Differentiation of Cells Isolated from Human Femoral Heads into Functional Osteoclasts
Abstract
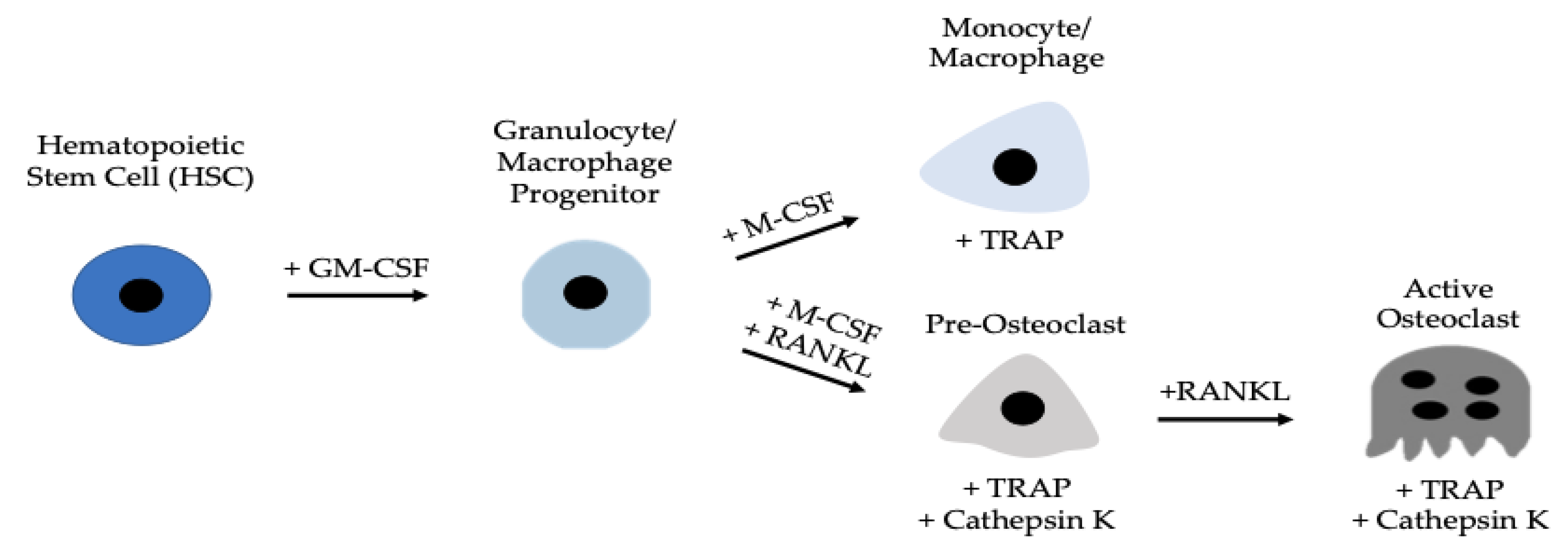
1. Introduction
2. Materials and Methods
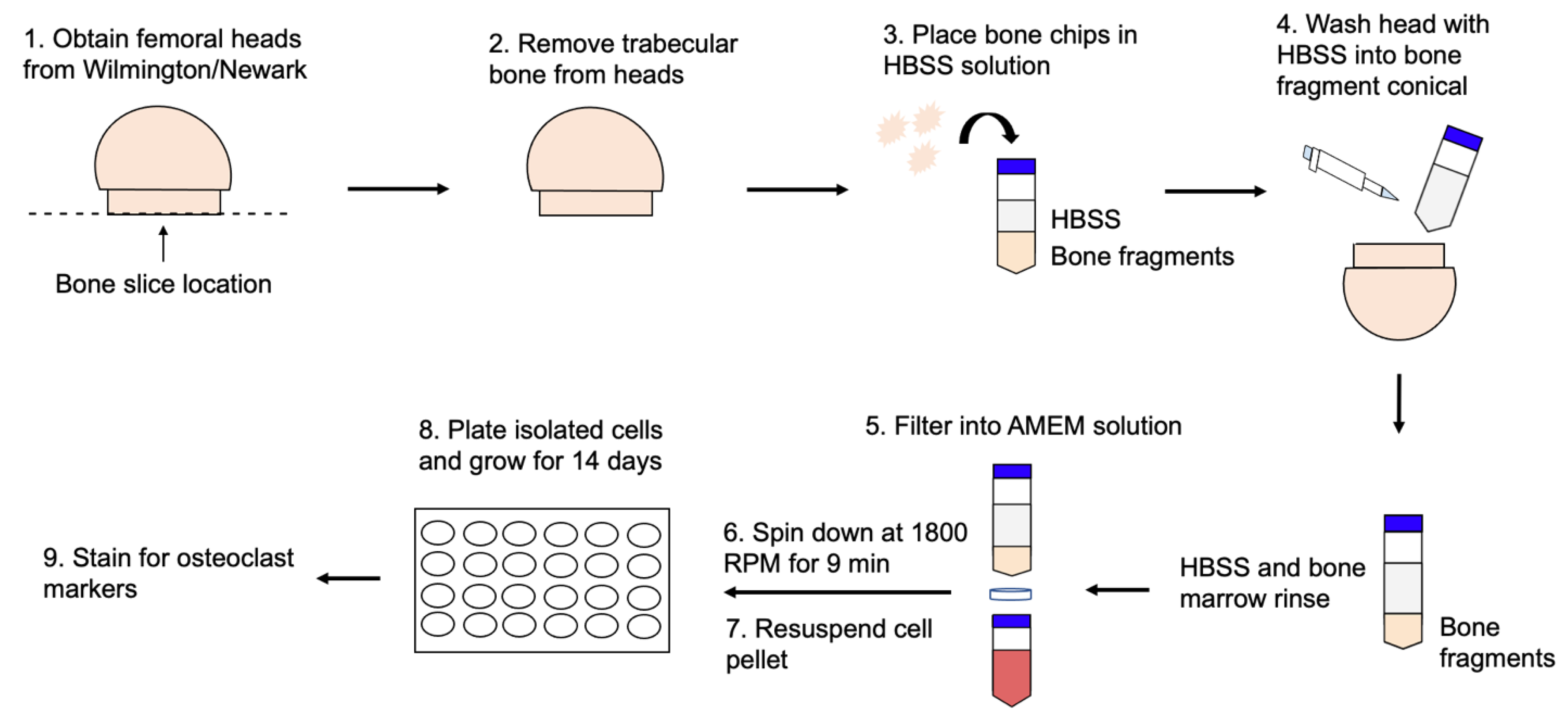
2.1. Femoral Head Retrieval
2.2. Cell Isolation from Femoral Heads
2.3. Cell Culture
2.4. Optimization of Differentiating Pre-Osteoclasts into Osteoclasts
2.5. Tartrate Resistant Acid Phosphatase (TRAP) Staining
2.6. Cell Viability and Proliferation Assay
2.7. Immunofluorescence
2.8. Statistical Analysis
3. Results
3.1. RAW264.7 Cells Do Not Differentiate Readily into Osteoclasts
3.2. Optimal Conditions for Osteoclastogenesis Utilizing M-CSF and RANKL
3.3. Cells isolated from Female and Male OA Patients Differentiate into Functional Osteoclasts
3.4. Cells Isolated from Female and Male OA Patients Are Viable and Do Not Proliferate
3.5. Osteoclasts Isolated from OA Patients Express TRAP and Cathepsin K
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Halloran, D.; Durbano, H.W.; Nohe, A. Bone Morphogenetic Protein-2 in Development and Bone Homeostasis. J. Dev. Biol. 2020, 8, 19. [Google Scholar] [CrossRef]
- Vortkamp, A. Defining the skeletal elements. Curr. Biol. 1997, 7, R104–R107. [Google Scholar] [CrossRef]
- Yang, Y. Skeletal morphogenesis during embryonic development. Crit. Rev. Eukaryot. Gene Expr. 2009, 19, 197–218. [Google Scholar] [CrossRef] [PubMed]
- Long, F.; Ornitz, D.M. Development of the endochondral skeleton. Cold Spring Harb. Perspect. Biol. 2013, 5, a008334. [Google Scholar] [CrossRef] [PubMed]
- Durbano, H.W.; Halloran, D.; Nguyen, J.; Stone, V.; McTague, S.; Eskander, M.; Nohe, A. Aberrant BMP2 Signaling in Patients Diagnosed with Osteoporosis. Int. J. Mol. Sci. 2020, 21, 6909. [Google Scholar] [CrossRef]
- Florencio-Silva, R.; Sasso, G.R.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. Biomed. Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef] [PubMed]
- Buckwalter, J.A.; Glimcher, M.J.; Cooper, R.R.; Recker, R. Bone biology. I: Structure, blood supply, cells, matrix, and mineralization. Instr. Course Lect. 1996, 45, 371–386. [Google Scholar]
- Sims, N.A.; Gooi, J.H. Bone remodeling: Multiple cellular interactions required for coupling of bone formation and resorption. Semin. Cell Dev. Biol. 2008, 19, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.; Gooi, J.H.; Sims, N.A. Molecular mechanisms in coupling of bone formation to resorption. Crit. Rev. Eukaryot. Gene Expr. 2009, 19, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Humphrey, M.B.; Nakamura, M.C. Osteoclasts—The innate immune cells of the bone. Autoimmunity 2008, 41, 183–194. [Google Scholar] [CrossRef]
