Laparoscopic Robotic Surgery: Current Perspective and Future Directions
Abstract
1. Introduction
2. Literature Review Methods
3. Literature Review Results
4. The Robotic-assisted surgery Systems
5. Imaging and Display Technology
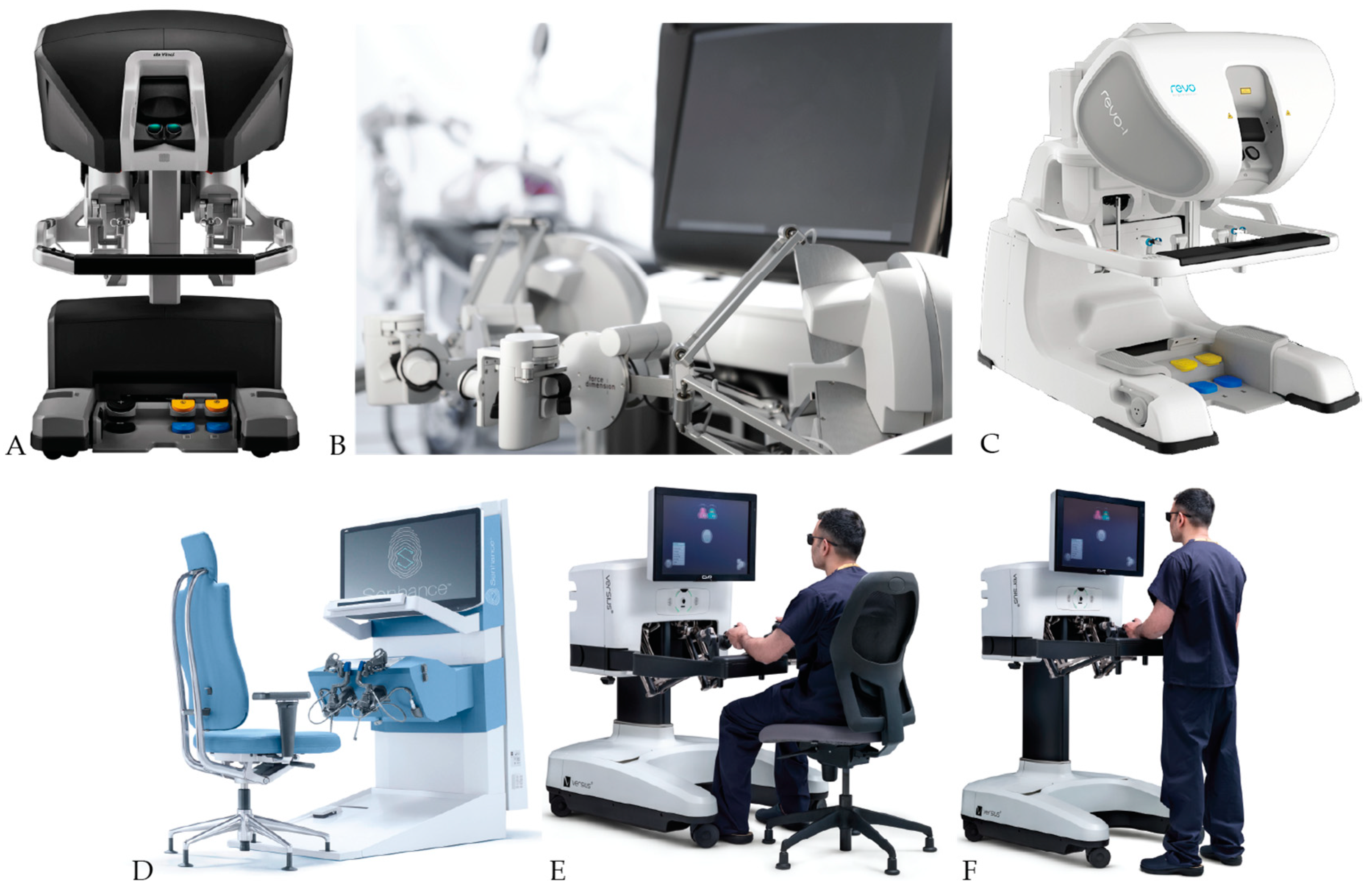
6. Surgeons Console
6.1. Seated or Standing
6.2. Hand Controllers
6.3. Haptic Feedback
6.4. Tremor Removal
6.5. Axillary Controls
7. Patient Interface
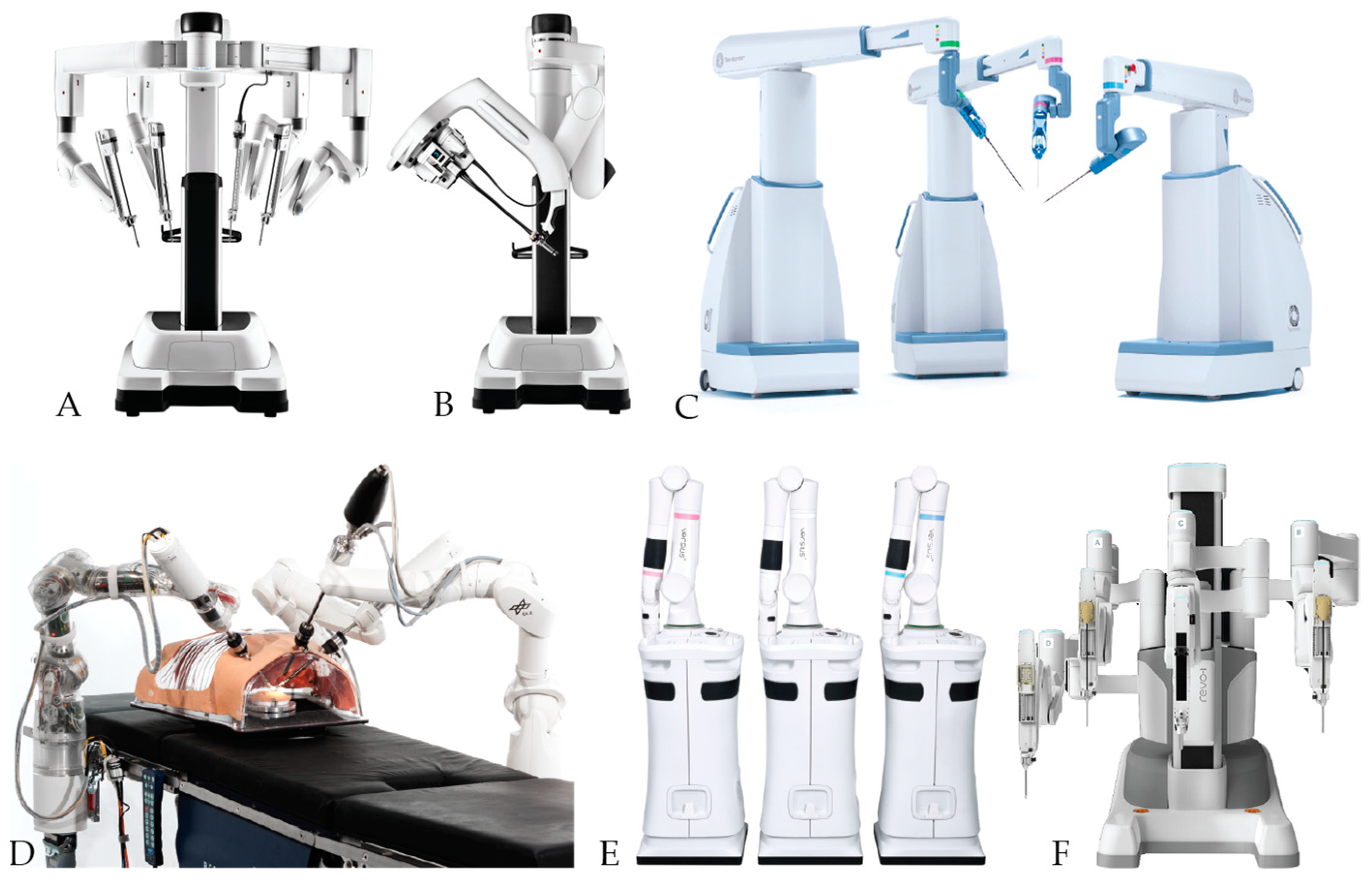
7.1. Patient Cart
7.2. Robot Arms
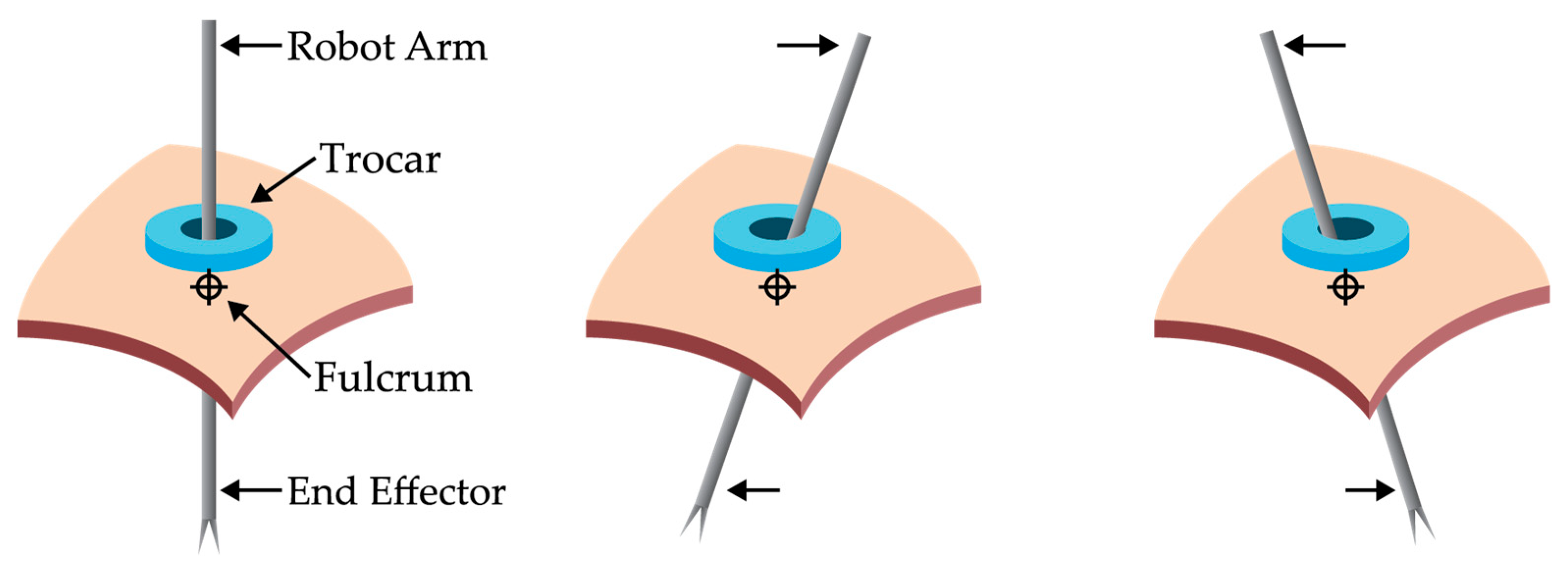
7.3. Trocar
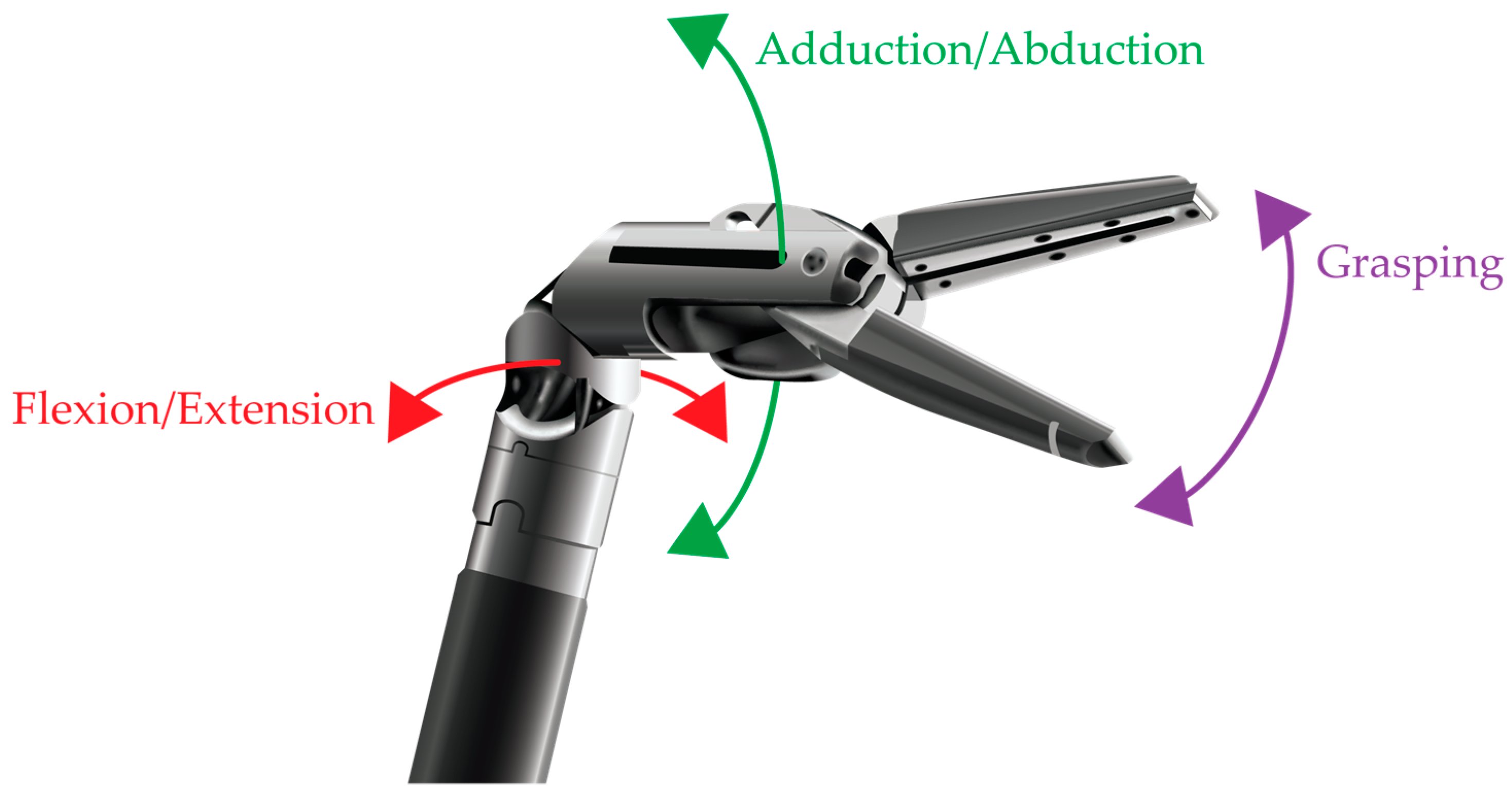
7.4. Instruments
8. Future Directions
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kwoh, Y.S.; Hou, J.; Jonckheere, E.A.; Hayati, S. A Robot with Improved Absolute Positioning Accuracy for CT Guided Stereotactic Brain Surgery. IEEE Trans. Biomed. Eng. 1988, 35, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Yates, D.R.; Vaessen, C.; Roupret, M. From Leonardo to da Vinci: The history of robot-assisted surgery in urology. BJU Int. 2011, 108, 1708–1713. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.P. Robotic surgery: New robots and finally some real competition. World J. Urol. 2018, 36, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Juric, S.; Flis, V.; Debevc, M.; Holzinger, A.; Zalik, B. Towards a low-cost mobile subcutaneous vein detection solution using near-infrared spectroscopy. Sci. World J. 2014, 2014, 365902. [Google Scholar] [CrossRef] [PubMed]
