The Story of the Finest Armor: Developmental Aspects of Reptile Skin
Abstract
1. Introduction
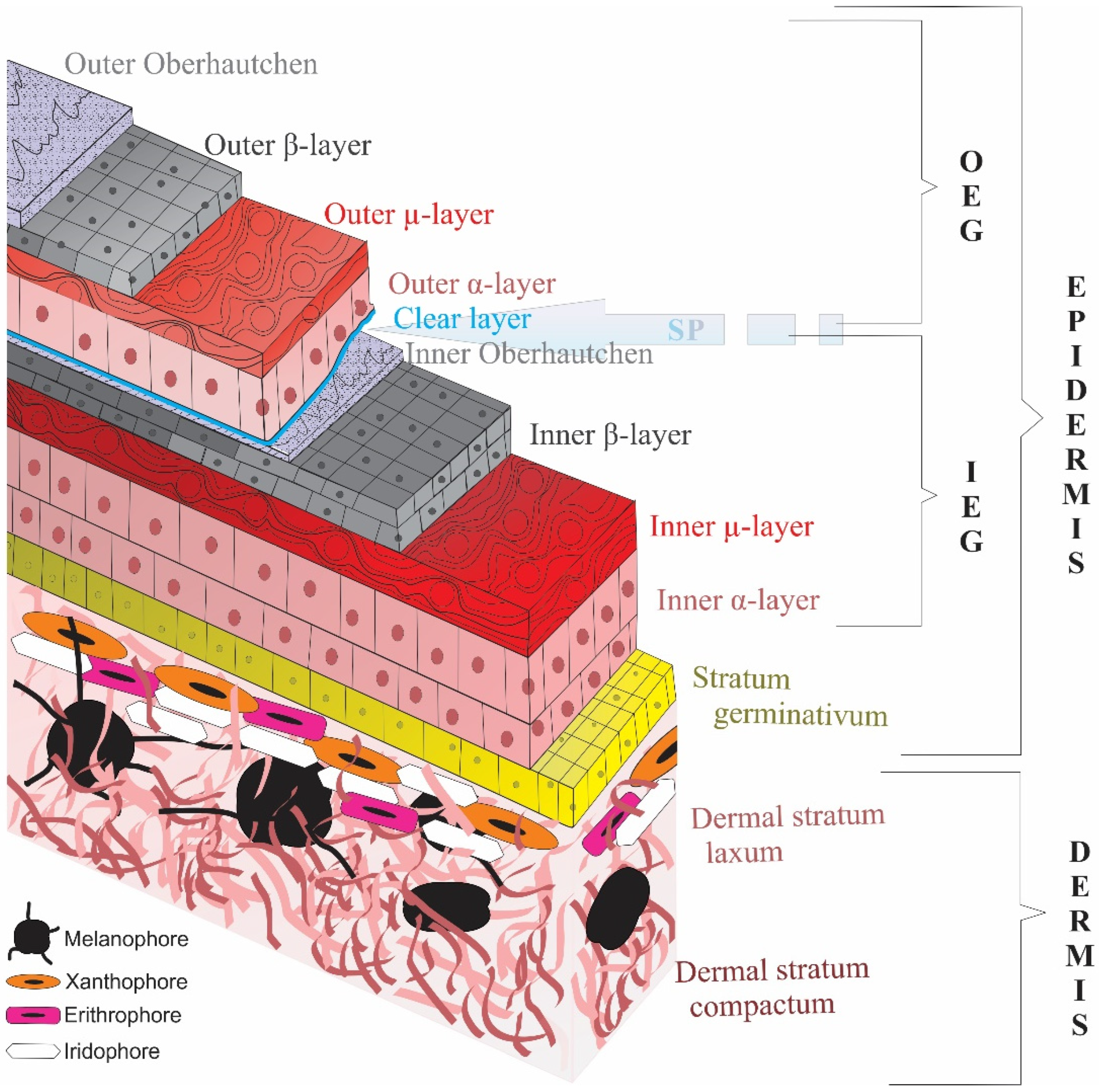
2. Evolution, the Armorer: The Cornified Epidermis
2.1. Making of the Armor: Proteins
2.2. Hardening the Armor: Cornification through Protein Interactions
3. Diversifying the Armor: Epidermal Appendages
3.1. Turtle Scutes
3.2. Turtle Beak (Rhamphotheca)
3.3. Lizard Setae
3.4. Claw
4. Armoring in Progress: Development of the Horny Epidermis
Renovating the Armor: Shedding Cycle
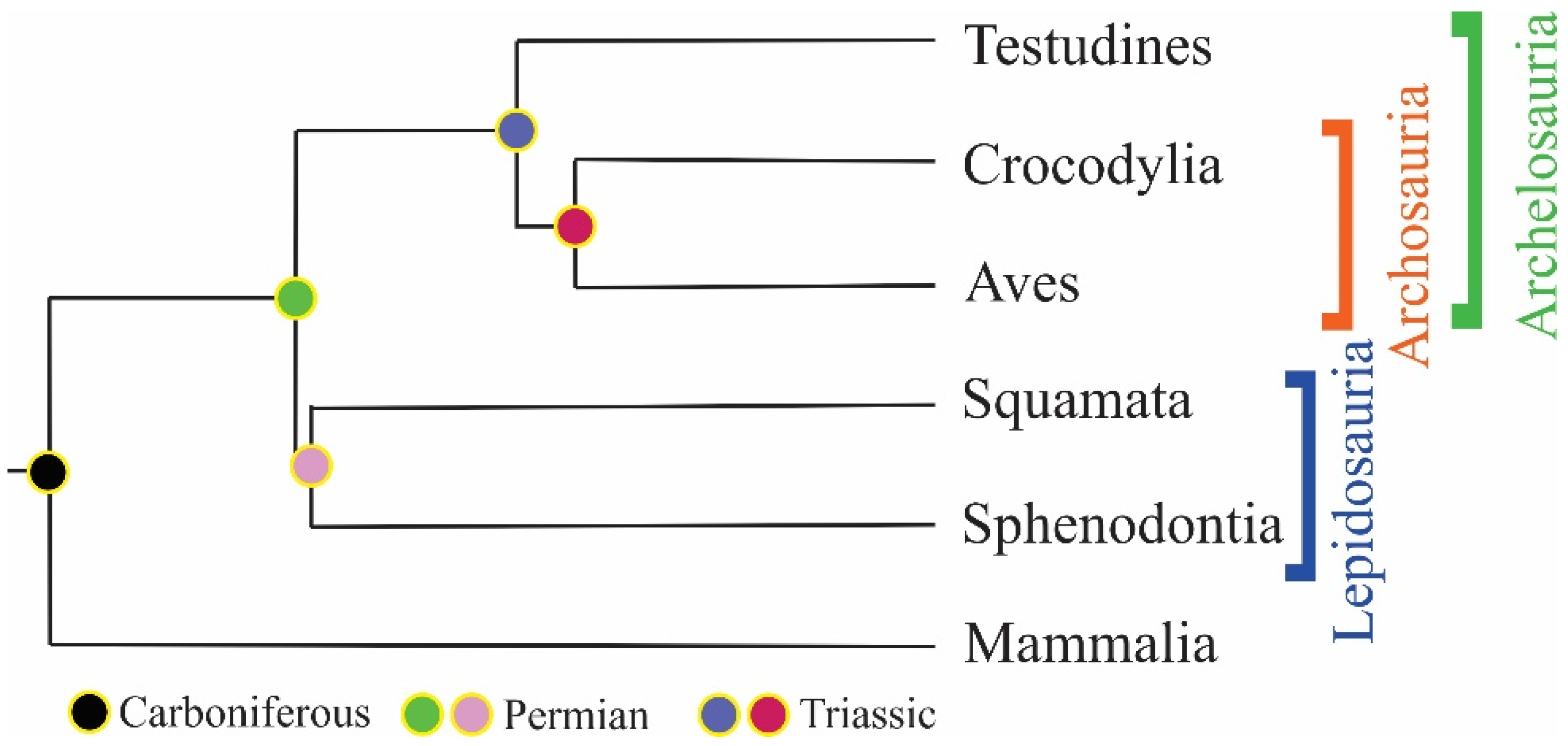
5. History of the Armor: Origin and Evolution
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eckhart, L.; Lippens, S.; Tschachler, E.; Declercq, W. Cell death by cornification. Biochim. Biophys. Acta 2013, 1833, 3471–3480. [Google Scholar] [CrossRef] [PubMed]
- Alibardi, L. Transition from embryonic to adult epidermis in reptiles occurs by the production of corneous beta-proteins. Int. J. Dev. Biol. 2014, 58, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Holthaus, K.B.; Eckhart, L.; Dalla Valle, L.; Alibardi, L. Review: Evolution and diversification of corneous beta-proteins, the characteristic epidermal proteins of reptiles and birds. J. Exp. Zool. 2020, 330, 438–453. [Google Scholar] [CrossRef] [PubMed]
- Akat, E.; Yenmiş, M.; Pombal, M.A.; Molist, P.; Megías, M.; Arman, S.; Veselỳ, M.; Anderson, R.; Ayaz, D. Comparison of vertebrate skin structure at class level: A review. Anat. Rec. 2022, 305, 3543–3608. [Google Scholar] [CrossRef] [PubMed]
- Shedlock, A.M.; Edwards, S.V. Amniotes (Amniota). In Timetree of Life; Hedges, S.B., Kumar, S., Eds.; Oxford University Press: New York, NY, USA, 2009; pp. 375–379. [Google Scholar]
- Crawford, N.G.; Parham, J.F.; Sellas, A.B.; Faircloth, B.C.; Glenn, T.C.; Papenfuss, T.J.; Henderson, J.B.; Hansen, M.H.; Simison, W.B. A phylogenomic analysis of turtles. Mol. Phylogenet. Evol. 2015, 83, 250–257. [Google Scholar] [CrossRef]
- Maderson, P.F. Some developmental problems of the reptilian integument. In Biology of the Reptilia, 1st ed.; Gans, C., Billett, F., Maderson, P.F.A., Eds.; John Wiley & Sons: New York, NY, USA, 1985; Volume 14, pp. 525–598. [Google Scholar]
- Landmann, L. The skin of Reptiles: Epidermis and dermis. In Biology of the Integument; Bereiter-Hahn, J., Matoltsy, A.G., Sylvia-Richards, K., Eds.; Springer Verlag: Berlin/Haidelberg, Germany; New York, NY, USA, 1986; Volume 2, pp. 150–187. [Google Scholar]
- Maderson, P.F.A.; Rabinowitz, T.; Tandler, B.; Alibardi, L. Ultrastructural contributions to an understanding of the cellular mechanisms involved in lizard skin shedding with comments on the function and evolution of a unique lepidosaurian phenomenon. J. Morphol. 1998, 236, 1–24. [Google Scholar] [CrossRef]
- Alibardi, L.; Maderson, P.F.A. Observations on the histochemistry and ultrastructure of the epidermis of the Tuatara, Sphenodon punctatus (Spehnodontida, Lepidosauri, Reptilis): A contribution to an understanding of the lepidosaurian epidermal generation and the evolutionary origin of the squamate shedding complex. J. Morphol. 2003, 256, 111–133. [Google Scholar] [PubMed]
