Using Multiple Scale Space-Time Patterns to Determine the Number of Replicates and Burn-In Periods in Spatially Explicit Agent-Based Modeling of Vector-Borne Disease Transmission
Abstract
1. Introduction
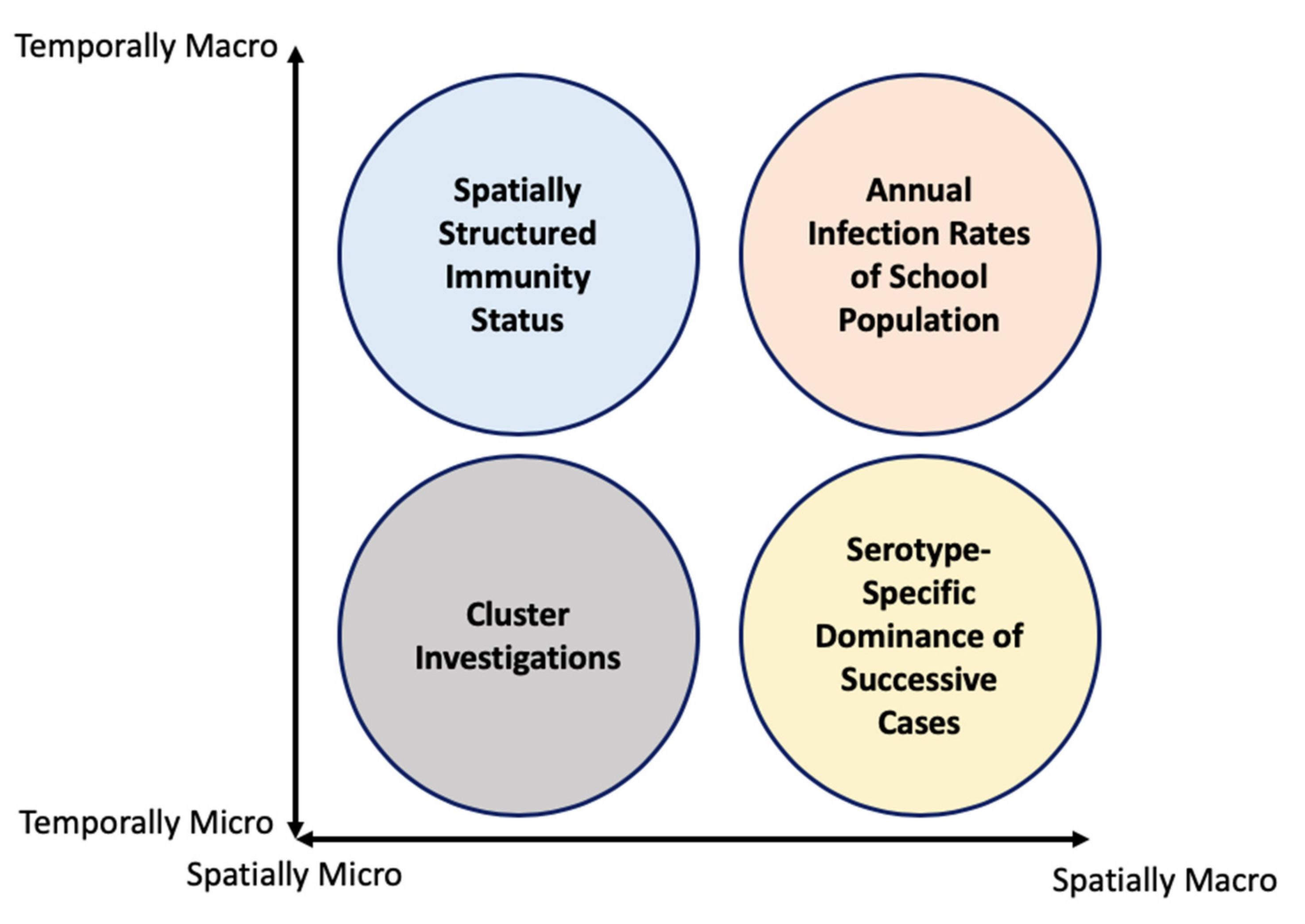
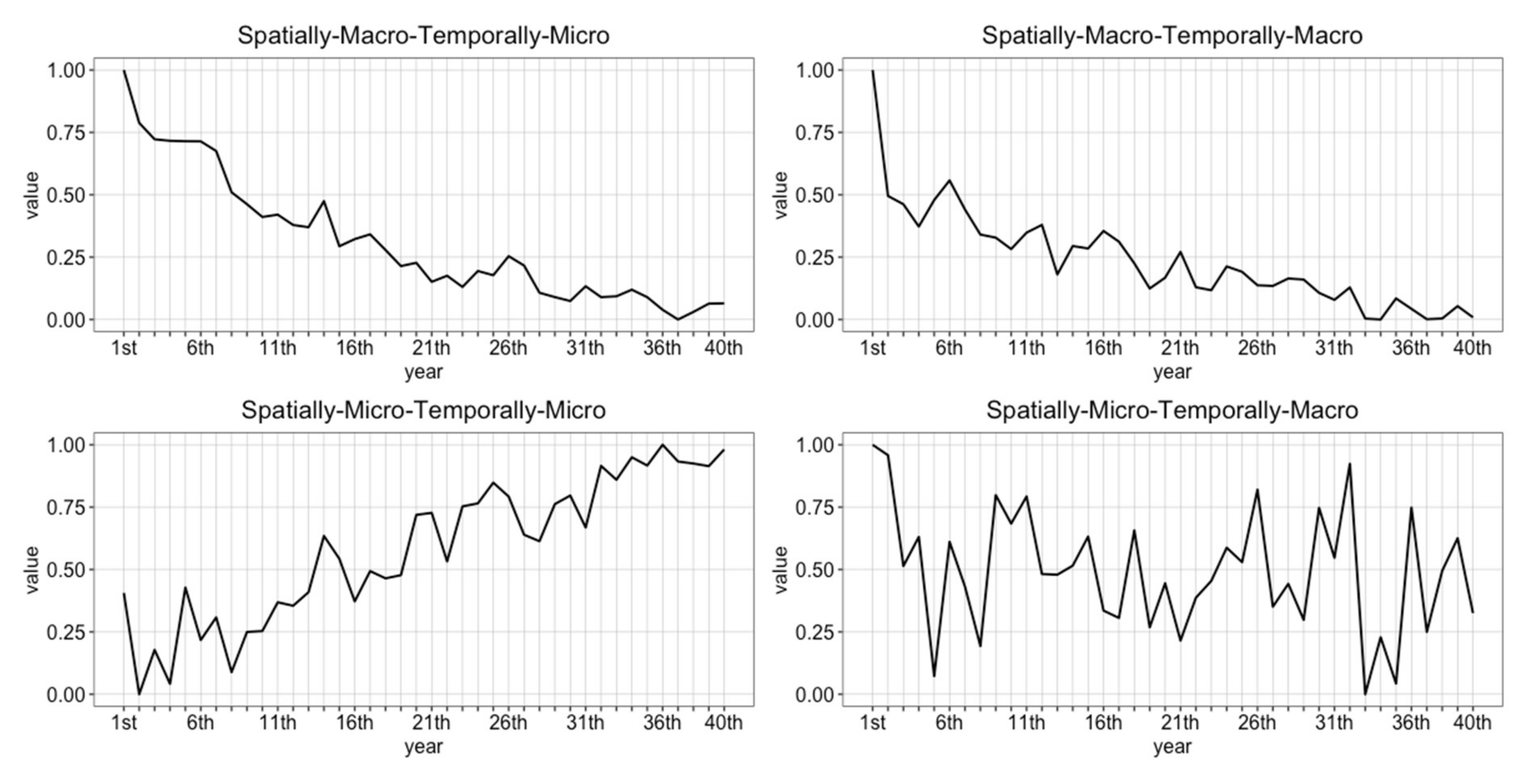
2. Multiple Scale Spacetime Patterns
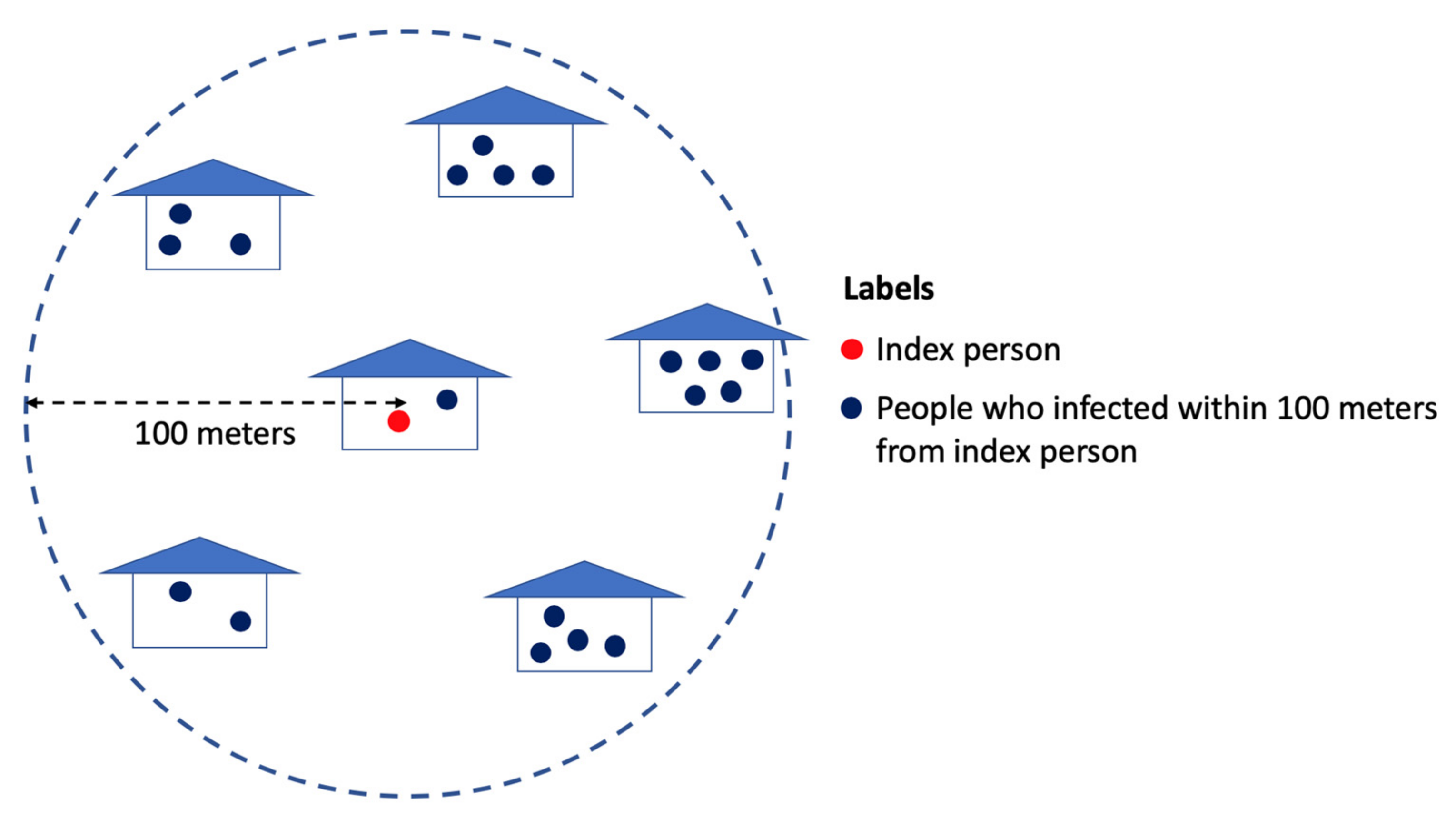
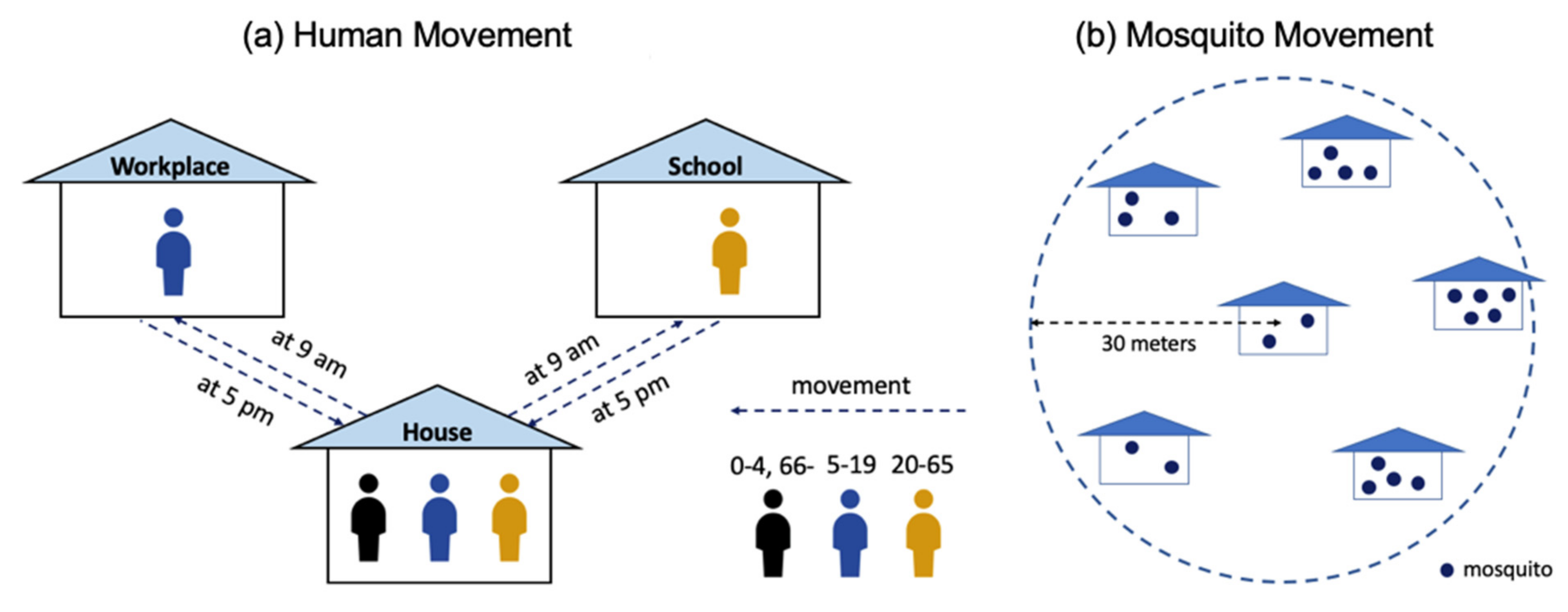
2.1. Spatially-Micro-Temporally-Macro Scale Pattern
2.2. Spatially-Macro-Temporally-Micro Scale Pattern
2.3. Spatially-Micro-Temporally-Macro Scale Pattern
2.4. Spatially-Micro-Temporally-Micro Scale Pattern
3. Materials and Methods
3.1. Agent-Based Model of DENV Transmission
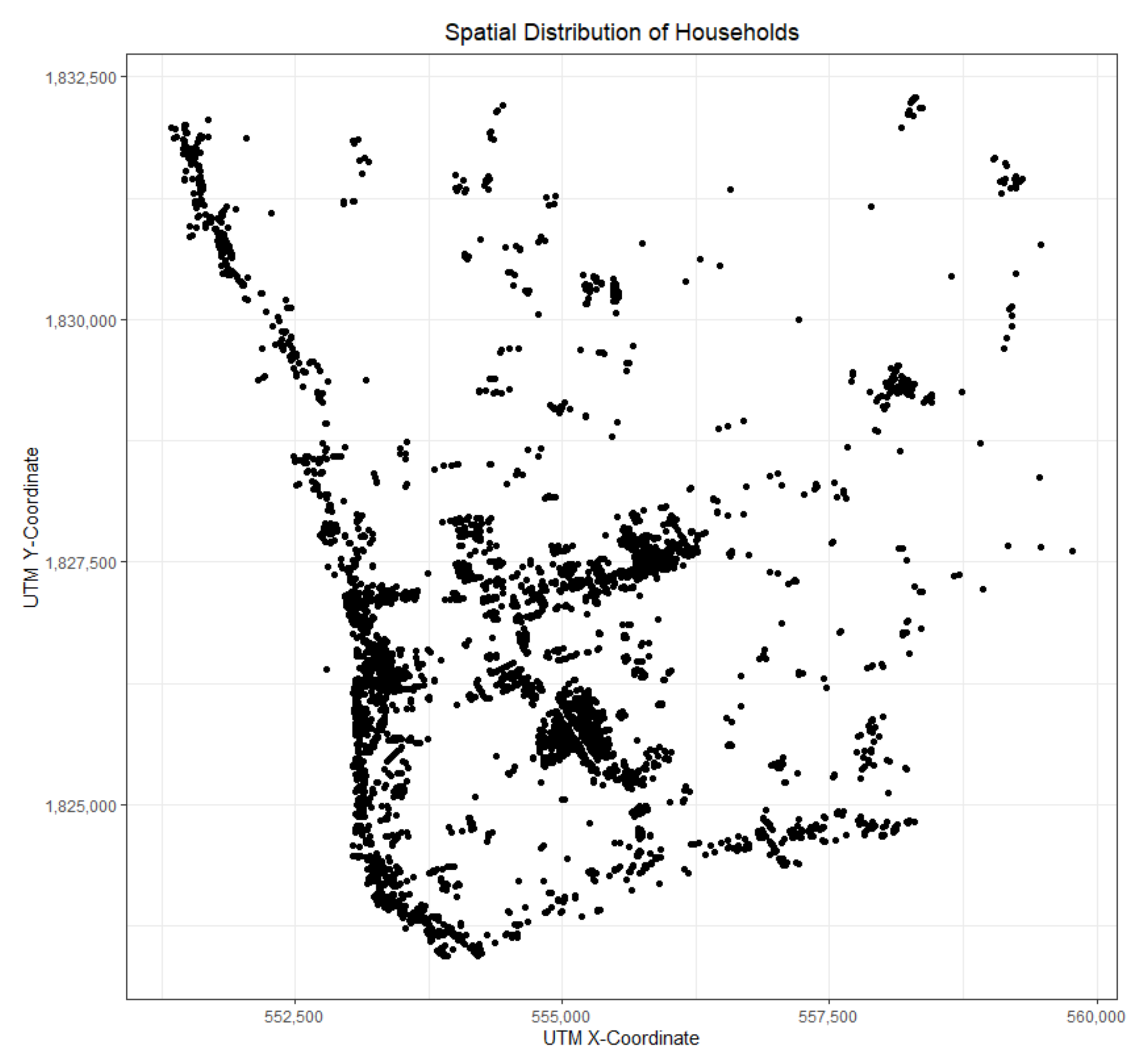
3.2. Study Area and Data
3.3. Measures
3.3.1. Multiple Scale Space-Time Patterns
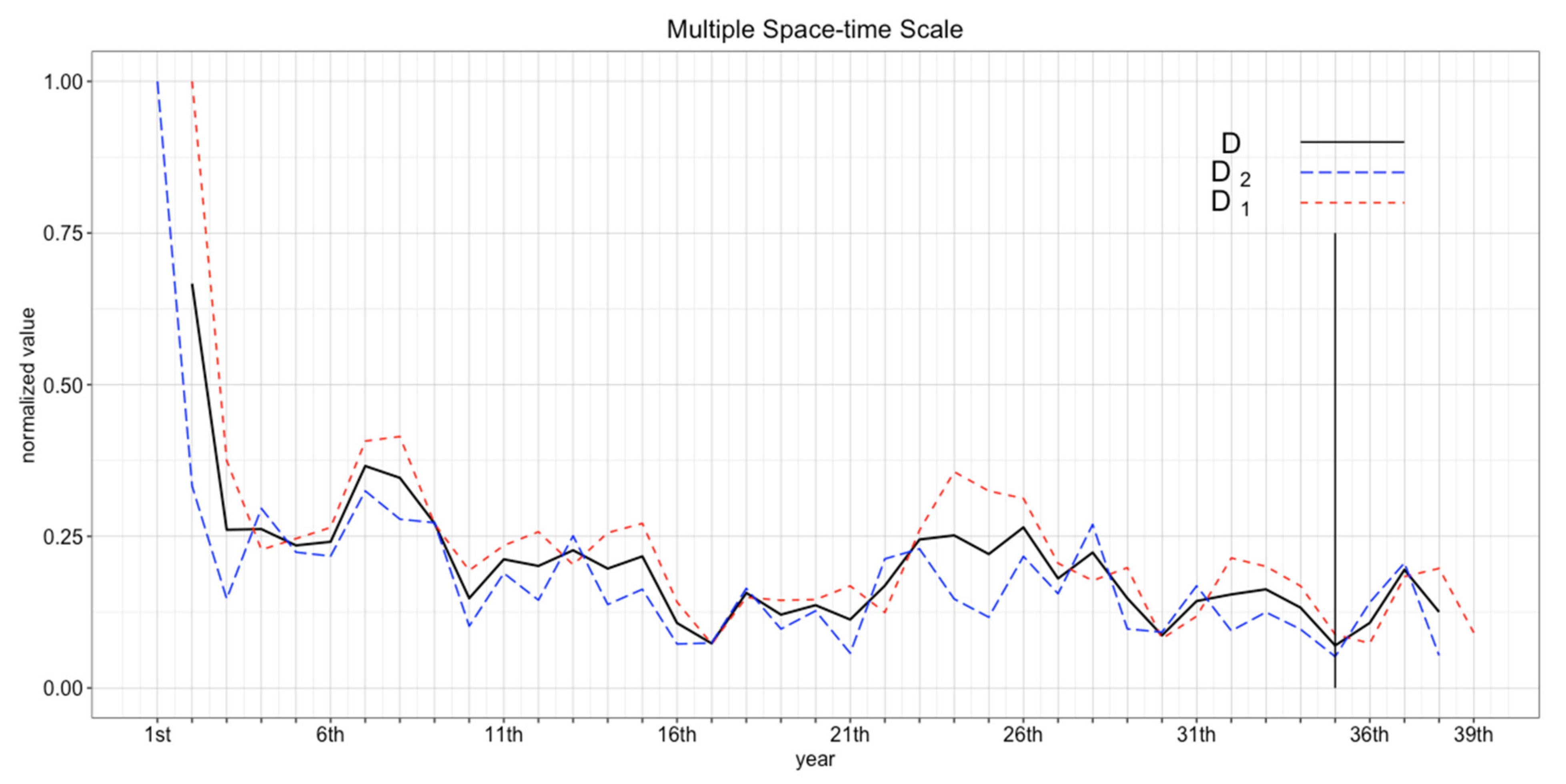
3.3.2. Coefficient of Variance
3.3.3. Incorporating Successive Coefficient of Variance
4. Results
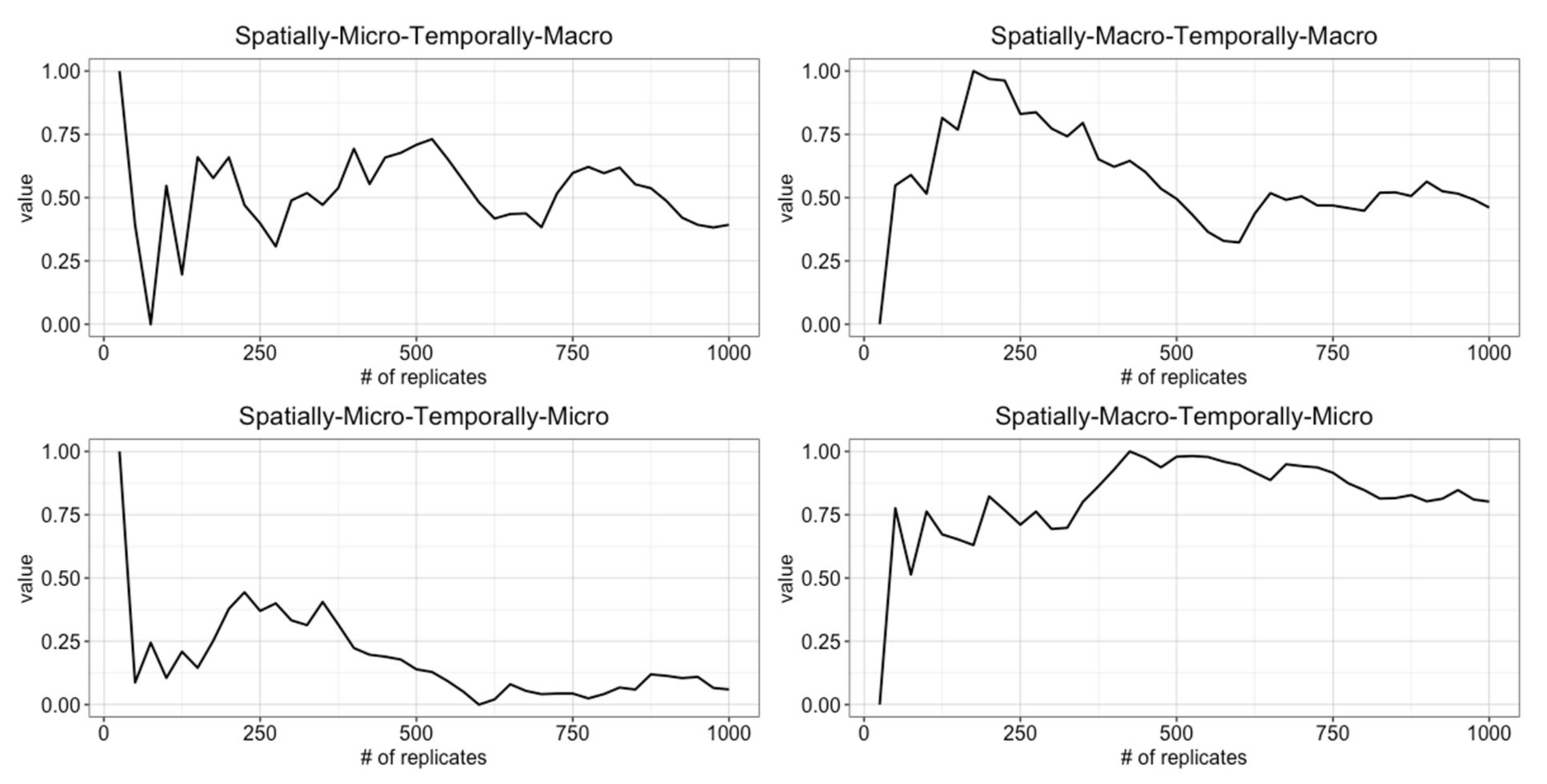
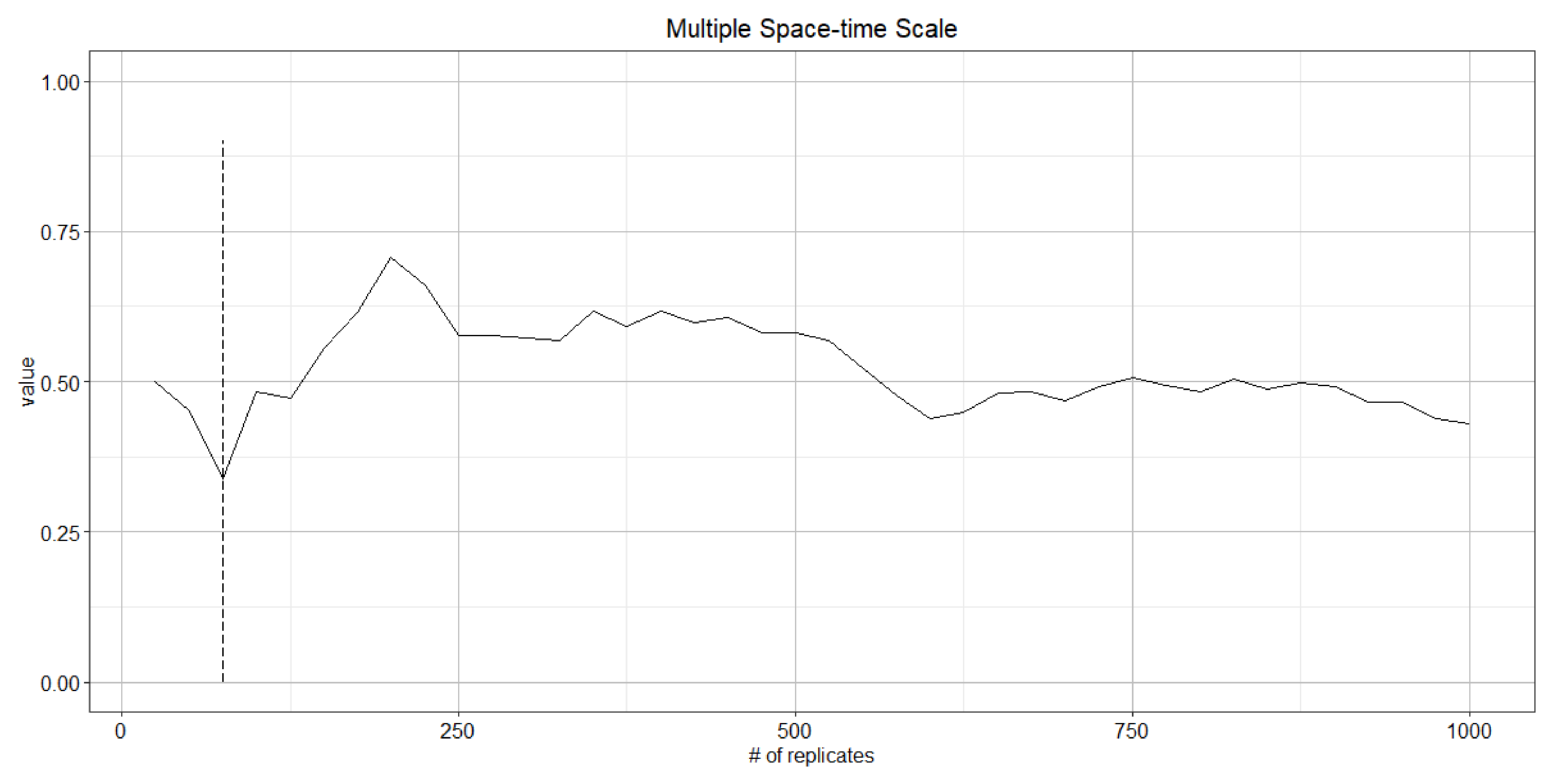
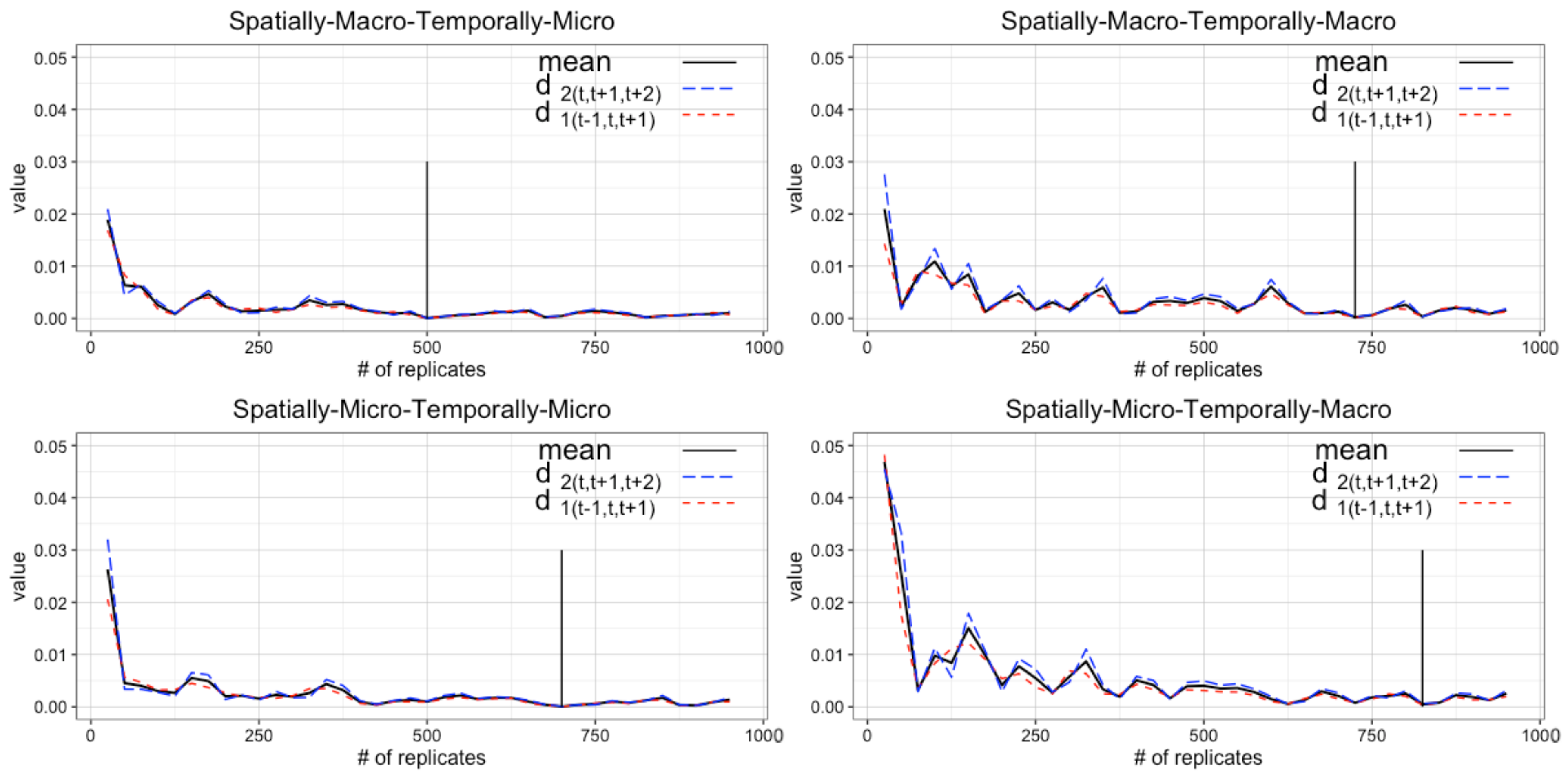
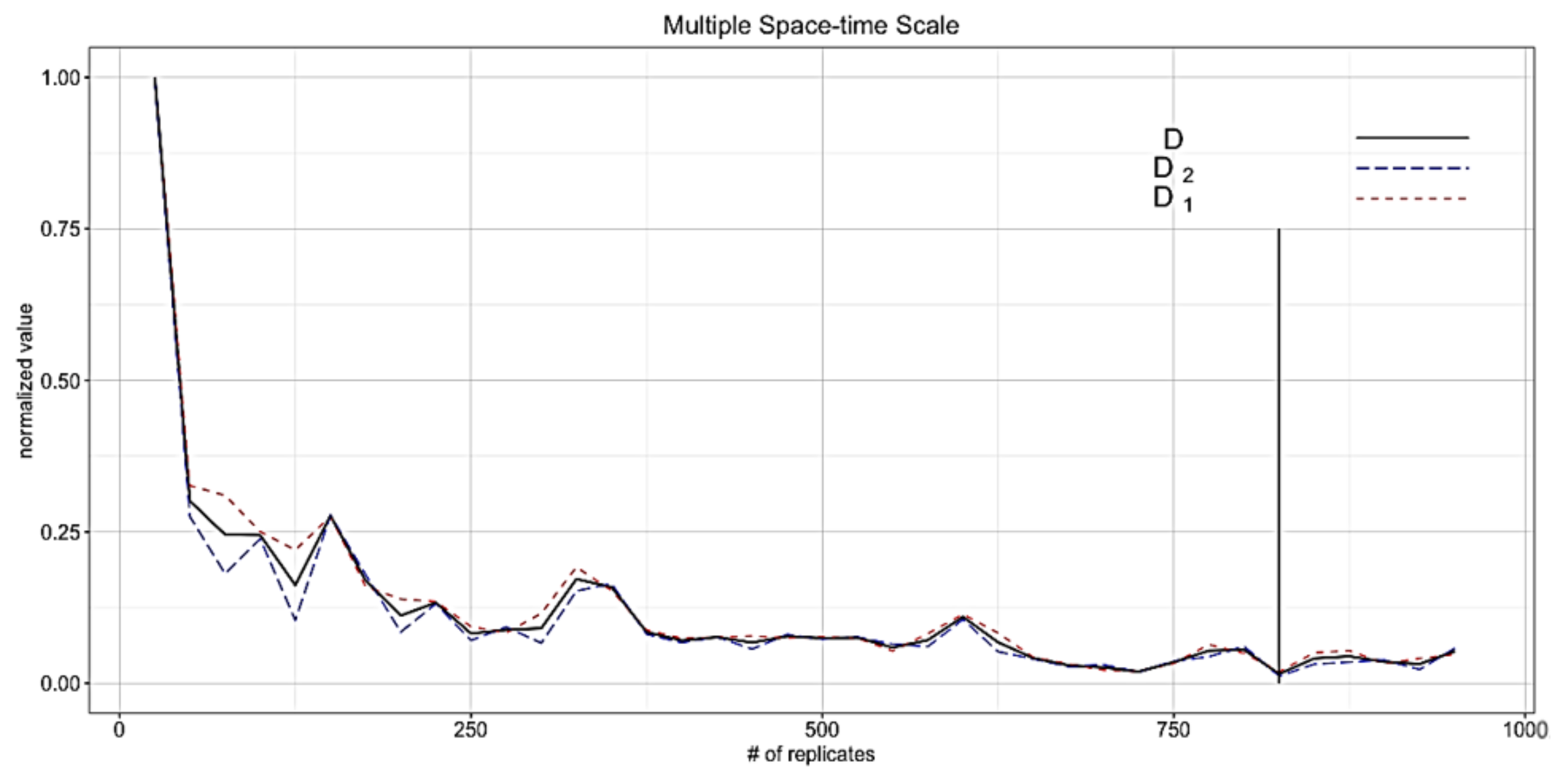
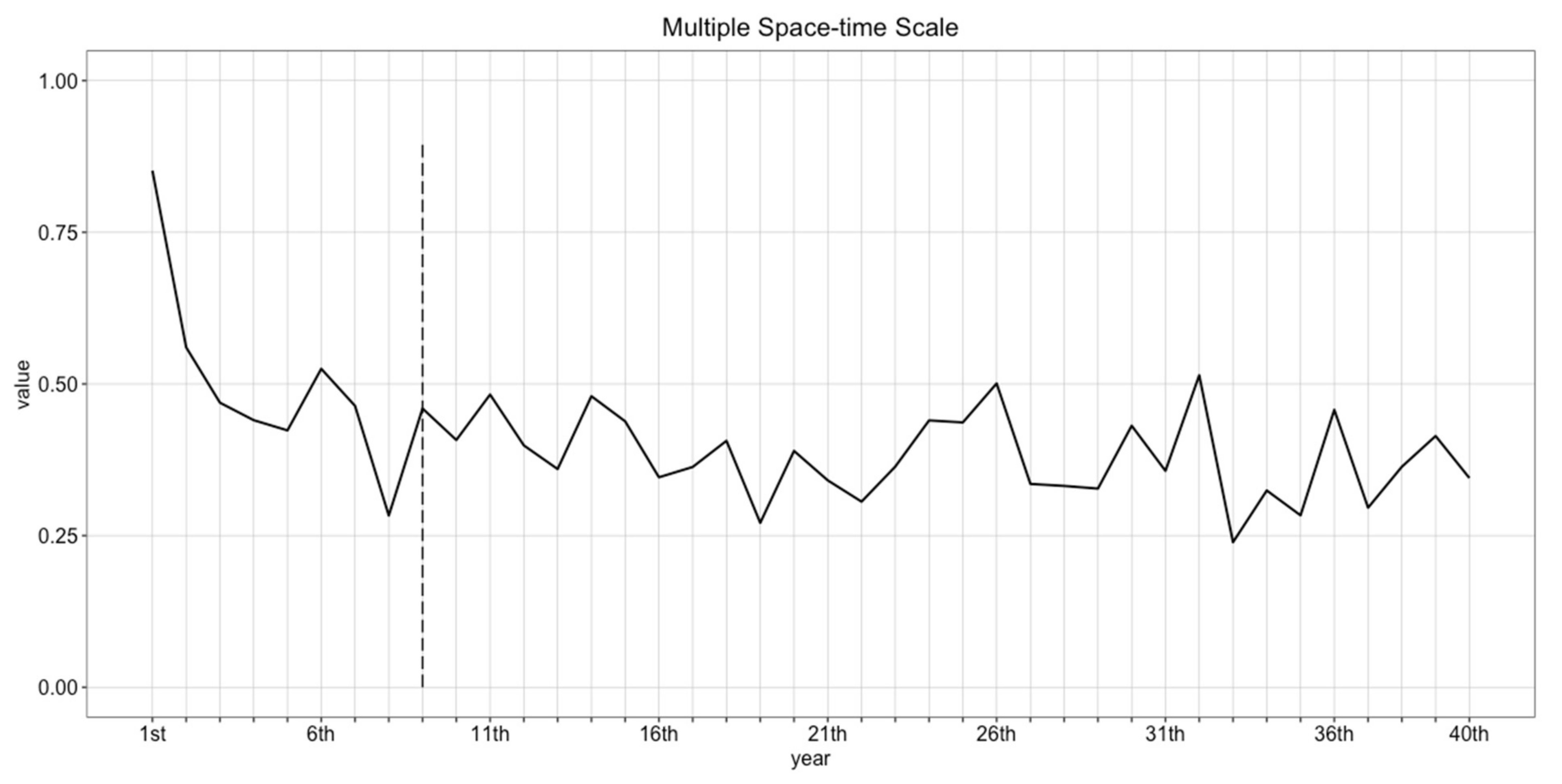
4.1. Determining the Number of Replicates
4.2. Determining Burn-in Period
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ligmann-Zielinska, A. Spatially-explicit sensitivity analysis of an agent-based model of land use change. Int. J. Geogr. Inf. Sci. 2013, 27, 1764–1781. [Google Scholar] [CrossRef]
- An, L.; Linderman, M.; Qi, J.; Shortridge, A.; Liu, J. Exploring Complexity in a Human–Environment System: An Agent-Based Spatial Model for Multidisciplinary and Multiscale Integration. Ann. Assoc. Am. Geogr. 2005, 95, 54–79. [Google Scholar] [CrossRef]
