Chamaejasmine Isolated from Wikstroemia dolichantha Diels Suppresses 2,4-Dinitrofluoro-benzene-Induced Atopic Dermatitis in SKH-1 Hairless Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Equipment Used
2.2. Plant Material and Extraction
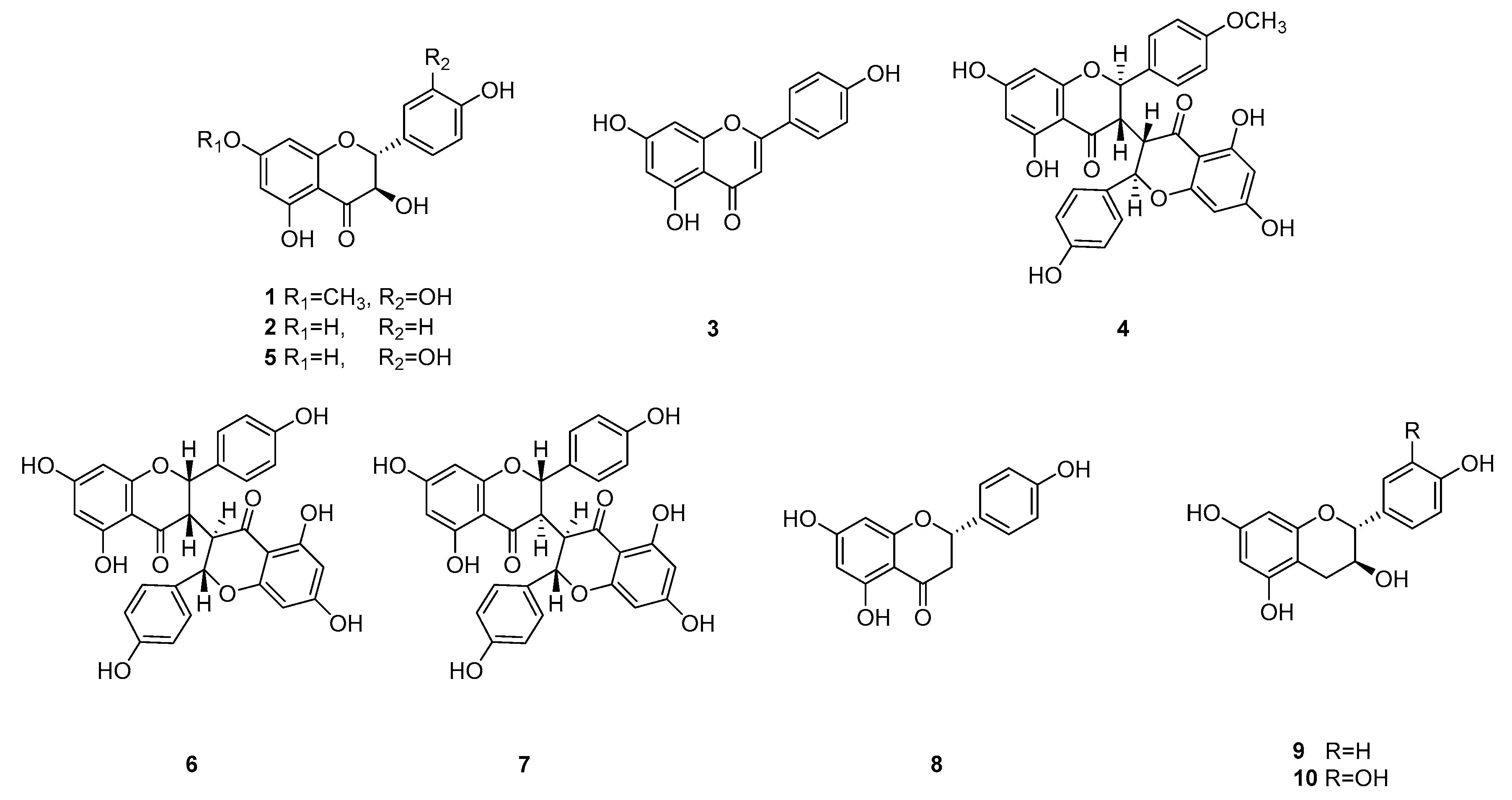
2.3. Compound Isolation
2.4. RBL-2H3 Cell Culture
2.6. Animals
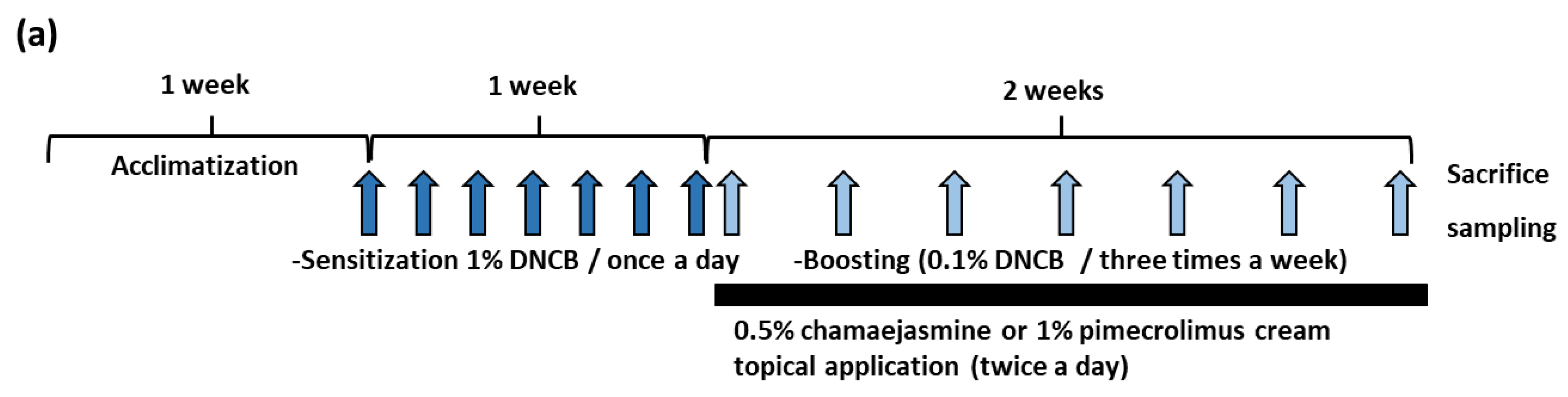
2.7. Induction of DNCB-Induced AD and Treatment with Chamaejamine from W. dolichantha
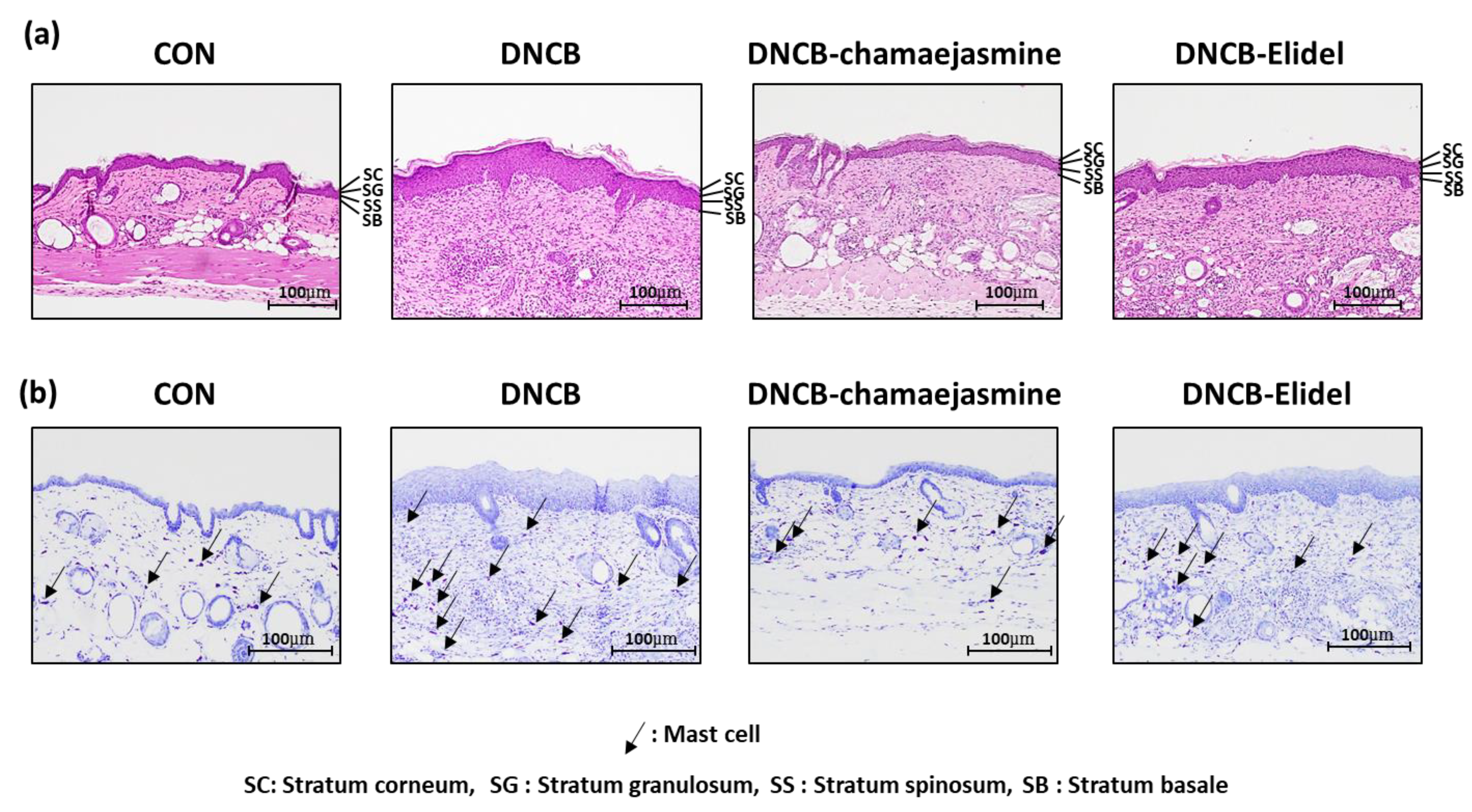
2.8. Histological Examination
2.9. Measurement of Transepidermal Water Loss (TEWL) and Skin Hydration
2.10. Measurement of Total Serum IgE and IL-4 Levels
2.11. Statistical Analysis
3. Results
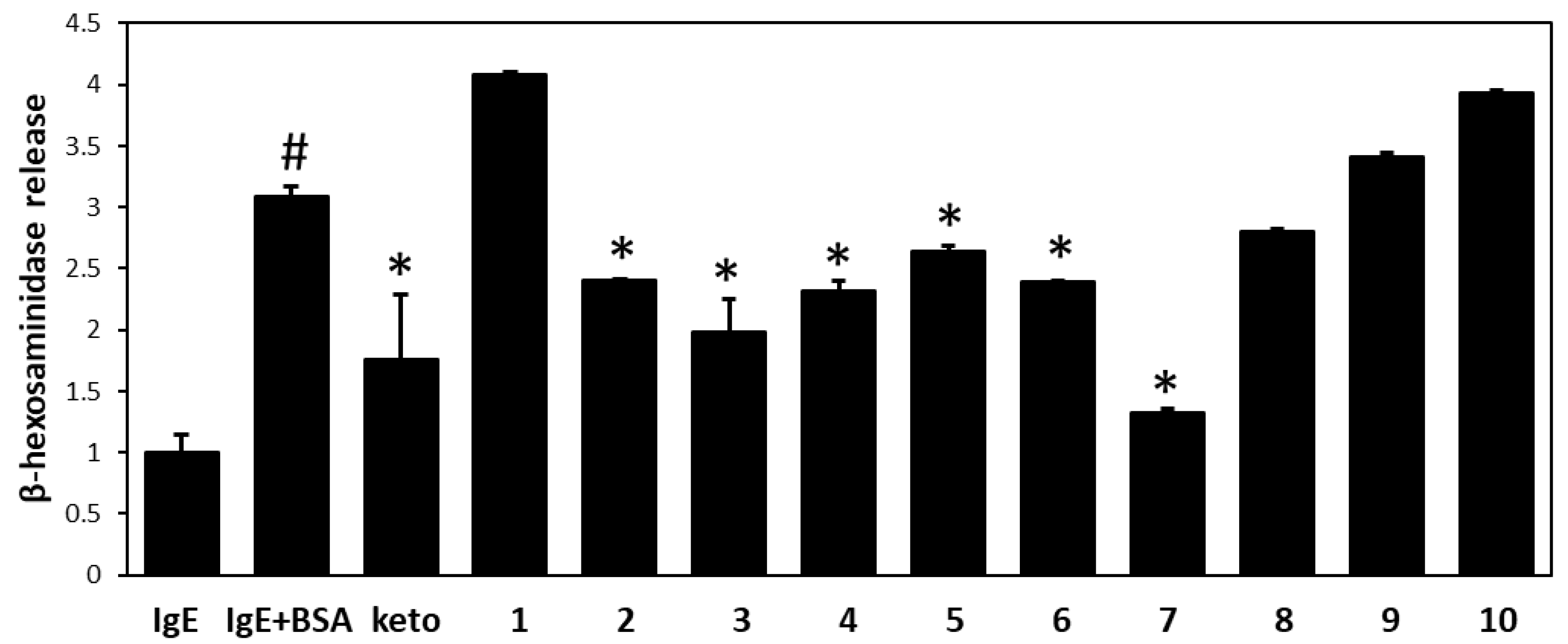
3.1. Isolation of Flavonoids from W. dolichantha and Their Effects on β-Hexosaminidase Release by RBL-2H3 Cells
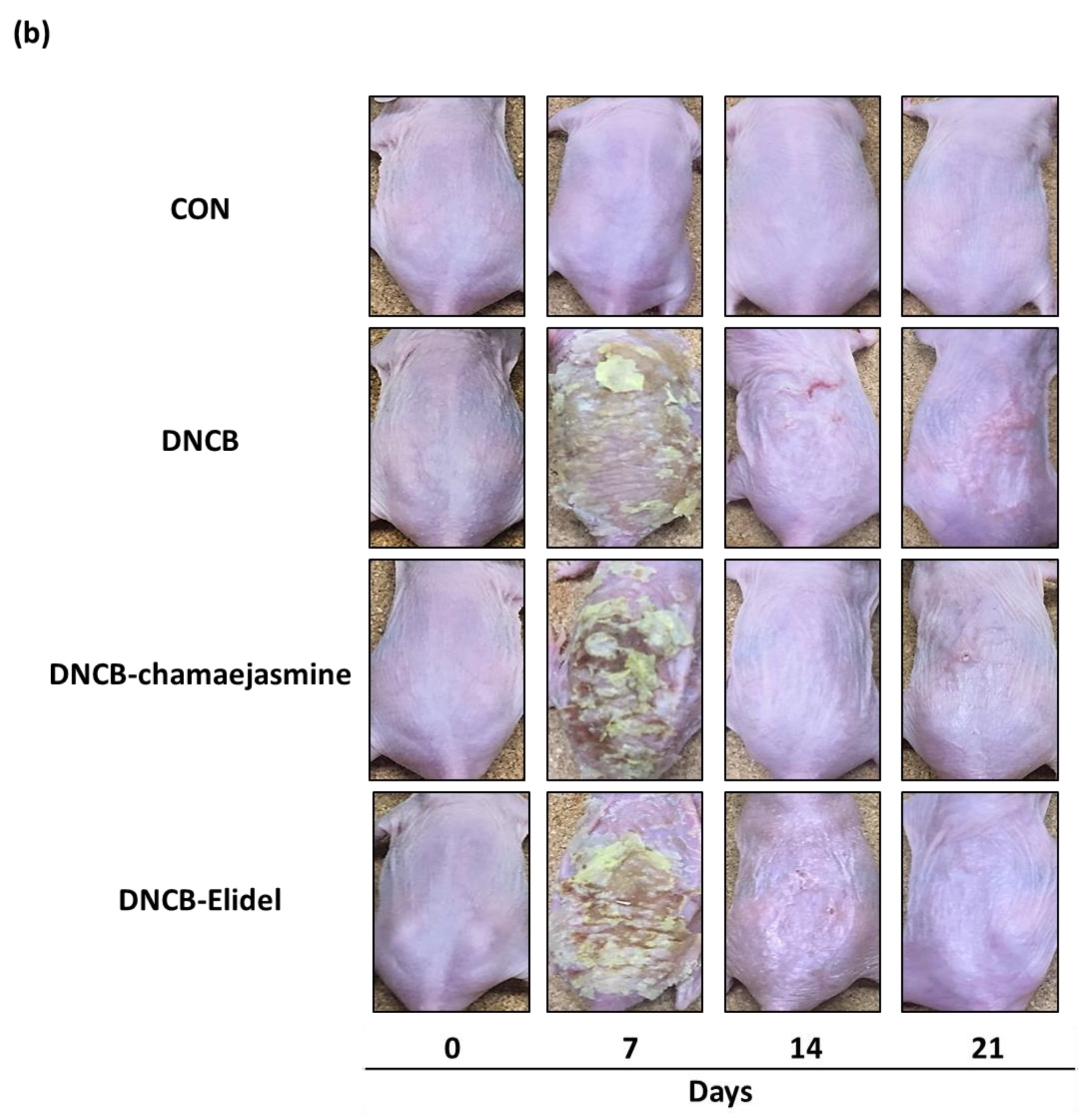
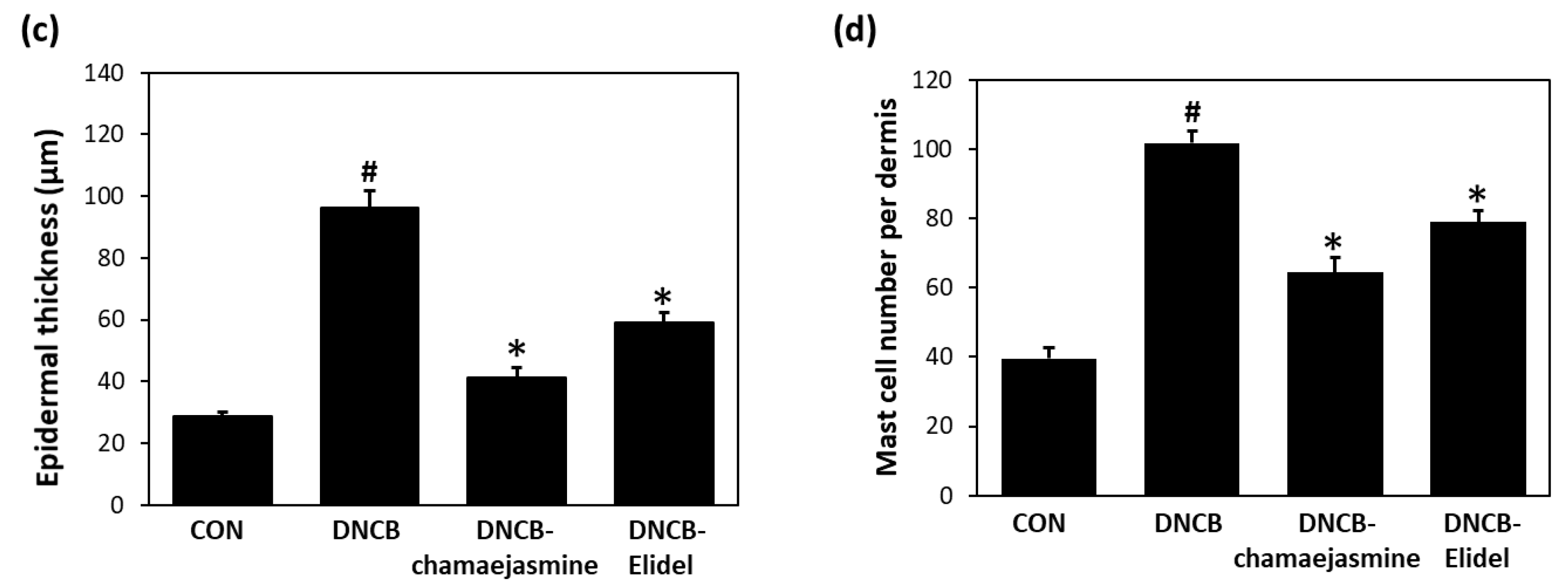
3.2. Chamaejasmine (7) from W. dolichantha Ameliorated AD-like Skin Symptoms in DNCB-Induced Atopic Mice
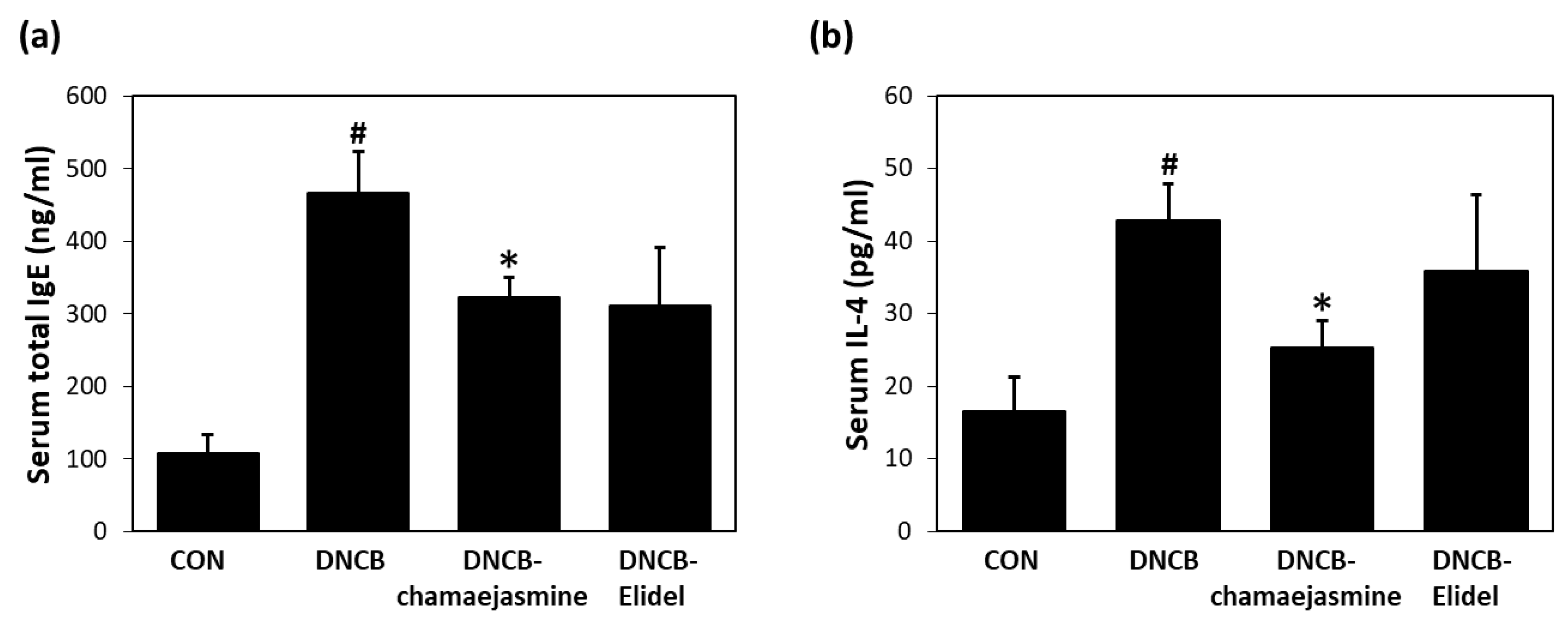
3.3. Chamaejasmine (7) Reduced Serum IgE and IL-4 Levels in DNCB-Induced Atopic Mice
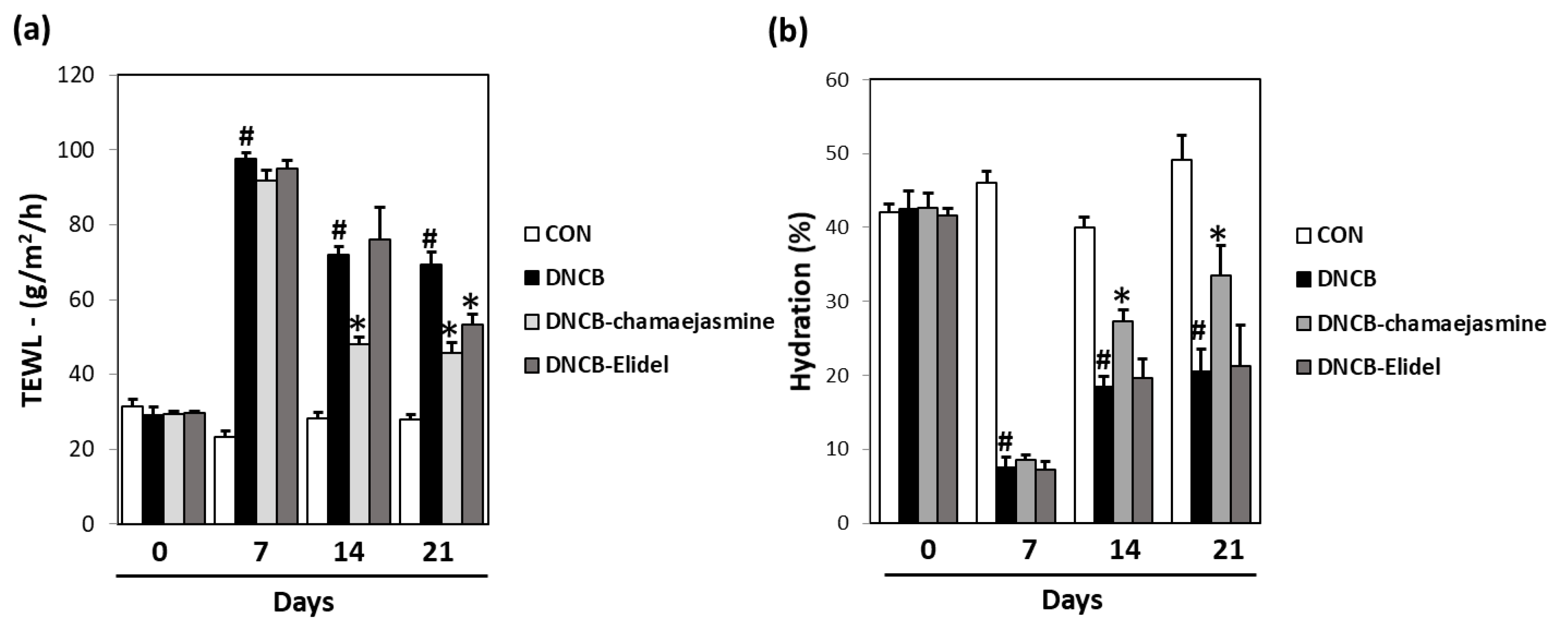
