Expression and Function of Toll Pathway Components in the Early Development of the Wasp Nasonia vitripennis
Abstract
1. Introduction
2. Materials and Methods
2.1. Discovery of Toll Pathway Orthologs
2.2. In Situ Hybridization
2.3. Parental RNA Interference (pRNAi)
2.4. Qualitative Polymerase Chain Reaction (qPCR)
2.5. Embryonic Lethality Screening
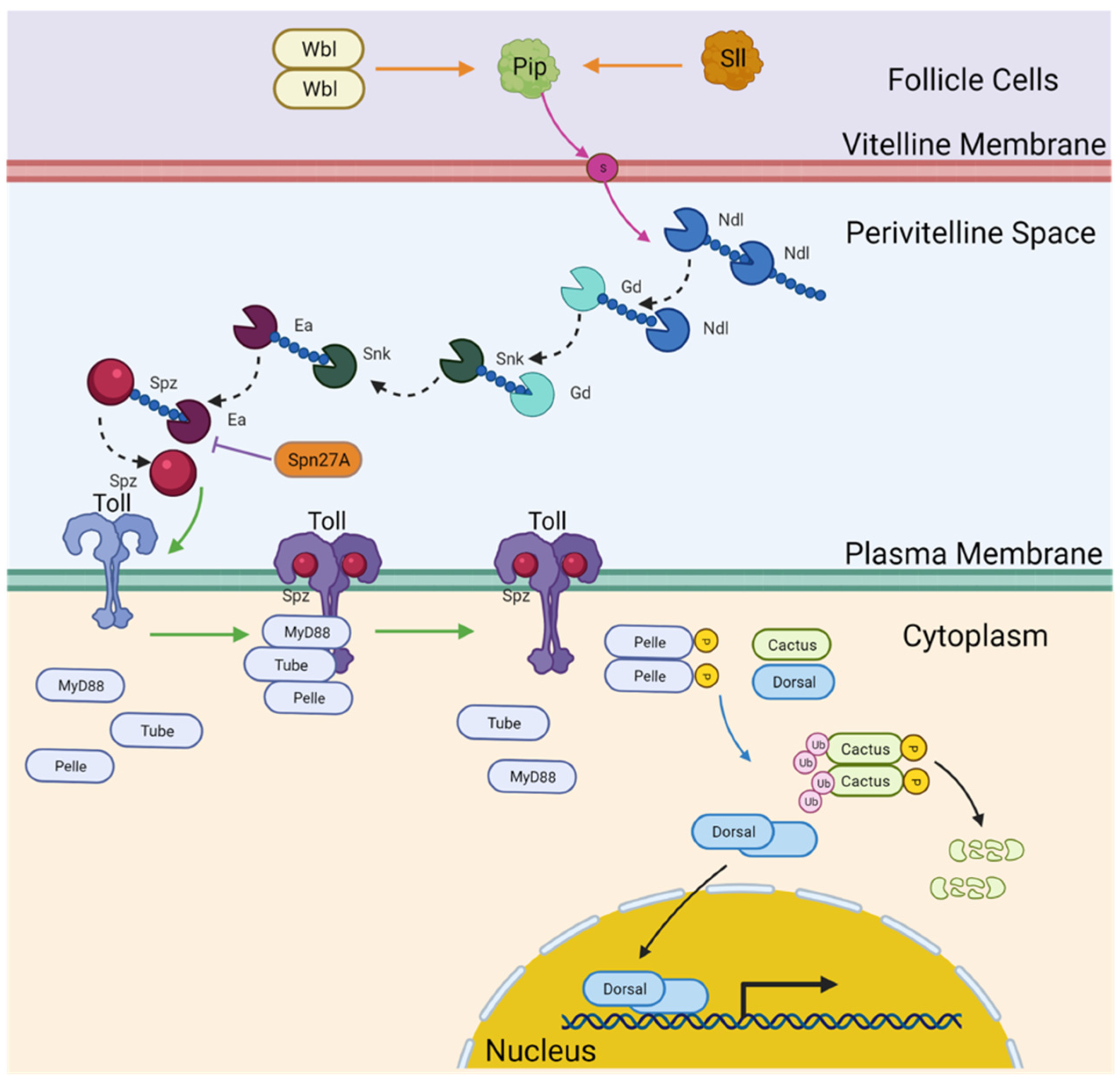
3. Results
3.1. Lack of Expression of Eggshell-Modifying Component Homologs
3.2. Novel Paralogs and Expression Patterns of Protease Cascade Components
3.3. Nudel
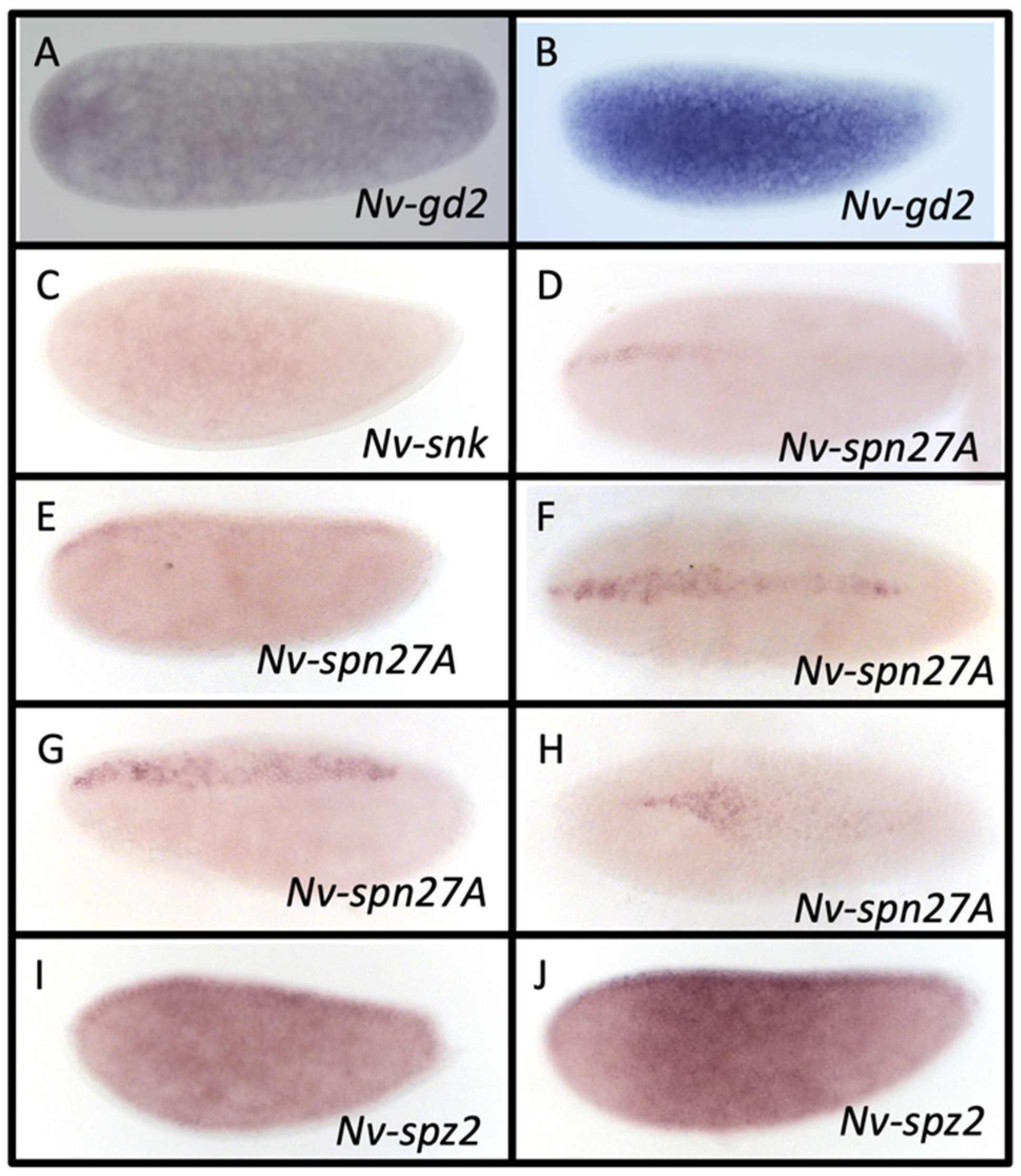
3.4. Gastrulation Defective
3.5. Snake
3.6. Easter
3.7. Serpin27A
3.8. Spätzle
3.9. Toll Receptors
3.10. Membrane-Interacting Mediators of Toll Signaling
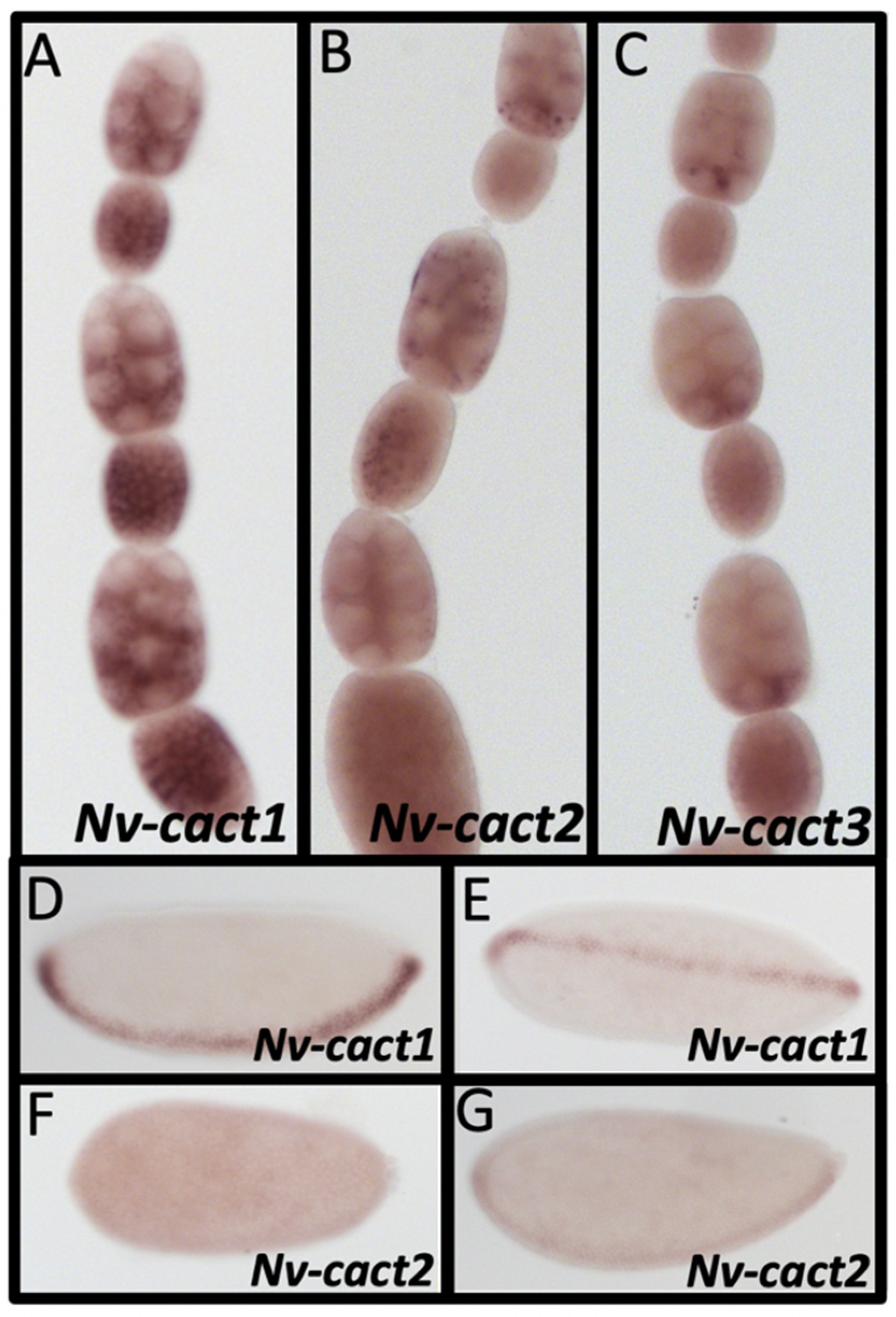
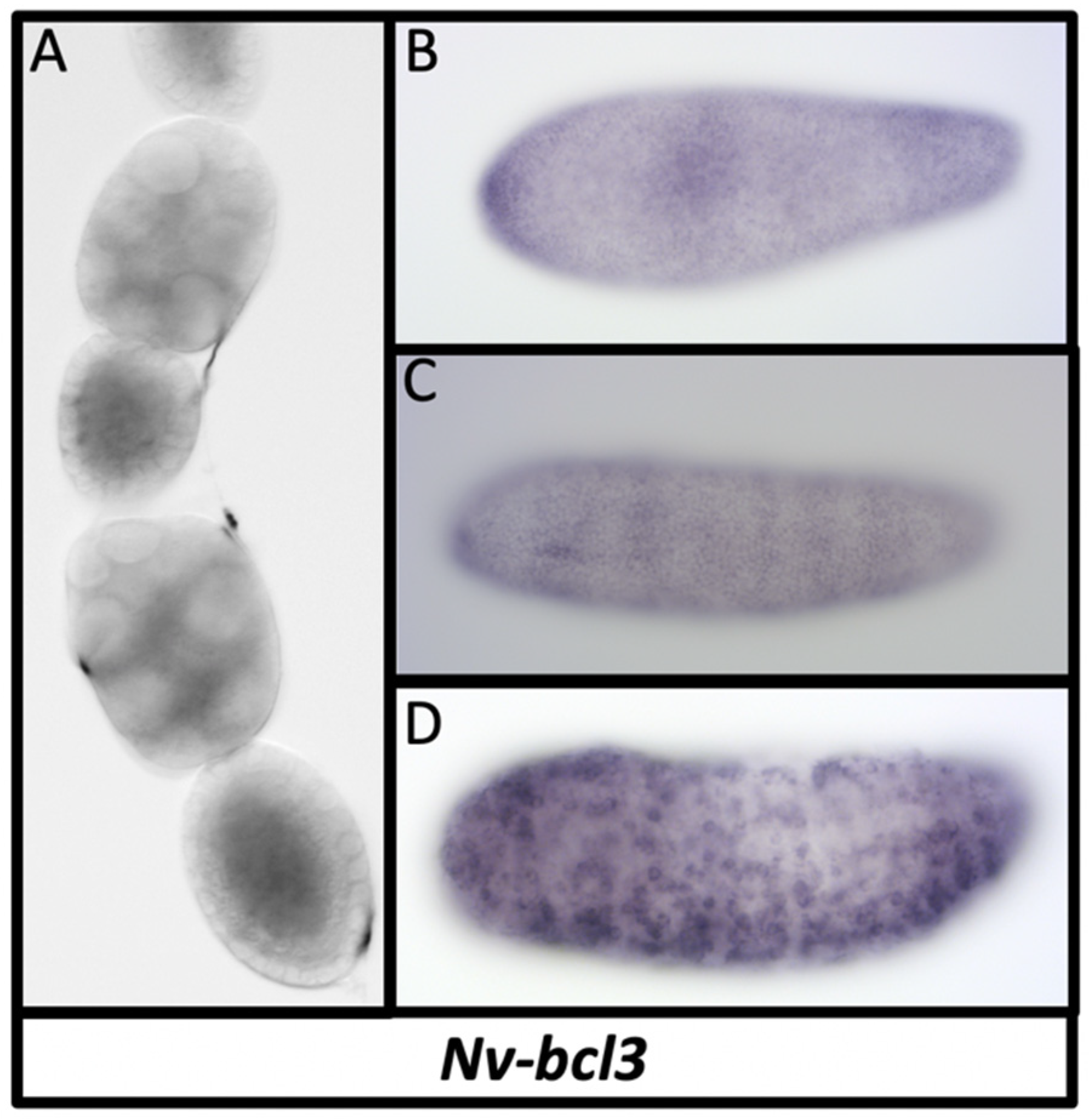
3.11. Dorsal Inhibitors
3.12. Duplications in Dorsal
3.13. Functional Analysis of Pathway Components
Functional Analysis of Nv-Dorsal Paralogs
3.14. Functional Analysis of Other DV Patterning Components
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Werren, J.H.; Richards, S.; Desjardins, C.A.; Niehuis, O.; Gadau, J.; Colbourne, J.K. Functional and evolutionary insights from the genomes of three parasitoid Nasonia species. Science 2010, 327, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Rago, A.; Gilbert, D.G.; Choi, J.H.; Sackton, T.B.; Wang, X.; Kelkar, Y.D.; Werren, J.H.; Colbourne, J.K. OGS2: Genome re-annotation of the jewel wasp Nasonia vitripennis. BMC Genom. 2016, 17, 1–25. [Google Scholar] [CrossRef]
- Werren, J.H.; Loehlin, D.W. The Parasitoid Wasp Nasonia: An Emerging Model System with Haploid Male Genetics. Cold Spring Harb. Protoc. 2009, 2009, pdb-emo134. [Google Scholar] [CrossRef]
- Lynch, J.A.; Brent, A.E.; Leaf, D.S.; Pultz, M.A.; Desplan, C. Localized maternal orthodenticle patterns anterior and posterior in the long germ wasp Nasonia. Nature 2006, 439, 728–732. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.A. The Expanding Genetic Toolbox of the Wasp Nasonia vitripennis and Its Relatives. Genetics 2015, 199, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Pultz, M.A.; Leaf, D.S. The jewel wasp Nasonia: Querying the genome with haplo-diploid genetics. Genesis 2003, 35, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Pultz, A.M.; Pitt, J.N.; Alto, N.M. Extensive zygotic control of the anteroposterior axis in the wasp Nasonia vitripennis. Development 1999, 126, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Buchta, T.; Ozuak, O.; Stappert, D.; Roth, S.; Lynch, J.A. Patterning the dorsal–ventral axis of the wasp Nasonia vitripennis. Dev. Biol. 2013, 381, 189–202. [Google Scholar] [CrossRef]