- Mao, L. Modeling triple-diffusions of infectious diseases, information, and preventive behaviors through a metropolitan social network—An agent-based simulation. Appl. Geogr. 2014, 50, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Crooks, A.T.; Hailegiorgis, A.B. An agent-based modeling approach applied to the spread of cholera. Environ. Model. Softw. 2014, 62, 164–177. [Google Scholar] [CrossRef]
- Boyd, R.; Roy, S.; Sibly, R.; Thorpe, R.; Hyder, K. A general approach to incorporating spatial and temporal variation in individual-based models of fish populations with application to Atlantic mackerel. Ecol. Model. 2018, 382, 9–17. [Google Scholar] [CrossRef]
- Malleson, N.; Birkin, M. Analysis of crime patterns through the integration of an agent-based model and a population microsimulation. Comput. Environ. Urban Syst. 2012, 36, 551–561. [Google Scholar] [CrossRef]
- Malleson, N.; Heppenstall, A.; See, L. Crime reduction through simulation: An agent-based model of burglary. Comput. Environ. Urban Syst. 2010, 34, 236–250. [Google Scholar] [CrossRef]
- Crooks, A.; Castle, C.; Batty, M. Key challenges in agent-based modelling for geo-spatial simulation. Comput. Environ. Urban Syst. 2008, 32, 417–430. [Google Scholar] [CrossRef]
- Rahmandad, H.; Sterman, J. Heterogeneity and network structure in the dynamics of diffusion: Comparing agent-based and differential equation models. Manag. Sci. 2008, 54, 998–1014. [Google Scholar] [CrossRef]
- Kang, J.-Y.; Aldstadt, J.; Vandewalle, R.; Yin, D.; Wang, S. A CyberGIS Approach to Spatiotemporally Explicit Uncertainty and Global Sensitivity Analysis for Agent-Based Modeling of Vector-Borne Disease Transmission. Ann. Am. Assoc. Geogr. 2020, 110, 1855–1873. [Google Scholar] [CrossRef]
- Ligmann-Zielinska, A.; Kramer, D.B.; Cheruvelil, K.S.; Soranno, P.A. Using uncertainty and sensitivity analyses in socioecological agent-based models to improve their analytical performance and policy relevance. PLoS ONE 2014, 9, e109779. [Google Scholar] [CrossRef]
