Selenoprotein N and SEPN1-Related Myopathies: Mechanisms, Models, and Therapeutic Perspectives
Abstract
1. Introduction
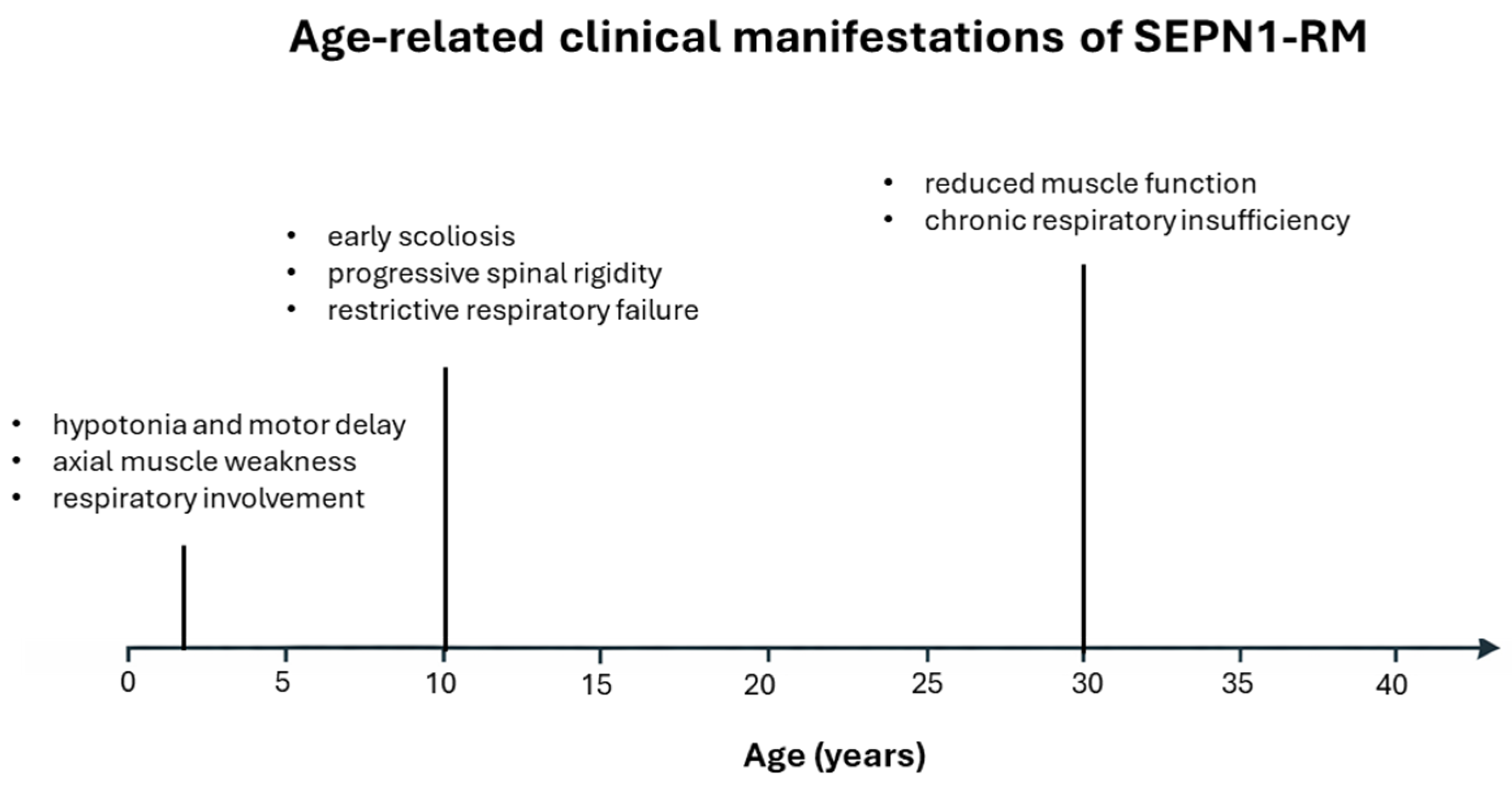
2. Clinical and Pathophysiological Features of SEPN1-RM
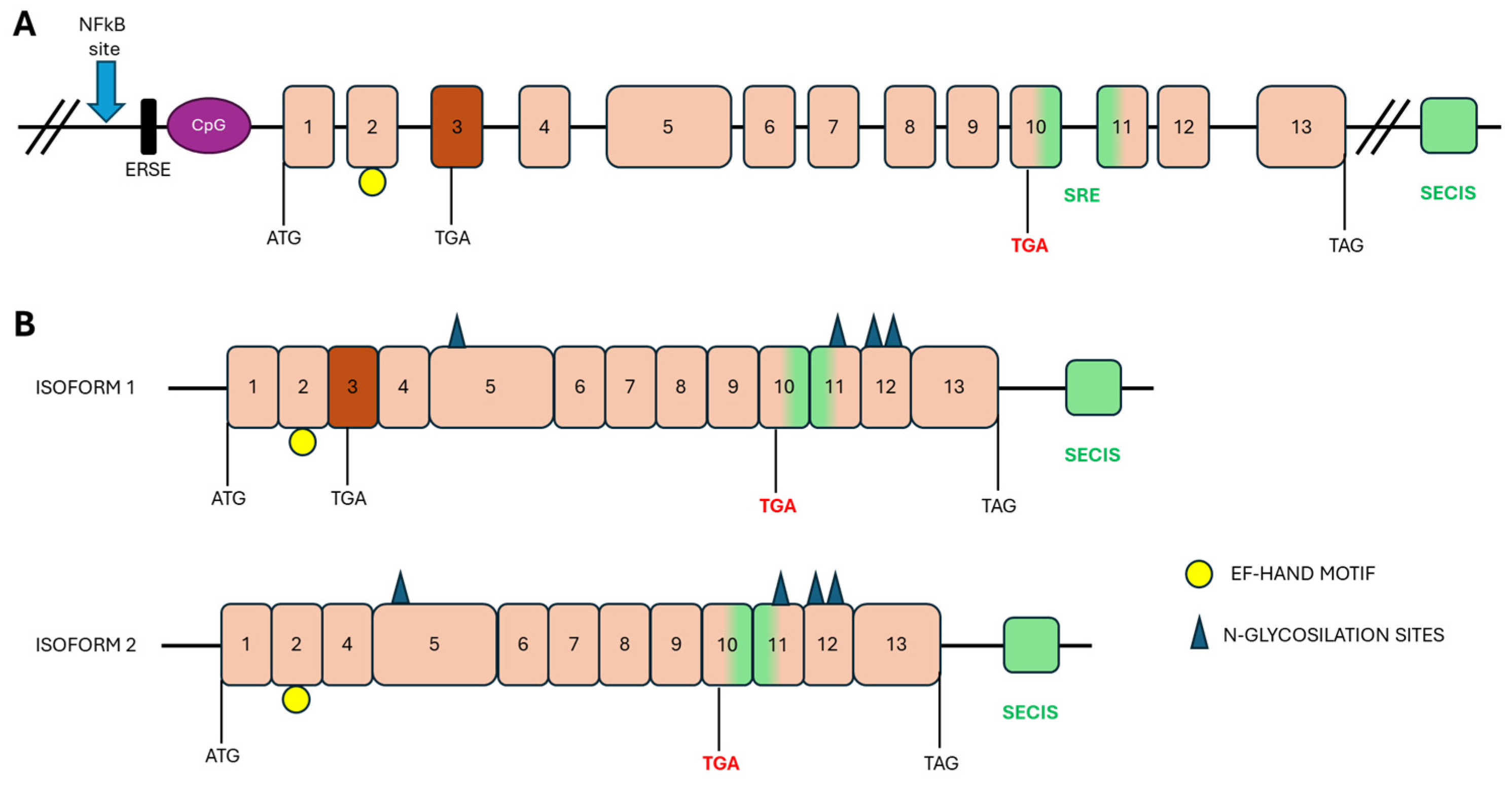
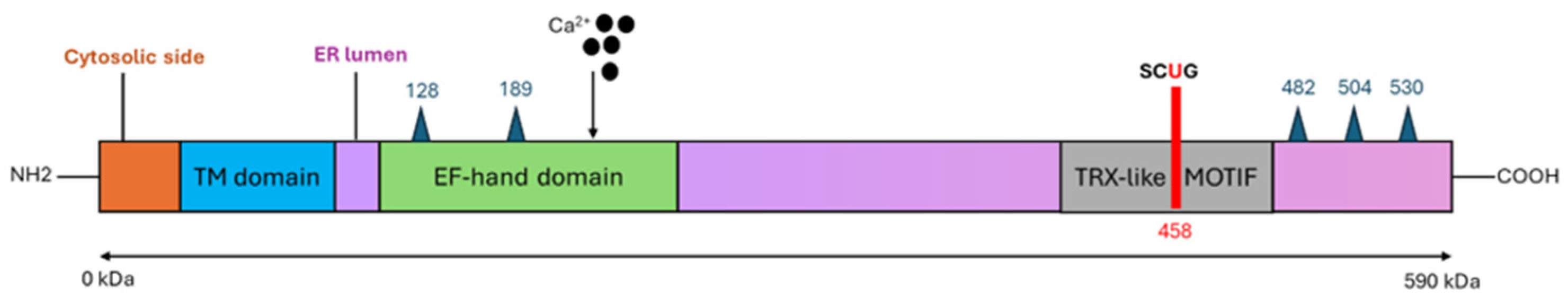
3. Synthesis, Structure and Localization of SelN
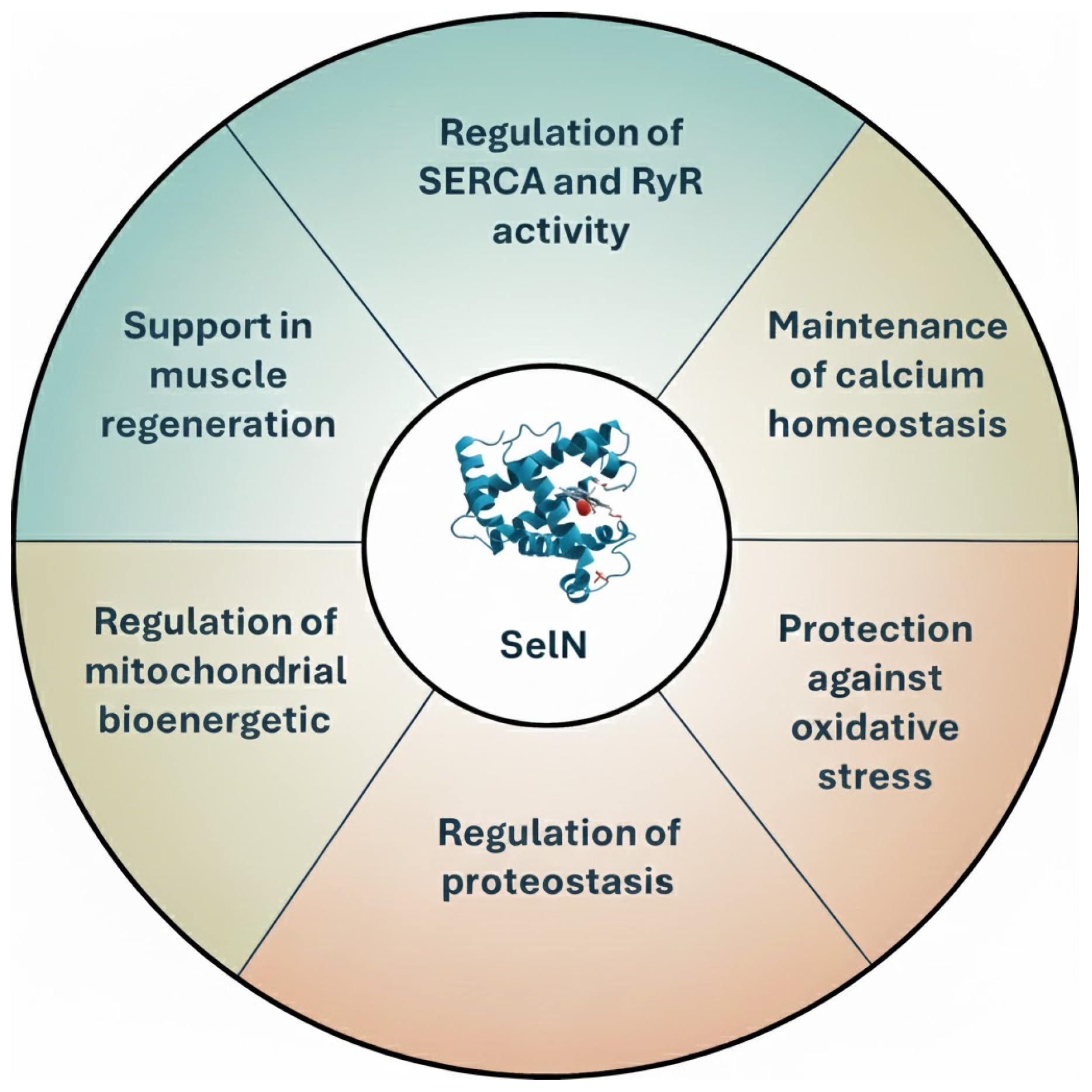
4. The Multifaceted Role of SelN in Cellular Function
4.1. Pathways Regulated by SelN and Related Dysfunctions in SEPN1-RM
4.1.1. ER Stress
4.1.2. Calcium Homeostasis
4.1.3. Metabolism
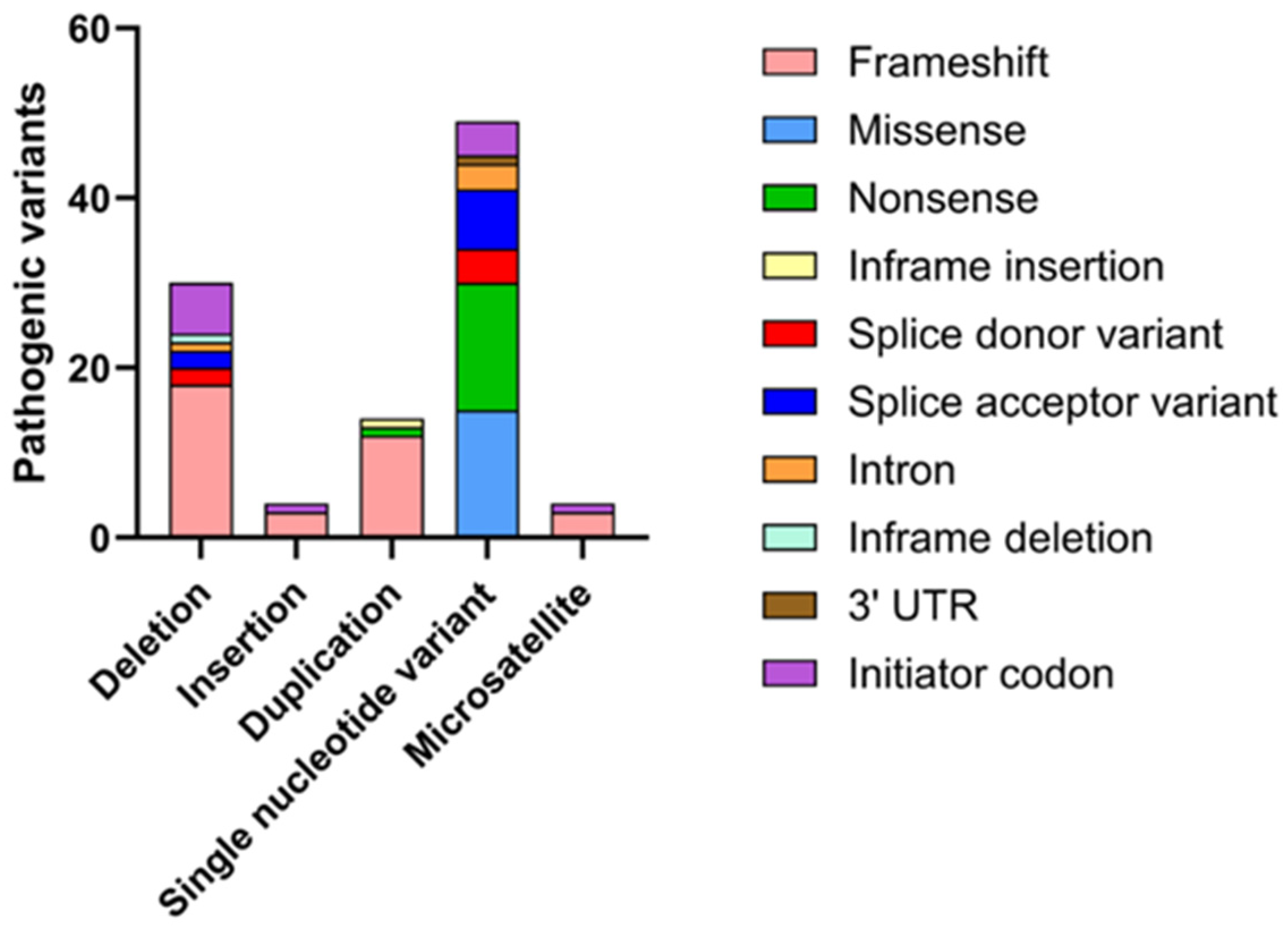
5. Mutations in SEPN1 and Clinical Spectrum of SEPN1-RM
6. SEPN1-RM Animal and Cellular Models
6.1. Animal Models
6.2. Cellular Models
7. Pharmacological Treatment Perspectives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Sec | Selenocysteine |
| SEPN1 or SELENON | Selenoprotein N |
| RSMD1 | Rigid spine muscular dystrophy |
| MmD | Multi-minicore disease |
| CFTD | Congenital fiber type disproportion |
| MB-DRMs | Desmin-related myopathy with Mallory body-like inclusions |
| SEPN1-RM | SEPN1-related myopathy |
| SECIS | Sec insertion sequence |
| SRE | Sec codon redefinition element |
| SBP2 | SECIS-binding protein 2 |
| EFSec | Sec elongation factor |
