Actin Filament in the First Cell Cycle Contributes to the Determination of the Anteroposterior Axis in Ascidian Development
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Experiments
2.2. Whole-Mount Immunofluorescent Staining
2.3. Phalloidin Staining
2.4. Whole-Mount RNA In Situ Hybridization
2.5. Image Acquisition and Data Analysis
3. Results
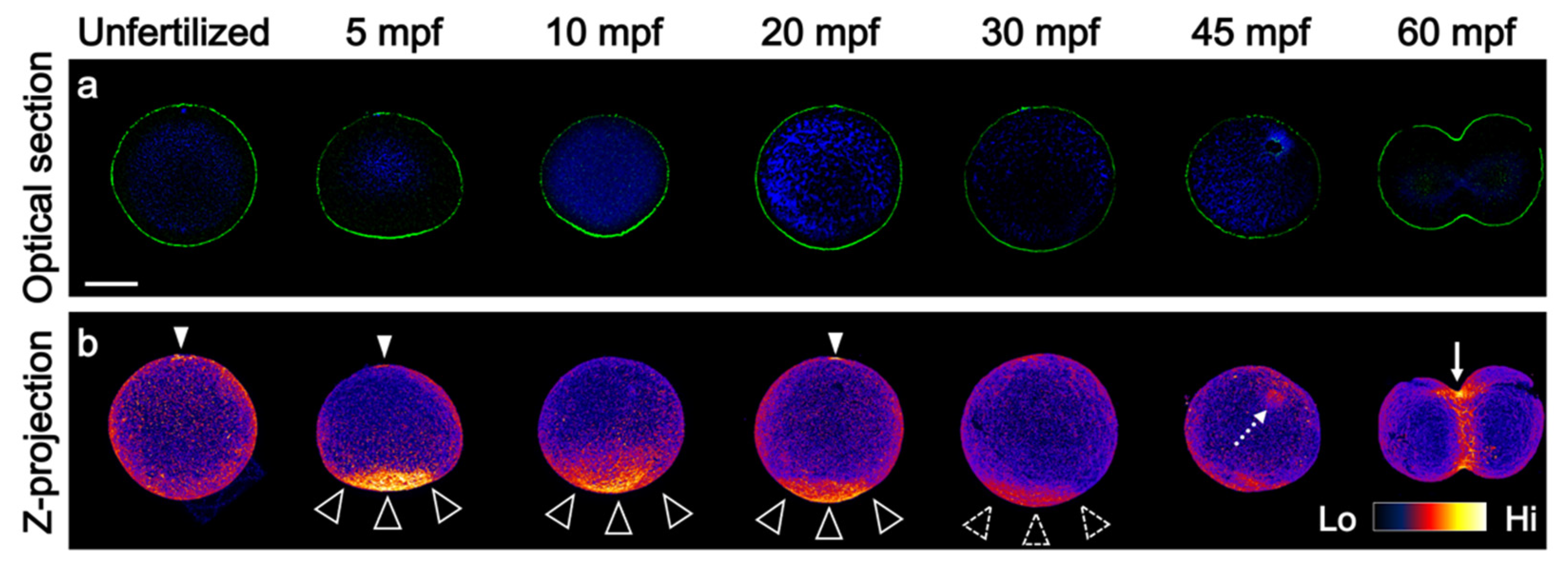
3.1. Prolonged F-Actin Accumulation at the Vegetal Cortex
3.2. Actin Depolymerization Induced Malformation of CAMP
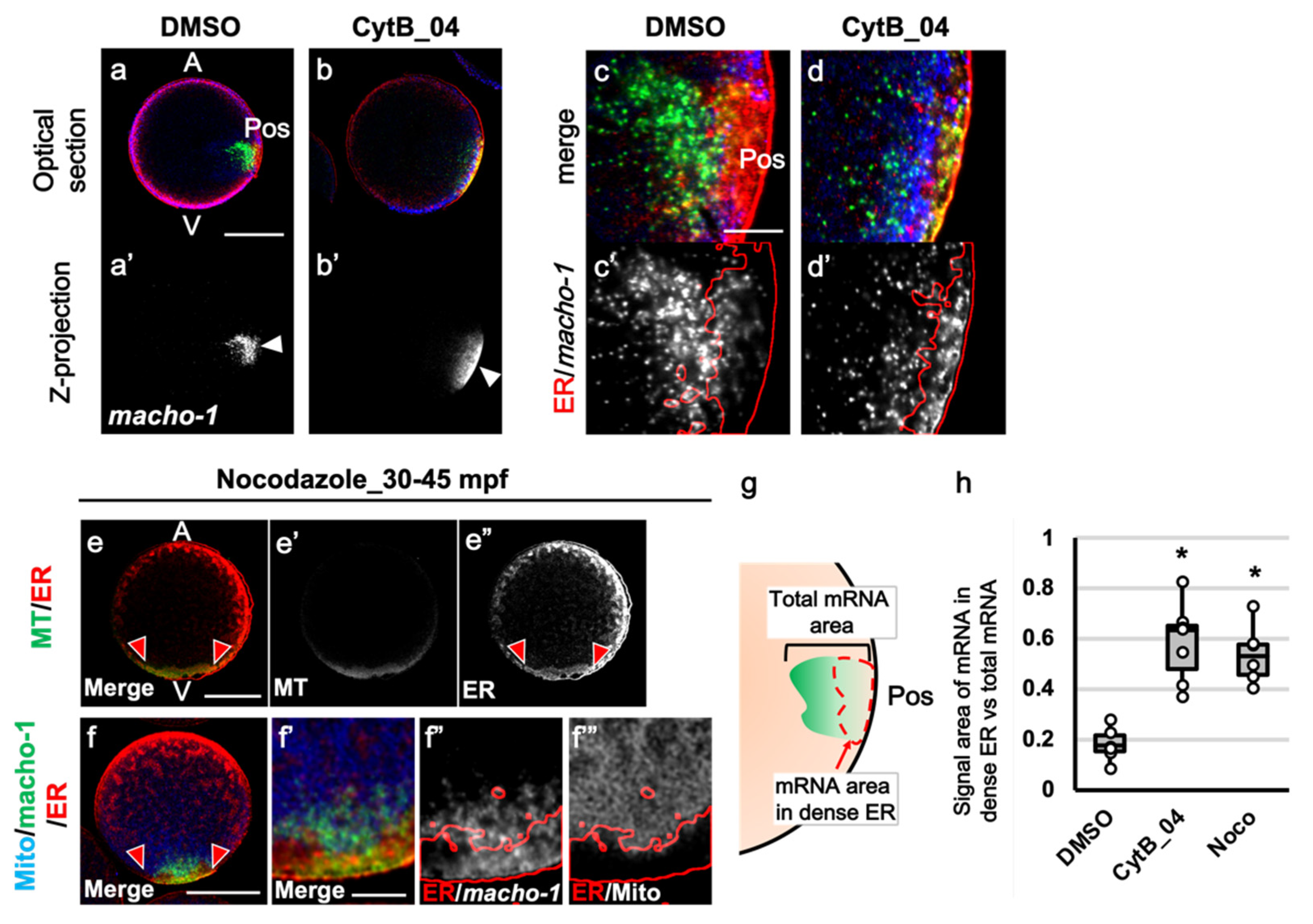
3.3. The Prolonged F-Actin Localization Contributed to the Midline Accumulation of Myoplasm and Maternal mRNA
3.4. The Role of F-Actin during the Second Phase of Ooplasmic Segregation
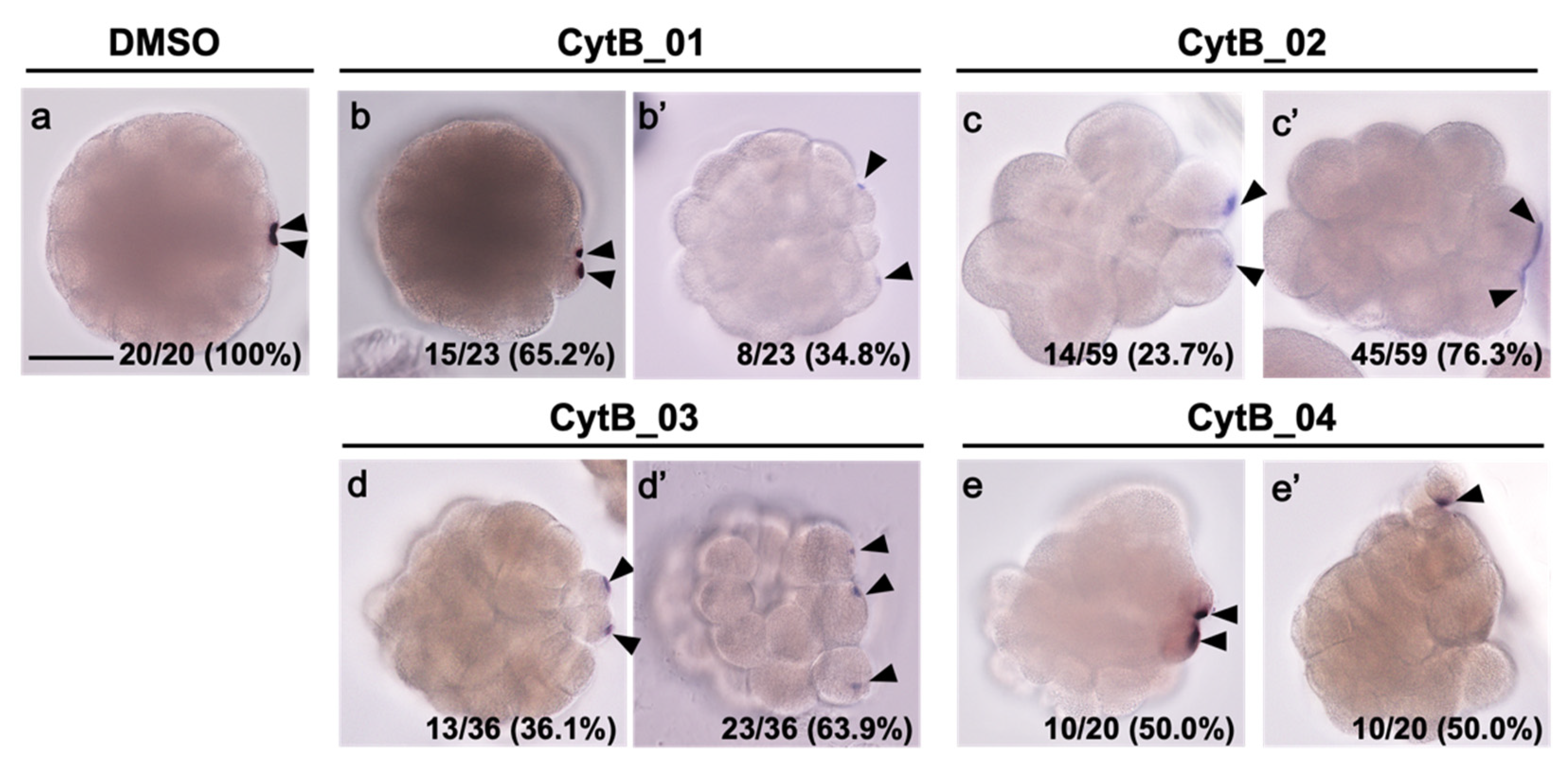
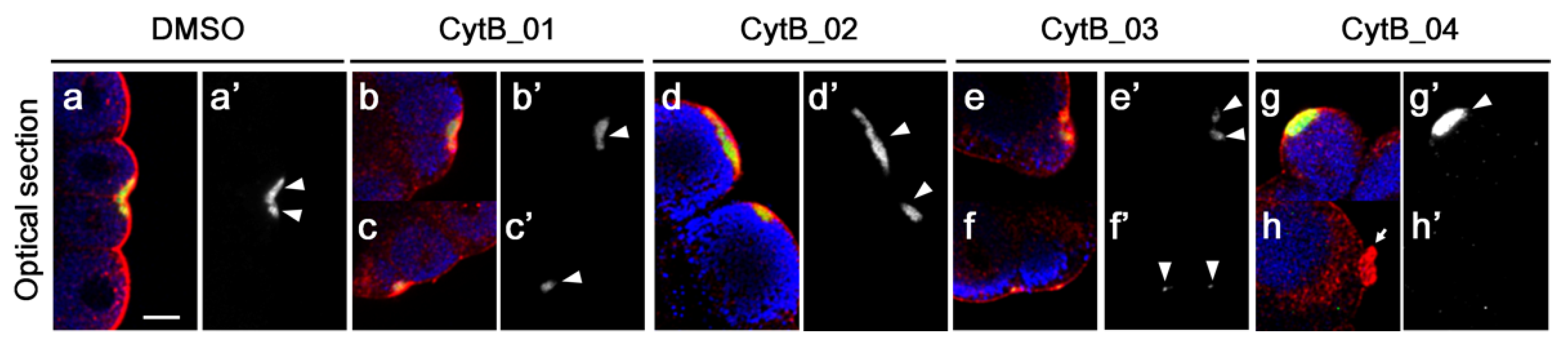
3.5. F-Actin Contributed to the Cleavage Patterning during the First Cell Cycle
3.6. The Disordered Morphogenesis Induced by Cytb Treatment during First-Cell Cycle
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miller, J.R.; Rowning, B.A.; Larabell, C.A.; Yang-Snyder, J.A.; Bates, R.L.; Moon, R.T. Establishment of the Dorsal–Ventral Axis in Xenopus Embryos Coincides with the Dorsal Enrichment of Dishevelled that Is Dependent on Cortical Rotation. J. Cell Biol. 1999, 146, 427–438. [Google Scholar] [CrossRef]
- Tao, Q.; Yokota, C.; Puck, H.; Kofron, M.; Birsoy, B.; Yan, D.; Asashima, M.; Wylie, C.C.; Lin, X.; Heasman, J. Maternal Wnt11 Activates the Canonical Wnt Signaling Pathway Required for Axis Formation in Xenopus Embryos. Cell 2005, 120, 857–871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, L.D.; Hino, H.; Quach, H.; Lim, S.; Shindo, A.; Mimori-Kiyosue, Y.; Mione, M.C.; Ueno, N.; Winkler, C.; Hibi, M.; et al. Dynamic Microtubules at the Vegetal Cortex Predict the Embryonic Axis in Zebrafish. Development 2012, 139, 3644–3652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyczak, R.; Gomes, J.E.; Bowerman, B. Heads or Tails: Cell Polarity and Axis Formation in the Early Caenorhabditis Elegans Embryo. Dev. Cell 2002, 3, 157–166. [Google Scholar] [CrossRef] [Green Version]