3.4. Chamaejasmine (7) from W. dolichantha Recovered Skin Barrier Function in DNCB-Induced Atopic Mice
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Leung, D.Y.; Boguniewicz, M.; Howell, M.D.; Nomura, I.; Hamid, Q.A. New insights into atopic dermatitis. J. Clin. Investig. 2004, 113, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.K.; Lin, D.; Tan, B.; Tudor, R.S.; Conley, D.B.; Peters, A.T.; Grammer, L.C.; Schleimer, R.P.; Kern, R.C. Chronic rhinosinusitis in the setting of other chronic inflammatory diseases. Am. J. Otolaryngol. 2011, 32, 388–391. [Google Scholar] [CrossRef] [PubMed]
- Boccardi, D.; D’Auria, E.; Turati, F.; DI, M.V.; Sortino, S.; Riva, E.; Cerri, A. Disease severity and quality of life in children with atopic dermatitis: PO-SCORAD in clinical practice. Minerva Pediatr. 2017, 69, 373–380. [Google Scholar] [PubMed]
- Tokura, Y. Extrinsic and intrinsic types of atopic dermatitis. J. Dermatol. Sci. 2010, 58, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.T.; Goodarzi, H.; Chen, H.Y. IgE, mast cells, and eosinophils in atopic dermatitis. Clin. Rev. Allergy Immunol. 2011, 41, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, T.; Ando, T.; Kimura, M.; Wilson, B.S.; Kawakami, Y. Mast cells in atopic dermatitis. Curr. Opin. Immunol. 2009, 21, 666–678. