Canadian Forest Fires and the Effects of Long-Range Transboundary Air Pollution on Hospitalizations among the Elderly
Abstract
:1. Introduction
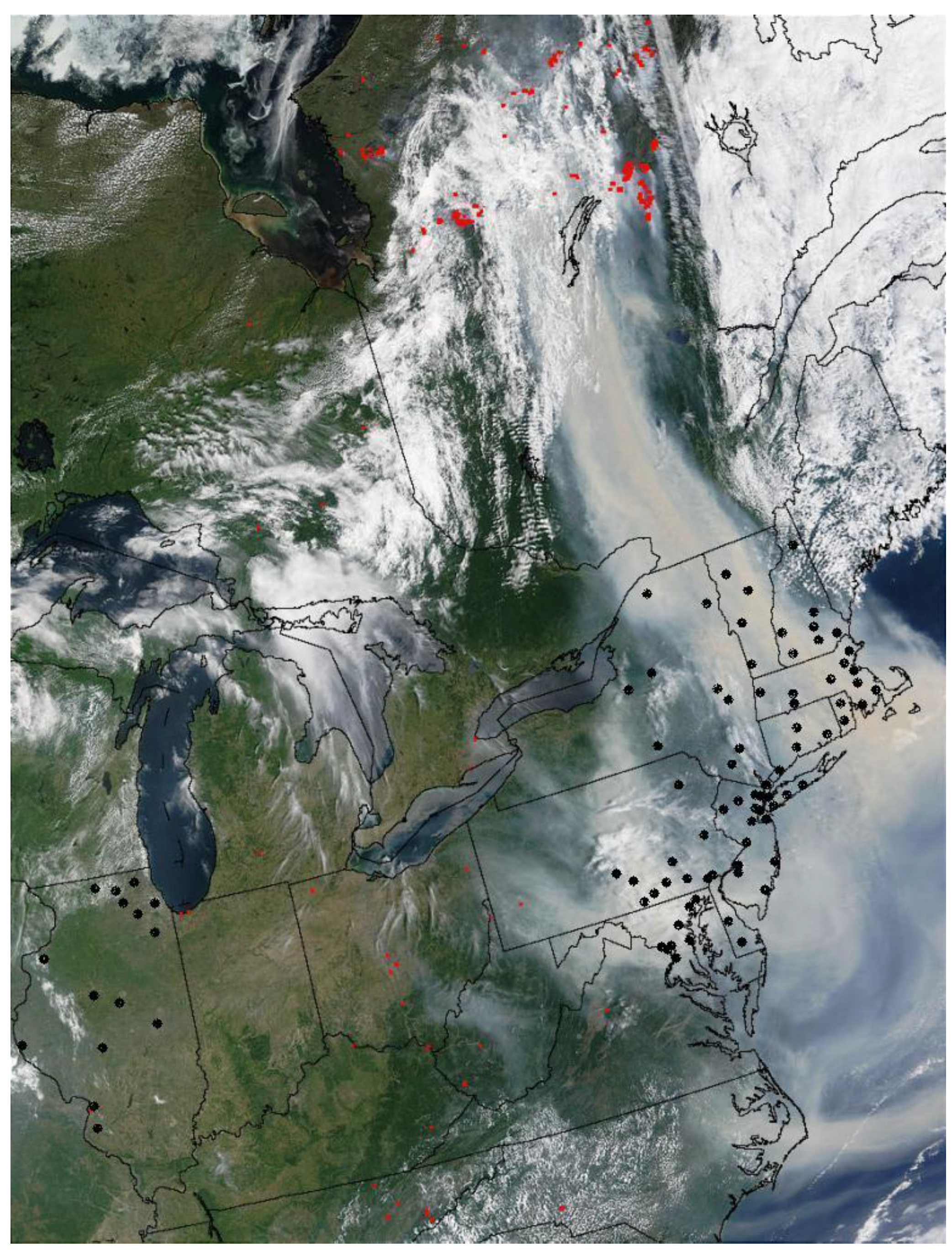
2. Methods
2.1. Study Area
2.2. Data Sources
2.3. Statistical Analysis
| Reason for Hospital Admission | ICD-9 Codes |
|---|---|
| Respiratory Outcomes | (460–519) |
| Chronic Obstructive Pulmonary Disease (COPD) | (490–492, 496) |
| Asthma | (493) |
| Respiratory Tract Infections | (464–466, 480–487) |
| Cardiovascular Outcomes | (390–492, 496) |
| Cerebrovascular Disease | (430–438) |
| Heart Failure | (440–448) |
| Stroke | (410) |
| Peripheral Vascular Disease | (410–414) |
| Myocardial Infarction | (426–427) |
| Ischemic Heart Disease | (426–428) |
| Heart Rhythm Disturbances | (46–427) |
| Heart Disease | (426–428) |
| Other Heart Disease | (420–425, 428) |
| Hypertension | (401–405) |
| Injury | (800–849) |
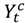 and
and 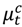 be the observed and expected daily number of cause-specific hospitalizations,
be the observed and expected daily number of cause-specific hospitalizations, 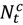 be the number of people at risk on day t in county c,
be the number of people at risk on day t in county c, 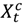 be the daily average PM2.5 for county c,
be the daily average PM2.5 for county c, 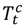 and
and  the daily average temperature and dew point temperature for county c. We fit the following log-linear regression model:
the daily average temperature and dew point temperature for county c. We fit the following log-linear regression model:
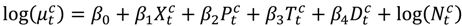
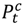 an indicator variable representing haze versus non-haze period and
an indicator variable representing haze versus non-haze period and 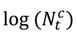 representing the regression offset. The parameter β1 denotes the log relative risk of cause-specific hospitalization associated with one unit (µg/m3) increase in daily average PM2.5 (block kriged county estimates) adjusted for temperature and dew-point temperature. The parameter β2 denotes the increase in log relative rate of hospitalizations during the haze period compared to that during the non-haze period.