- Ligmann-Zielinska, A.; Jankowski, P. Spatially-explicit integrated uncertainty and sensitivity analysis of criteria weights in multicriteria land suitability evaluation. Environ. Model. Softw. 2014, 57, 235–247. [Google Scholar] [CrossRef]
- Tang, W.; Jia, M. Global sensitivity analysis of a large agent-based model of spatial opinion exchange: A heterogeneous multi-GPU acceleration approach. Ann. Assoc. Am. Geogr. 2014, 104, 485–509. [Google Scholar] [CrossRef]
- Fachada, N.; Lopes, V.V.; Martins, R.C.; Rosa, A.C. Model-independent comparison of simulation output. Simul. Model. Pract. Theory 2017, 72, 131–149. [Google Scholar] [CrossRef]
- Lee, J.-S.; Filatova, T.; Ligmann-Zielinska, A.; Hassani-Mahmooei, B.; Stonedahl, F.; Lorscheid, I.; Voinov, A.; Polhill, G.; Sun, Z.; Parker, D.C. The complexities of agent-based modeling output analysis. J. Artif. Soc. Soc. Simul. 2015, 18, 4. [Google Scholar] [CrossRef]
- Kelton, W.D. Statistical analysis of simulation output. In Proceedings of the 29th Conference on Winter Simulation, Atlanta, GA, USA, 7–10 December 1997. [Google Scholar]
- Calisti, R.; Proietti, P.; Marchini, A. Promoting Sustainable Food Consumption: An Agent-Based Model About Outcomes of Small Shop Openings. J. Artif. Soc. Soc. Simul. 2019, 22, 2. [Google Scholar] [CrossRef]
- Hailegiorgis, A.; Crooks, A.; Cioffi-Revilla, C. An agent-based model of rural households’ adaptation to climate change. J. Artif. Soc. Soc. Simul. 2018, 21, 4. [Google Scholar] [CrossRef]
- Reinhardt, O.; Hilton, J.; Warnke, T.; Bijak, J.; Uhrmacher, A.M. Streamlining simulation experiments with agent-based models in demography. J. Artif. Soc. Soc. Simul. 2018, 21, 9. [Google Scholar] [CrossRef]