| ERSE | Endoplasmic reticulum stress response |
| ER | Endoplasmic reticulum |
| SR | Sarcoplasmic reticulum |
| MAMs | Mitochondria-associated membranes |
| NADH-TR | Nicotinamide Adenine Dinucleotide Tetrazolium Reductase |
| SDH | Succinate Dehydrogenase |
| UPR | Unfolded protein response |
| IRE1 | Inositol-requiring enzyme 1 |
| ATF6 | Activating transcription factor 6 |
| PERK | Protein kinase RNA-like ER kinase |
| OXPHOS | Oxidative phosphorylation |
| TUDCA | Tauroursodeoxycholic acid |
| NAC | N-acetylcysteine |
| iPSCs | pluripotent stem cells |
| BMI | body mass index |
References
- Barchielli, G.; Capperucci, A.; Tanini, D. The Role of Selenium in Pathologies: An Updated Review. Antioxidants 2022, 11, 251. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, S.; Dharmaraj, S. Selenium and Selenoproteins: It’s Role in Regulation of Inflammation. Inflammopharmacology 2020, 28, 667–695. [Google Scholar] [CrossRef]
- Di Gregorio, S. Selenium: A versatile trace element in life and environment. In Trace Elements in Human Health and Disease; Prasad, A.S., Ed.; Academic Press: New York, NY, USA, 2008; pp. 593–622. ISBN 978-0-470-18095-2. [Google Scholar]
