Figure 1.
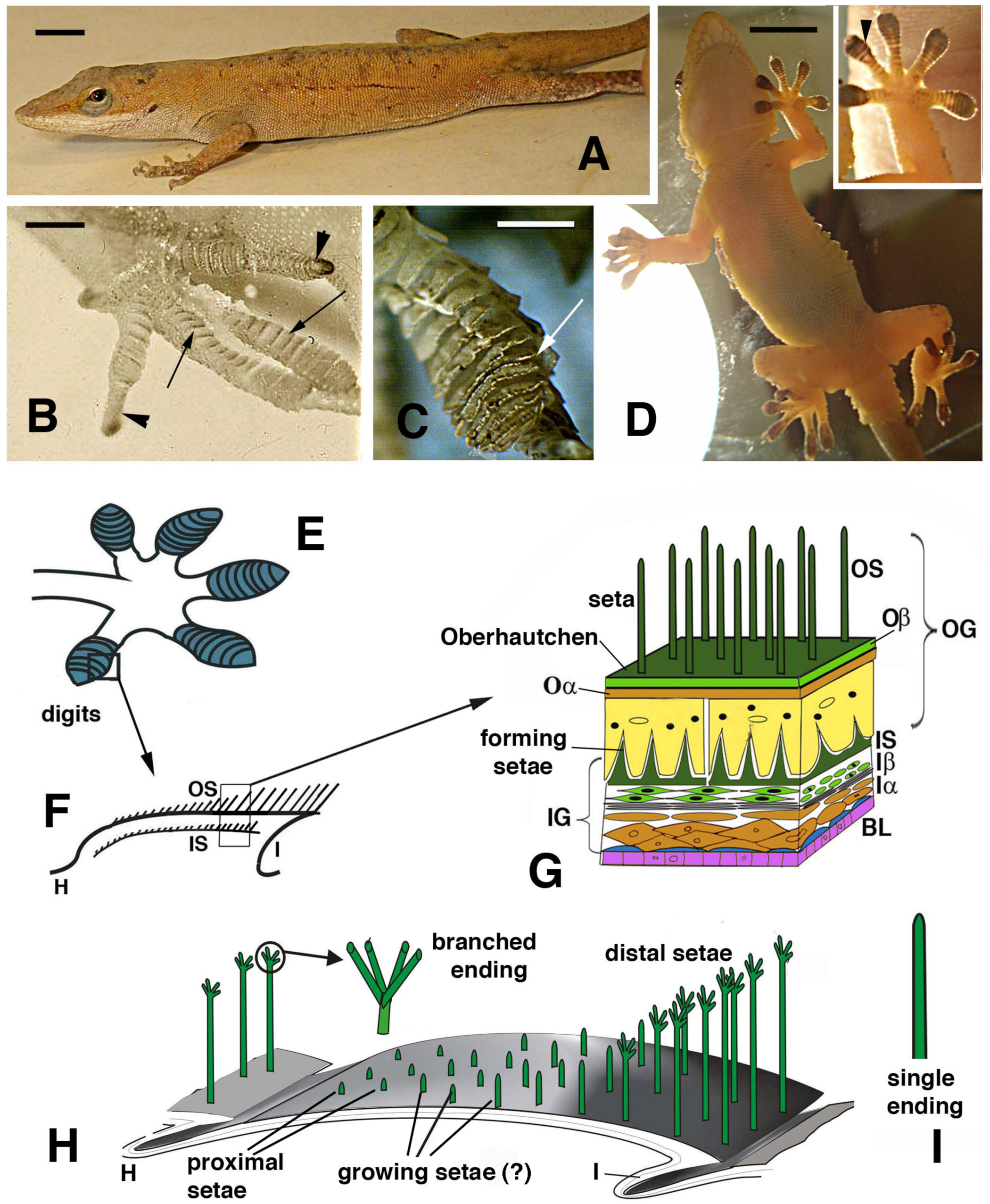
Lizards that can use digital adhesive pads (A–D) and schematic drawing of their histological and cellular structure (E–H). (A), Anolis carolinensis. Bar, 5 mm. (B), digits with developing lamellae (arrows) and claws (arrowheads) in an advanced embryo of A. lineatopus. Bar, 1 mm. (C), detail of overlapped lamellae (arrow) in adult A. carolinensis. Bar, 1 mm. (D), adult of Tarentola mauritanica climbing a glass wall as evidenced by a lamp-illuminated background. Bar, 10 mm. The inset details the adhesion of the hand pad lamellae (arrowhead) on the glass surface. (E), drawing of digits with pad lamellae (blue). (F), detail on the structure of a lamella sectioned longitudinally to show the two generations of setae: outer and the developing inner setae generation. (G), detail of the epidermal layers forming the outer and inner setae generations. (H), three-dimensional reconstruction of a pad lamella of geckos, evidencing the proximal–distal sequence of setae of different lengths (adhesion essentially occurs on the distal setae that do not overlap with the other lamellae). ? indicates that it is unknown whether setae growth on the outer setae takes place. The circle shows a magnification of the seta with its branching. (I), detail of an unbranched ending of a seta from A. carolinensis or A. lineatopus. Legend: BL, basal layer (in blue suprabasal cells); H, hinge (inter-scale) region; I, inner (ventral) scale/lamella surface; Iα, forming inner alpha-cells (differentiating); Iβ, forming inner beta-cells; IG, inner epidermal generation; IS, inner setae; Oα, outer alpha-layer; Oβ, outer beta-layer; OG, outer epidermal generation; OS, outer setae.
Figure 1.
Lizards that can use digital adhesive pads (A–D) and schematic drawing of their histological and cellular structure (E–H). (A), Anolis carolinensis. Bar, 5 mm. (B), digits with developing lamellae (arrows) and claws (arrowheads) in an advanced embryo of A. lineatopus. Bar, 1 mm. (C), detail of overlapped lamellae (arrow) in adult A. carolinensis. Bar, 1 mm. (D), adult of Tarentola mauritanica climbing a glass wall as evidenced by a lamp-illuminated background. Bar, 10 mm. The inset details the adhesion of the hand pad lamellae (arrowhead) on the glass surface. (E), drawing of digits with pad lamellae (blue). (F), detail on the structure of a lamella sectioned longitudinally to show the two generations of setae: outer and the developing inner setae generation. (G), detail of the epidermal layers forming the outer and inner setae generations. (H), three-dimensional reconstruction of a pad lamella of geckos, evidencing the proximal–distal sequence of setae of different lengths (adhesion essentially occurs on the distal setae that do not overlap with the other lamellae). ? indicates that it is unknown whether setae growth on the outer setae takes place. The circle shows a magnification of the seta with its branching. (I), detail of an unbranched ending of a seta from A. carolinensis or A. lineatopus. Legend: BL, basal layer (in blue suprabasal cells); H, hinge (inter-scale) region; I, inner (ventral) scale/lamella surface; Iα, forming inner alpha-cells (differentiating); Iβ, forming inner beta-cells; IG, inner epidermal generation; IS, inner setae; Oα, outer alpha-layer; Oβ, outer beta-layer; OG, outer epidermal generation; OS, outer setae.
![Jdb 11 00003 g001 Jdb 11 00003 g001]()
Figure 2.