representing the regression offset. The parameter β1 denotes the log relative risk of cause-specific hospitalization associated with one unit (µg/m3) increase in daily average PM2.5 (block kriged county estimates) adjusted for temperature and dew-point temperature. The parameter β2 denotes the increase in log relative rate of hospitalizations during the haze period compared to that during the non-haze period. 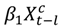 in the county-specific model (1) for
in the county-specific model (1) for 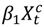 , where l = 0, 1, 2 days and
, where l = 0, 1, 2 days and 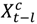 is the PM2.5 concentration for county c on day t at a lag of l days [63]. A lag of 0 days corresponds to the association between PM2.5 concentrations on a given day and the risk of hospitalization on the same day. We also applied distributed lag models [64,65] to estimate the relative risk of hospitalization associated with cumulative exposure over the current day and the previous two days. The distributed lag models are obtained by substituting
is the PM2.5 concentration for county c on day t at a lag of l days [63]. A lag of 0 days corresponds to the association between PM2.5 concentrations on a given day and the risk of hospitalization on the same day. We also applied distributed lag models [64,65] to estimate the relative risk of hospitalization associated with cumulative exposure over the current day and the previous two days. The distributed lag models are obtained by substituting  in the county-specific model (Equation (1)) for
in the county-specific model (Equation (1)) for 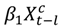 . Models with a PM2.5 by haze period interaction
. Models with a PM2.5 by haze period interaction 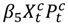 were also considered allowing for the relative risk of hospitalizations associated with PM2.5 exposure to change during the haze period compared to the non-haze period.