- Dubbelboer, J.; Nikolic, I.; Jenkins, K.; Hall, J. An agent-based model of flood risk and insurance. J. Artif. Soc. Soc. Simul. 2017, 20. [Google Scholar] [CrossRef]
- Moglia, M.; Podkalicka, A.; McGregor, J. An agent-based model of residential energy efficiency adoption. J. Artif. Soc. Soc. Simul. 2018, 21. [Google Scholar] [CrossRef]
- Gharakhanlou, N.M.; Hooshangi, N.; Helbich, M. A Spatial Agent-Based Model to Assess the Spread of Malaria in Relation to Anti-Malaria Interventions in Southeast Iran. ISPRS Int. J. Geo-Inf. 2020, 9, 549. [Google Scholar] [CrossRef]
- Sanchez, S.M. Output modeling: Abc’s of output analysis. In Proceedings of the 33nd Conference on Winter Simulation, WSC 2001, Arlington, VA, USA, 9–12 December 2001; IEEE Computer Society: Washington, DC, USA, 2001. [Google Scholar]
- Law, A.M. Statistical analysis of simulation output data: The practical state of the art. In Proceedings of the 2015 Winter Simulation Conference (WSC), Orlando, FL, USA, 14–18 December 2015. [Google Scholar]
- Garcia, R.; Rummel, P.; Hauser, J. Validating agent-based marketing models through conjoint analysis. J. Bus. Res. 2007, 60, 848–857. [Google Scholar] [CrossRef]
- Kang, J.-Y.; Aldstadt, J. Using multiple scale space-time patterns in variance-based global sensitivity analysis for spatially explicit agent-based models. Comput. Environ. Urban Syst. 2019, 75, 170–183. [Google Scholar] [CrossRef]
- Windrum, P.; Fagiolo, G.; Moneta, A. Empirical validation of agent-based models: Alternatives and prospects. J. Artif. Soc. Soc. Simul. 2007, 10, 8. [Google Scholar]
- Brown, D.; Page, S.; Riolo, R.; Zellner, M.; Rand, W. Path dependence and the validation of agent-based spatial models of land use. Int. J. Geogr. Inf. Sci. 2005, 19, 153–174. [Google Scholar] [CrossRef]