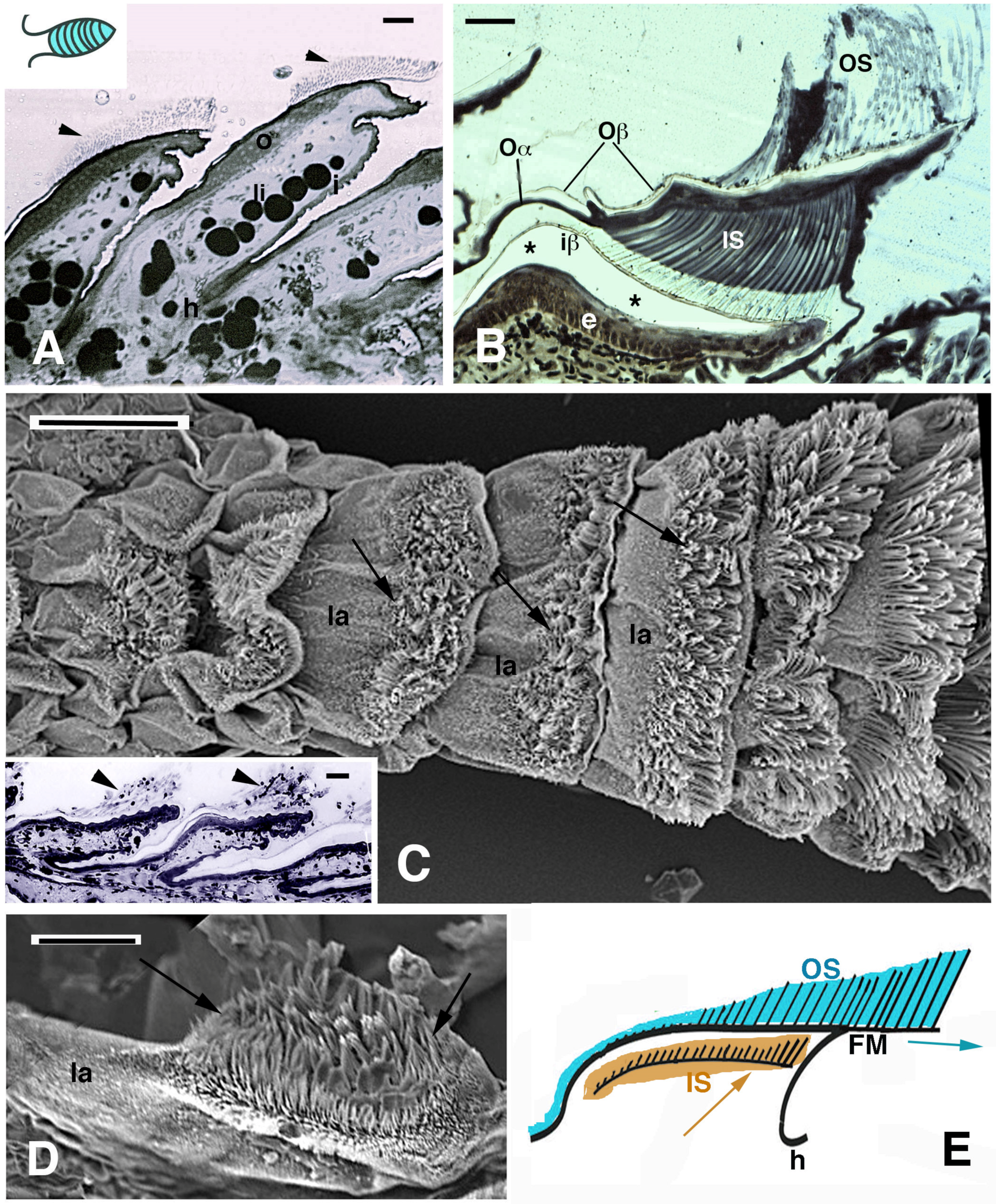
Histology (A,B) and SEM view (C–E) of digital lamellae in geckos. (A), longitudinal section of Phelsuma dubia showing a group of setae at the tip of the lamellae (arrowheads). Bar, 20 μm. (B), pad lamella of Hemidactylus turcicus featuring the outer and inner setae generations. Asterisks indicate an artifact detachment of the inner Oberhautchen-beta-layer from the epidermis. Bar, 20 μm. (C), SEM view of the ventral side of a digit in Tarentola mauritanica. Note the apical localization of the setae (arrows) in the lamellae. The longer setae are located in the distal lamellae. Bar, 200 μm. In the inset (Bar, 20 μm), the apical localization of setae (arrowheads) is evident in Anolis carolinensis lamellae. (D), apical collection of setae (arrows) in an isolated pad lamella of H. turcicus otherwise covered with spinulae. Bar, 50 μm. (E), schematic drawing of lamella with outer and inner generation of setae. The arrows indicate the presumed direction of shifting of the outer and inner setae. Legend: e, epidermis; FM, free margin (apical part of the Oberhautchen-beta-layer sustaining the apical outer setae); h, hinge (inter-scale/lamella) region; i, inner lamella surface; iβ, inner Oberhautchen-beta-layer; IS, inner setae generations; la, pad lamella; li, lipid droplets (cells); o, outer lamella surface; Oα, outer alpha-layer; Oβ, outer Oberhautchen-beta-layer; OS, outer setae generation.
Figure 2.
Histology (A,B) and SEM view (C–E) of digital lamellae in geckos. (A), longitudinal section of Phelsuma dubia showing a group of setae at the tip of the lamellae (arrowheads). Bar, 20 μm. (B), pad lamella of Hemidactylus turcicus featuring the outer and inner setae generations. Asterisks indicate an artifact detachment of the inner Oberhautchen-beta-layer from the epidermis. Bar, 20 μm. (C), SEM view of the ventral side of a digit in Tarentola mauritanica. Note the apical localization of the setae (arrows) in the lamellae. The longer setae are located in the distal lamellae. Bar, 200 μm. In the inset (Bar, 20 μm), the apical localization of setae (arrowheads) is evident in Anolis carolinensis lamellae. (D), apical collection of setae (arrows) in an isolated pad lamella of H. turcicus otherwise covered with spinulae. Bar, 50 μm. (E), schematic drawing of lamella with outer and inner generation of setae. The arrows indicate the presumed direction of shifting of the outer and inner setae. Legend: e, epidermis; FM, free margin (apical part of the Oberhautchen-beta-layer sustaining the apical outer setae); h, hinge (inter-scale/lamella) region; i, inner lamella surface; iβ, inner Oberhautchen-beta-layer; IS, inner setae generations; la, pad lamella; li, lipid droplets (cells); o, outer lamella surface; Oα, outer alpha-layer; Oβ, outer Oberhautchen-beta-layer; OS, outer setae generation.
![Jdb 11 00003 g002 Jdb 11 00003 g002]()
Figure 3.
Histology of developing pad lamellae in Anolis lineatopus after tritiated thymidine autoradiography ((Thy*), (A,B,E,F)) or 5BrdU immunolabeling ((5Br), (C)). Toluidine blue and low-stained sections. Bars, 10 μm in all images. (A), initial formation of a lamella 4 h post-injection (arrowhead on labeled nuclei). (B), elongating lamellae 2 days post-injection (arrowheads on some labeled cells). (C), labeled nuclei in the basal (arrows) and periderm (arrowheads) at 4 days post-injection. (D), stained section showing the forming Oberhautchen (arrow) and the initial setae underneath the granular (clear) layer (arrowheads). (E), elongating lamellae with labeled cells (arrowheads) in the basal layer 2 days post-injection. Arrows indicate melanophores. (F), lamellae with developed setae 2 days post-injection of tritiated thymidine with labeled cells (arrowheads) visible in the basal layer (tangentially cut). Dashes outline the epidermis. Large granules (arrows) are present in the clear layer. (G), well-developed lamella (late embryo) evidencing the formation of the free margin (arrowhead) at the tip. Arrows indicate cells of the corneous clear layer. Dashes outline the epidermis. Legend: β, beta-layer/cells; cl, clear (granulated) layer; de, dermis; h, forming hinge (inter-scale) region; oβ, Oberhautchen-beta-layer; se, setae; t, lamella tip.
Figure 3.
