10. https://doi.org/10.3390/jdb13020010
Chicago/Turabian StyleJiang, Ting-Xin, Ping Wu, Ang Li, Randall B. Widelitz, and Cheng-Ming Chuong. 2025. "Wound-Induced Regeneration in Feather Follicles: A Stepwise Strategy to Regenerate Stem Cells" Journal of Developmental Biology 13, no. 2: 10. https://doi.org/10.3390/jdb13020010
APA StyleJiang, T.-X., Wu, P., Li, A., Widelitz, R. B., & Chuong, C.-M. (2025). Wound-Induced Regeneration in Feather Follicles: A Stepwise Strategy to Regenerate Stem Cells. Journal of Developmental Biology, 13(2), 10. https://doi.org/10.3390/jdb13020010