- Sawyer, R.H.; Glenn, T.; French, J.O.; Mays, B.; Shames, R.B.; Barnes, G.L.; Rhodes, W.; Ishikawa, Y. The expression of beta (b) keratins in the epidermal appendages of reptiles and birds. Am. Zool. 2000, 40, 530–539. [Google Scholar] [CrossRef]
- Alibardi, L.; Toni, M. Cytochemical, biochemical and molecular aspects of the process of keratinization in the epidermis of reptilian scales. Prog. Histochem. Cytochem. 2006, 40, 73–134. [Google Scholar] [CrossRef]
- Alibardi, L. Structural and immunocytochemical characterization of keratinization in vertebrate epidermis and epidermal derivatives. Int. Rev. Cytol. 2006, 253, 177–259. [Google Scholar] [PubMed]
- Alibardi, L. Ultrastructural immunolocalization of alphakeratins and associated beta-proteins (beta-keratins) suggests a new interpretation on the process of hard and soft cornification in turtle epidermis. Micron 2013, 52, 8–15. [Google Scholar] [CrossRef]
- Fraser, R.D.; Parry, D.A. The role of b-sheets in the structure and assembly of keratins. Biophys. Rev. 2009, 1, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.C.; Buehler, M.J. Structure and mechanical properties of human trichocyte keratin intermediate filament protein. Biomacromolecules 2012, 13, 3522–3532. [Google Scholar] [CrossRef] [PubMed]
- Coulombe, P.A.; Omary, M.B. ‘Hard’ and ‘soft’ principles defining the structure, function and regulation of keratin intermediate filaments. Curr. Opin. Cell Biol. 2002, 14, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Alibardi, L.; Toni, M.; Dalla Valle, L. Review. Hard keratins in reptilian epidermis in comparison to those of birds and mammals. Exp. Dermatol. 2007, 16, 961–976. [Google Scholar] [CrossRef] [PubMed]
- Hallahan, D.L.; Keiper-Hrynko, N.M.; Shang, T.Q.; Ganzke, T.S.; Toni, M.; Dalla Valle, L.; Alibardi, L. Analysis of gene expression in gecko digital adhesive pads indicates significant production of cysteine- and glycine-rich beta-keratins. J. Exp. Zool. B 2008, 312, 58–73. [Google Scholar] [CrossRef]
- Alibardi, L. Review. The process of cornification evolved from the initial keratinization in the epidermis and epidermal derivatives of vertebrates: A new synthesis and the case of sauropsids. Int. Rev. Cell Mol. Biol. 2016, 327, 264–319. [Google Scholar]
- Alibardi, L. Keratinization and Cornification are not equivalent processes but keratinization in fish and amphibians evolved into cornification in terrestrial vertebrates. Exp. Dermatol. 2022, 31, 794–799. [Google Scholar] [CrossRef] [PubMed]
- Dalla Valle, L.; Nardi, A.; Bonazza, G.; Zuccal, C.; Emera, D.; Alibardi, L. Forty keratin-associated β–proteins (β-keratins) form the hard layers of scales, claws and adhesive pads in the green anole lizard, Anolis carolinensis. J. Exp. Zool. 2010, 314, 11–32. [Google Scholar] [CrossRef]