Histology of developing pad lamellae in Anolis lineatopus after tritiated thymidine autoradiography ((Thy*), (A,B,E,F)) or 5BrdU immunolabeling ((5Br), (C)). Toluidine blue and low-stained sections. Bars, 10 μm in all images. (A), initial formation of a lamella 4 h post-injection (arrowhead on labeled nuclei). (B), elongating lamellae 2 days post-injection (arrowheads on some labeled cells). (C), labeled nuclei in the basal (arrows) and periderm (arrowheads) at 4 days post-injection. (D), stained section showing the forming Oberhautchen (arrow) and the initial setae underneath the granular (clear) layer (arrowheads). (E), elongating lamellae with labeled cells (arrowheads) in the basal layer 2 days post-injection. Arrows indicate melanophores. (F), lamellae with developed setae 2 days post-injection of tritiated thymidine with labeled cells (arrowheads) visible in the basal layer (tangentially cut). Dashes outline the epidermis. Large granules (arrows) are present in the clear layer. (G), well-developed lamella (late embryo) evidencing the formation of the free margin (arrowhead) at the tip. Arrows indicate cells of the corneous clear layer. Dashes outline the epidermis. Legend: β, beta-layer/cells; cl, clear (granulated) layer; de, dermis; h, forming hinge (inter-scale) region; oβ, Oberhautchen-beta-layer; se, setae; t, lamella tip.
![Jdb 11 00003 g003 Jdb 11 00003 g003]()
Figure 4.
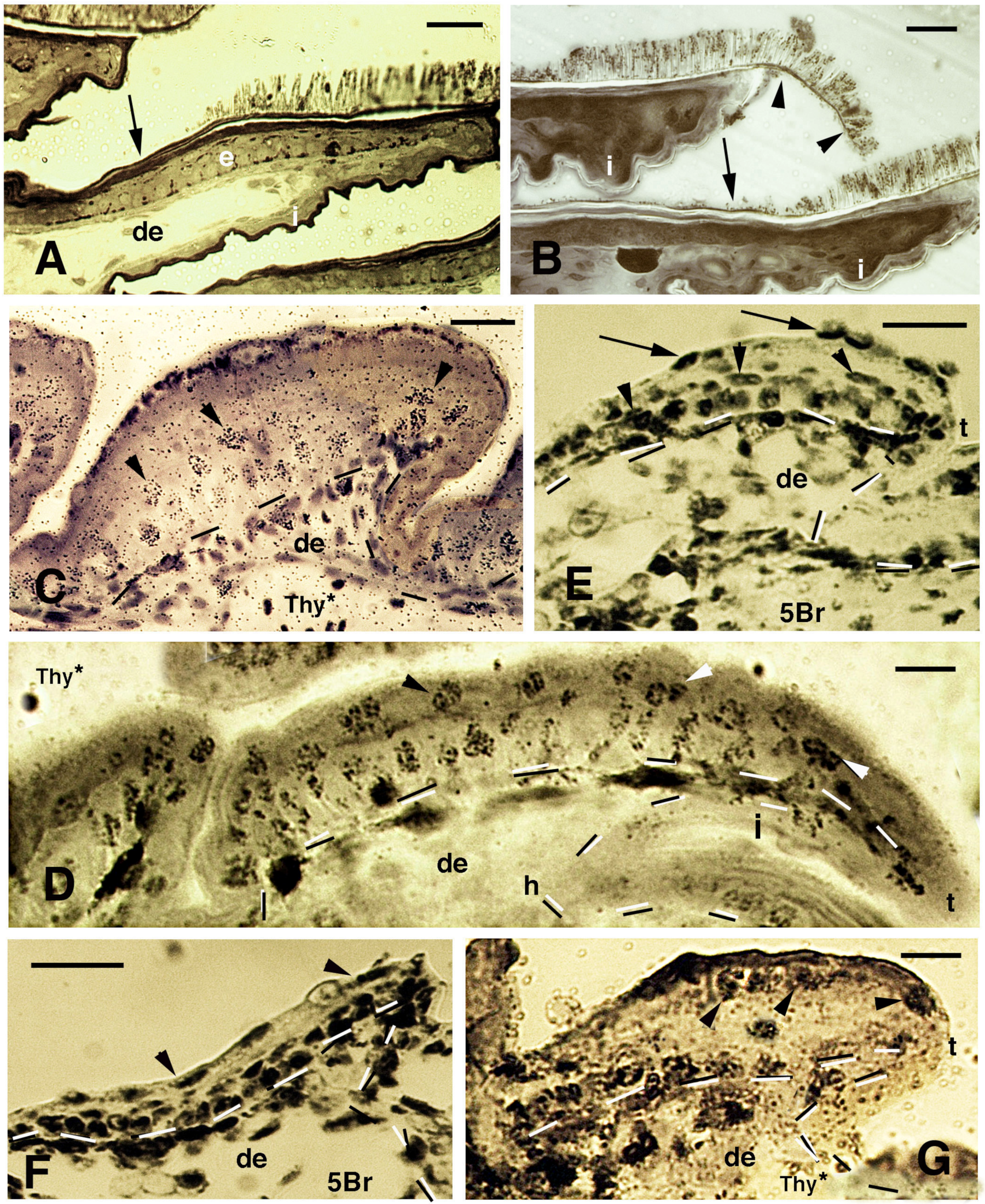
Histology of pads (A,B), sections after thymidine autoradiography ((Thy*), (C,D,G)) and after 5BrdU-immunohistochemistry ((5Br), (E,F)) in embryos of A. lineatopus. (A), pad with setae in the digit of a hatchling A. lineatopus. Setae are absent in the proximal part of the lamella (arrow) that is covered from the overlying lamella. Bar, 20 μm. (B), downfolded flexible free margin with distal setae (arrowheads) in the lamella of adult A. carolinensis. The arrow indicates lack of setae. Bar, 10 μm. (C), developing lamella of A. lineatopus 4 days post-injection showing basal and suprabasal (arrowheads) labeled cells. Bar, 20 μm. (D), elongating lamella (early stage) showing numerous labeled cells in most layers at 6–7 days post-injection, especially in the outer surface. Arrowheads indicate labeling in cells of the forming Oberhautchen. Bar, 10 μm. (E), short lamella located at the base (proximal part) of a digit, showing numerous labeled nuclei in the basal, suprabasal (arrowheads) and periderm (arrows) layers at 6–7 days post-injection. Bar, 20 μm. (F), other elongating lamella with most epidermal cells labeled at 4 days post-injection, including the periderm (arrowheads). Bar, 20 μm. (G), short proximal lamella with labeled cells in the basal layer and in clear cells (arrowheads) at 4 days post-injection. Bar, 10 μm. Legend: de, dermis; e, epidermis; i, inner surface of the lamella; t, tip of the lamella. Dashes outline the epidermis.
Figure 4.
Histology of pads (A,B), sections after thymidine autoradiography ((Thy*), (C,D,G)) and after 5BrdU-immunohistochemistry ((5Br), (E,F)) in embryos of A. lineatopus. (A), pad with setae in the digit of a hatchling A. lineatopus. Setae are absent in the proximal part of the lamella (arrow) that is covered from the overlying lamella. Bar, 20 μm. (B), downfolded flexible free margin with distal setae (arrowheads) in the lamella of adult A. carolinensis. The arrow indicates lack of setae. Bar, 10 μm. (C), developing lamella of A. lineatopus 4 days post-injection showing basal and suprabasal (arrowheads) labeled cells. Bar, 20 μm. (D), elongating lamella (early stage) showing numerous labeled cells in most layers at 6–7 days post-injection, especially in the outer surface. Arrowheads indicate labeling in cells of the forming Oberhautchen. Bar, 10 μm. (E), short lamella located at the base (proximal part) of a digit, showing numerous labeled nuclei in the basal, suprabasal (arrowheads) and periderm (arrows) layers at 6–7 days post-injection. Bar, 20 μm. (F), other elongating lamella with most epidermal cells labeled at 4 days post-injection, including the periderm (arrowheads). Bar, 20 μm. (G), short proximal lamella with labeled cells in the basal layer and in clear cells (arrowheads) at 4 days post-injection. Bar, 10 μm. Legend: de, dermis; e, epidermis; i, inner surface of the lamella; t, tip of the lamella. Dashes outline the epidermis.