- Lescure, A.; Gautheret, D.; Carbon, P.; Krol, A. Novel Selenoproteins Identified In Silico and In Vivo by Using a Conserved RNA Structural Motif. J. Biol. Chem. 1999, 274, 38147–38154. [Google Scholar] [CrossRef]
- Zito, E.; Ferreiro, A. Calcium and Redox Liaison: A Key Role of Selenoprotein N in Skeletal Muscle. Cells 2021, 10, 1116. [Google Scholar] [CrossRef]
- Chernorudskiy, A.; Varone, E.; Colombo, S.F.; Fumagalli, S.; Cagnotto, A.; Cattaneo, A.; Briens, M.; Baltzinger, M.; Kuhn, L.; Bachi, A.; et al. Selenoprotein N Is an Endoplasmic Reticulum Calcium Sensor That Links Luminal Calcium Levels to a Redox Activity. Proc. Natl. Acad. Sci. USA 2020, 117, 21288–21298. [Google Scholar] [CrossRef]
- Filipe, A.; Chernorudskiy, A.; Arbogast, S.; Varone, E.; Villar-Quiles, R.-N.; Pozzer, D.; Moulin, M.; Fumagalli, S.; Cabet, E.; Dudhal, S.; et al. Defective Endoplasmic Reticulum-Mitochondria Contacts and Bioenergetics in SEPN1-Related Myopathy. Cell Death Differ. 2021, 28, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Arbogast, S.; Ferreiro, A. Selenoproteins and Protection against Oxidative Stress: Selenoprotein N as a Novel Player at the Crossroads of Redox Signaling and Calcium Homeostasis. Antioxid. Redox Signal. 2010, 12, 893–904. [Google Scholar] [CrossRef]
