A Review of Robotic Interfaces for Post-Stroke Upper-Limb Rehabilitation: Assistance Types, Actuation Methods, and Control Mechanisms
Abstract
1. Introduction
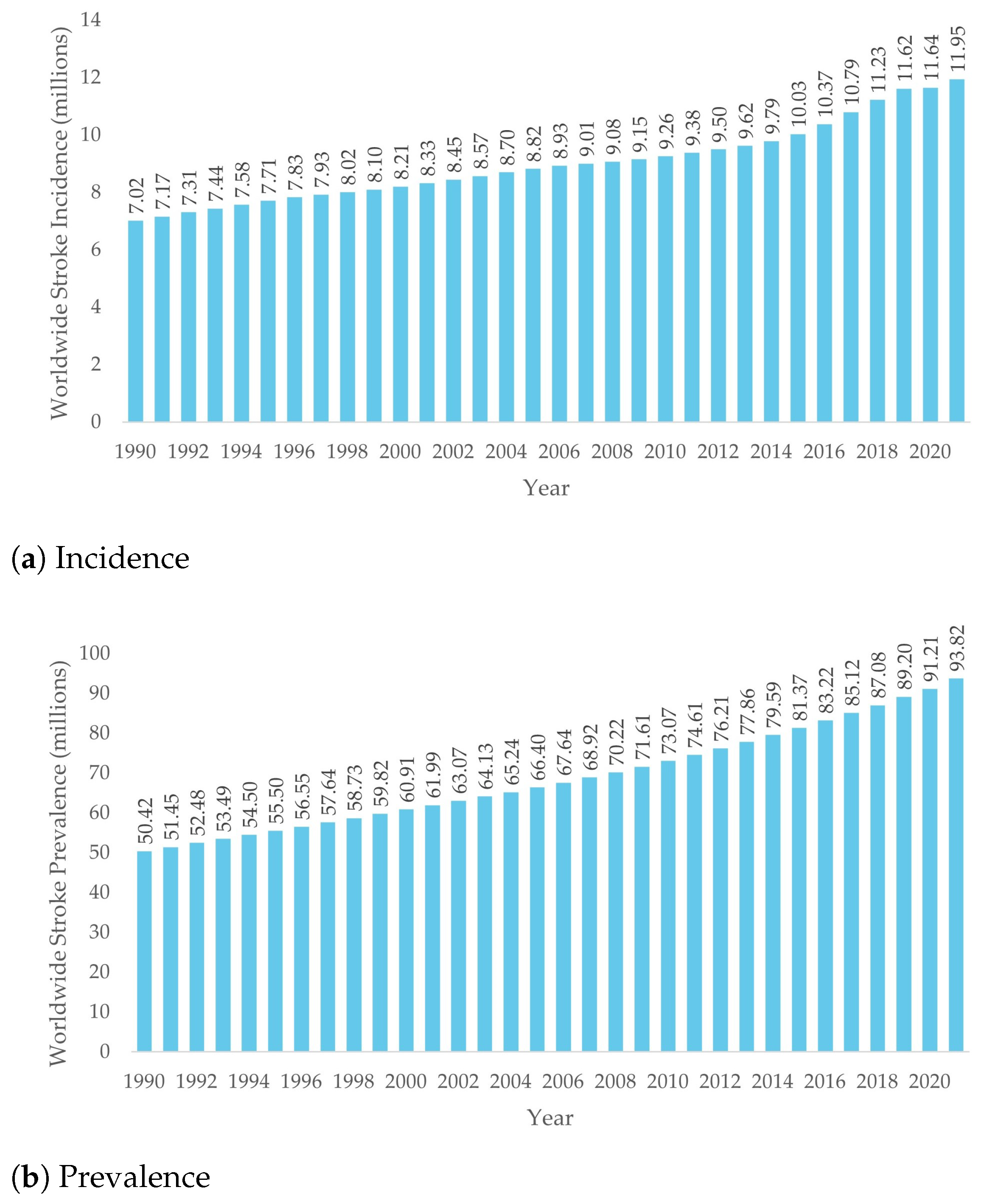
1.1. Stroke Incidence
1.2. Social and Economic Impact of Stroke
1.3. Stroke Repercussions
- Cognitive Challenges, such as memory loss and dementia [26];
- Emotional Issues, like dementia or low self-esteem [27];
- Sensorial Difficulties, because hearing and vision can deteriorate [28];
- Physical Effects, causing fatigue, incontinence of difficulty swallowing, among others [29]. The most common physical effects of strokes are hemiplegia or hemiparesis (total or partial weakness or loss of ability to produce movement of a limb, respectively), changes in muscle tension and loss of awareness of a limb position [30].
1.4. Upper-Limb Impairment and Robotic Rehabilitation
1.5. Study Methodology
- (upper limb OR upper-limb Or upper arm OR stroke OR funtion*) AND
- (robot OR robotic OR end-effector OR exoskeleton) AND
- (actuat* OR active OR control) AND
- (rehabilitation)
1.6. Contributions and Paper Structure
2. Fundamentals of Robot-Aided Upper-Limb Rehabilitation
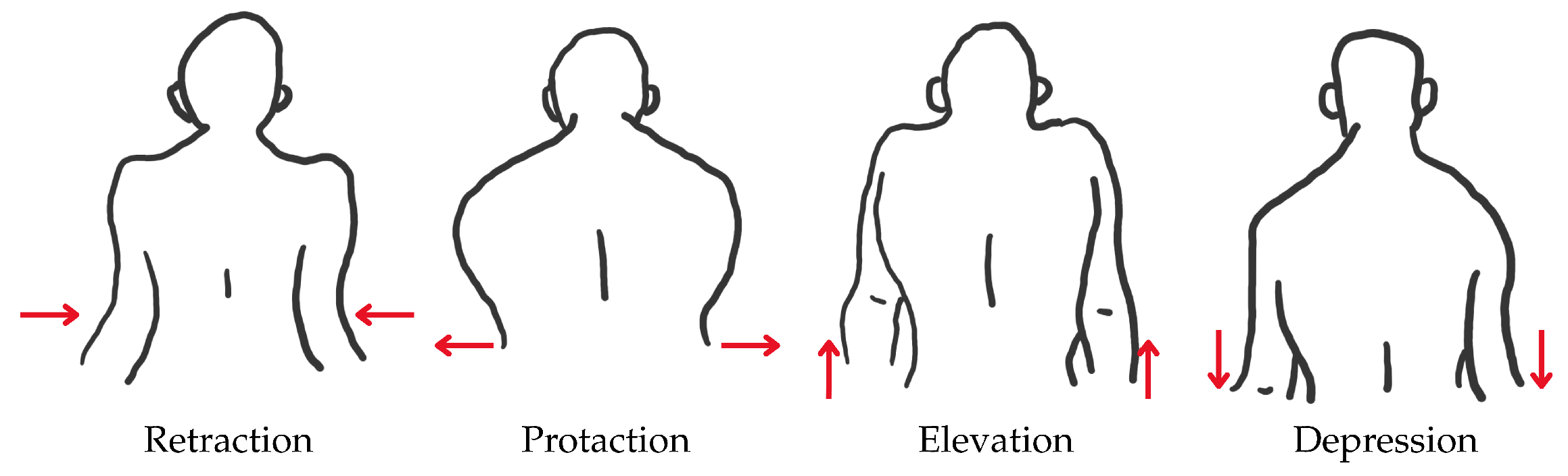
2.1. Human Upper-Limb Anatomy
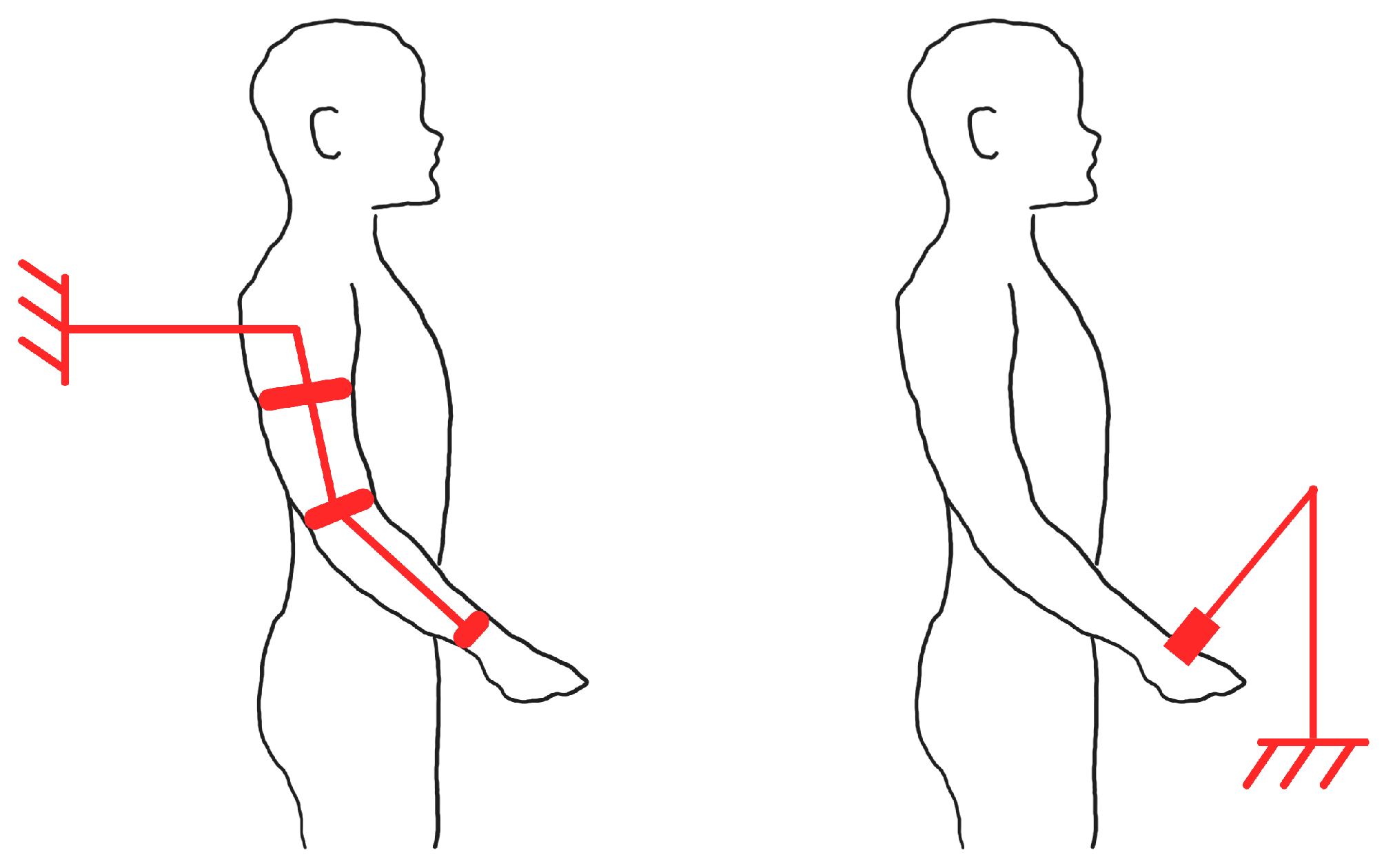
2.2. Exoskeleton-Based and End-Effector-Based Therapies
3. Assistance Types in Robot-Aided Physical Therapy
4. Rehabilitation Robotic Devices’ Control Philosophy
4.1. Assistive Control
4.1.1. Impedance-Based
4.1.2. Counter-Balanced-Based
4.1.3. EMG-Based
4.1.4. Performance-Based
4.2. Challenge-Based Control
4.2.1. Resistive-Based
4.2.2. Restraint-Induced-Based
4.2.3. Magnifying Error-Based
4.3. Haptic Interface Control
4.4. Non-Contact Monitoring Control
4.5. General Considerations on Control Philosophy
5. Actuation and Power Transmission in Robotic Devices
5.1. Electric Actuators
5.2. Pneumatic Actuators
5.3. Hydraulic Actuators
5.4. Comparison
6. Discussion of Robotic Devices for Upper-Limb Rehabilitation
6.1. End-Effector-Based Devices
6.2. Exoskeleton-Based Devices Actuated with Servomotors
6.3. Exoskeleton-Based Devices Actuated with Brushed DC Motors
6.4. Exoskeleton-Based Devices Actuated with Brushless DC Motors
6.5. Exoskeleton-Based Devices Actuated with SEAs
6.6. Non-Electrically Actuated Exoskeleton-Based Devices
6.7. Reviewed Devices Discussion and Comparison