![Jdb 11 00003 g004 Jdb 11 00003 g004]()
Figure 5.
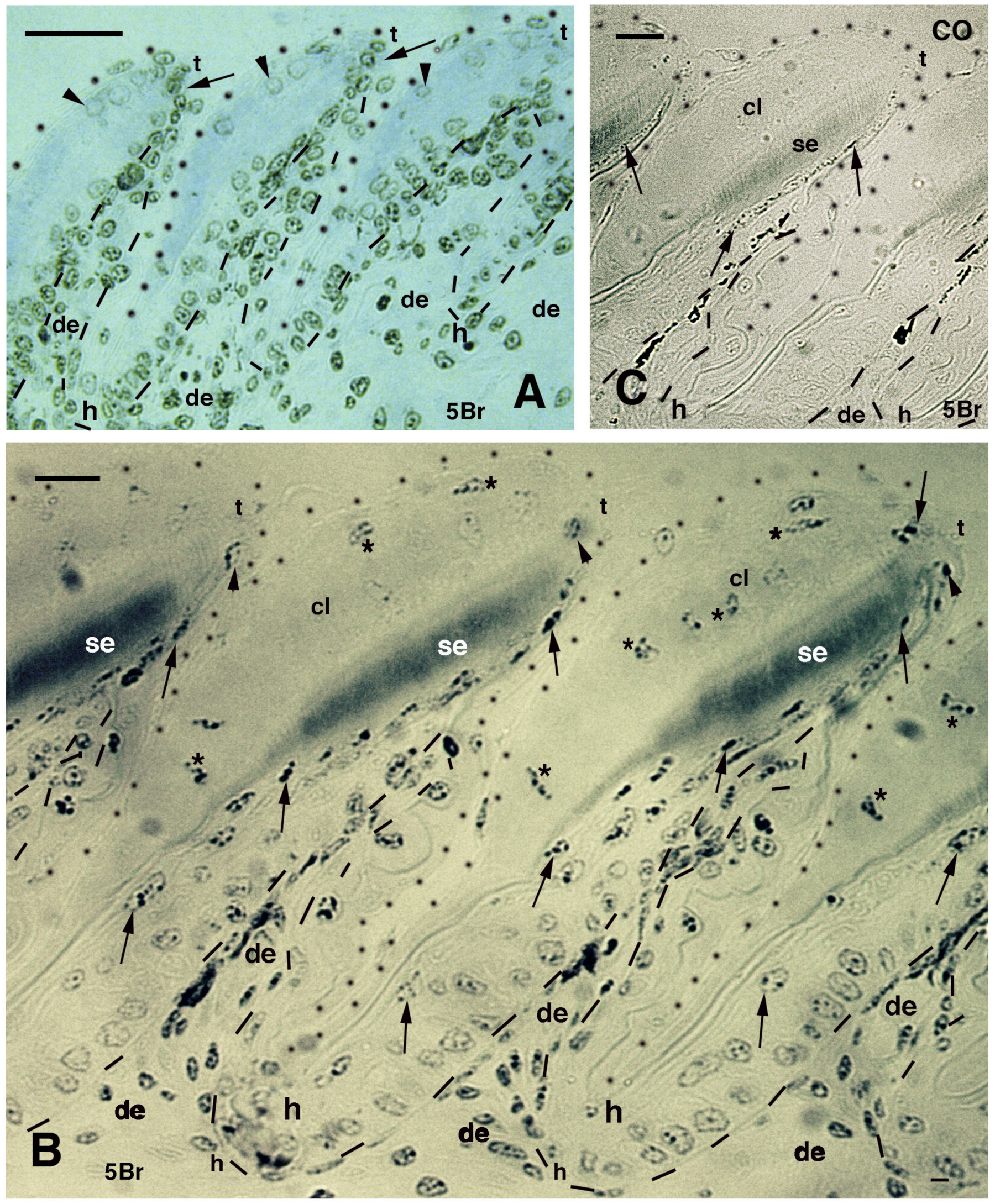
Immunolabeled pad lamellae of A. lineatopus at 8 days post-injection of 5BrdU (5Br). (A), early developing lamella with most labeled nuclei in the epidermis and dermis. Arrows indicate the relatively higher labeled nuclei by the tip of lamellae. Arrowheads point to labeled clear cell nuclei. Bar, 20 μm. (B), detail of labeled cells in the forming beta-layer (arrows pointing to flat nuclei). At the tip of the lamellae, labeled Oberhautchen cells are present (arrowheads). Asterisks indicate labeled nuclei of clear cells. Bar, 10 μm. (C), a control section does not show any labeled nuclei aside from finer grains of pigments (arrows). Bar, 10 μm. Legend: cl, clear layer; de, dermis; h, hinge (inter-scale) region; se, setae; t, tip (of the pad lamella). Dashes outline the epidermis. Dots outline most of the closely lined pad lamellae.
Figure 5.
Immunolabeled pad lamellae of A. lineatopus at 8 days post-injection of 5BrdU (5Br). (A), early developing lamella with most labeled nuclei in the epidermis and dermis. Arrows indicate the relatively higher labeled nuclei by the tip of lamellae. Arrowheads point to labeled clear cell nuclei. Bar, 20 μm. (B), detail of labeled cells in the forming beta-layer (arrows pointing to flat nuclei). At the tip of the lamellae, labeled Oberhautchen cells are present (arrowheads). Asterisks indicate labeled nuclei of clear cells. Bar, 10 μm. (C), a control section does not show any labeled nuclei aside from finer grains of pigments (arrows). Bar, 10 μm. Legend: cl, clear layer; de, dermis; h, hinge (inter-scale) region; se, setae; t, tip (of the pad lamella). Dashes outline the epidermis. Dots outline most of the closely lined pad lamellae.
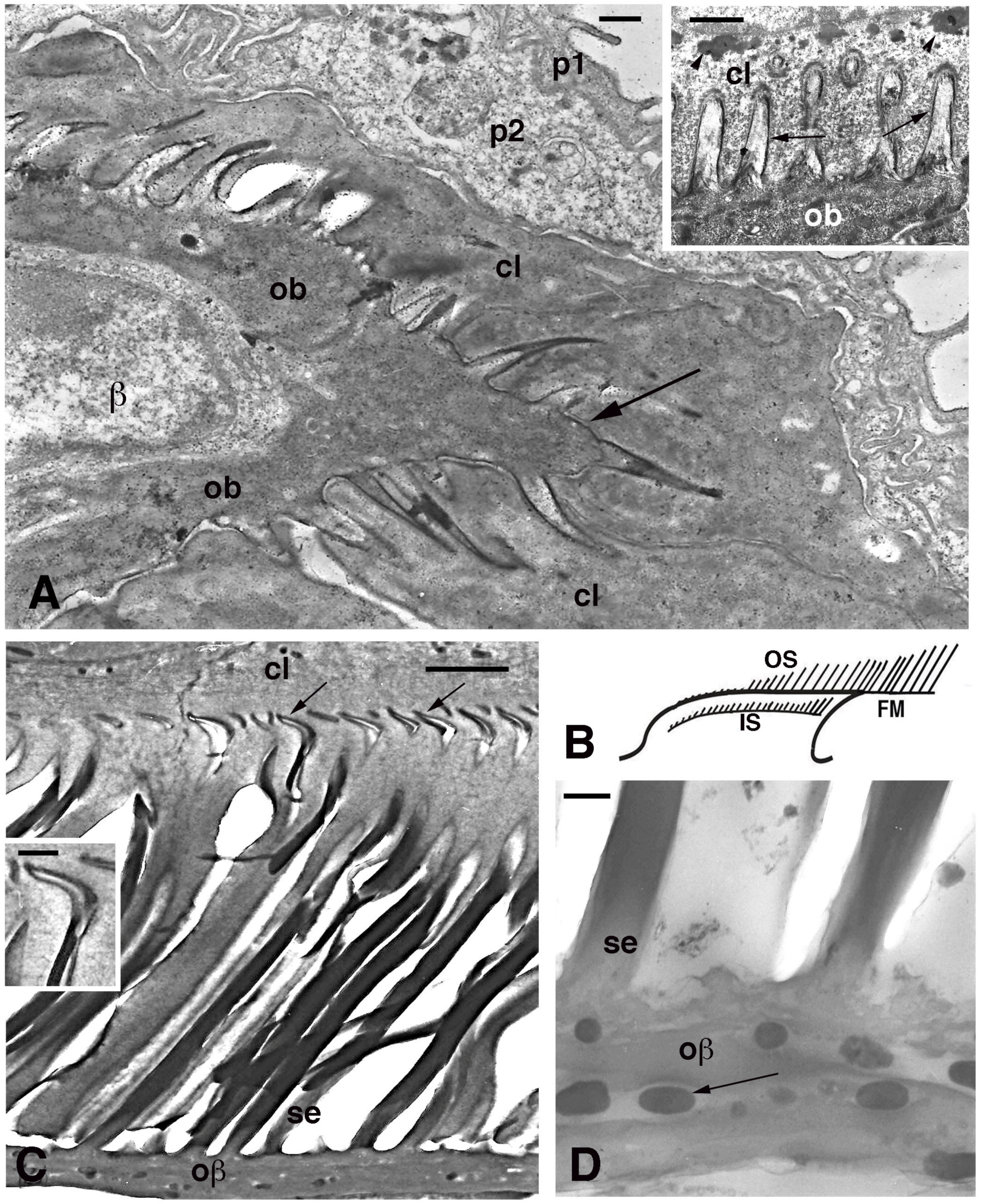
Figure 6.