- Nance, J.; Zallen, J.A. Elaborating Polarity: PAR Proteins and the Cytoskeleton. Development 2011, 138, 799–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sardet, C.; Nishida, H.; Prodon, F.; Sawada, K. Maternal mRNAs of PEM and Macho 1, the Ascidian Muscle Determinant, Associate and Move with a Rough Endoplasmic Reticulum Network in the Egg Cortex. Development 2003, 130, 5839–5849. [Google Scholar] [CrossRef] [Green Version]
- Prodon, F.; Yamada, L.; Shirae-Kurabayashi, M.; Nakamura, Y.; Sasakura, Y. Postplasmic/PEM RNAs: A Class of Localized Maternal mRNAs with Multiple Roles in Cell Polarity and Development in Ascidian Embryos. Dev. Dyn. 2007, 236, 1698–1715. [Google Scholar] [CrossRef]
- Sardet, C.; Paix, A.; Prodon, F.; Dru, P.; Chenevert, J. From Oocyte to 16-Cell Stage: Cytoplasmic and Cortical Reorganizations that Pattern the Ascidian Embryo. Dev. Dyn. 2007, 236, 1716–1731. [Google Scholar] [CrossRef] [PubMed]
- Sawada, T.-O.; Schatten, G. Effects of Cytoskeletal Inhibitors on Ooplasmic Segregation and Microtubule Organization during Fertilization and Early Development in the Ascidian Molgula Occidentalis. Dev. Biol. 1989, 132, 331–342. [Google Scholar] [CrossRef]
- Chiba, S.; Miki, Y.; Ashida, K.; Wada, M.R.; Tanaka, K.J.; Shibata, Y.; Nakamori, R.; Nishikata, T. Interactions Between Cyto-Skeletal Components during Myoplasm Rearrangement in Ascidian Eggs. Dev. Growth Differ. 1999, 41, 265–272. [Google Scholar] [CrossRef]
- Roegiers, F.; Djediat, C.; Dumollard, R.; Rouviere, C.; Sardet, C. Phases of Cytoplasmic and Cortical Reorganizations of the Ascidian Zygote between Fertilization and First Division. Development 1999, 126, 3101–3117. [Google Scholar] [CrossRef] [PubMed]