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.Y.; Chen, Y.L.; Huang, S.Z.; Kong, F.D.; Dai, H.F.; Hua, Y.; Zhao, Y.X. Two New Lignans from Wikstroemia dolichantha. Chem. Nat. Compd. 2018, 54, 22–25. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, J.; Shu, P.; Kong, L.; Hao, X.; Xue, Y.; Luo, Z.; Li, Y.; Li, G.; Yao, G.; et al. Two new diterpenoids from the buds of Wikstroemia chamaedaphne. Molecules 2012, 17, 6424–6433. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fu, W.W.; Sun, L.X.; Wang, Q.; Qi, W.; Yu, H. A new coumarin from Wikstroemia indica (l.) C.A. Mey. Chin. Chem. Lett. 2009, 20, 592–594. [Google Scholar] [CrossRef]
- Geng, L.D.; Zhang, C.; Xiao, Y.Q. Studies on the chemical constituents in stem rind of Wikstroemia indica. Zhongguo Zhong Yao Za Zhi 2006, 31, 817–819. [Google Scholar]
- Li, Y.M.; Zhu, L.; Jiang, J.G.; Yang, L.; Wang, D.Y. Bioactive components and pharmacological action of Wikstroemia indica (L.) CA Mey and its clinical application. Curr. Pharm. Biotechnol. 2009, 10, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Y.; Unehara, T.; Kitanaka, S. Anti-inflammatory activity of new guaiane type sesquiterpene from Wikstroemia indica. Chem. Pharm. Bull. 2005, 53, 137–139. [Google Scholar] [CrossRef] [PubMed]