- Grimm, V.; Revilla, E.; Berger, U.; Jeltsch, F.; Mooij, W.M.; Railsback, S.F.; Thulke, H.-H.; Weiner, J.; Wiegand, T.; Deangelis, D.L. Pattern-oriented modeling of agent-based complex systems: Lessons from ecology. Science 2005, 310, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-Y.; Aldstadt, J. Using multiple scale spatio-temporal patterns for validating spatially explicit agent-based models. Int. J. Geogr. Inf. Sci. 2019, 33, 193–213. [Google Scholar] [CrossRef]
- Mao, L.; Bian, L. Agent-based simulation for a dual-diffusion process of influenza and human preventive behavior. Int. J. Geogr. Inf. Sci. 2011, 25, 1371–1388. [Google Scholar] [CrossRef]
- Aldstadt, J. An incremental Knox test for the determination of the serial interval between successive cases of an infectious disease. Stoch. Environ. Res. Risk Assess. 2007, 21, 487–500. [Google Scholar] [CrossRef]
- Lorscheid, I.; Heine, B.-O.; Meyer, M. Opening the ‘black box’of simulations: Increased transparency and effective communication through the systematic design of experiments. Comput. Math. Organ. Theory 2012, 18, 22–62. [Google Scholar] [CrossRef]
- Chao, D.; Halstead, S.B.; Halloran, M.E.; Longini, I.M., Jr. Controlling dengue with vaccines in Thailand. PLoS Negl. Trop. Dis. 2012, 6, e1876. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Meaker, J.W.; Zhan, F.B. Agent-based modeling and analysis of hurricane evacuation procedures for the Florida Keys. Nat. Hazards 2006, 38, 321. [Google Scholar] [CrossRef]
- Goetz, M.; Zipf, A. Using crowdsourced geodata for agent-based indoor evacuation simulations. ISPRS Int. J. Geo-Inf. 2012, 1, 186–208. [Google Scholar] [CrossRef]