- Frazer, R.D.B.; Parry, D.A.D. The molecular structure of reptilian keratin. Int. J. Biol. Macromol. 1996, 19, 207–211. [Google Scholar] [CrossRef]
- Alibardi, L. Cornification in reptilian epidermis occurs through the deposition of keratin associated beta proteins (beta-keratins) onto a scaffold of intermediate filament keratins. J. Morphol. 2013, 274, 175–193. [Google Scholar] [CrossRef] [PubMed]
- Alibardi, L.; Toni, M. Beta keratins of reptilian epidermis share a conserved common epitope termed the core-box. Res. J. Biol. Sci. 2007, 2, 329–339. [Google Scholar]
- Strasser, B.; Mlitz, V.; Hermann, M.; Rice, R.H.; Eigenheer, R.A.; Alibardi, L.; Tschachler, E.; Eckhart, L. Evolutionary origin and diversification of epidermal barrier proteins in amniotes. Mol. Biol. Evol. 2014, 31, 3194–3205. [Google Scholar] [CrossRef]
- Calvaresi, M.; Eckhart, L.; Alibardi, L. The molecular organization of the beta-sheet region in corneous beta-proteins (beta-keratins) of sauropsids explains its stability and polymerization into filaments. J. Struct. Biol. 2016, 194, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Greenwold, M.J.; Sawyer, R.H. Molecular evolution and expression of archosaurian beta-keratins: Diversification and expansion of archosaurian beta-keratins and the origin of feather beta-keratins. J. Exp. Zool. B 2013, 320, 393–405. [Google Scholar] [CrossRef]
- Li, Y.I.; Kong, L.; Pontig, C.P.; Haerty, W. Rapid evolution of beta-keratin genes contributo to phenotypic differences that distinguish turtle and birds from other reptiles. Genome Biol. Evol. 2013, 5, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, Q.; Wang, Y.; Luo, L.; Yang, J.; Yang, L.; Liu, M.; Li, Y.; Qian, T.; Zheng, Y.; et al. Gekko japonicus genome reveals evolution of adhesive toe pads and tail regeneration. Nat. Commun. 2015, 6, 10033. [Google Scholar] [CrossRef] [PubMed]
- Mlitz, V.; Strasser, B.; Jaeger, K.; Hermann, M.; Ghannadan, M.; Buchberger, M.; Alibardi, L.; Tschachler, E.; Eckhart, L. Trichohyalin-like proteins have evolutionarily conserved roles in the morphogenesis of skin appendages. J. Investig. Dermatol. 2014, 134, 2685–2692. [Google Scholar] [CrossRef]
- Vandebergh, W.; Bossuyt, F. Radiation and functional diversification of alpha keratins during early vertebrate evolution. Mol. Biol. Evol. 2012, 29, 995–1004. [Google Scholar] [CrossRef]
- Ng, C.S.; Wu, P.; Fan, W.L.; Yan, J.; Chen, C.K.; Lai, Y.T.; Wu, S.M.; Mao, C.T.; Chen, J.J.; Ho, M.R.; et al. Genomic organization, transcriptomic analysis, and functional characterization of avian a- and b-keratins in diverse feather forms. Genome Biol. Evol. 2014, 6, 2258–2273. [Google Scholar] [CrossRef] [PubMed]
- Fraser, R.D.B.; Parry, D.A.D. The structural basis of the filament-matrix texture in the avian/reptilian group of hard beta-keratins. J. Struct. Biol. 2011, 173, 391–405. [Google Scholar] [CrossRef] [PubMed]