- Pers, D.; Buchta, T.; Özüak, O.; Wolff, S.; Pietsch, J.M.; Memon, M.B.; Roth, S.; Lynch, J.A. Global analysis of dorsoventral patterning in the wasp Nasonia reveals extensive incorporation of novelty in a regulatory network. BMC Biol. 2016, 14, 1–19. [Google Scholar] [CrossRef]
- Lynch, J.A.; Roth, S. The evolution of dorsal-ventral patterning mechanisms in insects. Genes Dev. 2011, 25, 107–118. [Google Scholar] [CrossRef]
- Moussian, B.; Roth, S. Dorsoventral Axis Formation in the Drosophila Embryo—Shaping and Transducing a Morphogen Gradient. Curr. Biol. 2005, 15, R887–R899. [Google Scholar] [CrossRef] [PubMed]
- Anderson, V.K.; Bokla, L.; Nusslein-Volhard, C. Establishment of Dorsal-Ventral Polarity in the Drosophila Embryo-the Induction of Polarity by the Toll Gene-Product. Cell 1985, 42, 791–798. [Google Scholar] [CrossRef]
- Anderson, V.K.; Jurgens, G.; Nusslein-Volhard, C. Establishment of Dorsal-Ventral Polarity in the Drosophila Embryo-Genetic-Studies on the Role of the Toll Gene-Product. Cell 1985, 42, 779–789. [Google Scholar] [CrossRef]
- Belvin, M.P.; Anderson, K.V. A conserved signaling pathway: The Drosophila Toll-Dorsal Pathway. Annu. Rev. Cell Dev. Biol. 1996, 12, 393–416. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, A.; Stein, D.; Geisler, R.; Hagenmaier, S.; Schmid, B.; Fernandez, N.; Schnell, B.; Nusslein-Volhard, C. A gradient of cytoplasmic Cactus degradation establishes the nuclear localization gradient of the dorsal morphogen in Drosophila. Mech. Dev. 1996, 60, 109–123. [Google Scholar] [CrossRef]
- Hong, J.W.; Hendrix, D.A.; Papatsenko, D.; Levine, M.S. How the Dorsal gradient works: Insights from postgenome technologies. Proc. Natl. Acad. Sci. USA 2008, 105, 20072–20076. [Google Scholar] [CrossRef]
- Steward, R. Dorsal, an Embryonic Polarity Gene in Drosophila, Is Homologous to the Vertebrate Proto-Oncogene, c- rel. Science 1987, 238, 692–694. [Google Scholar] [CrossRef]
- Roth, S.; Stein, D.; Nüsslein-Volhard, C. A gradient of nuclear localization of the dorsal protein determines dorsoventral pattern in the Drosophila embryo. Cell 1989, 59, 1189–1202. [Google Scholar] [CrossRef]
- Stathopoulos, A.; Levine, M. Dorsal Gradient Networks in the Drosophila Embryo. Dev. Biol. 2002, 246, 57–67. [Google Scholar] [CrossRef]
- Stathopoulos, A.; Van Drenth, M.; Erives, A.; Markstein, M.; Levine, M. Whole-Genome Analysis of Dorsal-Ventral Patterning in the Drosophila Embryo. Cell 2002, 111, 687–701. [Google Scholar] [CrossRef]
- Stein, D.S.; Stevens, L.M. Maternal control of the Drosophila dorsal-ventral body axis. Wiley Interdiscip Rev Dev Biol. 2014, 3, 301–330. [Google Scholar] [CrossRef] [PubMed]
- Reeves, T.G.; Stathopoulos, A. Graded dorsal and differential gene regulation in the Drosophila Embryo. Cold Spring Harb. Perspect. Biol. 2009, 1, a000836. [Google Scholar] [CrossRef] [PubMed]