- Vandewalle, R.; Kang, J.Y.; Yin, D.; Wang, S. Integrating CyberGIS-Jupyter and spatial agent-based modelling to evaluate emergency evacuation time. In Proceedings of the 2nd ACM SIGSPATIAL International Workshop on GeoSpatial Simulation, Chicago, IL, USA, 5 November 2019. [Google Scholar]
- Perez, L.; Dragicevic, S. An agent-based approach for modeling dynamics of contagious disease spread. Int. J. Health Geogr. 2009, 8, 50. [Google Scholar] [CrossRef]
- Endy, T.P.; Chunsuttiwat, S.; Nisalak, A.; Libraty, D.H.; Green, S.; Rothman, A.L.; Vaughn, D.W.; Ennis, F.A. Epidemiology of inapparent and symptomatic acute dengue virus infection: A prospective study of primary school children in Kamphaeng Phet, Thailand. Am. J. Epidemiol. 2002, 156, 40–51. [Google Scholar] [CrossRef]
- Kang, J.-Y.; Aldstadt, J. The Influence of Spatial Configuration of Residential Area and Vector Populations on Dengue Incidence Patterns in an Individual-Level Transmission Model. Int. J. Environ. Res. Public Health 2017, 14, 792. [Google Scholar] [CrossRef]
- Yoon, I.-K.; Getis, A.; Aldstadt, J.; Rothman, A.L.; Tannitisupawong, D.; Koenraadt, C.J.M.; Fansiri, T.; Jones, J.W.; Morrison, A.C.; Jarman, R.G.; et al. Fine scale spatiotemporal clustering of dengue virus transmission in children and Aedes aegypti in rural Thai villages. PLoS Negl. Trop. Dis. 2012, 6, e1730. [Google Scholar] [CrossRef] [PubMed]
- Grimm, V.; Berger, U.; DeAngelis, D.L.; Polhill, J.G.; Giske, J.; Railsback, S.F. The ODD protocol: A review and first update. Ecol. Model. 2010, 221, 2760–2768. [Google Scholar] [CrossRef]
- Gibbons, R.V.; Kalanarooj, S.; Jarman, R.G.; Nisalak, A.; Vaughn, D.W.; Endy, T.P.; Mammen, M.P., Jr.; Srikiatkhachorn, A. Analysis of repeat hospital admissions for dengue to estimate the frequency of third or fourth dengue infections resulting in admissions and dengue hemorrhagic fever, and serotype sequences. Am. J. Trop. Med. Hyg. 2007, 77, 910–913. [Google Scholar] [CrossRef]