- Zhou, N.; Li, Q.; Lin, X.; Hu, N.; Liao, J.Y.; Lin, L.B.; Zhao, C.; Hu, Z.M.; Liang, X.; Xu, W.; et al. BMP2 induces chondrogenic differentiation, osteogenic differentiation and endochondral ossification in stem cells. Cell Tissue Res. 2016, 366, 101–111. [Google Scholar] [CrossRef]
- Bonor, J.; Adams, E.L.; Bragdon, B.; Moseychuk, O.; Czymmek, K.J.; Nohe, A. Initiation of BMP2 signaling in domains on the plasma membrane. J. Cell Physiol. 2012, 227, 2880–2888. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Chen, G.; Li, Y.P. TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016, 4, 16009. [Google Scholar] [CrossRef] [PubMed]
- Bragdon, B.; Moseychuk, O.; Saldanha, S.; King, D.; Julian, J.; Nohe, A. Bone morphogenetic proteins: A critical review. Cell. Signal. 2011, 23, 609–620. [Google Scholar] [CrossRef]
- Charles, J.F.; Nakamura, M.C. Bone and the innate immune system. Curr. Osteoporos. Rep. 2014, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.; Nohe, A. Factors that Affect the Osteoclastogenesis of RAW264.7 Cells. 7 Cells. J. Biochem. Anal. Stud. 2017, 2. [Google Scholar] [CrossRef]
- Madel, M.B.; Ibáñez, L.; Wakkach, A.; de Vries, T.J.; Teti, A.; Apparailly, F.; Blin-Wakkach, C. Immune Function and Diversity of Osteoclasts in Normal and Pathological Conditions. Front. Immunol. 2019, 10, 1408. [Google Scholar] [CrossRef]
- Heubel, B.; Nohe, A. The Role of BMP Signaling in Osteoclast Regulation. J. Dev. Biol. 2021, 9, 24. [Google Scholar] [CrossRef]
- Maatouk, D.M.; Choi, K.S.; Bouldin, C.M.; Harfe, B.D. In the limb AER Bmp2 and Bmp4 are required for dorsal-ventral patterning and interdigital cell death but not limb outgrowth. Dev. Biol. 2009, 327, 516–523. [Google Scholar] [CrossRef]
- Witte, F.; Chan, D.; Economides, A.N.; Mundlos, S.; Stricker, S. Receptor tyrosine kinase-like orphan receptor 2 (ROR2) and Indian hedgehog regulate digit outgrowth mediated by the phalanx-forming region. Proc. Natl. Acad. Sci. USA 2010, 107, 14211–14216. [Google Scholar] [CrossRef]
- Bragdon, B.; D’Angelo, A.; Gurski, L.; Bonor, J.; Schultz, K.L.; Beamer, W.G.; Rosen, C.J.; Nohe, A. Altered plasma membrane dynamics of bone morphogenetic protein receptor type Ia in a low bone mass mouse model. Bone 2012, 50, 189–199. [Google Scholar] [CrossRef]
- Zhou, Y.; Lin, J.; Shao, J.; Zuo, Q.; Wang, S.; Wolff, A.; Nguyen, D.T.; Rintoul, L.; Du, Z.; Gu, Y.; et al. Aberrant activation of Wnt signaling pathway altered osteocyte mineralization. Bone 2019, 127, 324–333. [Google Scholar] [CrossRef]