- Fraser, R.D.B.; Parry, D.A. Amino acid sequence homologies in the hard keratins of birds and reptiles, and their implications for molecular structure and physical properties. J. Struct. Biol. 2014, 188, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Alibardi, L. Review: Mapping epidermal beta-proteins distribution in the lizard Anolis carolinensis shows a specific localization for the formation of scales, pads and claws. Protoplasma 2015, 6, 1405–1420. [Google Scholar] [CrossRef] [PubMed]
- Candi, E.; Schmidt, R.; Melino, G. The cornified envelope: A model of cell death in the skin. Nat. Rev. Mol. Cell Biol. 2005, 6, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Lippens, S.; Denecker, G.; Ovaere, P.; Vandenabeele, P.; Declercq, W. Death penalty for keratinocytes: Apoptosis versus cornification. Cell Death Differ. 2005, 12, 1497–1508. [Google Scholar] [CrossRef] [PubMed]
- Dalla Valle, L.; Nardi, A.; Alibardi, L. Isolation of a new class of cysteine-glycineproline rich beta-proteins (beta-keratins) and their expression in snake epidermis. J. Anat. 2009, 216, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Dalla Valle, L.; Nardi, A.; Toni, M.; Emeera, D.; Alibardi, L. Beta-keratins of turtle shell are glycine-proline-tyrosine-rich proteins similar to those of crocodilians and birds. J. Anat. 2009, 214, 284–300. [Google Scholar] [CrossRef]
- Alibardi, L. Vertebrate keratinization evolved into cornification mainly due to transglutaminase and sulfhydryl oxidase activities on epidermal proteins: An immunohistochemical survey. Anat. Rec. 2021, 305, 333–358. [Google Scholar] [CrossRef]
- Baden, H.P.; Maderson, P.F. Morphological and biophysical identification of fibrous proteins in the amniote epidermis. J. Exp. Zool. 1970, 174, 225–232. [Google Scholar] [CrossRef]
- Alibardi, L. Corneous beta proteins of the epidermal differentiation complex (EDC) form large part of the corneous material of claws and rhamphothecae in turtles. Protoplasma 2020, 257, 1123–1138. [Google Scholar] [CrossRef]
- Alibardi, L. Cell proliferation, adhesion and differentiation of keratinocytes in the developing beak and egg-tooth of the turtle Emydura macquarii. Protoplasma 2020, 5, 1433–1445. [Google Scholar] [CrossRef] [PubMed]
- Murata, T.; Honda, T.; Mostafa, A.; Kabashima, K. Stratum corneum as polymer sheet: Concept and cornification processes. Trends Mol. Med. 2022, 28, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Alibardi, L. Development, structure, and protein composition of reptilian claws and hypotheses of their evolution. Anat. Rec. 2021, 304, 732–757. [Google Scholar] [CrossRef] [PubMed]
- Moustakas-Verho, J.E.; Cherepanov, G.O. The integumental appendages of the turtle shell: An evo-devo perspective. J. Exp. Zool. B 2015, 324, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Dalla Valle, L.; Michieli, F.; Benato, F.; Skobo, T.; Alibardi, L. Molecular characterization of alpha-keratins in comparison to associated beta-proteins in soft-shelled and hard-shelled turtles produced during the process of epidermal differentiation. J. Exp. Zool. B 2013, 320, 428–441. [Google Scholar] [CrossRef] [PubMed]