- TransEnterix Inc. Media Kit. Available online: https://transenterix.com/media-kit (accessed on 24 February 2020).
- Brodie, A.; Vasdev, N. The future of robotic surgery. Ann. R. Coll. Surg. Engl. 2018, 100, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Beasley, R.A. Medical Robots: Current Systems and Research Directions. J. Rob. 2012, 2012. [Google Scholar] [CrossRef]
- Gueli Alletti, S.; Rossitto, C.; Cianci, S.; Perrone, E.; Pizzacalla, S.; Monterossi, G.; Vizzielli, G.; Gidaro, S.; Scambia, G. The Senhance™ surgical robotic system (“Senhance”) for total hysterectomy in obese patients: A pilot study. J. Robot. Surg. 2017, 12, 229–234. [Google Scholar] [CrossRef]
- Hutchins, A.R.; Manson, R.J.; Lerebours, R.; Farjat, A.E.; Cox, M.L.; Mann, B.P.; Zani, S. Objective Assessment of the Early Stages of the Learning Curve for the Senhance Surgical Robotic System. J. Surg. Educ. 2019, 76, 201–214. [Google Scholar] [CrossRef]
- Joseph, R.A.; Goh, A.C.; Cuevas, S.P.; Donovan, M.A.; Kauffman, M.G.; Salas, N.A.; Miles, B.; Bass, B.L.; Dunkin, B.J. “Chopstick” surgery: A novel technique improves surgeon performance and eliminates arm collision in robotic single-incision laparoscopic surgery. Surg. Endosc. 2010, 24, 1331–1335. [Google Scholar] [CrossRef]
- Samalavicius, N.E.; Janusonis, V.; Siaulys, R.; Jasėnas, M.; Deduchovas, O.; Venckus, R.; Ezerskiene, V.; Paskeviciute, R.; Klimaviciute, G. Robotic surgery using Senhance® robotic platform: Single center experience with first 100 cases. J. Robot. Surg. 2020, 14, 371–376. [Google Scholar] [CrossRef]
- Sheth, K.R.; Koh, C.J. The Future of Robotic Surgery in Pediatric Urology: Upcoming Technology and Evolution Within the Field. Front. Pediatr. 2019, 7, 259. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, A.; David, G.; Gidaro, S.; Carvello, M.; Sacchi, M.; Montorsi, M.; Montroni, I. First experience in colorectal surgery with a new robotic platform with haptic feedback. Colorectal Dis. 2018, 20, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Stark, M.; Pomati, S.; D’Ambrosio, A.; Giraudi, F.; Gidaro, S. A new telesurgical platform--preliminary clinical results. Minim. Invasive Ther. Allied Technol. 2015, 24, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Avateramedical GmbH. What Makes Avatera so Special? Available online: https://www.avatera.eu/en/avatera-system (accessed on 13 February 2020).