- Jegal, J.; Park, N.J.; Kim, T.Y.; Choi, S.; Lee, S.W.; Hang, J.; Kim, S.N.; Yang, M.H. Effect of topically applied Wikstroemia dolichantha Diels on the development of atopic dermatitis-like skin symptoms in mice. Nutrients 2019, 11, 914. [Google Scholar] [CrossRef] [PubMed]
- Ragab, E.A.; Raafat, M. A new monoterpene glucoside and complete assignments of dihydroflavonols of Pulicaria jaubertii: Potential cytotoxic and blood pressure lowering activity. Nat. Prod. Res. 2016, 30, 1280–1288. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.H.; Kim, H.J.; Song, Y.S.; Jin, C.; Lee, K.T.; Cho, J.; Lee, Y.S. Constituents of the stems and fruits of Opuntia ficus-indica var. saboten. Arch. Pharm. Res. 2003, 26, 1018–1023. [Google Scholar] [CrossRef]
- Fukui, Y.; Tanaka, Y.; Kusumi, T.; Iwashita, T.; Nomoto, K. A rationale for the shift in colour towards blue in transgenic carnation flowers expressing the flavonoid 3′,5′-hydroxylase gene. Phytochemistry 2003, 63, 15–23. [Google Scholar] [CrossRef]
- Chen, L.Y.; Chen, I.S.; Peng, C.F. Structural elucidation and bioactivity of biflavonoids from the stems of Wikstroemia taiwanensis. Int. J. Mol. Sci. 2012, 13, 1029–1038. [Google Scholar] [CrossRef]