were also considered allowing for the relative risk of hospitalizations associated with PM2.5 exposure to change during the haze period compared to the non-haze period.3. Results
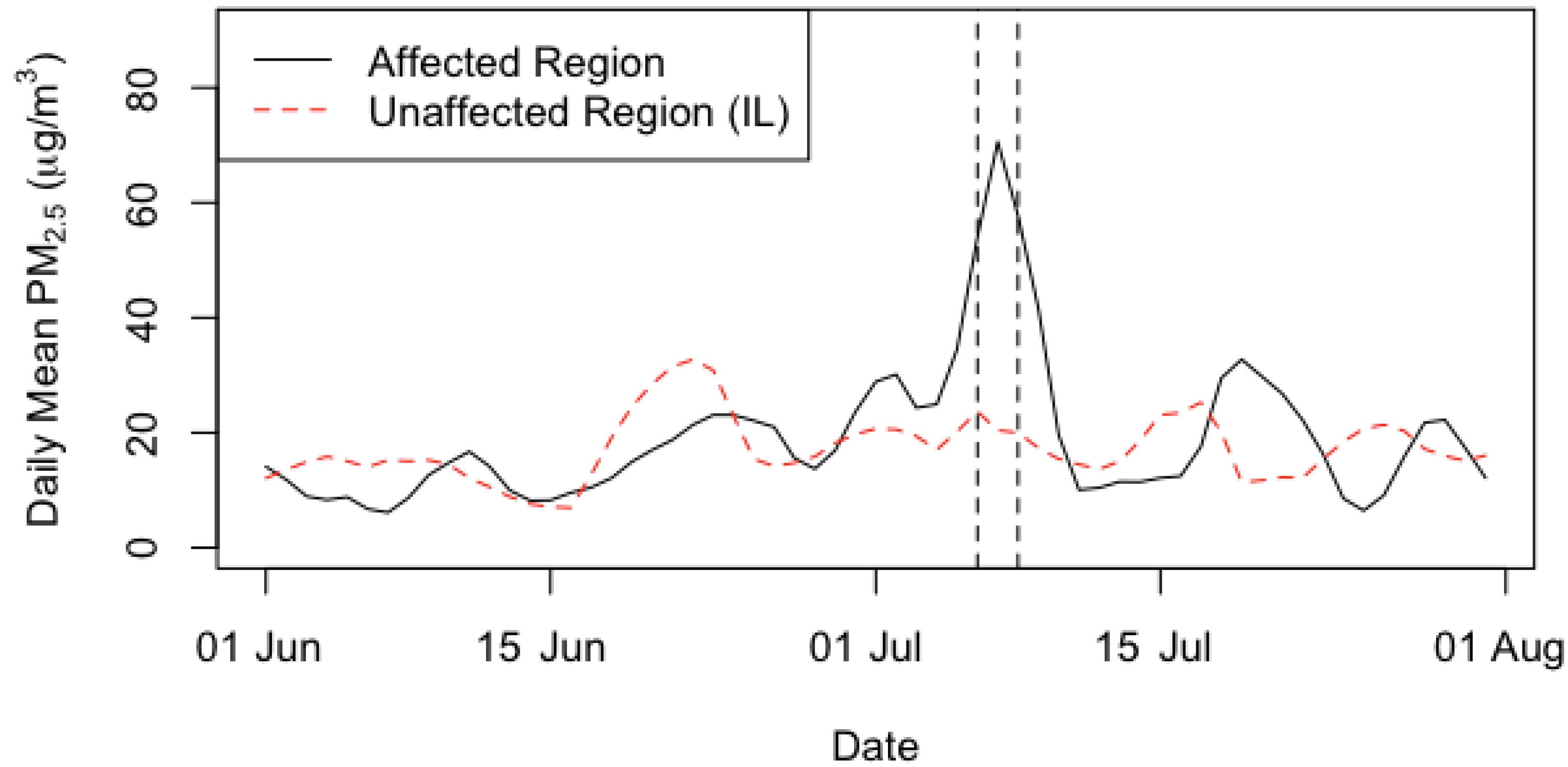
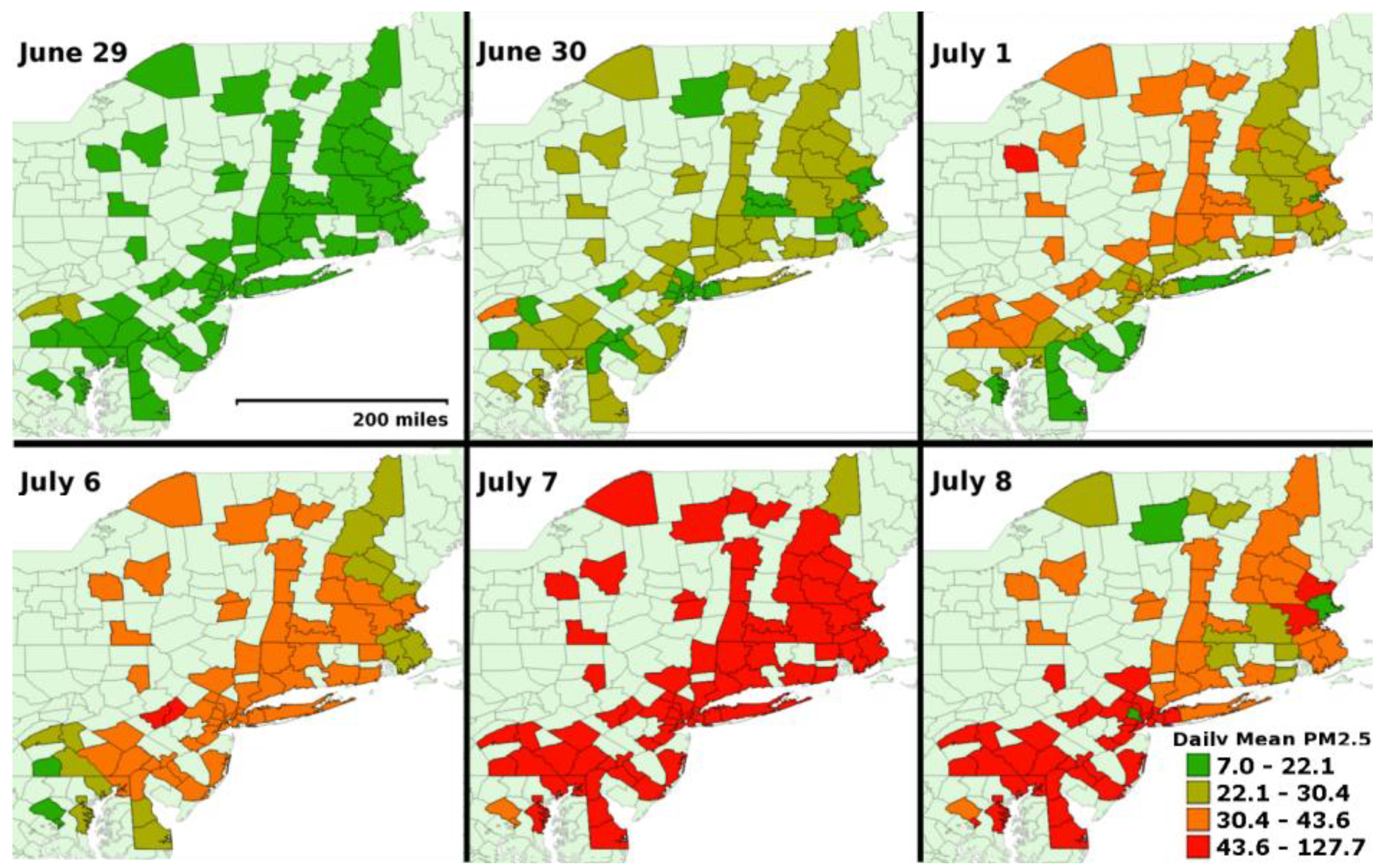
| Summary Statistics | Affected Region | Unaffected Region (IL) | ||
|---|---|---|---|---|
| Haze | Non-Haze | Haze | Non-Haze | |
| Min | 17.6 | 7.0 | 12.2 | 15.2 |
| 25th %tile | 35.6 | 13.7 | 16.0 | 19.0 |
| Median | 43.1 | 22.6 | 20.7 | 20.7 |
| Mean | 53.0 | 21.5 | 22.4 | 20.8 |
| 75th %tile | 69.1 | 25.3 | 29.7 | 22.1 |
| Max | 127.7 | 43.7 | 33.0 | 28.4 |
| SD | 25.0 | 10.3 | 7.2 | 5.0 |
| Hospitalization Codes | PM2.5 Model Lag | Percent Change | 95% Confidence Interval |
|---|---|---|---|
| All Respiratory | Lag 0 | 49.55 | (29.82, 72.29) |
| Lag 1 | 21.14 | (2.69, 42.90) | |
| Lag 2 | 23.36 | (10.20, 38.10) | |
| Dlag1 | 30.20 | (7.66, 57.46) | |
| Dlag2 | 6.48 | (−14.63, 32.81) | |
| All Cardiovascular | Lag 0 | 64.93 | (44.30, 88.51) |
| Lag 1 | 36.06 | (18.43, 56.32) | |
| Lag 2 | 49.28 | (34.39, 65.81) | |
| Dlag1 | 33.85 | (12.45, 59.33) | |
| Dlag2 | 8.31 | (−13.89, 36.24) | |
| Injury | Lag 0 | 3.04 | (−21.31, 34.92) |
| Lag 1 | 10.97 | (−14.04, 43.27) | |
| Lag 2 | 13.23 | (−5.64, 35.89) | |
| Dlag1 | 0.37 | (−25.69, 35.55) | |
| Dlag2 | −6.04 | (−30.50, 27.02) |
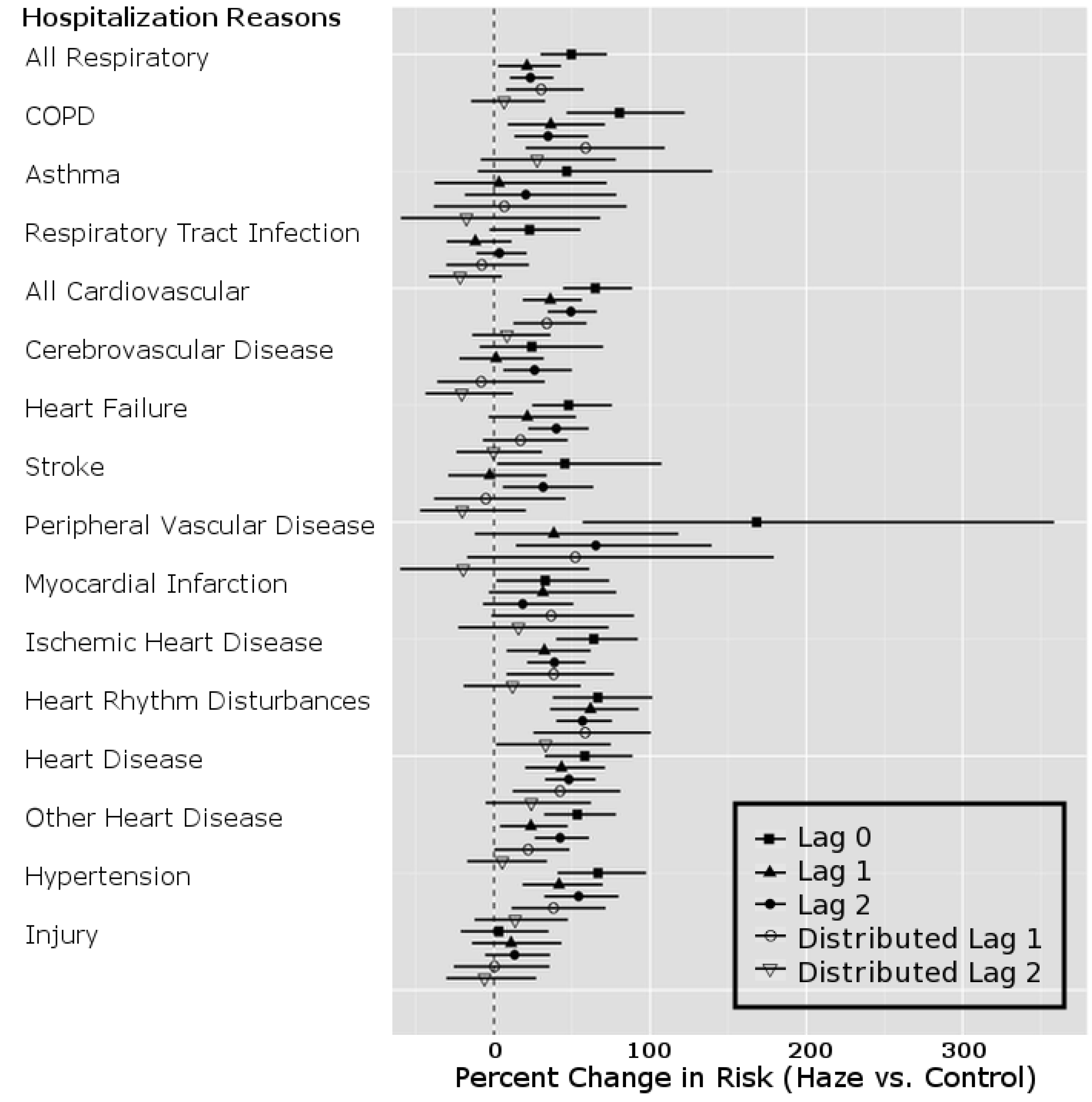
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ward, D. Health Guidelines for Vegetation Fire Events, Background Papers. In Smoke from Wildland Fires; WHO: Geneva, Switzerland, 1999; pp. 70–85. [Google Scholar]
- Miller, D.J. Assessing boreal forest fire smoke aerosol impacts on U.S. air quality: A case study using multiple data sets. J. Geophys. Res. 2011, 116, 1–19. [Google Scholar]
- Mathur, R. Estimating the impact of the 2004 Alaskan forest fires on episodic particulate matter pollution over the eastern Unites States through assimilation of satellite derived aerosol optical depths in a regional air quality model. J. Geophys. Res. 2008, 113, 1–14. [Google Scholar]