- Hanly, E.J.; Talamini, M.A. Robotic abdominal surgery. Am. J. Surg. 2004, 188, 19S–26S. [Google Scholar] [CrossRef]
- Kang, C.M.; Chong, J.U.; Lim, J.H.; Park, D.W.; Park, S.J.; Gim, S.; Ye, H.J.; Kim, S.H.; Lee, W.J. Robotic Cholecystectomy Using the Newly Developed Korean Robotic Surgical System, Revo-i: A Preclinical Experiment in a Porcine Model. Yonsei Med. J. 2017, 58, 1075–1077. [Google Scholar] [CrossRef]
- Palep, J.H. Robotic assisted minimally invasive surgery. J. Minimal Access Surg. 2009, 5, 1–7. [Google Scholar] [CrossRef]
- Tareq, D.; Shahab, E.; Luke, A.R.; Abhilash, P. Remote Presence: Development and Usability Evaluation of a Head-Mounted Display for Camera Control on the da Vinci Surgical System. Robotics 2019, 8, 31. [Google Scholar] [CrossRef]
- Gonzalez-Rivas, D.; Ismail, M. Subxiphoid or subcostal uniportal robotic-assisted surgery: Early experimental experience. J. Thorac. Dis. 2019, 11, 231–239. [Google Scholar] [CrossRef]
- Abdel Raheem, A.; Troya, I.S.; Kim, D.K.; Kim, S.h.; Won, P.D.; Joon, P.S.; Hyun, G.S.; Rha, K.H. Robot-assisted Fallopian tube transection and anastomosis using the new REVO-I robotic surgical system: Feasibility in a chronic porcine model. BJU Int. 2016, 118, 604–609. [Google Scholar] [CrossRef]
- Chang, K.D.; Abdel Raheem, A.; Choi, Y.D.; Chung, B.H.; Rha, K.H. Retzius-sparing robot-assisted radical prostatectomy using the Revo-i robotic surgical system: Surgical technique and results of the first human trial. BJU Int. 2018, 122, 441–448. [Google Scholar] [CrossRef]
- Kim, D.K.; Park, D.W.; Rha, K.H. Robot-assisted Partial Nephrectomy with the REVO-I Robot Platform in Porcine Models. Eur. Urol. 2016, 69, 541–542. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.H.; Lee, W.J.; Park, D.W.; Yea, H.J.; Kim, S.H.; Kang, C.M. Robotic cholecystectomy using Revo-i Model MSR-5000, the newly developed Korean robotic surgical system: A preclinical study. Surg. Endosc. 2016, 31, 3391–3397. [Google Scholar] [CrossRef] [PubMed]
- Schwaibold, H.; Wiesend, F.; Bach, C. The age of robotic surgery – Is laparoscopy dead? Arab J. Urol. 2018, 16, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Rassweiler, J.J.; Autorino, R.; Klein, J.; Mottrie, A.; Goezen, A.S.; Stolzenburg, J.-U.; Rha, K.H.; Schurr, M.; Kaouk, J.; Patel, V.; et al. Future of robotic surgery in urology. BJU Int. 2017, 120, 822–841. [Google Scholar] [CrossRef]
- Darwich, I.; Stephan, D.; Klöckner-Lang, M.; Scheidt, M.; Friedberg, R.; Willeke, F. A roadmap for robotic-assisted sigmoid resection in diverticular disease using a Senhance™ Surgical Robotic System: Results and technical aspects. J. Robot. Surg. 2020, 14, 297–304. [Google Scholar] [CrossRef]
- DLR Institute of Robotics and Mechatronics. MiroSurge. Available online: https://www.dlr.de/rm/en/desktopdefault.aspx/tabid-11674/#gallery/29787 (accessed on 14 February 2020).
- Konietschke, R.; Hagn, U.; Nickl, M.; Jorg, S.; Tobergte, A.; Passig, G.; Seibold, U.; Le-Tien, L.; Kubler, B.; Groger, M.; et al. The DLR MiroSurge - A robotic system for surgery. In Proceedings of the IEEE International Conference on Robotics and Automation, Kobe, Japan, 12–17 May 2009; pp. 1589–1590. [Google Scholar]
- Petroni, G.; Niccolini, M.; Caccavaro, S.; Quaglia, C.; Menciassi, A.; Schostek, S.; Basili, G.; Goletti, O.; Schurr, M.O.; Dario, P. A novel robotic system for single-port laparoscopic surgery: Preliminary experience. Surg. Endosc. 2013, 27, 1932–1937. [Google Scholar] [CrossRef] [PubMed]
- Petroni, G.; Niccolini, M.; Menciassi, A.; Dario, P.; Cuschieri, A. A novel intracorporeal assembling robotic system for single-port laparoscopic surgery. Surg. Endosc. 2013, 27, 665–670. [Google Scholar] [CrossRef]
- Atallah, S.; Parra-Davila, E.; Melani, A.G.F. Assessment of the Versius surgical robotic system for dual-field synchronous transanal total mesorectal excision (taTME) in a preclinical model: Will tomorrow’s surgical robots promise newfound options? Tech. Coloproctol. 2019, 23, 471–477. [Google Scholar] [CrossRef]
- Barret, E. V22 - Single-port radical prostatectomy with using SPORT Surgical System. Eur. Urol. Suppl. 2018, 17, e2142. [Google Scholar] [CrossRef]
- Carbone, M.; Turini, G.; Petroni, G.; Niccolini, M.; Menciassi, A.; Ferrari, M.; Mosca, F.; Ferrari, V. Computer guidance system for single-incision bimanual robotic surgery. Comput. Aided Surg. 2012, 17, 161–171. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Crouzet, S. PE49 - Single port robotic partial and hemi nephrectomy using a novel single port robotic platform: Pilot study in a pig model. Eur. Urol. Suppl. 2018, 17, e2319. [Google Scholar] [CrossRef]
- Gueli Alletti, S.; Rossitto, C.; Cianci, S.; Restaino, S.; Costantini, B.; Fanfani, F.; Fagotti, A.; Cosentino, F.; Scambia, G. Telelap ALF-X vs Standard Laparoscopy for the Treatment of Early-Stage Endometrial Cancer: A Single-Institution Retrospective Cohort Study. J. Minim. Invasive Gynecol. 2016, 23, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Khandalavala, K.; Shimon, T.; Flores, L.; Armijo, P.R.; Oleynikov, D. Emerging surgical robotic technology: A progression toward microbots. Ann. Laparosc. Endosc. Surg. 2019, 5, 1–18. [Google Scholar] [CrossRef]
- Medtronic. Robotic-Assisted Surgery (RAS) Analyst Update; Medtronic, Ed.; Medtronic: Dublin, Ireland, 2019. [Google Scholar]
- Peters, B.S.; Armijo, P.R.; Krause, C.; Choudhury, S.A.; Oleynikov, D. Review of emerging surgical robotic technology. Surg. Endosc. 2018, 32, 1636–1655. [Google Scholar] [CrossRef]
- Piccigallo, M.; Scarfogliero, U.; Quaglia, C.; Petroni, G.; Valdastri, P.; Menciassi, A.; Dario, P. Design of a Novel Bimanual Robotic System for Single-Port Laparoscopy. IEEE ASME Trans. Mechatron. 2010, 15, 871–878. [Google Scholar] [CrossRef]
- Sanchez, L.A.; Petroni, G.; Piccigallo, M.; Scarfogliero, U.; Niccolini, M.; Lui, C.; Stefanini, C.; Zemiti, N.; Menciassi, A.; Poignet, P.; et al. Real-time control and evaluation of a teleoperated miniature arm for Single Port Laparoscopy. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 7049–7053. [Google Scholar]
- Seeliger, B.; Diana, M.; Ruurda, J.P.; Konstantinidis, K.M.; Marescaux, J.; Swanström, L.L. Enabling single-site laparoscopy: The SPORT platform. Surg. Endosc. 2019, 33, 3696–3703. [Google Scholar] [CrossRef]
- Tamaki, A.; Rocco, J.W.; Ozer, E. The future of robotic surgery in otolaryngology – head and neck surgery. Oral Oncol. 2020, 101, 104510. [Google Scholar] [CrossRef]
- Titan Medical Inc. SPORT Surgical System. Available online: https://titanmedicalinc.com/technology/ (accessed on 16 February 2020).