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3D | Three-Dimensional |
| ADLs | Activities of Daily Living |
| AAN | Assist-as-Needed |
| AC | Alternating Current |
| ARAT | Action research arm test |
| BBT | Box and block test |
| CVA | Cerebrovascular Accident |
| DALYs | Disability-Adjusted Life Years |
| DC | Direct Current |
| DOF | Degrees of Freedom |
| EMG | Electromyography |
| FDA | Food and Drug Administration |
| FMA | Fugl–Myer assessment |
| HICs | High-Income Countries |
| IEEE | Institute of Electrical and Electronics Engineers |
| LMICs | Low/Middle-Income Countries |
| LSR | Life Science Robotics |
| NHS | National Health Service |
| PAMs | Pneumatic Artificial Muscles |
| SCI | Spinal Cord Injury |
| SEAs | Series Elastic Actuator |
| sEMG | Surface Electromyography |
| SMA | Shape Memory Alloy |
| UL | Upper-limb |
| WHO | World Health Organization |
| WSO | World Stroke Organization |
References
- National Health Service. Stroke. Available online: https://www.nhs.uk/conditions/stroke/ (accessed on 13 November 2014).
- John Hopkins Medicine. Arm Care After a Stroke. Available online: https://www.hopkinsmedicine.org/health/conditions-and-diseases/stroke/arm-care-after-a-stroke (accessed on 30 July 2025).
- World Stroke Organization. Impact of Stroke. Available online: https://www.world-stroke.org/world-stroke-day-campaign/about-stroke/impact-of-stroke (accessed on 4 November 2024).
- WHO Regional Director for South-East Asia Dr Poonam Khetrapal Singh. World Stroke Day, October 2021. Available online: https://www.who.int/southeastasia/news/detail/28-10-2021-world-stroke-day (accessed on 6 November 2024).
- Truelsen, T.; Piechowski-Jóźwiak, B.; Bonita, R.; Mathers, C.; Bogousslavsky, J.; Boysen, G. Stroke incidence and prevalence in europe: A review of available data. Eur. J. Neurol. 2006, 13, 581–598. [Google Scholar] [CrossRef]
- Rajsic, S.; Gothe, H.; Borba, H.H.; Sroczynski, G.; Vujicic, J.; Toell, T.; Siebert, U. Economic burden of stroke: A systematic review on post-stroke care. Eur. J. Health Econ. 2017, 20, 107–134. [Google Scholar] [CrossRef]
- World Health Organization. Ageing and Health, October 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 6 November 2024).
- Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2021 (gbd 2021) Results. 2022. Available online: https://vizhub.healthdata.org/gbd-results/ (accessed on 19 November 2024).