- Ishii, H.; Goto, T.; Nishikata, T. Microtubule Array Observed in the Posterior-Vegetal Cortex during Cytoplasmic and Cortical Reorganization of the Ascidian Egg. Dev. Growth Differ. 2017, 59, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Kanda, K.; Nishikata, T. Non-Centrosomal Microtubule Structures Regulated by Egg Activation Signaling Contribute to Cytoplasmic and Cortical Reorganization in the Ascidian Egg. Dev. Biol. 2018, 448, 161–172. [Google Scholar] [CrossRef]
- Goto, T.; Torii, S.; Kondo, A.; Kawakami, J.; Yagi, H.; Suekane, M.; Kataoka, Y.; Nishikata, T. Dynamic Changes in the Association between Maternal mRNAs and Endoplasmic Reticulum during Ascidian Early Embryogenesis. Dev. Genes Evol. 2021, 1–14. [Google Scholar] [CrossRef]
- Hibino, T.; Nishikata, T.; Nishida, H. Centrosome-Attracting Body: A Novel Structure Closely Related to Unequal Cleavages in the Ascidian Embryo. Dev. Growth Differ. 1998, 40, 85–95. [Google Scholar] [CrossRef]
- Nishikata, T.; Hibino, T.; Nishida, H. The Centrosome-Attracting Body, Microtubule System, and Posterior Egg Cytoplasm Are Involved in Positioning of Cleavage Planes in the Ascidian Embryo. Dev. Biol. 1999, 209, 72–85. [Google Scholar] [CrossRef] [Green Version]
- Ishii, H.; Kunihiro, S.; Tanaka, M.; Hatano, K.; Nishikata, T. Cytosolic Subunits of ATP Synthase are Localized to the Cortical Endoplasmic Reticulum-Rich Domain of the Ascidian Egg Myoplasm. Dev. Growth Differ. 2012, 54, 753–766. [Google Scholar] [CrossRef] [PubMed]
- Ishii, H.; Shirai, T.; Makino, C.; Nishikata, T. Mitochondrial Inhibitor Sodium Azide Inhibits the Reorganization of Mitochondria-Rich Cytoplasm and the Establishment of the Anteroposterior Axis in Ascidian Embryo. Dev. Growth Differ. 