- Titan Medical Inc. Investor Presentation; Titan Medical Inc.: Toronto, ON, Canada, 2016. [Google Scholar]
- Davies, B. Robotic Surgery – A Personal View of the Past, Present and Future. Int. J. Adv. Robot Syst. 2015, 12, 54–60. [Google Scholar] [CrossRef]
- Falk, V.; Mintz, D.; Grunenfelder, J.; Fann, J.I.; Burdon, T.A. Influence of three-dimensional vision on surgical telemanipulator performance. Surg. Endosc. 2001, 15, 1282–1288. [Google Scholar] [CrossRef]
- Kim, H.L.; Schulam, P. The PAKY, HERMES, AESOP, ZEUS, and da Vinci robotic systems. Urol. Clin. North Am. 2004, 31, 659–669. [Google Scholar] [CrossRef]
- Bodner, J.; Wykypiel, H.; Wetscher, G.; Schmid, T. First experiences with the da Vinci™ operating robot in thoracic surgery. Eur. J. Cardiothorac. Surg. 2004, 25, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Broeders, I.; Ruurda, J. Robots revolutionizing surgery: The Intuitive Surgical “Da Vinci” system. Ind. Robot 2001, 28, 387–391. [Google Scholar] [CrossRef]
- Falk, V.; Diegeler, A.; Walther, T.; Banusch, J.; Brucerius, J.; Raumans, J.; Autschbach, R.; Mohr, F.W. Total endoscopic computer enhanced coronary artery bypass grafting. Eur. J. Cardiothorac. Surg. 2000, 17, 38–45. [Google Scholar] [CrossRef]
- Schwab, K.; Smith, R.; Brown, V.; Whyte, M.; Jourdan, I. Evolution of stereoscopic imaging in surgery and recent advances. World J. Gastrointest. Endosc. 2017, 9, 368–377. [Google Scholar] [CrossRef] [PubMed]
- DeBeche-Adams, T.; Eubanks, W.S.; Fuente, S.G. Early experience with the Senhance®-laparoscopic/robotic platform in the US. J. Robot. Surg. 2019, 13, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Melling, N.; Barr, J.; Schmitz, R.; Polonski, A.; Miro, J.; Ghadban, T.; Wodack, K.; Izbicki, J.; Zani, S.; Perez, D. Robotic cholecystectomy: First experience with the new Senhance robotic system. J. Robot. Surg. 2019, 13, 495–500. [Google Scholar] [CrossRef]
- TransEnterix Inc. Fact Sheet: SENHANCE™ SURGICAL SYSTTEM HIGHLIGHTS; TransEnterix Inc.: Morrisville, NC, USA, 2018. [Google Scholar]
- Altobelli, E.; Gidaro, S.; Bove, A.M.; Falavolti, C.; Ruiz, E.; Rosa, T.; Stark, M.; Buscarini, M. TELELAP ALF-X: A NOVEL TELESURGICAL SYSTEM FOR THE 21ST CENTURY. J. Urol. 2013, 189, e575–e576. [Google Scholar] [CrossRef]
- Rumolo, V.; Rosati, A.; Tropea, A.; Biondi, A.; Scambia, G. Senhance robotic platform for gynecologic surgery: A review of literature. Updates Surg. 2019, 71, 419–427. [Google Scholar] [CrossRef]
- Kaouk, J.H.; Haber, G.-P.; Autorino, R.; Crouzet, S.; Ouzzane, A.; Flamand, V.; Villers, A. A Novel Robotic System for Single-port Urologic Surgery: First Clinical Investigation. Eur. Urol. 2014, 66, 1033–1043. [Google Scholar] [CrossRef]
- Simaan, N.; Bajo, A.; Reiter, A.; Wang, L.; Allen, P.; Fowler, D. Lessons learned using the insertable robotic effector platform (IREP) for single port access surgery. J. Robot. Surg. 2013, 7, 235–240. [Google Scholar] [CrossRef]
- Galvao Neto, M.; Ramos, A.; Campos, J. Single port laparoscopic access surgery. Tech. Gastrointest. Endosc. 2009, 11, 84–93. [Google Scholar] [CrossRef]
- Haber, G.-P.; White, M.A.; Autorino, R.; Escobar, P.F.; Kroh, M.D.; Chalikonda, S.; Khanna, R.; Forest, S.; Yang, B.; Altunrende, F.; et al. Novel Robotic da Vinci Instruments for Laparoendoscopic Single-site Surgery. Urology 2010, 76, 1279–1282. [Google Scholar] [CrossRef] [PubMed]
- Kaouk, J.H.; Goel, R.K.; Haber, G.P.; Crouzet, S.; Stein, R.J. Robotic single-port transumbilical surgery in humans: Initial report. BJU Int. 2009, 103, 366–369. [Google Scholar] [CrossRef]
- DLR Institute of Robotics and Mechatronics. MiroSurge. Available online: https://www.dlr.de/rm/en/desktopdefault.aspx/tabid-11674/#gallery/28728 (accessed on 14 February 2020).