- Jalava, P.; Salonen, R.; Halinen, A.; Penttinen, P.; Pennanen, A.; Sillanpaa, M.; Sandell, E.; Hillamo, R.; Hirvonen, M.R. In vitro inflammatory and cytotoxic effects of size-segregated particulate samples collected during long-range transport of wildfire smoke to Helsinki. Toxicol. Appl. Pharmacol. 2006, 215, 341–353. [Google Scholar] [CrossRef]
- Sapkota, A.; Symons, J.; Kleissl, J.; Wang, L.; Parlange, M.; Ondov, J.; Breysse, P.N.; Diette, G.B.; Eggleston, P.A.; Buckley, T.J. Impact of the 2002 Canadian forest fires on particulate matter air quality in Baltimore city. Environ. Sci. Technol. 2005, 39, 24–32. [Google Scholar] [CrossRef]
- Morris, G.A.; Hershey, S.; Thompson, A.M.; Stohl, A.; Colarco, P.R.; McMillan, W.W.; Warner, J.; Johnson, B.J.; Witte, J.C.; Kucsera, T.L.; et al. Alaskan and Canadian forest fires exacerbate ozone pollution in Houston, Texas, on 19 and 20 July. J. Geophys. Res. 2004, 111, 1–10. [Google Scholar]
- Ovadnevaite, J.; Kvietkus, K.; Marsalka, A. 2002 summer fires in Lithuania: Impact on the Vilnius city air quality and the inhabitants health. Sci. Total Environ. 2006, 356, 11–21. [Google Scholar] [CrossRef]
- Mott, J.; Mannino, D.M.; Alverson, C.; Kiyu, A.; Hashim, J.; Lee, T.; Falter, K.; Redd, S.C. Cardiorespiratory hospitalizations associated with smoke exposure during the 1997, southeast Asian forest fires. Int. J. Hyg. Environ. Health 2005, 208, 75–85. [Google Scholar] [CrossRef]
- Heil, A.; Goldammer, J. Smoke-haze pollution: A review of the 1997 episode in southeast Asia. Reg. Environ. Chang. 2001, 2, 24–37. [Google Scholar] [CrossRef]
- Wotawa, G.; Trainer, M. The influence of Canadian forest fires on pollutant concentrations in the United States. Science 2000, 288, 324–328. [Google Scholar] [CrossRef]
- Health Guidelines for Vegetation Fire Events. In Technical Report; World Health Organization: Geneva, Switzerland, 1999.