- Holthaus, K.B.; Strasser, B.; Sipos, W.; Schmidt, H.A.; Mlitz, V.; Sukseree, S.; Weissenbacher, A.; Tschachler, E.; Alibardi, L.; Eckhart, L. Comparative genomics identifies epidermal proteins associated with the evolution of the turtle shell. Mol. Biol. Evol. 2015, 33, 726–737. [Google Scholar] [CrossRef]
- Moustakas-Verho, J.E.; Zimm, R.; Cebra-Thomas, J.; Lempiainee, N.K.; Kallonen, A.; Mitchel, K.L.; Hamalainen, K.; Salazar-Ciudad, I.; Jernvall, J.; Gilbert, S.F. The origin and loss of periodic patterning in the turtle shell. Development 2014, 141, 3033–3039. [Google Scholar] [CrossRef]
- Alibardi, L.; Minelli, D. Sites of cell proliferation during scute morphogenesis in turtle and alligator are different from those of lepidosaurian scales. Acta Zool. 2015, 97, 127–141. [Google Scholar] [CrossRef]
- Cherepanov, G.O. Turtle Shell: Morphogenesis and Evolution; St Petersburg State University: St. Petersburg, Russia, 2005; p. 184. [Google Scholar]
- Cherepanov, G.O. Ontogenesis and evolution of horny parts of the turtle shell. Fossil Turtle Research. Suppl. Russ. J. Herpetol. 2006, 1, 19–33. [Google Scholar]
- Cherepanov, G.O. Patterns of scute development in turtle shell: Symmetry and asymmetry. Paleontol. J. 2014, 48, 1275–1283. [Google Scholar] [CrossRef]
- Milinchovitch, M.C.; Manukyan, L.; Debry, A.; Di-Poi, N.; Martin, S.; Sungh, D.; Lambert, D.; Zwicker, M. Crocodile head scales are not developmental units but emerge from physical cracking. Science 2012, 339, 78–81. [Google Scholar] [CrossRef]
- Wu, P.; Hou, L.; Plikus, M.; Hughes, M.; Scehnet, J.; Suksaweang, S.; Widelitz, R.; Jiang, T.X.; Chuong, C.M. Evo-Devo of amniote integuments and appendages. Int. J. Dev. Biol. 2004, 48, 249–270. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Jiang, T.X.; Shen, J.Y.; Widelitz, R.B.; Chuong, C.M. Morphoregulation of avian beaks: Comparative mapping of growth zone activities and morphological evolution. Dev. Dyn. 2006, 235, 1400–1412. [Google Scholar] [CrossRef] [PubMed]
- Alibardi, L. Development, comparative morphology and cornification of reptilian claws in relation to claws evolution in tetrapods. Contrib.Zool. 2009, 78, 25–42. [Google Scholar] [CrossRef]
- Alibardi, L. Embryonic keratinization in vertebrates in relation to land colonization. Acta Zool. 2009, 90, 1–17. [Google Scholar] [CrossRef]
- Alibardi, L. Microscopic and immunohistochemical study on the cornification of the developing beak in the turtle Emydura macquarii. J. Morphol. 2016, 277, 1309–1319. [Google Scholar] [CrossRef]
- Moldowan, P.; Litzgus, J.D.; Brooks, R.J. Turtles with teeth: Beak morphology of testudines with a focus on the tomiodonts of painted turtles (Chrysemys spp.). Zoomorphol. 2016, 135, 121–135. [Google Scholar] [CrossRef]