- Ding, J.; Goldman, R.E.; Xu, K.; Allen, P.K.; Fowler, D.L.; Simaan, N. Design and Coordination Kinematics of an Insertable Robotic Effectors Platform for Single-Port Access Surgery. IEEE ASME Trans. Mechatron. 2013, 18, 1612–1624. [Google Scholar] [CrossRef] [PubMed]
- Kai, X.; Goldman, R.E.; Jienan, D.; Allen, P.K.; Fowler, D.L.; Simaan, N. System design of an Insertable Robotic Effector Platform for Single Port Access (SPA) Surgery. In Proceedings of the 2009 IEEE/RSJ International Conference on Intelligent Robots and Systems, St. Louis, MO, USA, 11–15 October 2009; pp. 5546–5552. [Google Scholar]
- Falk, V.; McLoughlin, J.; Guthart, G.; Salisbury, J.K.; Walther, T.; Gummert, J.; Mohr, F.W. Dexterity enhancement in endoscopic surgery by a computer-controlled mechanical wrist. Minim. Invasive Ther. Allied Technol. 1999, 8, 235–242. [Google Scholar] [CrossRef]
- Burdea, G.C. Force and touch feedback for virtual reality; John Wiley and Sons Ltd: New York, NY, USA, 1996. [Google Scholar]
- King, C.H.; Culjat, M.O.; Franco, M.L.; Bisley, J.W.; Carman, G.P.; Dutson, E.P.; Grundfest, W.S. A Multielement Tactile Feedback System for Robot-Assisted Minimally Invasive Surgery. IEEE Trans. Haptics 2009, 2, 52–56. [Google Scholar] [CrossRef]
- Ballantyne, H.G. The Pitfalls of Laparoscopic Surgery: Challenges for Robotics and Telerobotic Surgery. Surgical Laparoscopy, Endoscopy Percutaneous Tech. 2002, 12, 1–5. [Google Scholar] [CrossRef]
- Callaghan, D.; McGrath, M.; Coyle, E. Force Measurement Methods in Telerobotic Surgery: Implications for End-Effector Manufacture. In Proceedings of the 25th International Manufacturing Conference (IMC25), Dublin, Ireland, 3 September 2008; pp. 389–398. [Google Scholar]
- Ehrampoosh, S.; Dave, M.; Kia, M.A.; Rablau, C.; Zadeh, M.H. Providing haptic feedback in robot-assisted minimally invasive surgery: A direct optical force-sensing solution for haptic rendering of deformable bodies. Comput. Aided Surg. 2013, 18, 129–141. [Google Scholar] [CrossRef][Green Version]
- Haouchine, N.; Winnie, K.; Cotin, S.; Yip, M. Vision-Based Force Feedback Estimation for Robot-Assisted Surgery Using Instrument-Constrained Biomechanical Three-Dimensional Maps. IIEE Robot. Autom. Lett. 2018, 3, 2160–2165. [Google Scholar] [CrossRef]
- Okamura, M.A. Haptic feedback in robot-assisted minimally invasive surgery. Curr. Opin. Urology 2009, 19, 102–107. [Google Scholar] [CrossRef]
- Reiley, C.E.; Akinbiyi, T.; Burschka, D.; Chang, D.C.; Okamura, A.M.; Yuh, D.D. Effects of visual force feedback on robot-assisted surgical task performance. J. Thorac. Cardiov. Surg. 2008, 135, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Trejos, A.L.; Escoto, A.; Naish, M.D.; Patel, R.V. Design and Evaluation of a Sterilizable Force Sensing Instrument for Minimally Invasive Surgery. IEEE Sensors J. 2017, 17, 3983–3993. [Google Scholar] [CrossRef]
- Yu, L.; Yan, Y.; Li, C.; Zhang, X. Three-dimensional nonlinear force-sensing method based on double microgrippers with E-type vertical elastomer for minimally invasive robotic surgery. Robotica 2018, 36, 865–881. [Google Scholar] [CrossRef]
- FUTEK Advanced Sensor Technology Inc. Challenges in Designing and Manufacturing Haptic Sensors for Robotic Surgery Platforms. Available online: https://media.futek.com/content/futek/files/pdf/quality/challenges-in-designing-and-manufacturing-sensors-for-robotic-surgery.pdf (accessed on 14 February 2020).
- Yang, C.; Xie, Y.; Liu, S.; Sun, D. Force Modeling, Identification, and Feedback Control of Robot-Assisted Needle Insertion: A Survey of the Literature. Sensors 2018, 18, 561. [Google Scholar] [CrossRef]
- Akinbiyi, T.; Reiley, C.E.; Saha, S.; Burschka, D.; Hasser, C.J.; Yuh, D.D.; Okamura, A.M. Dynamic augmented reality for sensory substitution in robot-assisted surgical systems. In Proceedings of the 2006 International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006; p. 567. [Google Scholar]
- Hagn, U.; Konietschke, R.; Tobergte, A.; Nickl, M.; Jörg, S.; Kübler, B.; Passig, G.; Gröger, M.; Fröhlich, F.; Seibold, U.; et al. DLR MiroSurge: A versatile system for research in endoscopic telesurgery. Int. J. Comput. Assist. Radiol. Surg. 2010, 5, 183–193. [Google Scholar] [CrossRef]
- Orekhov, A.; Abah, C.; Simaan, N. Snake-Like Robots for Minimally Invasive, Single Port, and Intraluminal Surgeries; World Scientific Publishing Co. Pte. Ltd.: Singapore, 2019. [Google Scholar]
- Culjat, M.; Franks, J.; King, C.H.; Franco, M.; Bisley, J.; Grundfest, W.; Dutson, E. Pneumatic balloon actuators for tactile feedback in robotic surgery. Ind. Robot 2008, 35, 449–455. [Google Scholar] [CrossRef]
- Amirabdollahian, F.; Livatino, S.; Vahedi, B.; Gudipati, R.; Sheen, P.; Gawrie-Mohan, S.; Vasdev, N. Prevalence of haptic feedback in robot-mediated surgery: A systematic review of literature. J. Robot. Surg. 2017, 12, 11–25. [Google Scholar] [CrossRef]
- Wagner, C.R.; Stylopoulos, N.; Howe, R.D. The Role of Force Feedback In Surgery: Analysis Of Blunt Dissection. In Proceedings of the 10th Symposium on Haptic Interfaces for Virtual Environment and Teleoperator Systems, HAPTICS 2002, Orlando, FL, USA, 24–25 March 2002; pp. 68–74. [Google Scholar]
- Tholey, P.G.; Desai, E.J.; Castellanos, E.A. Force Feedback Plays a Significant Role in Minimally Invasive Surgery: Results and Analysis. Ann. Surg. 2005, 241, 102–109. [Google Scholar] [CrossRef]
- Wagner, C.R.; Howe, R.D. Force Feedback Benefit Depends on Experience in Multiple Degree of Freedom Robotic Surgery Task. IEEE T. Robot 2007, 23, 1235–1240. [Google Scholar] [CrossRef]
- Zhou, M.; Tse, S.; Derevianko, A.; Jones, D.; Schwaitzberg, S.; Cao, C. Effect of haptic feedback in laparoscopic surgery skill acquisition. Surg. Endosc. 2012, 26, 1128–1134. [Google Scholar] [CrossRef]
- Kitagawa, M.; Dokko, D.; Okamura, A.M.; Yuh, D.D. Effect of sensory substitution on suture-manipulation forces for robotic surgical systems. J. Thorac. Cardiov. Surg. 2005, 129, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Oncohema Key. Robotic Surgery. Available online: https://oncohemakey.com/robotic-surgery/ (accessed on 4 February 2020).
- Intuitive Surgical Inc. Press Resources: Images: Da Vinci systems and simulation; Intuitive Surgical Inc.: Sunnyvale, CA, USA, 2017; Volume 2020. [Google Scholar]
- Revo Surgical Solutions. Surgical Solution, Revo. Available online: http://revosurgical.com/#/revo.html (accessed on 24 September 2019).
- CMR Surgical Ltd. Press Kit. Available online: https://cmrsurgical.com/press-kit/ (accessed on 24 February 2020).
- DLR Institute of Robotics and Mechatronics. Telemanipulation in Minimally Invasive Surgery. Available online: https://www.dlr.de/rm/en/desktopdefault.aspx/tabid-3795/16616_read-40529/ (accessed on 14 February 2020).