- Rusch, J.; Levine, M. Threshold responses to the dorsal regulatory gradient and the subdivision of primary tissue territories in the Drosophila embryo. Curr. Opin. Genet. Dev. 1996, 6, 416–423. [Google Scholar] [CrossRef]
- Sandmann, T.; Girardot, C.; Brehme, M.; Tongprasit, W.; Stolc, V.; Furlong, E.E. A core transcriptional network for early mesoderm development in Drosophila melanogaster. Genes Dev. 2007, 21, 436–449. [Google Scholar] [CrossRef]
- O’Connor, M.B.; Umulis, D.; Othmer, H.G.; Blair, S.S. Shaping BMP morphogen gradients in the Drosophila embryo and pupal wing. Development 2006, 133, 183–193. [Google Scholar] [CrossRef]
- Leptin, M. twist and snail as positive and negative regulators during Drosophila mesoderm development. Genes Dev. 1991, 5, 1568–1576. [Google Scholar] [CrossRef]
- Jazwinska, A.; Rushlow, C.; Roth, S. The role of brinker in mediating the graded response to Dpp in early Drosophila embryos. Development 1999, 126, 3323–3334. [Google Scholar] [CrossRef]
- Wharton, A.; Ray, R.P.; Gelbart, W.M. An Activity Gradient of Decapentaplegic Is Necessary for the Specification of Dorsal Pattern Elements in the Drosophila Embryo. Development 1993, 117, 807–822. [Google Scholar] [CrossRef]
- Irish, V.F.; Gelbart, W.M. The Decapentaplegic Gene Is Required for Dorsal Ventral Patterning of the Drosophila Embryo. Genes Dev. 1987, 1, 868–879. [Google Scholar] [CrossRef]
- Shimmi, O.; Umulis, D.; Othmer, H.; O’Connor, M.B. Facilitated transport of a Dpp/Scw heterodimer by Sog/Tsg leads to robust patterning of the Drosophila blastoderm embryo. Cell 2005, 120, 873–886. [Google Scholar] [CrossRef]
- Srinivasan, S.; Rashka, K.E.; Bier, E. Creation of a Sog morphogen gradient in the Drosophila embryo. Dev. Cell 2002, 2, 91–101. [Google Scholar] [CrossRef]
- Ashe, L.H.; Levine, M. Local inhibition and long-range enhancement of Dpp signal transduction by Sog. Nature 1999, 398, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Biehs, B.; Francois, V.; Bier, E. The Drosophila short gastrulation gene prevents Dpp from autoactivating and suppressing neurogenesis in the neuroectoderm. Genes Dev. 1996, 10, 2922–2934. [Google Scholar] [CrossRef] [PubMed]
- Marques, G.; Musacchio, M.; Shimell, M.J.; WunnenbergStapleton, K.; Cho, K.W.Y.; OConnor, M.B. Production of a DPP activity gradient in the early Drosophila embryo through the opposing actions of the SOG and TLD proteins. Cell 1997, 91, 417–426. [Google Scholar] [CrossRef]
- Shimell, M.J.; Ferguson, E.L.; Childs, S.R.; Oconnor, M.B. The Drosophila Dorsal-Ventral Patterning Gene Tolloid Is Related to Human Bone Morphogenetic Protein-1. Cell 1991, 67, 469–481. [Google Scholar] [CrossRef]