2014, 56, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Ojima, K.; Lin, Z.X.; Andrade, I.R.; Costa, M.L.; Mermelstein, C. Distinctive Effects of Cytochalasin B in Chick Primary My-oblasts and Fibroblasts. PLoS ONE 2016, 11, e0154109. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, T.; Lewis, A.; Hayashi, E.; Betenbaugh, M.J.; Su, T.P. Antigen Retrieval to Improve the Immunocytochemistry De-tection of Sigma-1 Receptors and ER Chaperones. Histochem. Cell Biol. 2011, 135, 627–637. [Google Scholar] [CrossRef] [Green Version]
- Prodon, F.; Hanawa, K.; Nishida, H. Actin Microfilaments Guide the Polarized Transport of Nuclear Pore Complexes and the Cytoplasmic Dispersal of Vasa mRNA during GVBD in the Ascidian Halocynthia Roretzi. Dev. Biol. 2009, 330, 377–388. [Google Scholar] [CrossRef]
- Ke, M.-T.; Fujimoto, S.; Imai, T. SeeDB: A Simple and Morphology-Preserving Optical Clearing Agent for Neuronal Circuit Reconstruction. Nat. Neurosci. 2013, 16, 1154–1161. [Google Scholar] [CrossRef]
- Satou, Y.; Satoh, N. Cataloging Transcription Factor and Major Signaling Molecule Genes for Functional Genomic Studies in Ciona Intestinalis. Dev. Genes Evol. 2005, 215, 580–596. [Google Scholar] [CrossRef]
- Chenevert, J.; Pruliere, G.; Ishii, H.; Sardet, C.; Nishikata, T. Purification of Mitochondrial Proteins HSP60 and ATP Synthase from Ascidian Eggs: Implications for Antibody Specificity. PLoS ONE 2013, 8, e52996. [Google Scholar] [CrossRef] [Green Version]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDougall, A.; Hebras, C.; Pruliere, G.; Burgess, D.; Costache, V.; Dumollard, R.; Chenevert, J. Role of PB1 Midbody Remnant Creating Tethered Polar Bodies during Meiosis II. Genes 2020, 11, 1394. [Google Scholar] [CrossRef]
- Jeffery, W.R.; Meier, S. A Yellow Crescent Cytoskeletal Domain in Ascidian Eggs and its Role in Early Development. Dev. Biol. 1983, 96, 125–143. [Google Scholar] [CrossRef]