- Vaughn, D.W.; Green, S.; Kalayanarooj, S.; Innis, B.L.; Nimmannitya, S.; Suntayakorn, S.; Endy, T.P.; Raengsakulrach, B.; Rothman, A.L.; Ennis, F.A.; et al. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J. Infect. Dis. 2000, 181, 2–9. [Google Scholar] [CrossRef]
- Harrington, L.C.; Françoisevermeylen, J.J.J.; Kitthawee, S.; Sithiprasasna, R.; Edman, J.D.; Scott, T.W. Age-dependent survival of the dengue vector Aedes aegypti (Diptera: Culicidae) demonstrated by simultaneous release–recapture of different age cohorts. J. Med. Entomol. 2008, 45, 307–313. [Google Scholar]
- Harrington, L.C.; Buonaccorsi, J.P.; Edman, J.D.; Costero, A.; Kittayapong, P.; Clark, G.G.; Scott, T.W. Analysis of survival of young and old Aedes aegypti (Diptera: Culicidae) from Puerto Rico and Thailand. J. Med. Entomol. 2001, 38, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Harrington, L.C.; Scott, T.W.; Lerdthusnee, K.; Coleman, R.C.; Costero, A.; Clark, G.G.; Jones, J.J.; Kitthawee, S.; Kittayapong, P.; Sithiprasasna, R.; et al. Dispersal of the dengue vector Aedes aegypti within and between rural communities. Am. J. Trop. Med. Hyg. 2005, 72, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.J.; Aldstadt, J.; Jarman, R.G.; Buddhari, D.; Yoon, I.-K.; Richardson, J.H.; Ponlawat, A.; Iamsirithaworn, S.; Scott, T.W.; Rothman, A.L.; et al. Improving dengue virus capture rates in humans and vectors in Kamphaeng Phet Province, Thailand, using an enhanced spatiotemporal surveillance strategy. Am. J. Trop. Med. Hyg. 2015, 93, 24–32. [Google Scholar] [CrossRef][Green Version]
- DeJong, T.M. A comparison of three diversity indices based on their components of richness and evenness. Oikos 1975, 26, 222–227. [Google Scholar] [CrossRef]