- Feng, B.; Pei, Y.; Hua, H.; Wang, T.; Zhang, Y. Biflavonoids from Stellera chamaejasme. Pharm. Biol. 2003, 41, 59–61. [Google Scholar] [CrossRef]
- Wan, S.B.; Chan, T.H. Enantioselective synthesis of afzelechin and epiafzelechin. Tetrahedron 2004, 60, 8207–8211. [Google Scholar] [CrossRef]
- Davis, A.L.; Cai, Y.; Davies, A.P.; Lewis, J.R. 1H and 13C NMR assignments of some green tea polyphenols. Magn. Reson. Chem. 1996, 34, 887–890. [Google Scholar] [CrossRef]
- Kaur, C.; Kapoor, H.C. Antioxidants in fruits and vegetables–the millennium’s health. Int. J. Food Sci. Technol. 2001, 36, 703–725. [Google Scholar] [CrossRef]
- Harborne, J.B.; Williams, C.A. Advances in flavonoid research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef]
- Cook, N.C.; Samman, S. Flavonoids–chemistry, metabolism, cardioprotective effects, and dietary sources. J. Nutr. Biochem. 1996, 7, 66–76. [Google Scholar] [CrossRef]
- Lien, E.J.; Ren, S.; Bui, H.H.; Wang, R. Quantitative structure-activity relationship analysis of phenolic antioxidants. Free Radic. Biol. Med. 1999, 26, 285–294. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Kim, H.P.; Mani, I.; Iversen, L.; Ziboh, V.A. Effects of naturally-occurring flavonoids and biflavonoids on epidermal cyclooxygenase and lipoxygenase from guinea-pigs. Prostaglandins Leukot. Essent. Fatty Acids 1998, 58, 17–24. [Google Scholar] [CrossRef]