- Downey, P.A.; Siegel, M.I. Bone biology and the clinical implications for osteoporosis. Phys. Ther. 2006, 86, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Weidner, H.; Yuan Gao, V.; Dibert, D.; McTague, S.; Eskander, M.; Duncan, R.; Wang, L.; Nohe, A. CK2.3, a Mimetic Peptide of the BMP Type I Receptor, Increases Activity in Osteoblasts over BMP2. Int. J. Mol. Sci. 2019, 20, 5877. [Google Scholar] [CrossRef]
- Chen, L.R.; Ko, N.Y.; Chen, K.H. Medical Treatment for Osteoporosis: From Molecular to Clinical Opinions. Int. J. Mol. Sci. 2019, 20, 2213. [Google Scholar] [CrossRef]
- Halloran, D.; Vrathasha, V.; Durbano, H.W.; Nohe, A. Bone Morphogenetic Protein-2 Conjugated to Quantum Dot. Nanomaterials 2020, 10, 1208. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.; Kelly, S.; Wood, R.; Heubel, B.; Nohe, A. A Synthetic Peptide, CK2.3, Inhibits RANKL-Induced Osteoclastogenesis through BMPRIa and ERK Signaling Pathway. J. Dev. Biol. 2020, 8, 12. [Google Scholar] [CrossRef]
- Weivoda, M.M.; Chew, C.K.; Monroe, D.G.; Farr, J.N.; Atkinson, E.J.; Geske, J.R.; Eckhardt, B.; Thicke, B.; Ruan, M.; Tweed, A.J.; et al. Identification of osteoclast-osteoblast coupling factors in humans reveals links between bone and energy metabolism. Nat. Commun. 2020, 11, 87. [Google Scholar] [CrossRef]
- Teti, A. Mechanisms of osteoclast-dependent bone formation. Bonekey Rep. 2013, 2, 449. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, Z.; Duan, N.; Zhu, G.; Schwarz, E.M.; Xie, C. Osteoblast-osteoclast interactions. Connect. Tissue Res. 2018, 59, 99–107. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, N. Signaling Pathways in Osteoclast Differentiation. Chonnam Med. J. 2016, 52, 12–17. [Google Scholar] [CrossRef]
- Mouline, C.C.; Quincey, D.; Laugier, J.P.; Carle, G.F.; Bouler, J.M.; Rochet, N.; Scimeca, J.C. Osteoclastic differentiation of mouse and human monocytes in a plasma clot/biphasic calcium phosphate microparticles composite. Eur. Cell Mater. 2010, 20, 379–392. [Google Scholar] [CrossRef]
- Yamashita, T.; Takahashi, N.; Udagawa, N. New roles of osteoblasts involved in osteoclast differentiation. World J. Orthop. 2012, 3, 175–181. [Google Scholar] [CrossRef]
- Chen, X.; Liu, H.; Focia, P.J.; Shim, A.H.; He, X. Structure of macrophage colony stimulating factor bound to FMS: Diverse signaling assemblies of class III receptor tyrosine kinases. Proc. Natl. Acad. Sci. USA 2008, 105, 18267–18272. [Google Scholar] [CrossRef]
- Bourette, R.P.; De Sepulveda, P.; Arnaud, S.; Dubreuil, P.; Rottapel, R.; Mouchiroud, G. Suppressor of cytokine signaling 1 interacts with the macrophage colony-stimulating factor receptor and negatively regulates its proliferation signal. J. Biol. Chem. 2001, 276, 22133–22139. [Google Scholar] [CrossRef]