- Alibardi, L. Immunocytochemical observations on the cornification of soft and hard epidermis in the turtle Chrysemis picta. Zoology 2002, 105, 31–44. [Google Scholar] [CrossRef]
- Alibardi, L. Immunolocalization of beta-proteins and alpha-keratins in the epidermis of the soft-shelled turtle explains the lack of formation of hard corneous material. Acta Zool. 2014, 96, 218–224. [Google Scholar] [CrossRef]
- Alibardi, L. Immunocytochemistry suggests that a prevalence of a sub-type of beta-proteins determines the hardness in the epidermis of the hard-shelled turtle. J. Exp. Zool. B 2014, 322, 54–63. [Google Scholar] [CrossRef]
- Maderson, P.F.A. Lizard glands and lizard hands: Models for evolutionary study. Forma Functio 1970, 3, 179–204. [Google Scholar]
- Russel, A.P. Integrative functional morphology of the gekkotan adhesive system (Reptilia, Gekkota). Int. Comp. Biol. 2002, 42, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Toni, M.; Dalla Valle, L.; Alibardi, L. Review. Beta-keratins in the epidermis of reptiles: Composition, sequence and molecular organization. J. Proteome Res. 2007, 6, 3377–3392. [Google Scholar] [CrossRef] [PubMed]
- Alibardi, L. Adhesive pads of gecko and anoline lizards utilize corneous and cytoskeletal proteins for setae development and renewal. J. Exp. Zool. B 2020, 334, 263–279. [Google Scholar] [CrossRef]
- Baden, H.P.; Kvedar, J.C. The nail. In Physiology, Biochemistry and Molecular Biology of the Skin; Goldsmith, L.A., Ed.; Oxford University Press: New York, NY, USA; Oxford, UK, 1983; Volume 1, pp. 697–711. [Google Scholar]
- Gillespie, J.M. The structural proteins of hair: Isolation, characterization and regulation of biosynthesis. In Physiology, Biochemistry and Molecular Biology of the Skin; Goldsmith, L., Ed.; Oxford University Press: Oxford, UK, 1991; pp. 625–659. [Google Scholar]
- Rogers, G.E. Hair follicle differentiation and regulation. Int. J. Dev. Biol. 2004, 48, 163–170. [Google Scholar] [CrossRef]
- Alibardi, L.; Toni, M. Cytochemical and molecular characteristics of the process of cornification during feather morphogenesis. Prog. Histochem. Cytochem. 2008, 43, 1–69. [Google Scholar] [CrossRef]
- Alibardi, L.; Toni, M. Immunocytochemistry and protein analysis suggest that reptilian claws contain small high cysteine-glycine proteins. Tissue Cell 2009, 41, 180–192. [Google Scholar] [CrossRef]
- Alibardi, L. Microscopic analysis of lizard claw morphogenesis and hypothesis on its evolution. Acta Zool. 2008, 89, 169–178. [Google Scholar] [CrossRef]
- Alibardi, L. Autoradiographic observations on developing and growing claws of reptiles. Acta Zool. 2010, 91, 233–241. [Google Scholar] [CrossRef]
- Byrne, C.; Hardman, M.; Nield, K. Covering the limb—Formation of the integument. J. Anat. 2003, 202, 113–124. [Google Scholar] [CrossRef]