- Da Silva, V.C.; de Sousa, P.T.; Dall’oglio, E.L.; Matiz, G.E.; de Carvalho, M.G. Anti-inflammatory activities of flavonoids from Luxemburgia octandra flowers. Chem. Nat. Compd. 2011, 46, 961–963. [Google Scholar] [CrossRef]
- Kim, H.P.; Son, K.H.; Chang, H.W.; Kang, S.S. Anti-inflammatory plant flavonoids and cellular action mechanisms. J. Pharmacol. Sci. 2004, 96, 229–245. [Google Scholar] [CrossRef]
- Jin, H.; He, R.; Oyoshi, M.; Geha, R.S. Animal models of atopic dermatitis. J. Investig. Dermatol. 2009, 129, 31–40. [Google Scholar] [CrossRef]
- Wolf, R.; Wolf, D. Abnormal epidermal barrier in the pathogenesis of atopic dermatitis. Clin. Dermatol. 2012, 30, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.Y.; Chen, A.Y.; Zhu, B.T. Mechanism of dinitrochlorobenzene-induced dermatitis in mice: Role of specific antibodies in pathogenesis. PLoS ONE 2009, 4, e7703. [Google Scholar] [CrossRef] [PubMed]
- Cork, M.J.; Robinson, D.A.; Vasilopoulos, Y.; Ferguson, A.; Moustafa, M.; MacGowan, A.; Duff, G.W.; Ward, S.J.; Tazi-Ahnini, R. New perspectives on epidermal barrier dysfunction in atopic dermatitis: Gene–environment interactions. J. Allergy Clin. Immunol. 