- Sframeli, M.; Sarkozy, A.; Bertoli, M.; Astrea, G.; Hudson, J.; Scoto, M.; Mein, R.; Yau, M.; Phadke, R.; Feng, L.; et al. Congenital muscular dystrophies in the UK population: Clinical and molecular spectrum of a large cohort diagnosed over a 12-year period. Neuromuscul. Disord. 2017, 27, 793–803. [Google Scholar] [CrossRef]
- Maggi, L.; Scoto, M.; Cirak, S.; Robb, S.A.; Klein, A.; Lillis, S.; Cullup, T.; Feng, L.; Manzur, A.Y.; Sewry, C.A.; et al. Congenital myopathies—Clinical features and frequency of individual subtypes diagnosed over a 5-year period in the United Kingdom. Neuromuscul. Disord. 2013, 23, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Villar-Quiles, R.N.; von der Hagen, M.; Métay, C.; Gonzalez, V.; Donkervoort, S.; Bertini, E.; Castiglioni, C.; Chaigne, D.; Colomer, J.; Cuadrado, M.L.; et al. The clinical, histologic, and genotypic spectrum of SEPN1-related myopathy: A case series. Neurology 2020, 95, e1512–e1527. [Google Scholar]
- Zhang, S.; Lei, L.; Fan, Z.; Su, S.; Duo, J.; Luan, Q.; Lu, Y.; Di, L.; Wang, M.; Da, Y. Delayed respiratory insufficiency and extramuscular abnormalities in selenoprotein N-related myopathies. Front. Neurol. 2021, 12, 766942. [Google Scholar] [CrossRef]