- Andreae, M. Biomass burning in the tropics: Impact on environmental quality and global climate. Popul. Dev. Rev. 1990, 16, 268–291. [Google Scholar] [CrossRef]
- Duclos, P.; Sanderson, L.M.; Lipsett, M. The 1987 forest fire disaster in California: Assessment of emergency room visits. Arch. Environ. Health 1990, 45, 53–58. [Google Scholar] [CrossRef]
- Emmanuel, S.C. Impact to lung health of haze from forest fires: The Singapore experience. Respirology 2000, 5, 175–182. [Google Scholar] [CrossRef]
- Johnston, F.H.; Webby, R.J.; Pilotto, L.S.; Bailie, R.S.; Parry, D.L.; Halpin, S.J. Vegetation fires, particulate air pollution and asthma: A panel study in the Australian monsoon tropics. Int. J. Environ. Health Res. 2006, 16, 391–404. [Google Scholar] [CrossRef]
- Rappold, A.G.; Stone, S.L.; Cascio, W.E.; Neas, L.M.; Kilaru, V.J.; Carraway, M.S.; Szykman, J.J.; Ising, A.; Cleve, W.E.; Meredith, J.T.; et al. Peat bog wildfire smoke exposure in rural north Carolina is associated with cardiopulmonary emergency department visits assessed through syndromic surveillance. Environ. Health Perspect. 2011, 119, 1415–1420. [Google Scholar] [CrossRef]
- Sastry, N. Forest fires, air pollution and mortality in southeast Asia. Demography 2002, 39, 1–23. [Google Scholar] [CrossRef]
- Larson, T.V.; Koenig, J.Q. Wood smoke: Emissions and noncancer respiratory effects. Annu. Rev. Public Health 1994, 15, 133–156. [Google Scholar] [CrossRef]
- Delfino, R.J.; Brummel, S.; Wu, J.; Stern, H.; Ostro, B.; Lipsett, M.; Winer, A.; Street, D.H.; Zhang, L.; Tjoa, T.; et al. The relationship of respiratory and cardiovascular hospital admissions to the southern California wildfires of 2003. Occup. Environ. Med. 2009, 66, 189–197. [Google Scholar] [CrossRef]
- Henderson, S.B.; Brauer, M.; MacNab, Y.C.; Kennedy, S.M. Three measures of forest fire smoke exposure and their associations with respiratory and cardiovascular health outcomes in a population-based cohort. Environ. Health Perspect. 2011, 119, 1266–1271. [Google Scholar] [CrossRef]
- Johnston, F.H.; Bailie, R.S.; Pilotto, L.S.; Hanigan, I.C. Ambient biomass smoke and cardio-respiratory hospital admissions in Darwin, Australia. BMC Public Health 2007, 7. [Google Scholar] [CrossRef]
- Kunii, O.; Kanagawa, S.; Yajima, I.; Hisamatsu, Y.; Yamamura, S.; Amagai, T.; Ismail, I.T. The 1997 haze disaster in Indonesia: Its air quality and health effects. Arch. Environ. Health 2002, 174, 16–22. [Google Scholar]
- Ostro, B.; Broadwin, R.; Green, S.; Feng, W.; Lipsett, M. Fine particulate air pollution and mortality in nine California counties: Results from Calfine. Environ. Health Perspect. 2006, 114, 29–33. [Google Scholar]
- Pope, C.A., III; Dockery, D.W. Health effects of particulate air pollution: Lines that connect. J. Air Waste Manag. Assoc. 2006, 56, 709–742. [Google Scholar] [CrossRef]
- Rückerl, R.; Schneider, A.; Breitner, S.; Cyrys, J.; Peters, A. Health effects of particulate air pollution: A review of epidemiological evidence. Inhal. Toxicol. 2011, 23, 555–592. [Google Scholar] [CrossRef]
- Samet, J.M.; Domnici, F.; Curriero, F.; Coursac, I.; Zeger, S. Fine particulate air pollution and mortality in 20 U.S. cities: 1987–1994. N. Engl. J. Med. 2000, 342, 1742–1757. [Google Scholar] [CrossRef]
- Schwartz, J.; Dockery, D.W.; Neas, L. Is daily mortality associated specifically with fine particles? J. Air Waste Manag. Assoc. 1996, 46, 927–939. [Google Scholar] [CrossRef]
- Zanobetti, A.; Schwartz, J. Air pollution and emergency admissions in Boston, MA, USA. J. Epidemiol. Community Health 2006, 60, 890–896. [Google Scholar] [CrossRef]
- Aditama, T. Impact of haze from forest fire to respiratory health: Indonesian experience. Respirology 2000, 5, 169–174. [Google Scholar] [CrossRef]
- Künzli, N.; Avol, E.; Wu, J.; Gauderman, W.J.; Rappaport, E.; Millstein, J.; Bennion, J.; McConnell, R.; Gilliland, F.D.; Berhane, K.; et al. Health effects of the 2003 Southern California wildfire on children. Amer. J. Respir. Crit. Care Med. 2006, 174, 1221–1228. [Google Scholar] [CrossRef]
- Moore, D.; Copes, R.; Fisk, R.; Joy, R.; Chan, K.; Brauer, M. Population health effects of air quality changes due to forest fires in British Columbia in 2003: Estimates from physician-visit billing data. Can. J. Public Health 2006, 97, 105–108. [Google Scholar]
- Naeher, L.P.; Brauer, M.; Lipsett, M.; Zelikoff, J.T.; Simpson, C.D.; Koenig, J.Q.; Smith, K.R. Woodsmoke health effects: A review. Inhal. Toxicol. 2007, 19, 67–106. [Google Scholar]