- Ozuak, O.; Buchta, T.; Roth, S.; Lynch, J.A. Dorsoventral polarity of the Nasonia embryo primarily relies on a BMP gradient formed without input from Toll. Curr. Biol. 2014, 24, 2393–2398. [Google Scholar] [CrossRef]
- Ozuak, O.; Buchta, T.; Roth, S.; Lynch, J.A. Ancient and diverged TGF-beta signaling components in Nasonia vitripennis. Dev. Genes Evol. 2014, 224, 223–233. [Google Scholar] [CrossRef][Green Version]
- Neumansilberberg, S.F.; Schupbach, T. The Drosophila Dorsoventral Patterning Gene Gurken Produces a Dorsally Localized Rna and Encodes a Tgf-Alpha-Like Protein. Cell 1993, 75, 165–174. [Google Scholar] [CrossRef]
- Neumansilberberg, S.F.; Schupbach, T. Dorsoventral Axis Formation in Drosophila Depends on the Correct Dosage of the Gene Gurken. Development 1994, 120, 2457–2463. [Google Scholar] [CrossRef]
- Roth, S. The origin of dorsoventral polarity in Drosophila. Philos. Trans. R. Soc. Lond. Ser. B-Biol. Sci. 2003, 358, 1317–1329. [Google Scholar] [CrossRef]
- Roth, S.; Schupbach, T. The Relationship between Ovarian and Embryonic Dorsoventral Patterning in Drosophila. Development 1994, 120, 2245–2257. [Google Scholar] [CrossRef] [PubMed]
- Gonzalezreyes, A.; Elliott, H.; Stjohnston, D. Polarization of Both Major Body Axes in Drosophila by Gurken-Torpedo Signaling. Nature 1995, 375, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.A.; Peel, A.D.; Drechsler, A.; Averof, M.; Roth, S. EGF Signaling and the Origin of Axial Polarity among the Insects. Curr. Biol. 2010, 20, 1042–1047. [Google Scholar] [CrossRef] [PubMed]
- Andreu, M.J.; Gonzalez-Perez, E.; Ajuria, L.; Samper, N.; Gonzalez-Crespo, S.; Campuzano, S.; Jimenez, G. Mirror represses pipe expression in follicle cells to initiate dorsoventral axis formation in Drosophila. Development 2012, 139, 1110–1114. [Google Scholar] [CrossRef] [PubMed]
- Sen, J.; Goltz, J.S.; Stevens, L.; Stein, D. Spatially restricted expression of pipe in the Drosophila egg chamber defines embryonic dorsal-ventral polarity. Cell 1998, 95, 471–481. [Google Scholar] [CrossRef]
- Konsolaki, M.; Schupbach, T. Windbeutel, a gene required for dorsoventral patterning in Drosophila, encodes a protein that has homologies to vertebrate proteins of the endoplasmic reticulum. Genes Dev. 1998, 12, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Sen, J.; Goltz, J.S.; Konsolaki, M.; Schupbach, T.; Stein, D. Windbeutel is required for function and correct subcellular localization of the Drosophila patterning protein Pipe. Development 2000, 127, 5541–5550. [Google Scholar] [CrossRef]
- Ma, Q.J.; Guo, C.S.; Barnewitz, K.; Sheldrick, G.M.; Soling, H.D.; Uson, I.; Ferrari, D.M. Crystal structure and functional analysis of Drosophila Wind, a protein-disulfide isomerase-related protein. J. Biol. Chem. 2003, 278, 44600–44607. [Google Scholar] [CrossRef]