2006, 118, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Kyselova, Z. Toxicological aspects of the use of phenolic compounds in disease prevention. Interdiscip. Toxicol. 2011, 4, 173–183. [Google Scholar] [CrossRef]
- Elias, P.M.; Schmuth, M. Abnormal skin barrier in the etiopathogenesis of atopic dermatitis. Allergy Clin. Immunol. 2009, 9, 437–446. [Google Scholar]
- Middleton, E. Effect of plant flavonoids on immune and inflammatory cell function. In Flavonoids in the Living System; Springer: Boston, MA, USA, 1998; pp. 175–182. [Google Scholar]
- Middleton, E., Jr.; Kandaswami, C. Effects of flavonoids on immune and inflammatory cell functions. Biochem. Pharmacol. 1992, 43, 1167–1179. [Google Scholar] [CrossRef]
- Cheong, H.; Ryu, S.Y.; Oak, M.H.; Cheon, S.H.; Yoo, G.S.; Kim, K.M. Studies of structure activity relationship of flavonoids for the anti-allergic actions. Arch. Pharm. Res. 1998, 21, 478–480. [Google Scholar] [CrossRef]
- Mastuda, H.; Morikawa, T.; Ueda, K.; Managi, H.; Yoshikawa, M. Structural requirements of flavonoids for inhibition of antigen-induced degranulation, TNF-α and IL-4 production from RBL-2H3 cells. Bioorg. Med. Chem. 2002, 10, 3123–3128. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, T.