- Vedal, S. Ambient particles and health: Lines that divide. J. Air Waste Manag. Assoc. 1997, 37, 551–581. [Google Scholar] [CrossRef]
- Mott, J.; Meyer, P.; Mannino, D.M.; Redd, S.; Smith, E.; Gotway-Crawford, C.; Chase, E. Wildland forest fire smoke: Health effects and intervention evaluation, Hoopa, California, 1999. West. J. Med. 2002, 176, 157–162. [Google Scholar] [CrossRef]
- Schreuder, A.; Larson, T.; Sheppard, L.; Claiborn, C. Ambient woodsmoke and associated respiratory emergency department visits in Spokane, Washington. Int. J. Occup. Environ. Health 2006, 12, 147–153. [Google Scholar] [CrossRef]
- Shusterman, D.; Kaplan, J.; Canabarro, C. Immediate health effects of an urban wildfire. West. J. Med. 1993, 158, 133–138. [Google Scholar]
- Sutherland, E.; Make, B.; Vedal, S.; Zhang, L.; Dutton, S.; Murphy, J.; Silkoff, P.E. Wildfire smoke and respiratory symptoms in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2005, 115, 420–422. [Google Scholar] [CrossRef]
- Viswanathan, S.; Eria, L.; Diunugala, N.; Johnson, J.; McClean, C. An analysis of effects of San Diego wildfire on ambient air quality. J. Air Waste Manag. Assoc. 2006, 56, 56–67. [Google Scholar] [CrossRef]
- Report of the Biregional Workshop on the Health Impacts of Haze Related Air Pollution. In Technical Report RS/98/GE/17(MAA); Regional Office for the Western Pacific, World Health Organization: Manila, Philippines, 1998.
- American Lung Association. State of the Air: 2004. In Technical Report; American Lung Association: New York, NY, USA, 2004. [Google Scholar]
- Anderson, H.; Atkinson, R.; Peacock, J.; Marston, L.; Konstantinou, K. Meta-Analysis of Time-Series Studies and Panel Studies of Particulate Matter (PM) and Ozone (O3). In Technical Report; World Health Organization (WHO): Geneva, Switzerland, 2004. [Google Scholar]
- Annesi-Maesano, I.; Agabiti, N.; Pistelli, R.; Couilliot, M.; Forastiere, F. Subpopulations at increased risk of adverse health outcomes from air pollution. Eur. Respir. J.Suppl. 2003, 40, S57–S63. [Google Scholar]
- Barnett, A.; Williams, G.; Schwartz, J.; Best, T.; Neller, A.; Petroeschevsky, A.; Simpson, R. The effects of air pollution on hospitalizations for cardiovascular disease in elderly people in Australian and New Zealand cities. Environ. Health Perspect. 2006, 114, 1018–1023. [Google Scholar] [CrossRef]
- Boezen, H.; Vonk, J.; van der Zee, S.; Gerritsen, J.; Hoek, G.; Brunekreef, B.; Schouten, J.P.; Postma, D.S. Susceptibility to air pollution in elderly males and females. Eur. Respir. J. 2005, 25, 1018–1024. [Google Scholar] [CrossRef]
- Brook, R.; Franklin, B.; Cascio, W.; Hong, Y.; Howard, G.; Lipsett, M.; Luepker, R.; Mittleman, M.; Samet, J.; Smith, S.C., Jr.; et al. Air pollution and cardiovascular disease: A statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation 2004, 109, 2655–2671. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Q.; Krewski, D.; Burnett, R.; Shi, Y.; McGrail, K. The effect of coarse ambient particulate matter on first, second, and overall hospital admissions for respiratory disease among the elderly. Inhal. Toxicol. 2005, 17, 649–655. [Google Scholar] [CrossRef]
- Goldberg, M.S.; Burnett, R.; Bailar, J., III; Brook, J.; Bonvalot, Y.; Tamblyn, R.; Singh, R.; Valois, M. Identifying Subgroups of 19 the General Population that may be Susceptible to Short-Term Increases in Particulate Air Pollution: A Time-Series Study in Montreal, Quebec. In Research Report 097; Health Effects Institute: Cambridge, MA, USA, 2000. [Google Scholar]
- Pope, C.A., III. Epidemiology of fine particulate air pollution and human health: Biologic mechanisms and who’s at risk? Environ. Health Perspect. 2000, 108, 713–723. [Google Scholar] [CrossRef]
- Pope, C.A., III; Burnett, R.; Thun, M.; Calle, E.; Krewski, D.; Ito, K.; Thurston, G.D. Lung cancer, cardiopulmonary mortality and long-term exposure to particulate air pollution. JAMA 2002, 287, 1132–1141. [Google Scholar] [CrossRef]
- Villeneuve, P.; Chen, L.; Stieb, D.; Rowe, B. Associations between outdoor air pollution and emergency department visits for stroke in Edmonton, Canada. Eur. J. Epidemiol. 2006, 21, 689–700. [Google Scholar] [CrossRef]
- DeBell, L.J.; Talbot, R.; Dibb, J. A major regional air pollution event in the northeastern United States caused by extensive forest fires in Quebec, Canada. J. Geophys. Res. 2004, 109, D19305. [Google Scholar] [CrossRef]
- NASA: Visible Earth. Wildfires in Quebec and Smoke over United States East Coast. Available online: http://visibleearth.nasa.gov/view.php?id=60610 (accessed on 1 February 2011).