- Altobelli, E.; Stefano, G.; Falavolti, C.; Bove, A.M.; Ruiz, E.M.; Stark, M.; Lazzaretti, S.S.; Buscarini, M. 1412 VESICO-URETHRAL ANASTOMOSIS USING A NOVEL TELESURGICAL SYSTEM WITH HAPTIC SENSATION: THE TELELAP ALF-X. A PILOT STUDY. J. Urol. 2013, 189, e578. [Google Scholar] [CrossRef]
- Chang, K.D.; Raheem, A.A.; Rha, K.H. Novel robotic systems and future directions. Indian J. Urol. 2018, 34, 110. [Google Scholar] [CrossRef]
- Dobbs, R.W.; Halgrimson, W.R.; Madueke, I.; Vigneswaran, H.T.; Wilson, J.O.; Crivellaro, S. Single-port robot-assisted laparoscopic radical prostatectomy: Initial experience and technique with the da Vinci® SP platform. BJU Int. 2019, 124, 1022–1027. [Google Scholar] [CrossRef]
- Zhao, J.; Feng, B.; Zheng, M.-H.; Xu, K. Surgical robots for SPL and NOTES: A review. Minim. Invasive Ther. Allied Technol. 2015, 24, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Gruijthuijsen, C.; Lin, D.; Morel, G.; Poorten, E.V. Leveraging the Fulcrum Point in Robotic Minimally Invasive Surgery. IIEE Robot. Autom. Lett. 2018, 3, 2071–2078. [Google Scholar] [CrossRef]
- Wang, Z.; Phee, S.J.; Wong, J.; Ho, K.-Y. Development of a robotic platform for natural orifice transluminal endoscopic surgery. Gastrointest. Interv. 2012, 1, 40–42. [Google Scholar] [CrossRef][Green Version]
- Phee, S.J.; Low, S.C.; Huynh, V.A.; Kencana, A.P.; Sun, Z.L.; Yang, K. Master and slave transluminal endoscopic robot (MASTER) for natural Orifice Transluminal Endoscopic Surgery (NOTES). In Proceedings of the 2009 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 3–6 September 2009; pp. 1192–1195. [Google Scholar]
- Hagn, U.; Nickl, M.; Jörg, S.; Passig, G.; Bahls, T.; Nothhelfer, A.; Hacker, F.; Le-Tien, L.; Albu-Schäffer, A.; Konietschke, R.; et al. The DLR MIRO: A versatile lightweight robot for surgical applications. Ind. Robot 2008, 35, 324–336. [Google Scholar] [CrossRef]
- Intuitive Surgical Inc. Davinci Xi, X - Instrument & Accessory Catalog - 19; Intuitive Surgical Inc.: Sunnyvale, CA, USA, 2019 20 January. [Google Scholar]
- King, C. Endoscopic electrosurgery an overview. Gastrointest. Nurs. 2011, 9, 28–33. [Google Scholar] [CrossRef]
- Van de Berg, N.; Van den Dobbelsteen, J.; Jansen, F.; Grimbergen, C.; Dankelman, J. Energetic soft-tissue treatment technologies: An overview of procedural fundamentals and safety factors. Surg. Endosc. 2013, 27, 3085–3099. [Google Scholar] [CrossRef]
- Bovo, F.; De Rossi, G.; Visentin, F. Surgical robot simulation with BBZ console. J. Vis. Surg. 2017, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Revo Surgical Solutions. Revo-i Overview; Revo Surgical Solutions: Seoul, Korea, 2019; Volume 2019. [Google Scholar]
- TransEnterix Inc. Senhance 3 mm Instruments for Hernia Repair - Dr. Dietmar Stephen; TransEnterix Inc: Morrisville, NC, USA, 2019; Volume 2020. [Google Scholar]
- TransEnterix Inc. RADIA Articulating Surgical Instrument; TransEnterix Inc.: Morrisville, NC, USA, 2017; Volume 2020. [Google Scholar]
- TransEnterix Inc. Senhance Robotic Creation of Fundoplication; Advanced Suturing - Porcine Model; TransEnterix Inc.: Morrisville, NC, USA, 2017; Volume 2020. [Google Scholar]
- TransEnterix Inc. Senhance Robotic Articulating Instrument in Colorectal Dissection; TransEnterix Inc.: Morrisville, NC, USA, 2017; Volume 2020. [Google Scholar]
- DLR Institute of Robotics and Mechatronics. MICA. Available online: https://www.dlr.de/rm/en/desktopdefault.aspx/tabid-11672/#gallery/29823 (accessed on 14 February 2020).
- Khor, W.S.; Baker, B.; Amin, K.; Chan, A.; Patel, K.; Wong, J. Augmented and virtual reality in surgery-the digital surgical environment: Applications, limitations and legal pitfalls. Ann. Transl. Med. 2016, 4, 454. [Google Scholar] [CrossRef]
- Nizamoglu, M.; Tan, A.; Gerrish, H.; Barnes, D.; Dziewulski, P. Infrared technology to improve efficacy of venous access in burns population. Eur. J. Plast. Surg. 2015, 39, 37–40. [Google Scholar] [CrossRef]
- Crisan, S.; Tarnovan, I.G.; Crisan, T.E. A Low Cost Vein Detection System Using Near Infrared Radiation. In Proceedings of the IEEE Sensors Applications Symposium, San Diego, CA, USA, 6–8 Feburary 2007. [Google Scholar]
- Chin, K.; Engelsman, A.F.; Chin, P.T.K.; Meijer, S.L.; Strackee, S.D.; Oostra, R.J.; Van Gulik, T.M. Evaluation of collimated polarized light imaging for real-time intraoperative selective nerve identification in the human hand. Biomed. Opt. Express 2017, 8, 4122–4134. [Google Scholar] [CrossRef] [PubMed]
| Manufacturer | Model | Science Direct | Ovid | Web of Science | Scopus | PubMED | Cochrane Library | Google Scholar | ProQuest Central | Mean | Search Term |
|---|---|---|---|---|---|---|---|---|---|---|---|
| avateramedical | Avatera | 4 | 2 | 1 | 1 | 1 | 0 | 22 | 3 | 4 | “Robot” AND “Surgery” AND “Avatera” |
| Intuitive Surgical | da Vinci | 4617 | 2858 | 1899 | 2652 | 1413 | 276 | 26,400 | 2513 | 5329 | “Robot” AND “Surgery” AND “da Vinci” |
| Intuitive Surgical | da Vinci S | 386 | 294 | 120 | 154 | 87 | 22 | 2640 | 302 | 501 | “Robot” AND “Surgery” AND “da Vinci S” |