- Yousufuddin, M.; Young, N. Aging and Ischemic Stroke. Aging 2019, 11, 2542–2544. [Google Scholar] [CrossRef]
- Feigin, V.L.; Brainin, M.; Norrving, B.; Martins, S.; Sacco, R.L.; Hacke, W.; Fisher, M.; Pandian, J.; Lindsay, P. World stroke organization (wso): Global stroke fact sheet 2022. Int. J. Stroke 2022, 17, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Rahbar, M.H.; Medrano, M.; Diaz-Garelli, F.; Villaman, C.G.; Saroukhani, S.; Kim, S.; Tahanan, A.; Franco, Y.; Castro-Tejada, G.; Diaz, S.A.; et al. Younger age of stroke in low-middle income countries is related to healthcare access and quality. Ann. Clin. Transl. Neurol. 2022, 9, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Thayabaranathan, T.; Kim, J.; Cadilhac, D.A.; Thrift, A.G.; Donnan, G.A.; Howard, G.; Howard, V.J.; Rothwell, P.M.; Feigin, V.; Norrving, B.; et al. Global stroke statistics 2022. Int. J. Stroke 2022, 17, 946–956. [Google Scholar] [CrossRef]
- Mathews, C. Population ageing in lower- and middle-income countries. Celebrating 40 years of helpage international. J. Popul. Ageing 2024, 17, 1–4. [Google Scholar] [CrossRef]
- Banzrai, C.; Bosookhuu, O.; Yadamsuren, E.; Dambasuren, B.; Turbat, S.; Erdenedalai, T.; Myadagsuren, M.; Munkhtur, U.; Baatar, K.; Boldbayar, P.; et al. Incidence and outcomes for stroke in ulaanbaatar, mongolia, during 2019–2021: A prospective population-based study. Lancet Glob. Health 2023, 11, e942–e952. [Google Scholar] [CrossRef]
- Jiang, K.; He, T.; Ji, Y.; Zhu, T.; Jiang, E. The perspective of hypertension and salt intake in chinese population. Front. Public Health 2023, 11, 1125608. [Google Scholar] [CrossRef]
- He, F.J.; MacGregor, G.A. Salt, blood pressure and cardiovascular disease. Curr. Opin. Cardiol. 2007, 22, 298–305. [Google Scholar] [CrossRef]
- Rayner, M.; Allender, S.; Scarborough, P.; Peto, V. European Cardiovascular Disease Statistics, 2008 Edition, September 2008. Available online: https://coachmikeblogs.com/wp-content/uploads/2014/08/European_cardiovascular_disease_statistics_2008.pdf (accessed on 6 November 2024).
- Instituto Nacional de Estatística. Causas de Morte 2022, May 2024. Available online: https://www.ine.pt/xportal/xmain?xpid=INE&xpgid=ine_destaques&DESTAQUESdest_boui=646029525&DESTAQUESmodo=2 (accessed on 4 November 2024).
- Centers for Disease Control and Prevention. Stroke Facts. Available online: https://www.cdc.gov/stroke/data-research/facts-stats/index.html (accessed on 7 February 2025).
- World Health Organization and World Bank. World Report on Disability, December 2011. Available online: https://www.who.int/publications/i/item/9789241564182 (accessed on 6 November 2024).
- Fonseca, L. O avc é a principal causa de morte e incapacidade em portugal. Soc. Port. De Med. Interna 2021. Available online: https://www.spmi.pt/o-avc-e-a-principal-causa-de-morte-e-incapacidade-em-portugal/ (accessed on 4 November 2024).
- Stroke Association. Stroke Research Means Everything. Available online: https://www.stroke.org.uk/stroke/support/materials/rebuilding-lives/stroke-research-means-everything (accessed on 6 January 2025).
- National Stroke Association. Effects of Stroke. Available online: https://www.stroke.org/en/about-stroke/effects-of-stroke (accessed on 5 February 2025).
- National Stroke Association. Communication Problems After Stroke. Available online: https://www.stroke.org/en/about-stroke/effects-of-stroke/communication-and-aphasia (accessed on 5 February 2025).
- Johns Hopkins Medicine. Aphasia. Available online: https://www.hopkinsmedicine.org/health/conditions-and-diseases/aphasia (accessed on 5 February 2025).
- National Stroke Association. Cognitive Challenges Post-Stroke. Available online: https://www.stroke.org/en/about-stroke/effects-of-stroke/cognitive-effects (accessed on 5 February 2025).
- National Stroke Association. Emotional and Behavioral Effects of Stroke. Available online: https://www.stroke.org/en/about-stroke/effects-of-stroke/emotional-effects (accessed on 5 February 2025).
- National Stroke Association. Vision and Hearing. Available online: https://www.stroke.org/en/about-stroke/effects-of-stroke/vision-and-hearing (accessed on 5 February 2025).
- National Stroke Association. Physical Effects of Stroke. Available online: https://www.stroke.org/en/about-stroke/effects-of-stroke/physical-effects (accessed on 5 February 2025).
- National Health Service. Physical Effects of Stroke. Available online: https://www.newcastle-hospitals.nhs.uk/services/stroke-unit/life-after-stroke/physical-effects-of-stroke/#common-physical-effects (accessed on 28 November 2024).
- World Health Organization. Disability-Adjusted Life Years (Dalys). Available online: https://www.who.int/data/gho/indicator-metadata-registry/imr-details/158 (accessed on 15 November 2024).
- Feigin, V.L.; Abate, M.D.; Abate, Y.H.; ElHafeez, S.A.; Abd-Allah, F.; Abdelalim, A.; Abdelkader, A.; Abdelmasseh, M.; Abd-Elsalam, S.; Abdi, P.; et al. Global, regional, and national burden of stroke and its risk factors, 1990–2021: A systematic analysis for the global burden of disease study 2021. Lancet Neurol. 2024, 23, 973–1003. [Google Scholar] [CrossRef]
- World Health Organization. The Global Burden of Disease: 2004 Update; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Bonita, R.; Solomon, N.; Broad, J.B. Prevalence of stroke and stroke-related disability. Stroke 1997, 28, 1898–1902. [Google Scholar] [CrossRef]
- American Stroke Association. Stroke Rehabilitation. Available online: https://www.stroke.org/en/life-after-stroke/stroke-rehab (accessed on 6 November 2024).
- Anwer, S.; Waris, A.; Gilani, S.O.; Iqbal, J.; Shaikh, N.; Pujari, A.N.; Niazi, I.K. Rehabilitation of upper limb motor impairment in stroke: A narrative review on the prevalence, risk factors, and economic statistics of stroke and state of the art therapies. Healthcare 2022, 10, 190. [Google Scholar] [CrossRef]
- Persson, H.C.; Parziali, M.; Danielsson, A.; Sunnerhagen, K.S. Outcome and upper extremity function within 72 h after first occasion of stroke in an unselected population at a stroke unit. A part of the salgot study. BMC Neurol. 2012, 12, 162. [Google Scholar] [CrossRef] [PubMed]
- Broeks, J.G.; Lankhorst, G.J.; Rumping, K.; Prevo, A.J.H. The long-term outcome of arm function after stroke: Results of a follow-up study. Disabil. Rehabil. 1999, 21, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Friedman, P.J. Gait recovery after hemiplegic stroke. Int. Disabil. Stud. 1990, 12, 119–122. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, D.; Ali, K. Recommendations for upper limb motor recovery: An overview of the uk and european rehabilitation after stroke guidelines (2023). Healthcare 2024, 12, 1433. [Google Scholar] [CrossRef]
- Bertani, R.; Melegari, C.; Cola, M.C.D.; Bramanti, A.; Bramanti, P.; Calabrò, R.S. Effects of robot-assisted upper limb rehabilitation in stroke patients: A systematic review with meta-analysis. Neurol. Sci. 2017, 38, 1561–1569. [Google Scholar] [CrossRef]
- Veerbeek, J.M.; Langbroek-Amersfoort, A.C.; van Wegen, E.E.H.; Meskers, C.G.M.; Kwakkel, G. Effects of robot-assisted therapy for the upper limb after stroke: A systematic review and meta-analysis. Neurorehabilit. Neural Repair 2017, 31, 107–121. [Google Scholar] [CrossRef]
- Fasoli, S.E.; Krebs, H.I.; Stein, J.; Frontera, W.R.; Hogan, N. Effects of robotic therapy on motor impairment and recovery in chronic stroke. Arch. Phys. Med. Rehabil. 2003, 84, 477–482. [Google Scholar] [CrossRef]
- Krebs, H.; Hogan, N.; Volpe, B.; Aisen, M.; Edelstein, L.; Diels, C. Overview of clinical trials with mit-manus: A robot-aided neuro- rehabilitation facility. Technol. Health Care Off. J. Eur. Soc. Eng. Med. 1999, 7, 419–423. [Google Scholar] [CrossRef]
- Lo, K.; Stephenson, M.; Lockwood, C. Effectiveness of robotic assisted rehabilitation for mobility and functional ability in adult stroke patients: A systematic review. JBI Database Syst. Rev. Implement. Rep. 2017, 15, 3049–3091. [Google Scholar] [CrossRef]
- Mehrholz, J.; Pohl, M.; Platz, T.; Kugler, J.; Elsner, B. Electromechanical and robot-assisted arm training for improving activities of daily living, arm function, and arm muscle strength after stroke. Cochrane Database Syst. Rev. 2018, 2018, CD006876. [Google Scholar] [CrossRef] [PubMed]
- Mathiowetz, V.; Volland, G.; Kashman, N.; Weber, K. Adult norms for the box and block test of manual dexterity. Am. J. Occup. Ther. 1985, 39, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wu, X.; Li, Y.; Yu, S. Efficacy of robot-assisted training on upper limb motor function after stroke: A systematic review and network meta-analysis. Arch. Rehabil. Res. Clin. Transl. 2025, 7, 100387. [Google Scholar] [CrossRef]
- Wagner, T.H.; Lo, A.C.; Peduzzi, P.; Bravata, D.M.; Huang, G.D.; Krebs, H.I.; Ringer, R.J.; Federman, D.G.; Richards, L.G.; Haselkorn, J.K.; et al. An economic analysis of robot-assisted therapy for long-term upper-limb impairment after stroke. Stroke 2011, 42, 2630–2632. [Google Scholar] [CrossRef]
- Gower, V.; Aprile, I.; Falchini, F.; Fasano, A.; Germanotta, M.; Randazzo, M.; Spinelli, F.; Trieste, L.; Gramatica, F.; Turchetti, G. Cost analysis of technological vs. conventional upper limb rehabilitation for patients with neurological disorders: An italian real-world data case study. Front. Public Health 2024, 12, 1445099. [Google Scholar] [CrossRef]
- Lo, K.; Stephenson, M. The economic cost of robotic rehabilitation for adult stroke patients: A systematic review. JBI Database Syst. Rev. Implement. Rep. 2019, 17, 520–547. [Google Scholar] [CrossRef]
- Jakob, I.; Kollreider, A.; Germanotta, M.; Benetti, F.; Cruciani, A.; Padua, L.; Aprile, I. Robotic and sensor technology for upper limb rehabilitation. PM&R 2018, 10 (Suppl. 2), S189–S197. [Google Scholar] [CrossRef]
- National Institute of Health. Neuroplasticity, May 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557811/ (accessed on 4 November 2024).