- Kamiyama, S.; Suda, T.; Ueda, R.; Yoshida, H.; Kikuchi, N.; Chiba, Y.; Goto, S.; Toyoda, H.; Narimatsu, H.; Jigami, Y.; et al. Molecular cloning and identification of 3′-phosphoadenosine 5′-phosphosulfate transporter. Glycobiology 2003, 13, 857. [Google Scholar] [CrossRef]
- Luders, F.; Segawa, H.; Stein, D.; Selva, E.M.; Perrimon, N.; Turco, S.J.; Hacker, U. slalom encodes an adenosine 3′-phosphate 5′-phosphosulfate transporter essential for development in Drosophila. Embo J. 2003, 22, 3635–3644. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Stevens, L.M.; Stein, D. Sulfation of Eggshell Components by Pipe Defines Dorsal-Ventral Polarity in the Drosophila Embryo. Curr. Biol. 2009, 19, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.Y.; Stevens, L.M.; Stein, D. Pipe-Dependent Ventral Processing of Easter by Snake Is the Defining Step in Drosophila Embryo DV Axis Formation. Curr. Biol. 2010, 20, 1133–1137. [Google Scholar] [CrossRef]
- LeMosy, E.K.; Hashimoto, C. The Nudel protease of Drosophila is required for eggshell biogenesis in addition to embryonic patterning. Mol. Biol. Cell 1999, 10, 341a. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.C.; Hashimoto, C. An Unusual Mosaic Protein with a Protease Domain, Encoded by the Nudel Gene, Is Involved in Defining Embryonic Dorsoventral Polarity in Drosophila. Cell 1995, 82, 785–794. [Google Scholar] [CrossRef]
- Konrad, K.D.; Goralski, T.J.; Mahowald, A.P.; Marsh, J.L. The gastrulation defective gene of Drosophila melanogaster is a member of the serine protease superfamily. Proc. Natl. Acad. Sci. USA 1998, 95, 6819–6824. [Google Scholar] [CrossRef] [PubMed]
- Han, J.F.; Lee, S.H.; Tan, Y.Q.; LeMosy, E.K.; Hashimoto, C. Gastrulation defective is a serine protease involved in activating the receptor Toll to polarize the Drosophila embryo. Proc. Natl. Acad. Sci. USA 2000, 97, 9093–9097. [Google Scholar] [CrossRef] [PubMed]
- DeLotto, R.; Spierer, P. A gene required for the specification of dorsal-ventral pattern in Drosophila appears to encode a serine protease. Nature 1986, 323, 688–692. [Google Scholar] [CrossRef] [PubMed]
- Chasan, R.; Anderson, K.V. The Role of Easter, an Apparent Serine Protease, in Organizing the Dorsal Ventral Pattern of the Drosophila Embryo. Cell 1989, 56, 391–400. [Google Scholar] [CrossRef]
- Chasan, R.; Jin, Y.; Anderson, K.V. Activation of the easter zymogen is regulated by five other genes to define dorsal-ventral polarity in the Drosophila embryo. Development 1992, 115, 607–616. [Google Scholar] [CrossRef]
- Haskel-Ittah, M.; Ben-Zvi, D.; Branski-Arieli, M.; Schejter, E.D.; Shilo, B.Z.; Barkai, N. Self-Organized Shuttling: Generating Sharp Dorsoventral Polarity in the Early Drosophila Embryo. Cell 2012, 150, 1016–1028. [Google Scholar] [CrossRef]