- GFMC (Global Fire Monitoring Center)/Fire Ecology Research Group. 2007 UN International Strategy for Disaster Reduction (ISDR). Available online: http://www.fire.uni-freiburg.de/ (accessed on 3 December 2010).
- International Classification of Diseases, Ninth Revision (ICD-9); World Health Organization: Geneva, Switzerland, 1977.
- U.S. Environmental Protection Agency. Air Quality System Data Mart (Internet Database). Available online: http://www.epa.gov/ttn/airs/aqsdatamart (accessed on 1 February 2011).
- National Climatic Data Center. NCDC Surface Airways, CD Database; EarthInfo Inc.: Boulder, CO, USA, 2011. [Google Scholar]
- Cressie, N.A.; Cassie, N.A. Statistics for Spatial Data; Wiley: New York, NY, USA, 1993. [Google Scholar]
- Young, L.J.; Gotway, C.A.; Yang, J.; Kearney, G.; DuClos, C. Linking health and environmental data in geographical analysis: It’s so much more than centroids. Spat. Spatiotemporal Epidemiol. 2009, 1, 73–84. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing, 2014. Available online: Available online: www.r-project.org (accessed on 1 February 2014).
- Dominici, F.; Peng, R.; Bell, M.; Pham, L.; McDermott, A.; Zeger, S.; Samet, J.M. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA 2006, 295, 1127–1134. [Google Scholar] [CrossRef]
- Eftim, S.E.; Janes, H.; McDermott, A.; Samet, J.; Dominici, F. A comparative analysis of the chronic effects of fine particulate matter. Epidemiology 2008, 19, 209–216. [Google Scholar] [CrossRef]
- Liang, K.-Y.; Zeger, S. Longitudinal data analysis using generalized linear models. Biometrika 1986, 73, 13–22. [Google Scholar] [CrossRef]
- Peng, D.; Dominici, F. Statistical Methods for Environmental Epidemiology with R; Springer Science, Business Media, LLC: New York, NY, USA, 2008. [Google Scholar]
- Schwartz, J. The distributed lag between air pollution and daily deaths. Epidemiology 2000, 1, 320–326. [Google Scholar] [CrossRef]
- Welty, L.; Zeger, S. Are the acute effects of particulate matter on mortality in the national morbidity, mortality, and air pollution study the result of inadequate control for weather and season? A sensitivity analysis using flexible distributed lag models. Amer. J. Epidemiol. 2005, 162, 80–88. [Google Scholar] [CrossRef]
- Morgan, G.; Sheppard, V.; Khalaj, B.; Ayyar, A.; Lincoln, D.; Jalaludin, B.; Beard, J.; Corbett, S.; Lumley, T. Effects of brushfire smoke on daily mortality and hospital admissions in Sidney, Australia. Epidemiology 2010, 21, 47–55. [Google Scholar] [CrossRef]
- Vedal, S.; Steven, J.D. Wildfire air pollution and daily mortality in a large urban area. Environ. Res. 2006, 102, 29–35. [Google Scholar] [CrossRef]
- Dennekamp, M.; Abramson, M.J. The effects of bushfire smoke on respiratory health. Respirology 2011, 16, 198–209. [Google Scholar] [CrossRef]
- Dockery, D.; Ware, J.; Ferris, B.J.; Speizer, F.; Cook, N.; Herman, S. Change in pulmonary function in children associated with air pollution episodes. J. Air Pollut. Control Assoc. 1982, 32, 937–942. [Google Scholar] [CrossRef]
- Roemer, W.; Hoek, G.; Brunekreef, B. Effect of ambient winter air pollution on respiratory health of children with chronic respiratory symptoms. Amer. Rev. Resp. Dis. 1993, 147, 118–124. [Google Scholar] [CrossRef]
- Hoek, G.; Brunekreef, B. Effect of photochemical air pollution on acute respiratory symptoms in children. Amer. J. Resp. Crit. Care Med. 1995, 151, 27–32. [Google Scholar] [CrossRef]
- Hoek, G.; Dockery, D.; Pope, C.A.; Neas, L.; Roemer, W.; Brunekreef, B. Association between PM10 and decrements in peak expiratory flow rates in children: Reanalysis of data from five panel studies. Eur. Resp. J. 1998, 11, 1307–1311. [Google Scholar] [CrossRef]
- Brook, R.D.; Rajagopalan, S.; Pope, C.A.; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Holguin, F.; Hong, Y.; Luepker, R.V.; Mittleman, M.A.; et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 2010, 121, 2331–2378. [Google Scholar] [CrossRef]
- Sin, D.; Man, S. Chronic obstructive pulmonary disease as a risk factor for cardiovascular morbidity and mortality. Proc. Amer. Thorac. Soc. 2005, 2, 8–11. [Google Scholar] [CrossRef]
- Sin, D.; Wu, L.; Man, S. The relationship between reduced lung function and cardiovascular mortality: A population-based study and a systematic review of the literature. Chest 2005, 127, 1952–1959. [Google Scholar] [CrossRef]
- Van Eeden, S.; Yeung, A.; Quinlam, K.; Hogg, J. Systemic response to ambient particulate matter: Relevance to chronic obstructive pulmonary disease. Proc. Amer. Thorac. Soc. 2005, 2, 61–67. [Google Scholar] [CrossRef]