- Bhujel, S.; Hasan, S. A comparative study of end-effector and exoskeleton type rehabilitation robots in human upper extremity rehabilitation. Hum.-Intell. Syst. Integr. 2023, 5, 11–42. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The prisma 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Narayan, J.; Kalita, B.; Dwivedy, S.K. Development of robot-based upper limb devices for rehabilitation purposes: A systematic review. Augment. Hum. Res. 2021, 6, 4. [Google Scholar] [CrossRef]
- Chang, L.; Anand, P.; Varacallo, M.A. Anatomy, Shoulder and Upper Limb, Glenohumeral Joint. Available online: https://www.ncbi.nlm.nih.gov/books/NBK537018/ (accessed on 30 July 2025).
- Physiopedia. Scapulohumeral Rhythm. Available online: https://www.physio-pedia.com/Scapulohumeral_Rhythm?lang=en (accessed on 30 July 2025).
- Lecturio. Elbow Joint: Anatomy. Available online: https://www.lecturio.com/concepts/elbow-joint/ (accessed on 6 February 2025).
- Physiopedia. Wrist and Hand. Available online: https://www.physio-pedia.com/Wrist_and_Hand (accessed on 30 July 2025).
- Narayan, R. Encyclopedia of Biomedical Engineering; Elsevier: Chantilly, VA, USA, 2018; Available online: http://ebookcentral.proquest.com/lib/kcl/detail.action?docID=5558494 (accessed on 1 December 2024).
- Ferris, D.P.; Schlink, B.R.; Young, A.J. Robotics: Exoskeletons; Elsevier: Oxford, UK, 2019. [Google Scholar] [CrossRef]
- Ruiz-Olaya, A.F.; Lopez-Delis, A.; da Rocha, A.F. Chapter Eight—Upper and Lower Extremity Exoskeletons; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar] [CrossRef]
- Maciejasz, P.; Eschweiler, J.; Gerlach-Hahn, K.; Jansen-Troy, A.; Leonhardt, S. A survey on robotic devices for upper limb rehabilitation. J. Neuroeng. Rehabil. 2014, 11, 3. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, G.; Cho, D.Y.; Kim, H.Y.; Lee, J.-Y.; Kim, S.; Park, S.-B.; Shin, J.-H. Comparisons between end-effector and exoskeleton rehabilitation robots regarding upper extremity function among chronic stroke patients with moderate-to-severe upper limb impairment. Sci. Rep. 2020, 10, 1806. [Google Scholar] [CrossRef] [PubMed]
- Lo, H.S.; Xie, S.Q. Exoskeleton robots for upper-limb rehabilitation: State of the art and future prospects. Med. Eng. Phys. 2012, 34, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.K.; Bhujel, S.B.; Niemiec, G.S. Emerging trends in human upper extremity rehabilitation robot. Cogn. Robot. 2024, 4, 174–190. [Google Scholar] [CrossRef]
- Meng, W.; Liu, Q.; Zhou, Z.; Ai, Q.; Sheng, B.; Xie, S. Recent development of mechanisms and control strategies for robot-assisted lower limb rehabilitation. Mechatronics 2015, 31, 132–145. [Google Scholar] [CrossRef]
- Rehacare. Mecharithm. Available online: https://mecharithm.com/news/article/training-modalities-in-rehabilitation-robotics-12 (accessed on 28 January 2025).
- Mounis, S.Y.A.; Azlan, N.Z.; Sado, F. Assist-as-needed control strategy for upper-limb rehabilitation based on subject’s functional ability. Meas. Control 2019, 52, 1354–1361. [Google Scholar] [CrossRef]
- Jeon, S.Y.; Ki, M.; Shin, J.-H. Resistive versus active assisted robotic training for the upper limb after a stroke: A randomized controlled study. Ann. Phys. Rehabil. Med. 2024, 67, 101789. [Google Scholar] [CrossRef]
- Marchal-Crespo, L.; Reinkensmeyer, D.J. Review of control strategies for robotic movement training after neurologic injury. J. Neuroeng. Rehabil. 2009, 6, 20. [Google Scholar] [CrossRef]
- Novak, V.; Riener, R. Control strategies and artificial intelligence in rehabilitation robotics. Ai Mag. 2015, 36, 23–33. [Google Scholar] [CrossRef]
- Britannica. Electrical Impedance. Available online: https://www.britannica.com/science/electrical-impedance (accessed on 4 January 2025).
- Akdogan, E.; Aktan, M.E. Chapter twelve—Impedance control applications in therapeutic exercise robots. In Control Systems Design of Bio-Robotics and Bio-Mechatronics with Advanced Applications; Azar, A.T., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 395–443. [Google Scholar] [CrossRef]
- Ghannadi, B.; Razavian, R.S.; McPhee, J. Configuration-dependent optimal impedance control of an upper extremity stroke rehabilitation manipulandum. Front. Robot. AI 2018, 5, 124. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, T.; Tao, H.; Liu, F.; Hu, B.; Wu, M.; Yu, H. Research on adaptive impedance control technology of upper limb rehabilitation robot based on impedance parameter prediction. Front. Bioeng. Biotechnol. 2024, 11, 1332689. [Google Scholar] [CrossRef]
- Stienen, A.H.A.; Hekman, E.E.G.; Van der Helm, F.C.T.; Prange, G.B.; Jannink, M.J.A.; Aalsma, A.M.M.; Van der Kooij, H. Freebal: Dedicated gravity compensation for the upper extremities. In Proceedings of the 2007 IEEE 10th International Conference on Rehabilitation Robotics, Noordwijk, The Netherlands, 13–15 June 2007; pp. 804–808. [Google Scholar] [CrossRef]
- Gasperina, S.D.; Roveda, L.; Pedrocchi, A.; Braghin, F.; Gandolla, M. Review on patient-cooperative control strategies for upper-limb rehabilitation exoskeletons. Front. Robot. AI 2021, 8, 745018. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zakerimanesh, A.; Ou, Y.; Badre, A.; Tavakoli, M. Iterative learning for gravity compensation in impedance control. IEEE/ASME Trans. Mechatron. 2025, 30, 119–130. [Google Scholar] [CrossRef]
- Dipietro, L.; Ferraro, M.; Palazzolo, J.J.; Krebs, H.I.; Volpe, B.T.; Hogan, N. Customized interactive robotic treatment for stroke: Emg-triggered therapy. IEEE Trans. Neural Syst. Rehabil. Eng. 2005, 13, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Özgünen, K.T.; Çelik, U.; Kurdak, S.S. Determination of an optimal threshold value for muscle activity detection in EMG analysis. J. Sport. Sci. Med. 2010, 9, 620–628. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3761824/ (accessed on 4 September 2025).