| Intuitive Surgical | da Vinci SI | 530 | 330 | 90 | 258 | 83 | 40 | 2850 | 358 | 568 | “Robot” AND “Surgery” AND “da Vinci SI” |
| Intuitive Surgical | da Vinci SP | 58 | 32 | 20 | 28 | 13 | 0 | 252 | 14 | 52 | “Robot” AND “Surgery” AND “da Vinci SP” |
| Intuitive Surgical | da Vinci XI | 249 | 136 | 83 | 186 | 78 | 15 | 1530 | 146 | 303 | “Robot” AND “Surgery” AND “da Vinci XI” |
| Medtronic | Hugo | 706 | 106 | 3 | 8 | 32 | 1 | 6320 | 357 | 942 | “Robot” AND “Surgery” AND (“Einstein” OR “Hugo”) |
| DLR | MiroSurge | 20 | 8 | 6 | 15 | 1 | 0 | 392 | 34 | 60 | “Robot” AND “Surgery” AND “MiroSurge” |
| Revo Surgical Solutions | Revo | 6 | 11 | 6 | 10 | 6 | 0 | 62 | 9 | 14 | “Robot” AND “Surgery” AND “Revo-I” |
| TransEnterix | Senhance A | 51 | 26 | 15 | 28 | 16 | 2 | 335 | 32 | 63 | “Robot” AND “Surgery” AND (“Senhance” OR “ALF-X”) |
| Titan Medical | SPORT Surgical System | 19 | 11 | 2 | 4 | 20 | 0 | 130 | 19 | 26 | “Robot” AND “Surgery” AND (“SPORT Surgical System” OR “Single Port Orifice Robotic Technology”) |
| ARKANES | SPRINT C | 102 | 34 | 5 | 6 | 3 | 0 | 1200 | 45 | 174 | “Robot” AND “Surgery” AND “SPRINT” |
| CMR Surgical B | Versius | 8 | 7 | 1 | 7 | 1 | 0 | 70 | 6 | 13 | “Robot” AND “Surgery” AND “Versius” |
| Robot | 2D | 3D | 3DS | Endoscope Control | Ref. |
|---|---|---|---|---|---|
| Avatera | A | B | D | [15,26] | |
| da Vinci (all versions) | A | B | D | [16,18,19,39,47,48,49,50,51,66] | |
| Hugo | A | C | N/A | [38] | |
| MiroSurge | C | N/A | [29,39,63] | ||
| Revo-I | B | N/A | [21,22,24] | ||
| Senhence | B | C | ◊ | [8,9,11,12,13,27,36,37,39,53,54,55,56,57] | |
| SPORT Surgical System | C | N/A | [33,35,39,42,44] | ||
| SPRINT | C | N/A | [30,31,34,40,41] | ||
| Versius | C | N/A | [6,32,37,39,43] |
| Robot | Instrument and Arm Control | Instrument Feedback | Tremor Removal | Clutching Arms | Arm Switching | Endoscope Control | Diathermy | Seated or Standing | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Avatera | A | F | N/A | N/A | N/A | H | H | S | [15] |
| da Vinci (all versions) | A | E | P | H | H | H | H | S | [14,16,17,18,19] |
| Hugo | B | N/A | N/A | N/A | N/A | N/A | H | S | [38] |
| MiroSurge | A | F | N/A | N/A | N/A | N/A | N/A | S | [28,63,93] |
| Revo-I | A | F | N/A | H | H | H | H | S | [17,22,24] |
| Senhence | C | F | P | N/A | N/A | I | N/A | S | [8,9,11,13,14,26,36,54,56,57,94] |
| SPORT Surgical System | A | N/A | N/A | N/A | NP | N/A | N/A | S | [33,35,42,44] |
| SPRINT | A | N/A | N/A | H | N/A | N/A | N/A | S | [30,31,34,40] |
| Versius | D | F | N/A | G | G | G | G | S/U | [6,12,32,37,39] |
| Robot | No. Arms | Instrument Arms B | DOF | Trocar | Cart Type | References |
|---|---|---|---|---|---|---|
| Avatera | 4 | 3 | 6 | 5 mm | Single | [15,25,26] |
| da Vinci (except SP) | 4 | 3 | 7 | 8 mm | Single | [16,18,49] |
| da Vinci SP | 1 | 2 | 7 | S 25 mm | Single | [20] |
| Hugo | 4 | 3 | N/A | N/A | Individual | [38] |
| MiroSurge | 3 | 2 | 7 | N/A | Individual A | [29,63,82,101] |
| Revo-I | 4 | 3 | 7 | 12 mm | Single | [17,21,22,23,24] |
| Senhence | 4 | 3 | 7 | I 5 mm E 10 mm | Individual | [8,11,12,13,14,37] |
| SPORT Surgical System | 1 | 2 | N/A | S 25 mm | Single | [42,44,45] |
| SPRINT | 1 | 2 | 6 | S 30 mm | Single | [40,41] |
| Versius | 5 | 4 | 7 | 5 mm | Individual | [6,12,22,37,39,43] |
| Robot | Cautery Hook | Cautery Spatula | Clip Applier | Dissector | Forceps | Grasper | Needle Drivers | Retractors | Scissors | Sheers | Stapler | Suction/Irrigator | Vessel Sealer | Reusability | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Avatera | B | S | S | S | 1 | [15] | |||||||||
| da Vinci Xi | M | M | S | B | SB | B | S | S | S | SM | S | S | S | ±10 | [16,102] |
| da Vinci SS 1 | M | S | S | SB | S | S | S | S | ±10 | [102] | |||||
| da Vinci SP 2 | S | S | S | N/A | [90] | ||||||||||
| Hugo 3 | I | N/A | [38] | ||||||||||||
| MiroSurge | I | I | S | I | N/A | [80,111] | |||||||||
| Revo-I | M | I | I | I | S | M | 20 | [17,21,23] | |||||||
| Senhence | M | B | I | I | I | ∞ | [8,9,11,13,14] | ||||||||
| SPORT Surgical System | M | B | SB | S | SM | 1 | [33,35,42,44] | ||||||||
| SPRINT 4 | I | N/A | [30,31] | ||||||||||||
| Versius | I | I | I | S | I | N/A | [12,39] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Longmore, S.K.; Naik, G.; Gargiulo, G.D. Laparoscopic Robotic Surgery: Current Perspective and Future Directions. Robotics 2020, 9, 42. https://doi.org/10.3390/robotics9020042
Longmore SK, Naik G, Gargiulo GD. Laparoscopic Robotic Surgery: Current Perspective and Future Directions. Robotics. 2020; 9(2):42. https://doi.org/10.3390/robotics9020042
Chicago/Turabian StyleLongmore, Sally Kathryn, Ganesh Naik, and Gaetano D. Gargiulo. 2020. "Laparoscopic Robotic Surgery: Current Perspective and Future Directions" Robotics 9, no. 2: 42. https://doi.org/10.3390/robotics9020042
APA StyleLongmore, S. K., Naik, G., & Gargiulo, G. D. (2020). Laparoscopic Robotic Surgery: Current Perspective and Future Directions. Robotics, 9(2), 42. https://doi.org/10.3390/robotics9020042