Electron microscopy of seta formation in late embryo of Anolis lineatopus (A) and adult of A. carolinensis (C,D). (A), tip of lamella with apical spinulae forming a rostrum that will produce the free margin (arrow) at a later stage. Bar, 0.5 μm. The inset (Bar, 0.5 μm) shows the initial formation of embryonic setae (arrows) penetrating the clear layer (arrowheads on keratohyalin-like granules). (B), schematic drawing illustrating the outer (OS) and inner (IS) setae generations of a mature lamella during the renewal phase. (C), detail on inner setae (refers to IS in (B)) that are almost mature with their terminal spatulae (arrows). Bar, 1 μm. The inset (Bar, 200 nm) shows the curved shape of the spatula. (D), detail on melanosomes (arrows) included within the Oberhautchen-beta-layer of the free margin (refers to FM in (B)). Bar, 0.5 μm. Legend: β, beta-cell (immature); cl, clear layer; IS, inner setae; ob, Oberhautchen cell; oβ, Oberhautchen merged with the beta-layer; OS, outer setae; p1, outer periderm; p2, inner periderm; se, setae.
Figure 6.
Electron microscopy of seta formation in late embryo of Anolis lineatopus (A) and adult of A. carolinensis (C,D). (A), tip of lamella with apical spinulae forming a rostrum that will produce the free margin (arrow) at a later stage. Bar, 0.5 μm. The inset (Bar, 0.5 μm) shows the initial formation of embryonic setae (arrows) penetrating the clear layer (arrowheads on keratohyalin-like granules). (B), schematic drawing illustrating the outer (OS) and inner (IS) setae generations of a mature lamella during the renewal phase. (C), detail on inner setae (refers to IS in (B)) that are almost mature with their terminal spatulae (arrows). Bar, 1 μm. The inset (Bar, 200 nm) shows the curved shape of the spatula. (D), detail on melanosomes (arrows) included within the Oberhautchen-beta-layer of the free margin (refers to FM in (B)). Bar, 0.5 μm. Legend: β, beta-cell (immature); cl, clear layer; IS, inner setae; ob, Oberhautchen cell; oβ, Oberhautchen merged with the beta-layer; OS, outer setae; p1, outer periderm; p2, inner periderm; se, setae.
![Jdb 11 00003 g006 Jdb 11 00003 g006]()
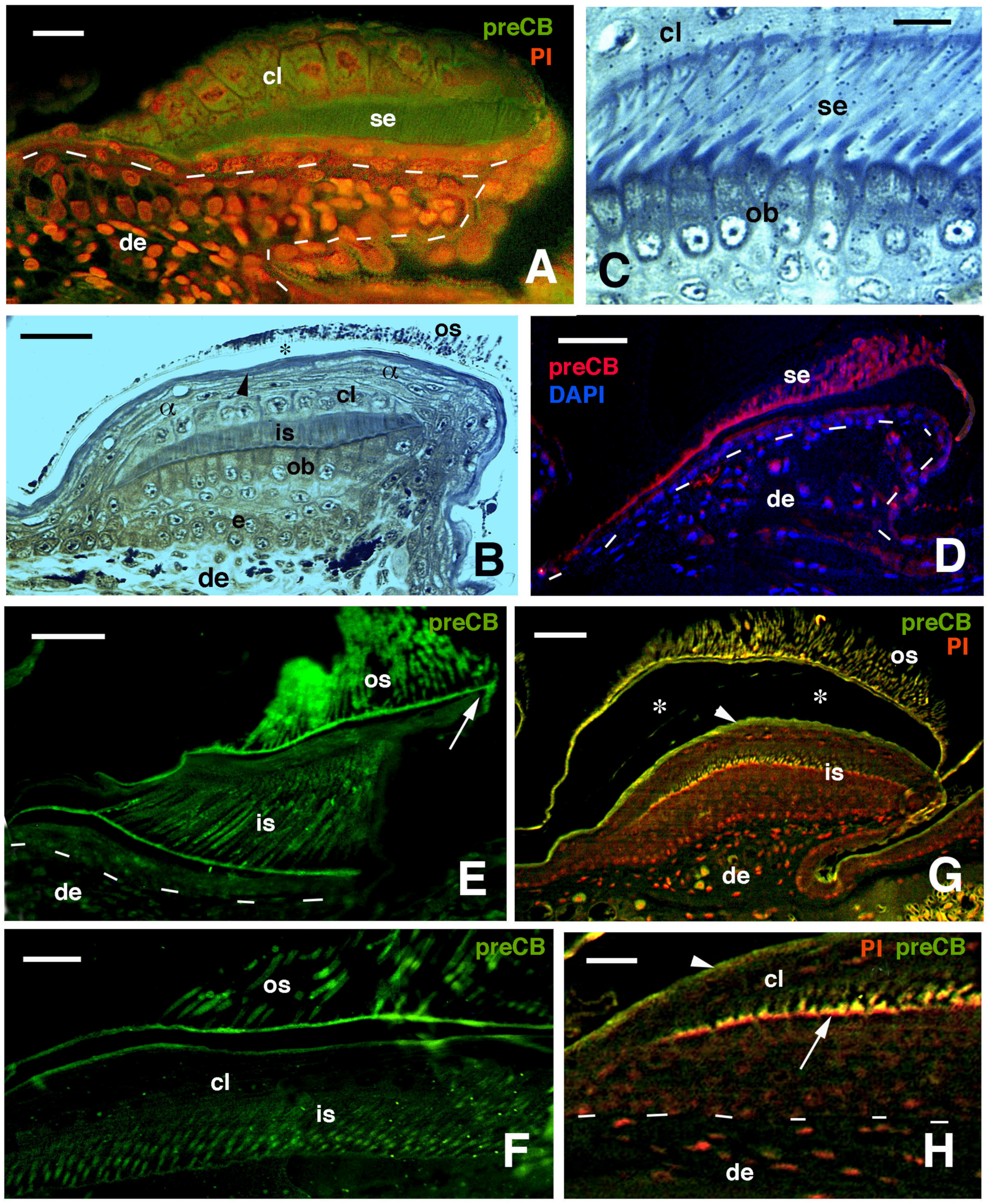
Figure 7.