- Cesqui, B.; Tropea, P.; Micera, S.; Krebs, H.I. Emg-based pattern recognition approach in post stroke robot-aided rehabilitation: A feasibility study. J. Neuroeng. Rehabil. 2013, 10, 75. [Google Scholar] [CrossRef]
- Hussain, I.; Jany, R. Interpreting stroke-impaired electromyography patterns through explainable artificial intelligence. Sensors 2024, 24, 1392. [Google Scholar] [CrossRef]
- Krebs, H.I.; Palazzolo, J.J.; Dipietro, L.; Ferraro, M.; Krol, J.; Rannekleiv, K.; Volpe, B.T.; Hogan, N. Rehabilitation robotics: Performance-based progressive robot-assisted therapy. Auton. Robot. 2003, 15, 7–20. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, H.; Tian, Y.; Caldwell, D.G. Task performance-based adaptive velocity assist-as-needed control for an upper limb exoskeleton. Biomed. Signal Process. Control 2022, 73, 103474. [Google Scholar] [CrossRef]
- Liang, X.; Yan, Y.; Dai, S.; Guo, Z.; Li, Z.; Liu, S.; Su, T. Multi-mode adaptive control strategy for a lower limb rehabilitation robot. Front. Bioeng. Biotechnol. 2024, 12, 1392599. [Google Scholar] [CrossRef] [PubMed]
- Metzger, J.C.; Lambercy, O.; Califfi, A.; Keller, T.; Gassert, R. Assessment-driven selection and adaptation of exercise difficulty in robot-assisted therapy: A pilot study with a hand rehabilitation robot. J. Neuroeng. Rehabil. 2014, 11, 154. [Google Scholar] [CrossRef]
- Furuhashi, Y.; Nagasaki, M.; Aoki, T.; Morita, Y.; Ukai, H.; Matsui, N. Development of rehabilitation support robot for personalized rehabilitation of upper limbs. In Proceedings of the 2009 IEEE International Conference on Rehabilitation Robotics, Kyoto, Japan, 23–26 June 2009; pp. 787–792. [Google Scholar] [CrossRef]
- Johnson, M.; Loos, H.; Burgar, C.; Shor, P.; Leifer, L. Design and evaluation of driver’s seat: A car steering simulation environment for upper limb stroke therapy. Robotica 2003, 21, 13–23. [Google Scholar] [CrossRef]
- Patton, J.L.; Stoykov, M.E.; Kovic, M.; Mussa-Ivaldi, F.A. Evaluation of robotic training forces that either enhance or reduce error in chronic hemiparetic stroke survivors. Exp. Brain Res. 2006, 168, 368–383. [Google Scholar] [CrossRef]
- Cambridge Dictionary. Haptic. Available online: https://dictionary.cambridge.org/dictionary/english/haptic-technology (accessed on 30 December 2024).
- Rocha, C.D.; Carneiro, I.; Torres, M.; Oliveira, H.P.; Pires, E.J.S.; Silva, M.F. Post-stroke upper limb rehabilitation: Clinical practices, compensatory movements, assessment, and trends. Prog. Biomed. Eng. 2025, 7, 042001. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.A.; Silva, M.F.; Oliveira, H.P.; Rocha, C.D. Developing a serious video game to engage the upper limb post-stroke rehabilitation. Appl. Sci. 2025, 15, 8240. [Google Scholar] [CrossRef]
- Matarić, M.J.; Eriksson, J.; Feil-Seifer, D.J.; Winstein, C.J. Socially assistive robotics for post-stroke rehabilitation. J. Neuroeng. Rehabil. 2007, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Feingold-Polak, R.; Barzel, O.; Levy-Tzedek, S. A robot goes to rehab: A novel gamified system for long-term stroke rehabilitation using a socially assistive robot—Methodology and usability testing. J. Neuroeng. Rehabil. 2021, 18, 122. [Google Scholar] [CrossRef]
- Abdullahi, A.M.; Haruna, A.; Chaichaowarat, R. Hybrid adaptive impedance and admittance control based on the sensorless estimation of interaction joint torque for exoskeletons: A case study of an upper limb rehabilitation robot. J. Sens. Actuator Netw. 2024, 13, 24. [Google Scholar] [CrossRef]
- Qian, Q.; Nam, C.; Guo, Z.; He, J.; Lee, J.; Li, W.; Hu, X. Distal versus proximal—An investigation on different supportive strategies by robots for upper limb rehabilitation after stroke: A randomized controlled trial. J. Neuroeng. Rehabil. 2019, 16, 64. [Google Scholar] [CrossRef] [PubMed]
- Lencioni, T.; Fornia, L.; Bowman, T.; Marzegan, A.; Caronni, A.; Turolla, A.; Jonsdottir, J.; Carpinella, I.; Ferrarin, M. A randomized controlled trial on the effects induced by robot-assisted and usual-care rehabilitation on upper limb muscle synergies in post-stroke subjects. Sci. Rep. 2021, 11, 5323. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Herath, D. What Makes Robots? Sensors, Actuators, and Algorithms; Springer Nature: Singapore, 2022. [Google Scholar] [CrossRef]
- Whitesides, G.M. Soft robotics. Angew. Chem. Int. Ed. 2018, 57, 4258–4273. [Google Scholar] [CrossRef]
- Masia, L.; Xiloyannis, M.; Khanh, D.B.; Wilson, A.C.; Contu, S.; Yongtae, K.G. Chapter 4—Actuation for Robot-Aided Rehabilitation: Design and Control Strategies; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar] [CrossRef]
- Scheinman, V.; McCarthy, J.M.; Song, J.-B. Mechanism and Actuation; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Autsou, S.; Kudelina, K.; Vaimann, T.; Rassõlkin, A.; Kallaste, A. Principles and methods of servomotor control: Comparative analysis and applications. Appl. Sci. 2024, 14, 2579. [Google Scholar] [CrossRef]
- RS. Servo Motors. Available online: https://uk.rs-online.com/web/c/automation-control-gear/electric-motors/servo-motors/ (accessed on 29 January 2025).
- Garofalo, S.; Morano, C.; Perrelli, M.; Pagnotta, L.; Carbone, G.; Mundo, D.; Bruno, L. A critical review of transitioning from conventional actuators to artificial muscles in upper-limb rehabilitation devices. J. Intell. Mater. Syst. Struct. 2024, 35, 1263–1290. [Google Scholar] [CrossRef]
- SKYMotor. Hat Are the Key Differences Between ac and dc Servo Motors? Available online: https://www.skysmotor.co.uk/article-18-What-are-the-key-differences-between-AC-and-DC-servo-motors.html (accessed on 29 January 2025).
- Millett, P. Brushless vs. Brushed DC Motors: When and Why to Choose One over the Other. Available online: https://www.monolithicpower.com/en/learning/resources/brushless-vs-brushed-dc-motors (accessed on 29 January 2025).
- RS. The Complete Guide to Stepper Motors. Available online: https://uk.rs-online.com/web/content/discovery/ideas-and-advice/stepper-motors-guide (accessed on 29 January 2025).