- Swiston, J.R.; Davidson, W.; Attridge, S.; Li, G.T.; Brauer, M.; van Eeden, S.F. Wood smoke exposure induces a pulmonary and systemic inflammatory response in fire fighters. Eur. Resp. J. 2008, 32, 129–138. [Google Scholar] [CrossRef]
- Peters, A.; Döring, A.; Wichmann, H.E.; Koenig, W. Increased plasma viscosity during an air pollution episode: A link to mortality? Lancet 1997, 349, 1582–1587. [Google Scholar] [CrossRef]
- Dominici, F.; Sheppard, L.; Clyde, M. Statistical review of air pollution. Int. Statist. Rev. 2003, 71, 243–276. [Google Scholar] [CrossRef]
- Baicker, K.; Chandra, A.; Skinner, J.S.; Wennberg, J.E. Who you are and where you live: How race and geography affect the treatment of Medicare beneficiaries. Health Affair. 2004, 23. [Google Scholar] [CrossRef]
- Fisher, E.; Whaley, F.; Krushat, W.; Malenka, D.; Fleming, C.; Baron, J.A.; Hsia, D.C. The accuracy of Medicare’s hospital claims data: Progress has been made, but problems remain. Amer. J. Public Health 1992, 82, 243–248. [Google Scholar] [CrossRef]
- Havranek, E.; Wolfe, P.; Masoudi, F.; Rathore, S.; Krumholz, H.; Ordin, D. Provider and hospital characteristics associated with geographic variation in the evaluation and management of elderly patients with heart failure. Arch. Intern. Med. 2004, 164, 1186–1191. [Google Scholar]
- Kiyota, Y.; Schneeweiss, S.; Glynn, R.; Cannuscio, C.; Avorn, J.; Solomon, D. Accuracy of Medicare claims-based diagnosis of acute myocardial infarction: Estimating positive predictive value on the basis of review of hospital records. Amer. Heart J. 2004, 148, 99–104. [Google Scholar] [CrossRef]
- Janssen, N.A.H.; Schwartz, J.; Zanobetti, A.; Suh, H.H. Air conditioning and source-specific particles as modifiers of the effect of PM10 on hospital admissions for heart and lung disease. Environ. Health 2002, 110, 43–49. [Google Scholar]
- Analitis, A.; Georgiadis, I.; Katsouyanni, K. Forest fires are associated with elevated mortality in a dense urban setting. Occup. Environ. Med. 2012, 69, 158–162. [Google Scholar]
- Hanigan, I.C.; Johnston, F.H.; Morgan, G.G. Vegetation fire smoke, indigenous status and cardio-respiratory hospital admissions in Darwin, Australia, 1996–2005: A time-series study. Environ. Health 2008, 7. [Google Scholar] [CrossRef]
- See, S.; Balasubramanian, R.; Rianawati, E.; Karthikevyan, S.; Streets, D. Characterization and source apportionment of particulate matter ≤2.5 μm in Sumatra, Indonesia, during a recent peat fire episode. Environ. Sci. Technol. 2007, 41, 3488–3494. [Google Scholar] [CrossRef]
- IPCC 2007: Climate Change: Synthesis Report. Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team; Pachauri, R.K.; Reisinger, A. (Eds.) IPCC: Geneva, Switzerland, 2007; p. 104.
- Westerling, A.L.; Hidalgo, H.G.; Cayan, D.R.; Swetnam, T.W. Warming and earlier spring increases western U.S. forest wildfire activity. Science 2006, 313, 940–943. [Google Scholar] [CrossRef]
- Pechony, O.; Shindell, D.T. Driving forces of global wildfires over the past millennium and the forthcoming century. Proc. Nat. Acad. Sci. USA 2010, 107, 19167–19170. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Le, G.E.; Breysse, P.N.; McDermott, A.; Eftim, S.E.; Geyh, A.; Berman, J.D.; Curriero, F.C. Canadian Forest Fires and the Effects of Long-Range Transboundary Air Pollution on Hospitalizations among the Elderly. ISPRS Int. J. Geo-Inf. 2014, 3, 713-731. https://doi.org/10.3390/ijgi3020713
Le GE, Breysse PN, McDermott A, Eftim SE, Geyh A, Berman JD, Curriero FC. Canadian Forest Fires and the Effects of Long-Range Transboundary Air Pollution on Hospitalizations among the Elderly. ISPRS International Journal of Geo-Information. 2014; 3(2):713-731. https://doi.org/10.3390/ijgi3020713
Chicago/Turabian StyleLe, George E., Patrick N. Breysse, Aidan McDermott, Sorina E. Eftim, Alison Geyh, Jesse D. Berman, and Frank C. Curriero. 2014. "Canadian Forest Fires and the Effects of Long-Range Transboundary Air Pollution on Hospitalizations among the Elderly" ISPRS International Journal of Geo-Information 3, no. 2: 713-731. https://doi.org/10.3390/ijgi3020713
APA StyleLe, G. E., Breysse, P. N., McDermott, A., Eftim, S. E., Geyh, A., Berman, J. D., & Curriero, F. C. (2014). Canadian Forest Fires and the Effects of Long-Range Transboundary Air Pollution on Hospitalizations among the Elderly. ISPRS International Journal of Geo-Information, 3(2), 713-731. https://doi.org/10.3390/ijgi3020713