- Towb, P.; Galindo, R.L.; Wasserman, S.A. Recruitment of Tube and Pelle to signaling sites at the surface of the Drosophila embryo. Development 1998, 125, 2443–2450. [Google Scholar] [CrossRef] [PubMed]
- Towb, P.; Bergmann, A.; Wasserman, S.A. The protein kinase Pelle mediates feedback regulation in the Drosophila Toll signaling pathway. Development 2001, 128, 4729–4736. [Google Scholar] [CrossRef] [PubMed]
- Towb, P.; Sun, H.; Wasserman, S.A. Tube Is an IRAK-4 homolog in a Toll pathway adapted for development and immunity. J. Innate Immun. 2009, 1, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Yagi, Y.; Tanji, T.; Zhou, S.; Ip, Y.T. Multimerization and interaction of Toll and Spatzle in Drosophila. Proc. Natl. Acad. Sci. USA 2004, 101, 9369–9374. [Google Scholar] [CrossRef]
- Grosshans, J.; Bergmann, A.; Haffter, P.; Nusslein-Volhard, C. Activation of the Kinase Pelle by Tube in the Dorsoventral Signal.-Transduction Pathway of Drosophila Embryo. Nature 1994, 372, 563–566. [Google Scholar] [CrossRef]
- Drier, A.E.; Huang, L.H.; Steward, R. Nuclear import of the Drosophila Rel protein Dorsal is regulated by phosphorylation. Genes Dev. 1999, 13, 556–568. [Google Scholar] [CrossRef]
- Drier, E.A.; Govind, S.; Steward, R. Cactus-independent regulation of Dorsal nuclear import by the ventral signal. Curr. Biol. 2000, 10, 23–26. [Google Scholar] [CrossRef]
- Edwards, D.N.; Towb, P.; Wasserman, S.A. An activity-dependent network of interactions links the Rel protein Dorsal with its cytoplasmic regulators. Development 1997, 124, 3855–3864. [Google Scholar] [CrossRef]
- Reach, M.; Galindo, R.L.; Towb, P.; Allen, J.L.; Karin, M.; Wasserman, S.A. A gradient of cactus protein degradation establishes dorsoventral polarity in the Drosophila embryo. Dev. Biol. 1996, 180, 353–364. [Google Scholar] [CrossRef]
- Fernandez, N.Q.; Grosshans, J.; Goltz, J.S.; Stein, D. Separable and redundant regulatory determinants in Cactus mediate its dorsal group dependent degradation. Development 2001, 128, 2963–2974. [Google Scholar] [CrossRef]
- Daigneault, J.; Klemetsaune, L.; Wasserman, S.A. The IRAK Homolog Pelle Is the Functional Counterpart of IκB Kinase in the Drosophila Toll Pathway. PLoS ONE 2013, 8, e75150. [Google Scholar] [CrossRef] [PubMed]
- Uv, A.E.; Roth, P.; Xylourgidis, N.; Wickberg, A.; Cantera, R.; Samakovlis, C. Members only encodes a Drosophila nucleoporin required for Rel protein import and immune response activation. Genes Dev. 2000, 14, 1945–1957. [Google Scholar] [CrossRef] [PubMed]
- Minakhina, S.; Yang, J.; Steward, R. Tamo selectively modulates nuclear import in Drosophila. Genes Cells 2003, 8, 299–310. [Google Scholar] [CrossRef]