- Junior, A.G.L.; de Andrade, R.M.; Filho, A.B. Series elastic actuator: Design, analysis and comparison. Recent Adv. Robot. Syst. 2016, 1. [Google Scholar] [CrossRef]
- Pratt, G.A.; Williamson, M.M. Series elastic actuators. In Proceedings of the Proceedings 1995 IEEE/RSJ International Conference on Intelligent Robots and Systems. Human Robot Interaction and Cooperative Robots, Pittsburgh, PA, USA, 5–9 August 1995; Volume 1, pp. 399–406. [Google Scholar] [CrossRef]
- Belforte, G.; Eula, G.; Ivanov, A.; Sirolli, S. Soft pneumatic actuators for rehabilitation. Actuators 2014, 3, 84–106. [Google Scholar] [CrossRef]
- Pan, M.; Yuan, C.; Liang, X.; Dong, T.; Liu, T.; Zhang, J.; Zou, J.; Yang, H.; Bowen, C. Soft actuators and robotic devices for rehabilitation and assistance. Adv. Intell. Syst. 2021, 4, 2100140. [Google Scholar] [CrossRef]
- Lyle, R.C. A performance test for assessment of upper limb function in physical rehabilitation treatment and research. Int. J. Rehabil. Res. 1981, 4, 483–492. [Google Scholar] [CrossRef]
- Life Science Robotics. Available online: https://www.lifescience-robotics.com/ (accessed on 30 December 2024).
- MEDICA. Medica Fair. Available online: https://www.medica-tradefair.com/ (accessed on 11 March 2025).
- KUKA. Robot-Assisted Rehabilitation—Robert® and Kuka Facilitate Mobilization. Available online: https://www.kuka.com/en-de/industries/solutions-database/2019/08/robert-from-life-science-robotics (accessed on 30 December 2024).
- Life Science Robotics. Emg-Triggered Fes. Available online: https://www.lifescience-robotics.com/solutions/sas/ (accessed on 30 December 2024).
- Wang, Y.; Xu, Q. Design and testing of a soft parallel robot based on pneumatic artificial muscles for wrist rehabilitation. Sci. Rep. 2021, 11, 1273. [Google Scholar] [CrossRef]
- Dorin, P.; Horatiu, R.; Liviu, M.F.; Carmen, P.L.; Petre, C.C. Design and development of an upper limb rehabilitation robotic system. In Proceedings of the 2018 19th International Carpathian Control Conference (ICCC), Szilvasvarad, Hungary, 28–31 May 2018; pp. 265–270. [Google Scholar] [CrossRef]
- Florin, M.L.; Dorin, P.; Horatiu, R.; Petre, C.C. Kinematic analysis and control for upper limb robotic rehabilitation system. In Proceedings of the 2018 19th International Carpathian Control Conference (ICCC), Szilvasvarad, Hungary, 28–31 May 2018; pp. 179–184. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Zhu, A.; Zheng, Z.; Zhu, H. Design and evaluation of a surface electromyography-controlled lightweight upper arm exoskeleton rehabilitation robot. Int. J. Adv. Robot. Syst. 2021, 18, 17298814211003461. [Google Scholar] [CrossRef]
- Wearables. Myo. Available online: https://wearables.com/products/myo (accessed on 4 January 2025).
- González-Mendoza, A.; Quiñones-Urióstegui, I.; Salazar-Cruz, S.; Perez-Sanpablo, A.; López-Gutiérrez, R.; Lozano, R. Design and implementation of a rehabilitation upper-limb exoskeleton robot controlled by cognitive and physical interfaces. J. Bionic Eng. 2022, 19, 1374–1391. [Google Scholar] [CrossRef]
- Zhang, L.; Tian, X.; Fan, Y.; Jiang, T.; Gu, S.; Xu, L. Development and efficacy assessment of an angle sensor-integrated upper limb exoskeleton system for autonomous rehabilitation training. Sensors 2025, 25, 3984. [Google Scholar] [CrossRef]
- Nef, T.; Riener, R. Armin iii—Arm therapy exoskeleton with an ergonomic shoulder actuation. Appl. Bionics Biomech. 2009, 6, 127–142. [Google Scholar] [CrossRef]
- Nef, T.; Klamroth-Marganska, V.; Riener, R. Armin-exoskeleton robot for stroke rehabilitation. In Proceedings of the 3rd International Conference on Robot Therapy, Munich, Germany, 7–12 September 2009; Springer: Munich, Germany, 2010; pp. 313–319. [Google Scholar] [CrossRef]
- Lambelet, C.; Lyu, M.; Woolley, D.; Gassert, R.; Wenderoth, N. The ewrist—A wearable wrist exoskeleton with semg-based force control for stroke rehabilitation. In Proceedings of the 2017 International Conference on Rehabilitation Robotics (ICORR), London, UK, 17–20 July 2017; pp. 726–733. [Google Scholar] [CrossRef]
- Fitle, K.D.; Pehlivan, A.U.; O’Malley, M.K. A robotic exoskeleton for rehabilitation and assessment of the upper limb following incomplete spinal cord injury. In Proceedings of the 2015 IEEE International Conference on Robotics and Automation (ICRA), Seattle, WA, USA, 26–30 May 2015; pp. 4960–4966. [Google Scholar] [CrossRef]
- McDonald, C.G.; Dennis, T.A.; O’Malley, M.K. Characterization of surface electromyography patterns of healthy and incomplete spinal cord injury subjects interacting with an upper-extremity exoskeleton. In Proceedings of the 2017 International Conference on Rehabilitation Robotics (ICORR), London, UK, 17–20 July 2017; pp. 164–169. [Google Scholar] [CrossRef]
- McDonald, C.G.; Sullivan, J.L.; Dennis, T.A.; O’Malley, M.K. A myoelectric control interface for upper-limb robotic rehabilitation following spinal cord injury. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 978–987. [Google Scholar] [CrossRef]
- Hocoma. Hocoma. Available online: https://www.hocoma.com/us/ (accessed on 31 December 2024).
- Hocoma. About Us. Available online: https://www.hocoma.com/us/hocoma/about-us/ (accessed on 31 December 2024).
- Hocoma. Armeo®Power. Available online: https://www.hocoma.com/us/solutions/armeo-power/ (accessed on 31 December 2024).
- Hocoma. Technical Data Sheet. Available online: https://www.hocoma.com/us/solutions/armeo-power/technical-data-sheet/ (accessed on 31 December 2024).
- Hocoma. Armeo®Power Modules. Available online: https://www.hocoma.com/us/solutions/armeo-power/modules/ (accessed on 31 December 2024).
- Bergamasco, M.; Salsedo, F.; Lenzo, B. An Exoskeleton Structure for Physical Interaction with a Human Being. Patent No. WO2013186705A3; International Patent, 11 June 2013. Available online: https://patents.google.com/patent/WO2013186705A3/en (accessed on 31 December 2024).
- Pirondini, E.; Coscia, M.; Marcheschi, S.; Roas, G.; Salsedo, F.; Frisoli, A.; Bergamasco, M.; Micera, S. Evaluation of a New Exoskeleton for Upper Limb Post-Stroke Neuro-Rehabilitation: Preliminary Results; Springer International Publishing: Cham, Switzerland, 2014; pp. 637–645. [Google Scholar]
- Pirondini, E.; Coscia, M.; Marcheschi, S.; Roas, G.; Salsedo, F.; Frisoli, A.; Bergamasco, M.; Micera, S. Evaluation of the effects of the arm light exoskeleton on movement execution and muscle activities: A pilot study on healthy subjects. J. Neuroeng. Rehabil. 2016, 13, 9. [Google Scholar] [CrossRef]
- Zeiaee, A.; Zarrin, R.S.; Langari, R.; Tafrershi, R. Design and kinematic analysis of a novel upper limb exoskeleton for rehabilitation of stroke patients. In Proceedings of the 2017 International Conference on Rehabilitation Robotics (ICORR), London, UK, 17–20 July 2017; pp. 759–764. [Google Scholar] [CrossRef]
- Zeiaee, A.; Zarrin, R.S.; Eib, A.; Langari, R.; Tafreshi, R. Cleverarm: A lightweight and compact exoskeleton for upper-limb rehabilitation. IEEE Robot. Autom. Lett. 2022, 7, 1880–1887. [Google Scholar] [CrossRef]
- Islam, M.R.; Assad-Uz-Zaman, M.; Brahmi, B.; Bouteraa, Y.; Wang, I.; Rahman, M.H. Design and development of an upper limb rehabilitative robot with dual functionality. Micromachines 2021, 12, 870. [Google Scholar] [CrossRef]
- Crea, S.; Cempini, M.; Moisè, M.; Baldoni, A.; Trigili, E.; Marconi, D.; Cortese, M.; Giovacchini, F.; Posteraro, F.; Vitiello, N. A novel shoulder-elbow exoskeleton with series elastic actuators. In Proceedings of the 2016 6th IEEE International Conference on Biomedical Robotics and Biomechatronics (BioRob), Singapore, 26–29 June 2016; pp. 1248–1253. [Google Scholar] [CrossRef]
- Kim, B.; Deshpande, A.D. An upper-body rehabilitation exoskeleton harmony with an anatomical shoulder mechanism: Design, modeling, control, and performance evaluation. Int. J. Robot. Res. 2017, 36, 414–435. [Google Scholar] [CrossRef]
- Harmonic Bionics. Introducing Harmony Shr. Available online: https://harmonicbionics.com/ (accessed on 10 February 2025).