Immunofluorescence for CBPs (corneous beta proteins) using the pre-core box antibody (preCB) on pad lamellae of lizards. (A), pad lamella of late embryo of A. lineatopus with immunolabeled setae (green) and nuclei fluorescent (red) with propidium iodide (PI). Dashes underline the epidermis. Bar, 10 μm. (B), longitudinal and tangential section of lamella of H. turcicus showing numerous epidermal layers. The arrowhead indicates the alpha-layer. The asterisk indicates artifact separation (due to sectioning) between outer setae and the alpha-layer. Toluidine blue stain. Bar, 20 μm. (C), detail on the shedding complex of T. mauritanica with forming setae derived from Oberhautchen cells. Toluidine blue stain. Bar, 10 μm. (D), immunolabeled setae (red) and nuclei with DAPI (blue) in lamella of T. mauritanica. Dashes underline the epidermis. Bar, 20 μm. (E), immunolabeled (green) outer corneous layer and setae and inner corneous layer with its setae in H. turcicus. The arrow points to the immunofluorescent free margin. Bar, 20 μm. (F), immunolabeled setae and outer corneous layer with setae in T. mauritanica. Bar, 10 μm. (G), double labeling in outer and early-forming inner setae of T. mauritanica. The arrowhead indicates the alpha-layer and the asterisks the artifactual split of the epidermis under sectioning. Bar, 20 μm. (H), close-up view of inner setae (arrow) at an early phase of their growth. The arrowhead indicates the alpha-layer. Dashes underline the epidermis. Bar, 10 μm. Legend: α, alpha-layer; cl, clear layer; de, dermis; e, epidermis; is, inner setae; ob, Oberhautchen-beta-layer; os, outer setae; se, setae.
Figure 7.
Immunofluorescence for CBPs (corneous beta proteins) using the pre-core box antibody (preCB) on pad lamellae of lizards. (A), pad lamella of late embryo of A. lineatopus with immunolabeled setae (green) and nuclei fluorescent (red) with propidium iodide (PI). Dashes underline the epidermis. Bar, 10 μm. (B), longitudinal and tangential section of lamella of H. turcicus showing numerous epidermal layers. The arrowhead indicates the alpha-layer. The asterisk indicates artifact separation (due to sectioning) between outer setae and the alpha-layer. Toluidine blue stain. Bar, 20 μm. (C), detail on the shedding complex of T. mauritanica with forming setae derived from Oberhautchen cells. Toluidine blue stain. Bar, 10 μm. (D), immunolabeled setae (red) and nuclei with DAPI (blue) in lamella of T. mauritanica. Dashes underline the epidermis. Bar, 20 μm. (E), immunolabeled (green) outer corneous layer and setae and inner corneous layer with its setae in H. turcicus. The arrow points to the immunofluorescent free margin. Bar, 20 μm. (F), immunolabeled setae and outer corneous layer with setae in T. mauritanica. Bar, 10 μm. (G), double labeling in outer and early-forming inner setae of T. mauritanica. The arrowhead indicates the alpha-layer and the asterisks the artifactual split of the epidermis under sectioning. Bar, 20 μm. (H), close-up view of inner setae (arrow) at an early phase of their growth. The arrowhead indicates the alpha-layer. Dashes underline the epidermis. Bar, 10 μm. Legend: α, alpha-layer; cl, clear layer; de, dermis; e, epidermis; is, inner setae; ob, Oberhautchen-beta-layer; os, outer setae; se, setae.
![Jdb 11 00003 g007 Jdb 11 00003 g007]()
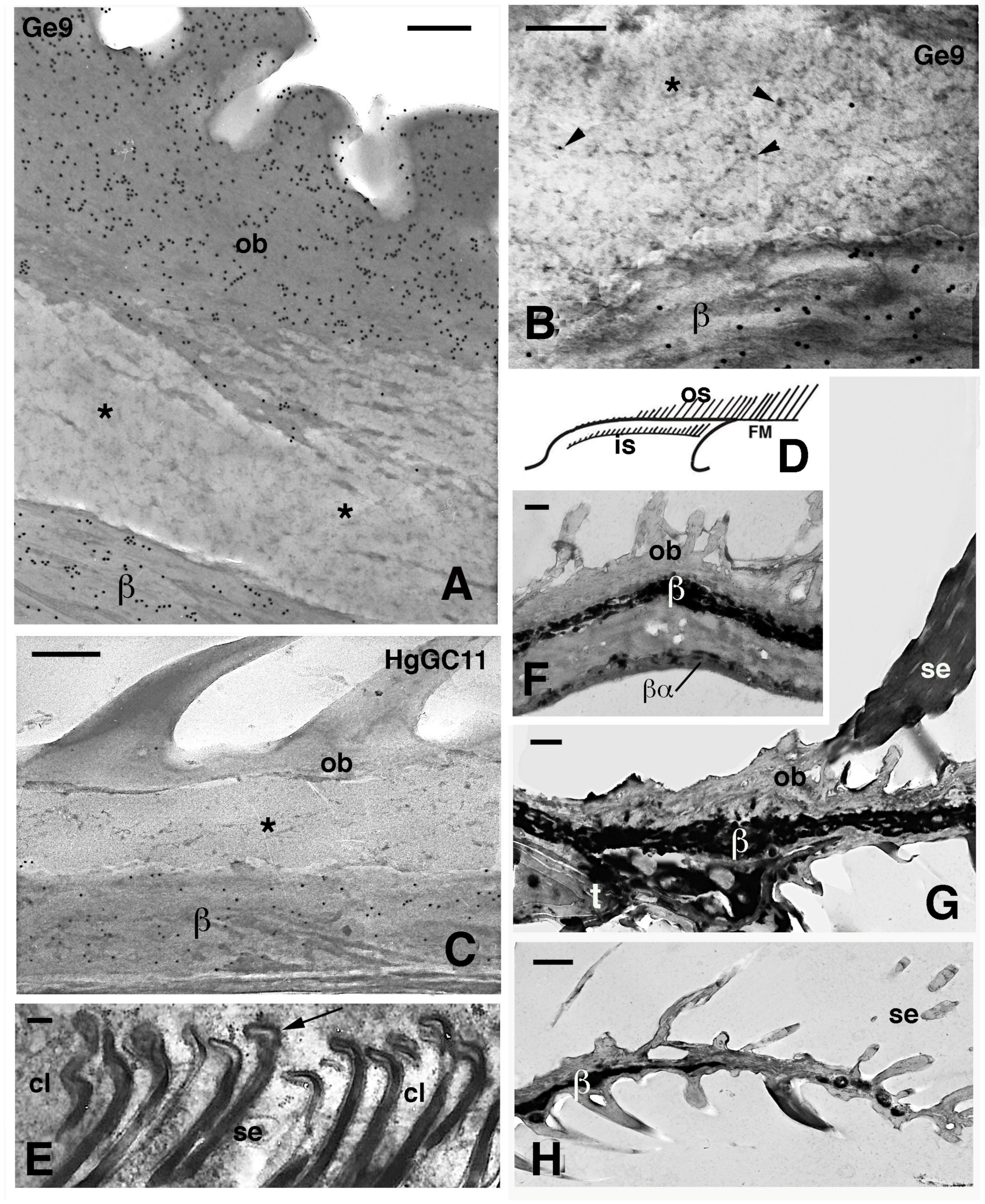
Figure 8.