- Kylmäoja, E.; Nakamura, M.; Turunen, S.; Patlaka, C.; Andersson, G.; Lehenkari, P.; Tuukkanen, J. Peripheral blood monocytes show increased osteoclast differentiation potential compared to bone marrow monocytes. Heliyon 2018, 4, e00780. [Google Scholar] [CrossRef] [PubMed]
- Lari, R.; Kitchener, P.D.; Hamilton, J.A. The proliferative human monocyte subpopulation contains osteoclast precursors. Arthritis Res. Ther. 2009, 11, R23. [Google Scholar] [CrossRef]
- Boyce, B.F.; Xing, L. Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res. Ther. 2007, 9 (Suppl. S1), S1. [Google Scholar] [CrossRef] [PubMed]
- Boyce, B.F.; Xing, L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch. Biochem. Biophys. 2008, 473, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Madel, M.B.; Ibáñez, L.; Rouleau, M.; Wakkach, A.; Blin-Wakkach, C. A Novel Reliable and Efficient Procedure for Purification of Mature Osteoclasts Allowing Functional Assays in Mouse Cells. Front. Immunol. 2018, 9, 2567. [Google Scholar] [CrossRef] [PubMed]
- Susa, M.; Luong-Nguyen, N.H.; Cappellen, D.; Zamurovic, N.; Gamse, R. Human primary osteoclasts: In vitro generation and applications as pharmacological and clinical assay. J. Transl. Med. 2004, 2, 6. [Google Scholar] [CrossRef]
- McDonald, M.M.; Khoo, W.H.; Ng, P.Y.; Xiao, Y.; Zamerli, J.; Thatcher, P.; Kyaw, W.; Pathmanandavel, K.; Grootveld, A.K.; Moran, I.; et al. Osteoclasts recycle via osteomorphs during RANKL-stimulated bone resorption. Cell 2021, 184, 1940. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.; Cornetta, K.G.; Econs, M.J. Efficient and stable gene expression into human osteoclasts using an HIV-1-based lentiviral vector. DNA Cell Biol. 2008, 27, 315–320. [Google Scholar] [CrossRef]
- Li, Z.H.; Si, Y.; Xu, G.; Chen, X.M.; Xiong, H.; Lai, L.; Zheng, Y.Q.; Zhang, Z.G. High-dose PMA with RANKL and MCSF induces THP-1 cell differentiation into human functional osteoclasts in vitro. Mol. Med. Rep. 2017, 16, 8380–8384. [Google Scholar] [CrossRef]
- Lowell, C.A.; Soriano, P. Knockouts of Src-family kinases: Stiff bones, wimpy T cells, and bad memories. Genes Dev. 1996, 10, 1845–1857. [Google Scholar] [CrossRef]
- Laitala-Leinonen, T. Unsatisfactory gene transfer into bone-resorbing osteoclasts with liposomal transfection systems. J. Negat. Results Biomed. 2005, 4, 5. [Google Scholar] [CrossRef][Green Version]
- Seta, N.; Okazaki, Y.; Kuwana, M. Human circulating monocytes can express receptor activator of nuclear factor-kappaB ligand and differentiate into functional osteoclasts without exogenous stimulation. Immunol. Cell Biol. 2008, 86, 453–459. [Google Scholar] [CrossRef]