- Sawyer, R.H.; Knapp, L.W. Avian skin development and the evolutionary origin of feathers. J. Exp. Zool. B 2003, 298, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Alibardi, L. Adaptation to the land: The skin of reptiles in comparison to that of amphibians and endotherm amniotes. J. Exp. Zool. 2003, 298, 12–41. [Google Scholar] [CrossRef] [PubMed]
- Dhouailly, D.; Maderson, P.F.A. Ultrastructural observations on the embryonic development of the integument of Lacerta muralis (Lacertilia, Reptilia). J. Morphol. 1984, 179, 203–228. [Google Scholar] [CrossRef] [PubMed]
- Carver, W.E.; Sawyer, R.H. Development and keratinisation of the epidermis of the common lizard, Anolis carolinensis. J. Exp. Zool. 1987, 243, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Holthaus, K.B.; Mlitz, V.; Strasser, B.; Tschachler, E.; Alibardi, L.; Eckhart, L. Identification and comparative analysis of the epidermal differentiation complex in snakes. Sci. Rep. 2017, 7, 45338. [Google Scholar] [CrossRef] [PubMed]
- Richardson, K.C.; Park, J.Y.; Webb, J.W.; Manolis, S.C. Skin histology of embryonic and hatchling estuarine and australian freshwater crocodiles. In Crocodilian Biology and Evolution; Grigg, G., Seebacher, F., Franklin, C.E., Eds.; Surrey Beatty & Sons: Chipping Norton, NSW, Australia, 2000; pp. 188–196. [Google Scholar]
- Alibardi, L.; Thompson, M.B. Fine structure of the developing epidermis in the embryo of the American alligator (Alligator mississippiensis, Crocodilia, Reptilia). J. Anat. 2001, 198, 265–282. [Google Scholar] [CrossRef]
- Alibardi, L.; Dipietrangelo, L. Differentiation of the epidermis of scutes in embryos and juveniles of the tortoise Testudo hermanni with emphasis on beta-keratinization. Acta Zool. 2005, 86, 205–216. [Google Scholar] [CrossRef]
- Wang, Z.; Pascual-Anaya, J.; Zadissa, A.; Li, W.; Niimura, Y.; Huang, Z.; Li, C.; White, S.; Xiong, Z.; Fang, D.; et al. The draft genomes of soft-shell turtle and green sea turtle yield insights into the development and evolution of the turtle-specific body plan. Nat. Genet. 2013, 45, 701–706. [Google Scholar] [CrossRef]
- Holthaus, K.B.; Alibardi, L.; Tschachler, E.; Eckhart, L. Identification of epidermal differentiation genes of the tuatara provides insights into the early evolution of lepidosaurian skin. Sci. Rep. 2020, 10, 12844. [Google Scholar] [CrossRef] [PubMed]
- Dalla Valle, L.; Nardi, A.; Belvedere, P.; Toni, M.; Alibardi, L. Betakeratins of differentiating epidermis of snake comprise glycineproline- serine-rich proteins with an avian-like gene organization. Dev. Dynam. 2007, 236, 1939–1953. [Google Scholar] [CrossRef]
- Yenmiş, M. İki Agamid Kertenkelesinin Karşılaştırmalı Ekomorfolojik Analizi: Stellagama stellio ve Trapelus lessonae Popülasyonlarında Habitat Farklılıklarının Fenotipik Etkisi. Ph.D. Thesis, Ege University, İzmir, Turkey, 26 August 2022. [Google Scholar]