- Hoffmann, A.; Levchenko, A.; Scott, M.L.; Baltimore, D. The IkappaB-NF-kappaB signaling module: Temporal control and selective gene activation. Science 2002, 298, 1241–1245. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Söding, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 2006, 22, 2688–2690. [Google Scholar] [CrossRef]
- Lynch, J.A.; Desplan, C. A method for parental RNA interference in the wasp Nasonia vitripennis. Nat. Protoc. 2006, 1, 486–494. [Google Scholar] [CrossRef]
- Pers, D.; Lynch, J.A. Ankyrin domain encoding genes from an ancient horizontal transfer are functionally integrated into Nasonia developmental gene regulatory networks. Genome Biol. 2018, 19, 148. [Google Scholar] [CrossRef]
- Pechmann, M.; Kenny, N.J.; Pott, L.; Heger, P.; Chen, Y.-T.; Buchta, T.; Özüak, O.; Lynch, J.; Roth, S. Striking parallels between dorsoventral patterning in Drosophila and Gryllus reveal a complex evolutionary history behind a model gene regulatory network. eLife 2021, 10, e68287. [Google Scholar] [CrossRef]
- Wilson, J.M.; Kenny, N.J.; Dearden, P.K. Components of the dorsal-ventral pathway also contribute to anterior-posterior patterning in honeybee embryos (Apis mellifera). EvoDevo 2014, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- DeLotto, Y.; DeLotto, R. Proteolytic processing of the Drosophila Spätzle protein by easter generates a dimeric NGF-like molecule with ventralising activity. Mech. Dev. 1998, 72, 141–148. [Google Scholar] [CrossRef]
- Morisato, D.; Anderson, K.V. The spätzle gene encodes a component of the extracellular signaling pathway establishing the dorsal-ventral pattern of the Drosophila embryo. Cell 1994, 76, 677–688. [Google Scholar] [CrossRef]
- Anthoney, N.; Foldi, I.; Hidalgo, A. Toll and Toll-like receptor signalling in development. Development. 2018, 145, dev156018. [Google Scholar] [CrossRef]
- Benton, M.A.; Pechmann, M.; Frey, N.; Stappert, D.; Conrads, K.H.; Chen, Y.-T.; Stamataki, E.; Pavlopoulos, A.; Roth, S. Toll Genes Have an Ancestral Role in Axis Elongation. Curr. Biol. 2016, 26, 1609–1615. [Google Scholar] [CrossRef]
- Paré, A.C.; Vichas, A.; Fincher, C.T.; Mirman, Z.; Farrell, D.L.; Mainieri, A.; Zallen, J.A. A positional Toll receptor code directs convergent extension in Drosophila. Nature 2014, 515, 523–527. [Google Scholar] [CrossRef]
- Gerttula, S.; Jin, Y.; Anderson, K.V. Zygotic Expression and Activity of the Drosophila Toll Gene, a Gene Required Maternally for Embryonic Dorsal-Ventral Pattern-Formation. Genetics 1988, 119, 123–133. [Google Scholar] [CrossRef]
- Fisher, B.; Weiszmann, R.; Frise, E.; Hammonds, A.; Tomancak, P.; Beaton, A.; Berman, B.; Quan, E.; Shu, S.; Lewis, S.; et al. BDGP Insitu Homepage. 2012. Available online: http://insitu.fruitfly.org/cgi-bin/ex/insitu.pl (accessed on 11 July 2014).