- Arbogast, S.; Beuvin, M.; Fraysse, B.; Zhou, H.; Muntoni, F.; Ferreiro, A. Oxidative stress in SEPN1-related myopathy: From pathophysiology to treatment. Ann. Neurol. 2009, 65, 677–686. [Google Scholar] [CrossRef]
- Rederstorff, M.; Castets, P.; Arbogast, S.; Lainé, J.; Vassilopoulos, S.; Beuvin, M.; Dubourg, O.; Vignaud, A.; Ferry, A.; Krol, A.; et al. Increased Muscle Stress-Sensitivity Induced by Selenoprotein N Inactivation in Mouse: A Mammalian Model for SEPN1-Related Myopathy. PLoS ONE 2011, 6, e23094. [Google Scholar] [CrossRef] [PubMed]
- Zambon, A.A.; Muntoni, F. Congenital Muscular Dystrophies: What Is New? Neuromuscul. Disord. 2021, 31, 931–942. [Google Scholar] [CrossRef]
- Varone, E.; Pozzer, D.; Di Modica, S.; Chernorudskiy, A.; Nogara, L.; Baraldo, M.; Cinquanta, M.; Fumagalli, S.; Villar-Quiles, R.N.; De Simoni, M.G.; et al. SELENON (SEPN1) protects skeletal muscle from saturated fatty acid-induced ER stress and insulin resistance. Redox Biol. 2019, 24, 101176. [Google Scholar] [CrossRef]
- Pozzer, D.; Varone, E.; Chernorudskiy, A.; Schiarea, S.; Missiroli, S.; Giorgi, C.; Pinton, P.; Canato, M.; Germinario, E.; Nogara, L.; et al. A maladaptive ER stress response triggers dysfunction in highly active muscles of mice with SELENON loss. Redox Biol. 2019, 20, 354–366. [Google Scholar] [CrossRef]