- Dogterom, M.; Koenderink, G.H. Actin–Microtubule Crosstalk in Cell Biology. Nat. Rev. Mol. Cell Biol. 2018, 20, 38–54. [Google Scholar] [CrossRef]
- Noordstra, I.; Liu, Q.; Nijenhuis, W.; Hua, S.; Jiang, K.; Baars, M.; Remmelzwaal, S.; Martin, M.; Kapitein, L.C.; Akhmanova, A. Control of Apico-Basal Epithelial Polarity by the Microtubule Minus-End Binding Protein CAMSAP3 and Spectraplakin ACF7. J. Cell Sci. 2016, 129, 4278–4288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koppers, M.; Özkan, N.; Farías, G.G. Complex Interactions between Membrane-Bound Organelles, Biomolecular Condensates and the Cytoskeleton. Front. Cell Dev. Biol. 2020, 8, 1661. [Google Scholar] [CrossRef] [PubMed]
- Frey, S.; Pool, M.; Seedorf, M. Scp160p, an RNA-Binding, Polysome-Associated Protein, Localizes to the Endoplasmic Reticulum of Saccharomyces Cerevisiae in a Microtubule-Dependent Manner. J. Biol. Chem. 2001, 276, 15905–15912. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, K.J.; Matsumoto, K.; Tsujimoto, M.; Nishikata, T. CiYB1 Is a Major Component of Storage mRNPs in Ascidian Oocytes: Implications in Translational Regulation of Localized mRNAs. Dev. Biol. 2004, 272, 217–230. [Google Scholar] [CrossRef] [Green Version]
- Paix, A.; Yamada, L.; Dru, P.; Lecordier, H.; Pruliere, G.; Chenevert, J.; Satoh, N.; Sardet, C. Cortical Anchorages and Cell Type Segregations of Maternal Postplasmic/PEM RNAs in Ascidians. Dev. Biol. 2009, 336, 96–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costache, V.; Hebras, C.; Pruliere, G.; Besnardeau, L.; Failla, M.; Copley, R.R.; Burgess, D.; Chenevert, J.; McDougall, A. Kif2 Localizes to a Subdomain of Cortical Endoplasmic Reticulum that Drives Asymmetric Spindle Position. Nat. Commun. 