- Tomancak, P.; Beaton, A.; Weiszmann, R.; Kwan, E.; Shu, S.; Lewis, S.E.; Richards, S.; Ashburner, M.; Hartenstein, V.; Celniker, S.E.; et al. Systematic determination of patterns of gene expression during Drosophila embryogenesis. Genome Biol. 2002, 3, 1–14. [Google Scholar] [CrossRef]
- Weiszmann, R.; Hammonds, A.S.; Celniker, S.E. Determination of gene expression patterns using high-throughput RNA in situ hybridization to whole-mount Drosophila embryos. Nat. Protoc. 2009, 4, 605–618. [Google Scholar] [CrossRef]
- Sachs, L.; Chen, Y.-T.; Drechsler, A.; Lynch, J.A.; Panfilio, K.A.; Lässig, M.; Berg, J.; Roth, S. Dynamic BMP signaling polarized by Toll patterns the dorsoventral axis in a hemimetabolous insect. eLife 2015, 4, e05502. [Google Scholar] [CrossRef]
- Nunes da Fonseca, R.; von Levetzow, C.; Kalscheuer, P.; Basal, A.; van der Zee, M.; Roth, S. Self-Regulatory Circuits in Dorsoventral Axis Formation of the Short-Germ Beetle Tribolium castaneum. Dev. Cell 2008, 14, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Wulczyn, G.F.; Naumann, M.; Scheidereit, C. Candidate proto-oncogene bcl-3 encodes a subunit-specific inhibitor of transcription factor NF-κB. Nature 1992, 358, 597–599. [Google Scholar] [CrossRef] [PubMed]
- Morisato, D. Spatzle regulates the shape of the Dorsal gradient in the Drosophila embryo. Development 2001, 128, 2309–2319. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.E.; Tuffery, J.; Bakopoulos, D.; Lequeux, S.; Warr, C.G.; Johnson, T.K.; Dearden, P.K. The torso-like gene functions to maintain the structure of the vitelline membrane in Nasonia vitripennis, implying its co-option into Drosophila axis formation. Biol. Open 2019, 8, bio046284. [Google Scholar] [CrossRef] [PubMed]
- Pultz, M.A.; Zimmerman, K.K.; Alto, N.M.; Kaeberlein, M.; Lange, S.K.; Pitt, J.N.; Reeves, N.L.; Zehrung, D.L. A genetic screen for zygotic embryonic lethal mutations affecting cuticular morphology in the wasp Nasonia vitripennis. Genetics 2000, 154, 1213–1229. [Google Scholar] [CrossRef] [PubMed]
- DeLotto, R. Gastrulation defective, a Complement factor C2/B-like protease, interprets a ventral prepattern in Drosophila. EMBO Rep. 2001, 2, 721–726. [Google Scholar] [CrossRef]







| Drosophila Protein | Nasonia Ortholog | Drosophila Transcript Expression | Nasonia Transcript Expression | RNAi | ||||
|---|---|---|---|---|---|---|---|---|
| Follicular Epithelium Components | Follicle cells | Oocyte | Early Embryo | Follicle cells | Oocyte | Early Embryo | Phenotype | |
| Windbeutel | Nv-Wbl | YES | NO | NO | NO | NO | NO | X |
| Slalom | Nv-Sll | YES | NO | NO | NO | NO | NO | N/A |
| Pipe | Nv-Pip | YES | NO | NO | NO | NO | NO | X |
| Perivitelline Space Components | ||||||||
| Nudel | Nv-Ndl | YES | NO | NO | YES | NO | NO | F |
| Gastrulation defective | Nv-Gd | YES | YES | YES | YES | YES | NO | X |
| Nv-Gd2 | - | - | - | NO | NO | YES | X | |
| Snake | Nv-Snk | NO | YES | YES | NO | NO | YES | X |
| Nv-Snk2 | - | - | - | NO | NO | NO | N/A | |
| Easter | Nv-Ea1 | NO | YES | YES | NO | YES | NO | X |
| Nv-Ea2 | - | - | - | NO | NO | NO | X | |
| Serpin27A | Nv-Spn27A | NO | YES | YES | NO | NO | YES | N/A |
| Spatzle | Nv-Spz1 | NO | YES | YES | NO | YES | NO | D |
| Nv-Spz2 | - | - | - | NO | NO | YES | D | |
| Toll Receptors | ||||||||
| Toll | Nv-TollA | NO | YES | YES | NO | YES | YES | D |
| Nv-TollB | - | - | - | NO | NO | NO | X | |
| Nv-TollC | - | - | - | NO | YES | YES | X | |
| Nv-TollD | - | - | - | NO | YES | YES | I | |
| Intracellular Components | ||||||||
| Tube | Nv-Tub | NO | YES | YES | NO | YES | YES | I |
| Pelle | Nv-Pll1 | NO | YES | YES | NO | NO | YES | I |
| Nv-Pll2 | - | - | - | NO | NO | YES | D | |
| Myd88 | Nv-Myd88 | NO | YES | YES | NO | NO | YES | I |
| Cactus | Nv-Cact1 | NO | YES | YES | YES | YES | YES | X |
| Nv-Cact2 | - | - | - | YES | YES | YES | X | |
| Nv-Cact3 | - | - | - | YES | YES | NO | X | |
| B-cell lymphoma 3 (mammalian) | Nv-Bcl3 | N/A | N/A | N/A | NO | YES | YES | X |
| Dorsal | Nv-Dl1 | NO | YES | YES | NO | YES | YES | X |
| Nv-Dl2 | - | - | - | NO | YES | YES | X | |
| Nv-Dl3 | - | - | - | NO | YES | YES | X | |
| Nv-Dl4 | - | - | - | NO | YES | YES | X | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pers, D.; Buchta, T.; Özüak, O.; Roth, S.; Lynch, J.A. Expression and Function of Toll Pathway Components in the Early Development of the Wasp Nasonia vitripennis. J. Dev. Biol. 2022, 10, 7. https://doi.org/10.3390/jdb10010007
Pers D, Buchta T, Özüak O, Roth S, Lynch JA. Expression and Function of Toll Pathway Components in the Early Development of the Wasp Nasonia vitripennis. Journal of Developmental Biology. 2022; 10(1):7. https://doi.org/10.3390/jdb10010007
Chicago/Turabian StylePers, Daniel, Thomas Buchta, Orhan Özüak, Siegfried Roth, and Jeremy A. Lynch. 2022. "Expression and Function of Toll Pathway Components in the Early Development of the Wasp Nasonia vitripennis" Journal of Developmental Biology 10, no. 1: 7. https://doi.org/10.3390/jdb10010007
APA StylePers, D., Buchta, T., Özüak, O., Roth, S., & Lynch, J. A. (2022). Expression and Function of Toll Pathway Components in the Early Development of the Wasp Nasonia vitripennis. Journal of Developmental Biology, 10(1), 7. https://doi.org/10.3390/jdb10010007