- Grimm, V.; Railsback, S.F. Pattern-oriented modelling: A ‘multi-scope’ for predictive systems ecology. Philos. Trans. R. Soc. B 2012, 367, 298–310. [Google Scholar] [CrossRef]
- Tang, W.; Bennett, D.A. Agent-based modeling of animal movement: A review. Geogr. Compass 2010, 4, 682–700. [Google Scholar] [CrossRef]
- Sanchez-Cartas, J.M. Agent-based models and industrial organization theory. A price-competition algorithm for agent-based models based on Game Theory. Complex Adapt. Syst. Model. 2018, 6, 1–30. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, J.-Y.; Aldstadt, J. Using Multiple Scale Space-Time Patterns to Determine the Number of Replicates and Burn-In Periods in Spatially Explicit Agent-Based Modeling of Vector-Borne Disease Transmission. ISPRS Int. J. Geo-Inf. 2021, 10, 604. https://doi.org/10.3390/ijgi10090604
Kang J-Y, Aldstadt J. Using Multiple Scale Space-Time Patterns to Determine the Number of Replicates and Burn-In Periods in Spatially Explicit Agent-Based Modeling of Vector-Borne Disease Transmission. ISPRS International Journal of Geo-Information. 2021; 10(9):604. https://doi.org/10.3390/ijgi10090604
Chicago/Turabian StyleKang, Jeon-Young, and Jared Aldstadt. 2021. "Using Multiple Scale Space-Time Patterns to Determine the Number of Replicates and Burn-In Periods in Spatially Explicit Agent-Based Modeling of Vector-Borne Disease Transmission" ISPRS International Journal of Geo-Information 10, no. 9: 604. https://doi.org/10.3390/ijgi10090604
APA StyleKang, J.-Y., & Aldstadt, J. (2021). Using Multiple Scale Space-Time Patterns to Determine the Number of Replicates and Burn-In Periods in Spatially Explicit Agent-Based Modeling of Vector-Borne Disease Transmission. ISPRS International Journal of Geo-Information, 10(9), 604. https://doi.org/10.3390/ijgi10090604