Electron microscopic details of external corneous layers of pad lamellae. (A), immunolabeled Oberhautchen and beta-layer in G. gecko using the Ge9 antibody for CBPs. Asterisks indicate a pale layer with scarce corneous material sandwiched between the labeled corneous layers. Bar, 200 nm. (B), detail of the pale layer (asterisk) containing sparse ribosomes (arrowheads). Ge9 immunolabeling in G. gecko. Bar, 100 nm. (C), detail of the corneous layer of lamella in H. turcicus with the pale layer (asterisk). Immunolabeling using the HgGC11 antibody for CBPs. Bar, 100 nm. (D), schematic drawing of lamella evidencing outer and inner setae. (E), forming spatula ends (arrows) of the inner setae in A. carolinensis. Bar, 200 nm. (F), detail of the corneous layer by the apex of the lamella in G. gecko. Bar, 0.5 μm. (G), detail of the corneous layer at the beginning of the free margin in G. gecko. Bar, 0.5 μm. (H), tip of the free margin in G. gecko. Bar, 0.5 μm. Legend: β, beta-layer/corneous material; βα, likely alpha-layer merged with the beta-layer of the lamella (mesos layer absent); cl, clear layer (cell); FM, free margin; is, inner setae; os, outer setae; ob, Oberhautchen; se, setae; t, distal tip of the pad lamella.
Figure 8.
Electron microscopic details of external corneous layers of pad lamellae. (A), immunolabeled Oberhautchen and beta-layer in G. gecko using the Ge9 antibody for CBPs. Asterisks indicate a pale layer with scarce corneous material sandwiched between the labeled corneous layers. Bar, 200 nm. (B), detail of the pale layer (asterisk) containing sparse ribosomes (arrowheads). Ge9 immunolabeling in G. gecko. Bar, 100 nm. (C), detail of the corneous layer of lamella in H. turcicus with the pale layer (asterisk). Immunolabeling using the HgGC11 antibody for CBPs. Bar, 100 nm. (D), schematic drawing of lamella evidencing outer and inner setae. (E), forming spatula ends (arrows) of the inner setae in A. carolinensis. Bar, 200 nm. (F), detail of the corneous layer by the apex of the lamella in G. gecko. Bar, 0.5 μm. (G), detail of the corneous layer at the beginning of the free margin in G. gecko. Bar, 0.5 μm. (H), tip of the free margin in G. gecko. Bar, 0.5 μm. Legend: β, beta-layer/corneous material; βα, likely alpha-layer merged with the beta-layer of the lamella (mesos layer absent); cl, clear layer (cell); FM, free margin; is, inner setae; os, outer setae; ob, Oberhautchen; se, setae; t, distal tip of the pad lamella.
![Jdb 11 00003 g008 Jdb 11 00003 g008]()
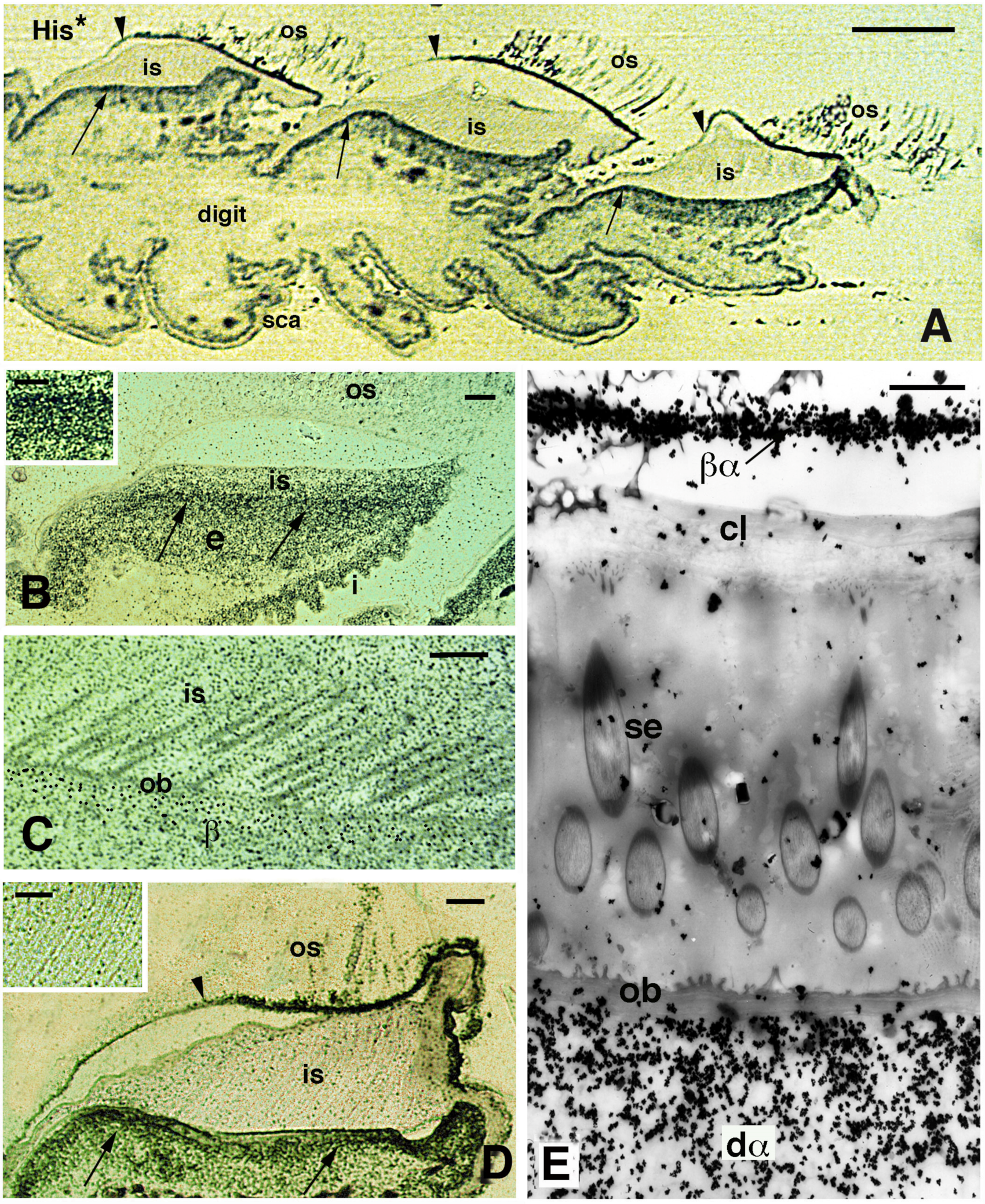
Figure 9.
Autoradiographical view of pad lamellae and setae of H. turcicus 4 h post-injection of tritiated histidine (His*). (A), longitudinal section showing a digit with three pad lamellae (the ventral side is downward). The inner Oberhautchen-beta-layer (arrows) is intensely labeled, while the outer Oberhautchen-beta-layer is mainly labeled in the distal part (indicated by arrowheads). Bar, 100 μm. (B), intensely labeled epidermal area occupied by differentiating inner Oberhautchen and beta-cells (arrows). Bar, 10 μm. The inset (Bar, 5 μm) provides detail on the intense labeling of these layers. (C), detail of the diffuse autoradiographic labeling in inner setae and Oberhautchen layer in the renewal phase of the lamella epidermis. Bar, 10 μm. (D), labeling in the outer Oberhautchen-corneous layer (arrowhead) and inner Oberhautchen-beta-layer (arrows) at a late renewal phase of the epidermis, with inner setae only diffusely labeled. Bar, 10 μm. The inset (Bar, 0.5 μm) shows that the inner setae are almost unlabeled at this stage of the renewal phase. (E), electron microscopic autoradiography showing unlabeled inner setae, Oberhautchen-beta-layer and clear layer, while the differentiating inner alpha-cells underneath are heavily labeled. Moreover, numerous silver grains are present over the thin beta-alpha-layer forming the corneous layer sustaining the outer setae. Bar, 1 μm. Legend: β, forming beta-layer; βα, corneous layer formed by a thin beta-layer with the alpha-layer; cl, clear layer; dα, differentiating alpha-cells; e, epidermis; i, inner surface of the lamella; is, inner setae; ob, Oberhautchen-beta-layer; os, outer setae; sca, scales; se, setae (inner).