2017, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Negishi, T.; Takada, T.; Kawai, N.; Nishida, H. Localized PEM mRNA and Protein Are Involved in Cleavage-Plane Orientation and Unequal Cell Divisions in Ascidians. Curr. Biol. 2007, 17, 1014–1025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumano, G.; Nishida, H. Patterning of an Ascidian Embryo along the Anterior–Posterior Axis through Spatial Regulation of Competence and Induction Ability by Maternally Localized PEM. Dev. Biol. 2009, 331, 78–88. [Google Scholar] [CrossRef] [Green Version]
- Paix, A.; Le Nguyen, P.N.; Sardet, C. Bi-Polarized Translation of Ascidian Maternal mRNA Determinant Pem-1 Associated with Regulators of the Translation Machinery on Cortical Endoplasmic Reticulum (cER). Dev. Biol. 2011, 357, 211–226. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goto, T.; Torii, S.; Kondo, A.; Kanda, K.; Kawakami, J.; Kataoka, Y.; Nishikata, T. Actin Filament in the First Cell Cycle Contributes to the Determination of the Anteroposterior Axis in Ascidian Development. J. Dev. Biol. 2022, 10, 10. https://doi.org/10.3390/jdb10010010
Goto T, Torii S, Kondo A, Kanda K, Kawakami J, Kataoka Y, Nishikata T. Actin Filament in the First Cell Cycle Contributes to the Determination of the Anteroposterior Axis in Ascidian Development. Journal of Developmental Biology. 2022; 10(1):10. https://doi.org/10.3390/jdb10010010
Chicago/Turabian StyleGoto, Toshiyuki, Shuhei Torii, Aoi Kondo, Kazumasa Kanda, Junji Kawakami, Yosky Kataoka, and Takahito Nishikata. 2022. "Actin Filament in the First Cell Cycle Contributes to the Determination of the Anteroposterior Axis in Ascidian Development" Journal of Developmental Biology 10, no. 1: 10. https://doi.org/10.3390/jdb10010010
APA StyleGoto, T., Torii, S., Kondo, A., Kanda, K., Kawakami, J., Kataoka, Y., & Nishikata, T. (2022). Actin Filament in the First Cell Cycle Contributes to the Determination of the Anteroposterior Axis in Ascidian Development. Journal of Developmental Biology, 10(1), 10. https://doi.org/10.3390/jdb10010010