- Deshpande, A.W.; Kim, B. Upper-Body Robotic Exoskeleton. U.S. Patent 20160206497 A1 [Online], 21 July 2016. Available online: https://patents.google.com/patent/US20160206497A1/en (accessed on 31 July 2025).
- Harmonic Bionics. Patents. Available online: https://harmonicbionics.com/patents/ (accessed on 10 February 2025).
- Ogden, E.M.; Chiu, D.; Clearman, R.; Card, C.; Deshpande, A.D. Evaluation of the harmony exoskeleton as an upper extremity rehabilitation tool after stroke. In Proceedings of the 2017 International Symposium on Wearable Robotics and Rehabilitation (WeRob), Houston, TX, USA, 5–8 November 2017; pp. 1–2. [Google Scholar] [CrossRef]
- Vlachos, E.; Jochum, E.; Demers, L.-P. Heat: The harmony exoskeleton self-assessment test. In Proceedings of the 2018 27th IEEE International Symposium on Robot and Human Interactive Communication (RO-MAN), Nanjing, China, 27–31 August 2018; pp. 577–582. [Google Scholar] [CrossRef]
- de Oliveira, A.C.; Rose, C.G.; Warburton, K.; Ogden, E.M.; Whitford, B.; Lee, R.K.; Deshpande, A.D. Exploring the capabilities of harmony for upper-limb stroke therapy. In Proceedings of the 2019 IEEE 16th International Conference on Rehabilitation Robotics (ICORR), Toronto, ON, Canada, 24–28 June 2019; pp. 637–643. [Google Scholar] [CrossRef]
- Hailey, R.O.; Oliveira, A.C.D.; Ghonasgi, K.; Whitford, B.; Lee, R.K.; Rose, C.G.; Deshpande, A.D. Impact of gravity compensation on upper extremity movements in harmony exoskeleton. In Proceedings of the 2022 International Conference on Rehabilitation Robotics (ICORR), Rotterdam, The Netherlands, 25–29 July 2022; pp. 1–6. [Google Scholar] [CrossRef]
- Harmonic Bionics. University of Houston Purchases Harmony Shr™ Upper Body Exoskeleton for Stroke Rehab Research. Available online: https://harmonicbionics.com/about/news/university-of-houston-purchases-harmony-shr-upper-body-exoskeleton-for-stroke-rehab-research/ (accessed on 10 February 2025).
- Harmonic Bionics. Harmonic Bionics Announces Commercial Launch of the Harmony Shr™ Robotic Rehabilitation System Following Fda Registration. 2025. Available online: https://harmonicbionics.com/about/news/harmonic-bionics-announces-commercial-launch-of-the-harmony-shr-robotic-rehabilitation-system-following-fda-registration/ (accessed on 10 February 2025).
- Harmonic Bionics. Harmonic Bionics Installs First of Two Harmony Shr™ Robotic Rehabilitation Systems Purchased by Top Neurological Hospital in Phoenix. Available online: https://harmonicbionics.com/about/news/harmonic-bionics-installs-first-of-two-harmony-shr-robotic-rehabilitation-systems-purchased-by-top-neurological-hospital-in-phoenix/ (accessed on 10 February 2025).
- Harmonic Bionics. First International Harmony Shr® Robotic Rehabilitation System Installed at Prestigious Chinese University of Hong Kong Medical Centre. Available online: https://harmonicbionics.com/about/news/first-international-harmony-shr-robotic-rehabilitation-system-installed-at-prestigious-chinese-university-of-hong-kong-medical-centre/ (accessed on 10 February 2025).
- Chen, T.; Casas, R.; Lum, P.S. An elbow exoskeleton for upper limb rehabilitation with series elastic actuator and cable-driven differential. IEEE Trans. Robot. 2019, 35, 1464–1474. [Google Scholar] [CrossRef]
- Zimmermann, Y.; Forino, A.; Riener, R.; Hutter, M. Anyexo: A versatile and dynamic upper-limb rehabilitation robot. IEEE Robot. Autom. Lett. 2019, 4, 3649–3656. [Google Scholar] [CrossRef]
- Zimmermann, Y.; Sommerhalder, M.; Wolf, P.; Riener, R.; Hutter, M. Anyexo 2.0: A fully actuated upper-limb exoskeleton for manipulation and joint-oriented training in all stages of rehabilitation. IEEE Trans. Robot. 2023, 39, 2131–2150. [Google Scholar] [CrossRef]
- Polygerinos, P.; Wang, Z.; Galloway, K.C.; Wood, R.J.; Walsh, C.J. Soft robotic glove for combined assistance and at-home rehabilitation. Robot. Auton. Syst. 2015, 73, 135–143. [Google Scholar] [CrossRef]
- Polygerinos, P.; Galloway, K.C.; Sanan, S.; Herman, M.; Walsh, C.J. Emg controlled soft robotic glove for assistance during activities of daily living. In Proceedings of the 2015 IEEE International Conference on Rehabilitation Robotics (ICORR), Singapore, 11–14 August 2015; pp. 55–60. [Google Scholar] [CrossRef]
- MathWorks. Biomechanics of Bodies (bob). Available online: https://www.mathworks.com/products/connections/product_detail/biomechanics-of-bodies.html (accessed on 12 February 2025).
- Villoslada, A.; Flores, A.; Copaci, D.; Blanco, D.; Moreno, L. High-displacement flexible shape memory alloy actuator for soft wearable robots. Robot. Auton. Syst. 2015, 73, 91–101. [Google Scholar] [CrossRef]
- Copaci, D.; Cano, E.; Moreno, L.; Blanco, D. New design of a soft robotics wearable elbow exoskeleton based on shape memory alloy wire actuators. Appl. Bionics Biomech. 2017, 2017, 1605101. [Google Scholar] [CrossRef] [PubMed]
- Copaci, D.; Serrano, D.; Moreno, L.; Blanco, D. A high-level control algorithm based on semg signalling for an elbow joint sma exoskeleton. Sensors 2018, 18, 2522. [Google Scholar] [CrossRef]
- Copaci, D.; Martín, F.; Moreno, L.; Blanco, D. Sma based elbow exoskeleton for rehabilitation therapy and patient evaluation. IEEE Access 2019, 7, 31473–31484. [Google Scholar] [CrossRef]
- Copaci, D.; Blanco, D.; Moreno, L.E. Flexible shape-memory alloy-based actuator: Mechanical design optimization according to application. Actuators 2019, 8, 63. [Google Scholar] [CrossRef]
- Beato, P.S.; Poologasundarampillai, G.; Nommeots-Nomm, A.; Kalaskar, D.M. 3—Materials for 3d printing in medicine: Metals, polymers, ceramics, and hydrogels. In 3D Printing in Medicine, 2nd ed.; Kalaskar, D.M., Ed.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Sawston, UK, 2023; pp. 59–103. [Google Scholar] [CrossRef]
- Copaci, D.; Cerro, D.S.D.; Guadalupe, J.A.; Lorente, L.M.; Rojas, D.B. semg-controlled soft exo-glove for assistive rehabilitation therapies. IEEE Access 2024, 12, 43506–43518. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Y.; Zhu, C.; Guo, X.; Meng, W.; Ai, Q.; Hu, J. Design and control of a reconfigurable upper limb rehabilitation exoskeleton with soft modular joints. IEEE Access 2021, 9, 166815–166824. [Google Scholar] [CrossRef]
- Life Science Robotics. Robert®—Upper Extremity Module. Available online: https://www.lifescience-robotics.com/solutions/upper-extremities/ (accessed on 30 December 2024).