Figure 9.
Autoradiographical view of pad lamellae and setae of H. turcicus 4 h post-injection of tritiated histidine (His*). (A), longitudinal section showing a digit with three pad lamellae (the ventral side is downward). The inner Oberhautchen-beta-layer (arrows) is intensely labeled, while the outer Oberhautchen-beta-layer is mainly labeled in the distal part (indicated by arrowheads). Bar, 100 μm. (B), intensely labeled epidermal area occupied by differentiating inner Oberhautchen and beta-cells (arrows). Bar, 10 μm. The inset (Bar, 5 μm) provides detail on the intense labeling of these layers. (C), detail of the diffuse autoradiographic labeling in inner setae and Oberhautchen layer in the renewal phase of the lamella epidermis. Bar, 10 μm. (D), labeling in the outer Oberhautchen-corneous layer (arrowhead) and inner Oberhautchen-beta-layer (arrows) at a late renewal phase of the epidermis, with inner setae only diffusely labeled. Bar, 10 μm. The inset (Bar, 0.5 μm) shows that the inner setae are almost unlabeled at this stage of the renewal phase. (E), electron microscopic autoradiography showing unlabeled inner setae, Oberhautchen-beta-layer and clear layer, while the differentiating inner alpha-cells underneath are heavily labeled. Moreover, numerous silver grains are present over the thin beta-alpha-layer forming the corneous layer sustaining the outer setae. Bar, 1 μm. Legend: β, forming beta-layer; βα, corneous layer formed by a thin beta-layer with the alpha-layer; cl, clear layer; dα, differentiating alpha-cells; e, epidermis; i, inner surface of the lamella; is, inner setae; ob, Oberhautchen-beta-layer; os, outer setae; sca, scales; se, setae (inner).
![Jdb 11 00003 g009 Jdb 11 00003 g009]()
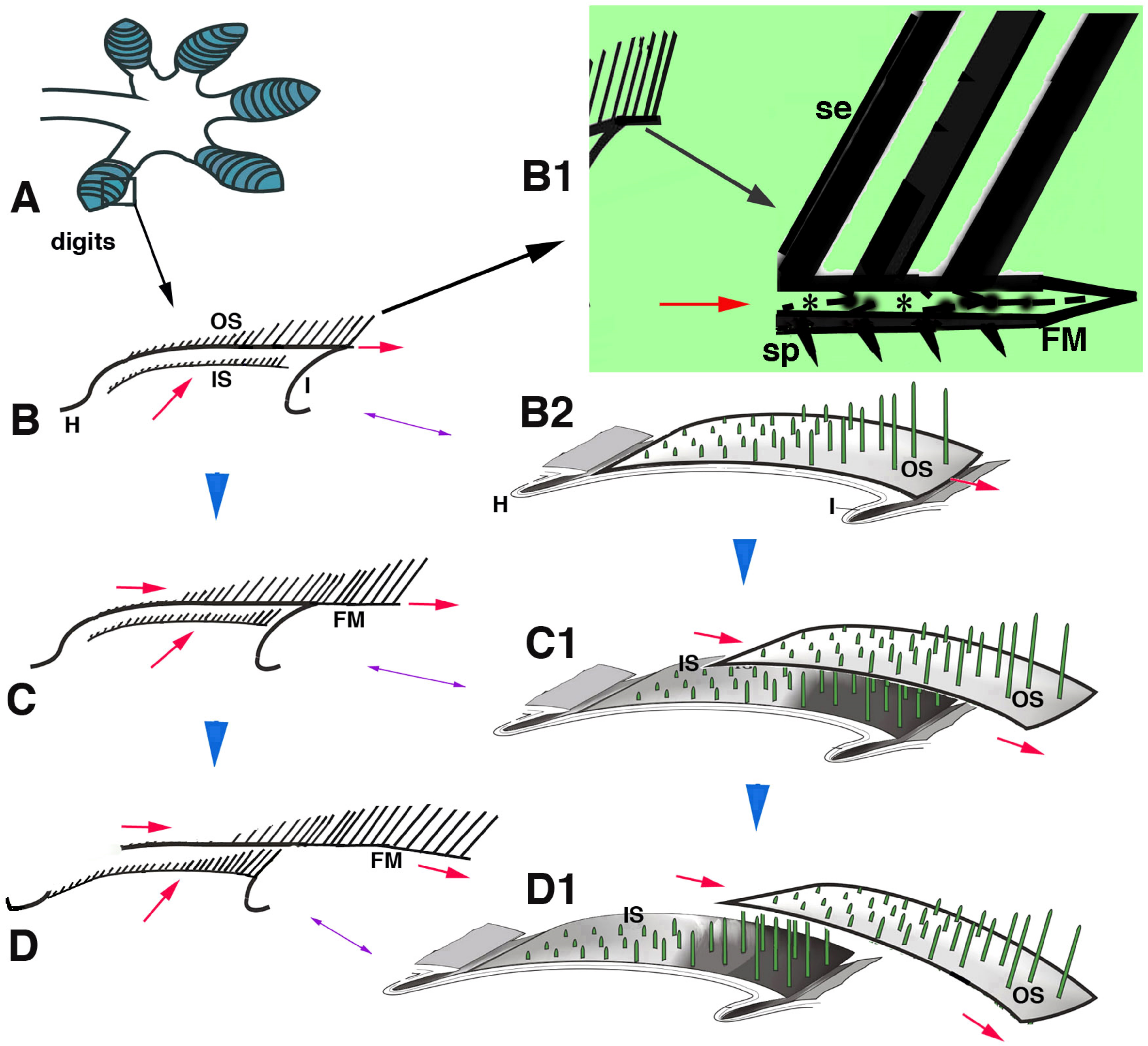
Figure 10.
Schematic drawing summarizing the hypothesis of setae replacement in pad lamellae from phase (B–D) (blue arrowheads). (A), digit with lamellae (blue). (B), detail of a longitudinally sectioned lamellae with two setae generations. (B1), detail of the free margin (large arrow). Asterisks in B1 indicate the pale layer within the Oberhautchen and beta-layer that sustain the setae. (B2), three-dimensional representation of a lamella. (C), in a following phase, the outer setae move toward the lamella tip, while the inner setae grow underneath (red arrows). (C1) shows the distal displacement of the outer setae and the sustaining Oberhautchen-beta-layer. (D), in a final phase of shedding, the outer setae are lost from the lamella tip and the inner setae emerge underneath. (D1), three-dimensional view of outer setae shedding. Legend: FM, free margin; H, hinge region; I, inner lamella surface; IS, inner setae; OS, outer setae; se, setae; sp, spinulae; The red arrows indicate the upper and distal movements of outer and inner setae.
Figure 10.
Schematic drawing summarizing the hypothesis of setae replacement in pad lamellae from phase (B–D) (blue arrowheads). (A), digit with lamellae (blue). (B), detail of a longitudinally sectioned lamellae with two setae generations. (B1), detail of the free margin (large arrow). Asterisks in B1 indicate the pale layer within the Oberhautchen and beta-layer that sustain the setae. (B2), three-dimensional representation of a lamella. (C), in a following phase, the outer setae move toward the lamella tip, while the inner setae grow underneath (red arrows). (C1) shows the distal displacement of the outer setae and the sustaining Oberhautchen-beta-layer. (D), in a final phase of shedding, the outer setae are lost from the lamella tip and the inner setae emerge underneath. (D1), three-dimensional view of outer setae shedding. Legend: FM, free margin; H, hinge region; I, inner lamella surface; IS, inner setae; OS, outer setae; se, setae; sp, spinulae; The red arrows indicate the upper and distal movements of outer and inner setae.
![Jdb 11 00003 g010 Jdb 11 00003 g010]()