- Karabinos, A.; Riemer, D.; Erber, A.; Weber, K. Homologues of vertebrate type I, II and III intermediate filaments (IF) proteins in an invertebrate: The IF multigene family of the cephalocordate Branchiostoma. FEBS Lett. 1998, 437, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Schaffeld, M.; Schultess, J. Genes coding for intermediate filament proteins closely related to the hagfish “thread keratins (TK)” alpha and gamma also exist in lamprey, teleosts and amphibians. Exp. Cell Res. 2006, 312, 1447–1462. [Google Scholar] [CrossRef] [PubMed]
- Vanhoutteghem, A.; Djian, P.; Green, H. Ancient origin of the gene encoding involucrin, a precursor of the cross-linked envelope of epidermis and related epithelia. Proc. Natl. Acad. Sci. USA 2008, 105, 15481–15486. [Google Scholar] [CrossRef]
- Martin, L.D.; Czerkas, S.A. The fossil record of feather evolution in the mesozoic. Am. Zool. 2000, 40, 687–694. [Google Scholar] [CrossRef]
- Maddin, H.C.; Reisz, R.R. The morphology of the terminal phalanges in Permo- Carboniferous synapsids: An evolutionary perspective. Can. J. Earth Sci. 2007, 44, 267–274. [Google Scholar] [CrossRef]
- Eckhart, L.; Dalla Valle, L.; Jaeger, K.; Ballaun, C.; Szabo, S.; Nardi, A.; Buchberger, M.; Hermann, M.; Alibardi, L.; Tschachler, E. Identification of reptilian genes encoding hair keratin-like proteins suggests a new scenario for the evolutionary origin of hair. Proc. Natl. Acad. Sci. USA 2008, 105, 18419–18423. [Google Scholar] [CrossRef] [PubMed]
- Alibardi, L. Immunolocalization of keratin associated beta-proteins (beta-keratins) in the regenerating lizard epidermis indicates a new process for the differentiation of the epidermis in lepidosaurians. J. Morphol. 2012, 273, 1272–1279. [Google Scholar] [CrossRef]
- Alibardi, L.; Rogers, G. Observations on fur development in echidna (monotremata, Mammalia) indicate that spines precede hairs in ontogeny. Anat. Rec. 2015, 298, 761–770. [Google Scholar] [CrossRef]
- Greenwold, M.J.; Sawyer, R.H. Linking the molecular evolution of avian beta keratins to the evolution of feathers. J. Exp. Zool. 2011, 316, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Gregg, K.; Rogers, G. Feather keratins: Composition, structure and biogenesis. In Biology of the Integument, Vertebrates; Bereiter-Hahn, J., Ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1986; pp. 666–694. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yenmiş, M.; Ayaz, D. The Story of the Finest Armor: Developmental Aspects of Reptile Skin. J. Dev. Biol. 2023, 11, 5. https://doi.org/10.3390/jdb11010005
Yenmiş M, Ayaz D. The Story of the Finest Armor: Developmental Aspects of Reptile Skin. Journal of Developmental Biology. 2023; 11(1):5. https://doi.org/10.3390/jdb11010005
Chicago/Turabian StyleYenmiş, Melodi, and Dinçer Ayaz. 2023. "The Story of the Finest Armor: Developmental Aspects of Reptile Skin" Journal of Developmental Biology 11, no. 1: 5. https://doi.org/10.3390/jdb11010005
APA StyleYenmiş, M., & Ayaz, D. (2023). The Story of the Finest Armor: Developmental Aspects of Reptile Skin. Journal of Developmental Biology, 11(1), 5. https://doi.org/10.3390/jdb11010005