- Ferreiro, A.; Quijano-Roy, S.; Pichereau, C.; Moghadaszadeh, B.; Goemans, N.; Bönnemann, C.; Jungbluth, H.; Straub, V.; Villanova, M.; Leroy, J.-P.; et al. Mutations of the selenoprotein N gene, which is implicated in RSMD, cause the classical phenotype of multiminicore disease: Reassessing the nosology of early onset myopathies. Am. J. Hum. Genet. 2002, 71, 739–749. [Google Scholar] [CrossRef]
- Caggiano, S.; Khirani, S.; Dabaj, I.; Cavassa, E.; Amaddeo, A.; Arroyo, J.O.; Desguerre, I.; Richard, P.; Cutrera, R.; Ferreiro, A.; et al. Diaphragmatic dysfunction in SEPN1-related myopathy. Neuromuscul. Disord. 2017, 27, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Alhajaji, R.; Dhafar, H.O.; Al Saadi, M.M.; BaHammam, A.S. Resilience story of managing severe obstructive sleep apnea with hypoventilation secondary to SELENON (SEPN1)-related myopathy. Respirol. Case Rep. 2025, 13, e70327. [Google Scholar] [CrossRef]
- Moghadaszadeh, B.; Desguerre, I.; Topaloglu, H.; Muntoni, F.; Pavek, S.; Sewry, C.; Mayer, M.; Fardeau, M.; Tomé, F.M.; Guicheney, P. Identification of a New Locus for a Peculiar Form of Congenital Muscular Dystrophy with Early Rigidity of the Spine, on Chromosome 1p35-36. Am. J. Hum. Genet. 1998, 62, 1439–1445. [Google Scholar] [CrossRef]