-Y.; Park, N.-J.; Jegal, J.; Choi, S.; Lee, S.W.; Hang, J.; Kim, S.-N.; Yang, M.H. Chamaejasmine Isolated from Wikstroemia dolichantha Diels Suppresses 2,4-Dinitrofluoro-benzene-Induced Atopic Dermatitis in SKH-1 Hairless Mice. Biomolecules 2019, 9, 697. https://doi.org/10.3390/biom9110697
Kim T-Y, Park N-J, Jegal J, Choi S, Lee SW, Hang J, Kim S-N, Yang MH. Chamaejasmine Isolated from Wikstroemia dolichantha Diels Suppresses 2,4-Dinitrofluoro-benzene-Induced Atopic Dermatitis in SKH-1 Hairless Mice. Biomolecules. 2019; 9(11):697. https://doi.org/10.3390/biom9110697
Chicago/Turabian StyleKim, Tae-Young, No-June Park, Jonghwan Jegal, Sangho Choi, Sang Woo Lee, Jin Hang, Su-Nam Kim, and Min Hye Yang. 2019. "Chamaejasmine Isolated from Wikstroemia dolichantha Diels Suppresses 2,4-Dinitrofluoro-benzene-Induced Atopic Dermatitis in SKH-1 Hairless Mice" Biomolecules 9, no. 11: 697. https://doi.org/10.3390/biom9110697
APA StyleKim, T.-Y., Park, N.-J., Jegal, J., Choi, S., Lee, S. W., Hang, J., Kim, S.-N., & Yang, M. H. (2019). Chamaejasmine Isolated from Wikstroemia dolichantha Diels Suppresses 2,4-Dinitrofluoro-benzene-Induced Atopic Dermatitis in SKH-1 Hairless Mice. Biomolecules, 9(11), 697. https://doi.org/10.3390/biom9110697