- Molteni, F.; Gasperini, G.; Cannaviello, G.; Guanziroli, E. Exoskeleton and end-effector robots for upper and lower limbs rehabilitation: Narrative review. PM&R 2018, 10, S174–S188. [Google Scholar] [CrossRef]
- Meng, Q.; Xie, Q.; Yu, H. Upper-Limb Rehabilitation Robot: State of the Art and Existing Problems. In Proceedings of the 12th International Convention on Rehabilitation Engineering and Assistive Technology (i-CREATe 2018), Shanghai, China, 14–16 July 2018; Singapore Therapeutic, Assistive & Rehabilitative Technologies (START) Centre: Singapore, 2018; pp. 155–158. [Google Scholar]
- Moggio, L.; de Sire, A.; Marotta, N.; Demeco, A.; Ammendolia, A. Exoskeleton versus end-effector robot-assisted therapy for finger-hand motor recovery in stroke survivors: Systematic review and meta-analysis. Top. Stroke Rehabil. 2022, 29, 539–550. [Google Scholar] [CrossRef] [PubMed]







| Assistance Type | User Involvement | Role of Robot | Therapy Stage | Goal | References |
|---|---|---|---|---|---|
| Passive Assist | None | Moves limb entirely | Early rehabilitation | Prevent stiffness, stimulate muscles | [69] |
| Active Assist | Partial | Helps only if user cannot complete movement | Intermediate | Improve muscle power and coordination | [70] |
| Active Resist | Full | Applies resistance | Advanced | Increase strength and endurance | [71] |
| Motor Type | Advantages | Disadvantages | References |
|---|---|---|---|
| AC Servomotors | High-speed applications Efficient | Heavy | [104,105,106,107] |
| DC Servomotors | Low-speed, high-torque applications | Less efficient (compared to AC servomotors) | [104,105,106,107] |
| Brushed DC Motors | Simple Low-cost | Noisy Short lifespan | [108] |
| Brushless DC Motors | Quiet Efficient Long lifespan | Expensive | [108] |
| Stepper Motors | Accurate position control | Less efficient for continuous operations | [109] |
| Electric | Pneumatic | Hydraulic | |
|---|---|---|---|
| Advantages | High amounts of torque High precision Low impedance (with SEAs) | Flexible Lightweight Comfortable High force/weight ratio Durable | Very high power density |
| Disadvantages | Heavyweight | Compressed air requirements | Heavyweight Bulky Prone to leaks |
| References | [102,103] | [102,103,112] | [102,103,113] |
| Device Name | Year | Type | Target | DOFs | Control Philosophy and Sensors | Actuation | Assistance | Testing status |
|---|---|---|---|---|---|---|---|---|
| Armin III [126] | 2009 | Exoskeleton | Upper-Limb | 6 | Impedance Position control | Electric | AA AR PA | Tested on stroke patients |
| Armeo®Power [134] | 2011 | Exoskeleton | Upper-Limb | 7 (8 with Manovo®Power handle) | ND | Electric | AA PA | Commercially available |
| ALEx [137] | 2012 | Exoskeleton | Upper-Limb | 6 | Angular position sensors | Electric | AA | Tested on healthy subjects |
| Polygerinos et al. [159] | 2015 | Exoskeleton | Fingers | 15 | Fluidic pressure sensors Force/Torque sensors Movement sensors Position sensors | Hydraulic | AA | Tested on patient with muscular dystrophy |
| NESM [143] | 2016 | Exoskeleton | Shoulder Elbow | 4 (plus 8 passive) | Angular sensors Position sensors Torque sensors | Electric | AA PA | ND |
| eWrist [128] | 2017 | Exoskeleton | Wrist | 1 | Admittance Angle sensors Force/Torque sensors sEMG Velocity sensors | Electric | AA | Tested in healthy subjects |
| Harmony SHR® [144] | 2017 | Exoskeleton | Shoulder Elbow | 7 | Impedance Force/Torque sensors Movement sensors Position sensors | Electric | AA PA | Tested on stroke patients |
| ROBERT® [170] | 2018 | End-effector | Upper-Limb | 1 (end-effector only) | ND sEMG | Electric | AA AR PA | Commercially Available |
| Popescu et al. [120] | 2018 | Exoskeleton | Elbow Wrist | 3 | ND | Electric | PA | ND |
| Chen et al. [156] | 2019 | Exoskeleton | Elbow | 2 | Angle sensors Force/Torque sensors Impedance | Electric | AR | ND |
| Copaci et al. [165] | 2019 | Exoskeleton | Elbow | 2 | Angle sensors Force sensors Position sensors Pressure sensors sEMG Temperature sensors | SMA | AA | ND |
| MAHI Exo-II [131] | 2020 | Exoskeleton | Elbow Wrist | 4 | sEMG | Electric | AA AR PA | Tested on patients with SCI |
| CLEVERarm [141] | 2021 | Exoskeleton | Upper-Limb | 8 | Force/Torque sensors Movement sensors Position sensors | Electric | Anti-gravity | Tested on healthy subjects |
| Quan Liu et al. [169] | 2021 | Exoskeleton | Elbow Wrist | 3 | Angle sensors Force/Torque sensors | Pneumatic | AA | ND |
| u-Rob [142] | 2021 | Both | Upper-Limb | 7 (plus 2 passive) | Force sensors Position sensors Velocity sensors | Electric | PA | Tested on healthy subjects |
| Wang and Xu [119] | 2021 | End-effector | Wrist | 3 | sEMG | Pneumatic | ND | Tested on healthy subjects |
| Yang Liu et al. [122] | 2021 | Exoskeleton | Elbow | 1 | sEMG | Electric | AR | Tested on healthy subjects |
| González-Mendoza et al. [124] | 2022 | Exoskeleton | Elbow Wrist | 3 | Admittance Force/Torque sensors sEMG | Electric | AA PA | ND |
| ANYexo 2.0 [158] | 2023 | Exoskeleton | Upper-Limb | 9 | Force/Torque sensors Position sensors | Electric | ND | Tested on healthy subjects |
| Zhang et al. [125] | 2025 | Exoskeleton | Elbow Wrist | 2 | Angle Sensors Force sensors | Electric | AA PA | Tested on healthy subjects |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, A.; Silva, M.F.; Mendonça, H.; Rocha, C.D. A Review of Robotic Interfaces for Post-Stroke Upper-Limb Rehabilitation: Assistance Types, Actuation Methods, and Control Mechanisms. Robotics 2025, 14, 141. https://doi.org/10.3390/robotics14100141
Gonçalves A, Silva MF, Mendonça H, Rocha CD. A Review of Robotic Interfaces for Post-Stroke Upper-Limb Rehabilitation: Assistance Types, Actuation Methods, and Control Mechanisms. Robotics. 2025; 14(10):141. https://doi.org/10.3390/robotics14100141
Chicago/Turabian StyleGonçalves, André, Manuel F. Silva, Hélio Mendonça, and Cláudia D. Rocha. 2025. "A Review of Robotic Interfaces for Post-Stroke Upper-Limb Rehabilitation: Assistance Types, Actuation Methods, and Control Mechanisms" Robotics 14, no. 10: 141. https://doi.org/10.3390/robotics14100141
APA StyleGonçalves, A., Silva, M. F., Mendonça, H., & Rocha, C. D. (2025). A Review of Robotic Interfaces for Post-Stroke Upper-Limb Rehabilitation: Assistance Types, Actuation Methods, and Control Mechanisms. Robotics, 14(10), 141. https://doi.org/10.3390/robotics14100141