- Kirstein, B.; Chambers, T.J.; Fuller, K. Secretion of tartrate-resistant acid phosphatase by osteoclasts correlates with resorptive behavior. J. Cell Biochem. 2006, 98, 1085–1094. [Google Scholar] [CrossRef]
- Podgorski, I.; Linebaugh, B.E.; Sloane, B.F. Cathepsin K in the bone microenvironment: Link between obesity and prostate cancer? Biochem. Soc. Trans. 2007, 35, 701–703. [Google Scholar] [CrossRef] [PubMed]
- Herroon, M.K.; Rajagurubandara, E.; Rudy, D.L.; Chalasani, A.; Hardaway, A.L.; Podgorski, I. Macrophage cathepsin K promotes prostate tumor progression in bone. Oncogene 2013, 32, 1580–1593. [Google Scholar] [CrossRef] [PubMed]
- How, J.; Brown, J.R.; Saylor, S.; Rimm, D.L. Macrophage expression of tartrate-resistant acid phosphatase as a prognostic indicator in colon cancer. Histochem. Cell Biol. 2014, 142, 195–204. [Google Scholar] [CrossRef]
- Wang, R.N.; Green, J.; Wang, Z.; Deng, Y.; Qiao, M.; Peabody, M.; Zhang, Q.; Ye, J.; Yan, Z.; Denduluri, S.; et al. Bone Morphogenetic Protein (BMP) signaling in development and human diseases. Genes Dis. 2014, 1, 87–105. [Google Scholar] [CrossRef]
- Ornitz, D.M.; Marie, P.J. Fibroblast growth factor signaling in skeletal development and disease. Genes Dev. 2015, 29, 1463–1486. [Google Scholar] [CrossRef]
- Bacchetta, J.; Wesseling-Perry, K.; Gilsanz, V.; Gales, B.; Pereira, R.C.; Salusky, I.B. Idiopathic juvenile osteoporosis: A cross-sectional single-centre experience with bone histomorphometry and quantitative computed tomography. Pediatr. Rheumatol. Online J. 2013, 11, 6. [Google Scholar] [CrossRef]
- Taciak, B.; Białasek, M.; Braniewska, A.; Sas, Z.; Sawicka, P.; Kiraga, Ł.; Rygiel, T.; Król, M. Evaluation of phenotypic and functional stability of RAW 264.7 cell line through serial passages. PLoS ONE 2018, 13, e0198943. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, X.; Xu, X.L.; Yuan, X.L.; Gou, W.L.; Wang, A.Y.; Guo, Q.Y.; Peng, J.; Lu, S.B. Bone microstructure and regional distribution of osteoblast and osteoclast activity in the osteonecrotic femoral head. PLoS ONE 2014, 9, e96361. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Huang, G.T.; Chiang, H.; Chiou, L.L.; Chen, M.H.; Hsieh, C.H.; Jiang, C.C. Multipotential mesenchymal stem cells from femoral bone marrow near the site of osteonecrosis. Stem Cells 2003, 21, 190–199. [Google Scholar] [CrossRef]
- Bernhardt, A.; Skottke, J.; von Witzleben, M.; Gelinsky, M. Triple Culture of Primary Human Osteoblasts, Osteoclasts and Osteocytes as an In Vitro Bone Model. Int. J. Mol. Sci. 2021, 22, 7316. [Google Scholar] [CrossRef]
- Sabokbar, A.; Fujikawa, Y.; Neale, S.; Murray, D.W.; Athanasou, N.A. Human arthroplasty derived macrophages differentiate into osteoclastic bone resorbing cells. Ann. Rheum. Dis. 1997, 56, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Arai, F.; Miyamoto, T.; Ohneda, O.; Inada, T.; Sudo, T.; Brasel, K.; Miyata, T.; Anderson, D.M.; Suda, T. Commitment and differentiation of osteoclast precursor cells by the sequential expression of c-Fms and receptor activator of nuclear factor kappaB (RANK) receptors. J. Exp. Med. 1999, 190, 1741–1754. [Google Scholar] [CrossRef]
- Yi, F.; Huang, J.; Yang, L.; Xie, Y.; Xiao, G. Automatic extraction of cell nuclei from H&E-stained histopathological images. J. Med. Imaging 2017, 4, 027502. [Google Scholar] [CrossRef]
- Ortiz-Hidalgo, C.; Pina-Oviedo, S. Hematoxylin: Mesoamerica’s Gift to Histopathology. Palo de Campeche (Logwood Tree), Pirates’ Most Desired Treasure, and Irreplaceable Tissue Stain. Int. J. Surg. Pathol. 2019, 27, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Logar, D.B.; Komadina, R.; Prezelj, J.; Ostanek, B.; Trost, Z.; Marc, J. Expression of bone resorption genes in osteoarthritis and in osteoporosis. J. Bone Miner. Metab. 2007, 25, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Dai, R.; Wu, Z.; Chu, H.Y.; Lu, J.; Lyu, A.; Liu, J.; Zhang, G. Cathepsin K: The Action in and Beyond Bone. Front. Cell Dev. Biol. 2020, 8, 433. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halloran, D.R.; Heubel, B.; MacMurray, C.; Root, D.; Eskander, M.; McTague, S.P.; Pelkey, H.; Nohe, A. Differentiation of Cells Isolated from Human Femoral Heads into Functional Osteoclasts. J. Dev. Biol. 2022, 10, 6. https://doi.org/10.3390/jdb10010006
Halloran DR, Heubel B, MacMurray C, Root D, Eskander M, McTague SP, Pelkey H, Nohe A. Differentiation of Cells Isolated from Human Femoral Heads into Functional Osteoclasts. Journal of Developmental Biology. 2022; 10(1):6. https://doi.org/10.3390/jdb10010006
Chicago/Turabian StyleHalloran, Daniel R., Brian Heubel, Connor MacMurray, Denise Root, Mark Eskander, Sean P. McTague, Heather Pelkey, and Anja Nohe. 2022. "Differentiation of Cells Isolated from Human Femoral Heads into Functional Osteoclasts" Journal of Developmental Biology 10, no. 1: 6. https://doi.org/10.3390/jdb10010006
APA StyleHalloran, D. R., Heubel, B., MacMurray, C., Root, D., Eskander, M., McTague, S. P., Pelkey, H., & Nohe, A. (2022). Differentiation of Cells Isolated from Human Femoral Heads into Functional Osteoclasts. Journal of Developmental Biology, 10(1), 6. https://doi.org/10.3390/jdb10010006