- Flanigan, K.M.; Kerr, L.; Bromberg, M.B.; Leonard, C.; Tsuruda, J.; Zhang, P.; Gonzalez-Gomez, I.; Cohn, R.; Campbell, K.P.; Leppert, M. Congenital Muscular Dystrophy with Rigid Spine Syndrome: A Clinical, Pathological, Radiological, and Genetic Study. Ann. Neurol. 2000, 47, 152–161. [Google Scholar] [CrossRef]
- Lobanov, A.V.; Hatfield, D.L.; Gladyshev, V.N. Eukaryotic Selenoproteins and Selenoproteomes. Biochim. Biophys. Acta 2009, 1790, 1424–1428. [Google Scholar] [CrossRef]
- Petit, N.; Lescure, A.; Rederstorff, M.; Krol, A.; Moghadaszadeh, B.; Wewer, U.M.; Guicheney, P. Selenoprotein N: An Endoplasmic Reticulum Glycoprotein with an Early Developmental Expression Pattern. Hum. Mol. Genet. 2003, 12, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Makałowski, W.; Mitchell, G.A.; Labuda, D. Alu Sequences in the Coding Regions of MRNA: A Source of Protein Variability. Trends Genet. 1994, 10, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Moghadaszadeh, B.; Petit, N.; Jaillard, C.; Brockington, M.; Quijano Roy, S.; Merlini, L.; Romero, N.; Estournet, B.; Desguerre, I.; Chaigne, D.; et al. Mutations in SEPN1 Cause Congenital Muscular Dystrophy with Spinal Rigidity and Restrictive Respiratory Syndrome. Nat. Genet. 2001, 29, 17–18. [Google Scholar] [CrossRef]
- Howard, M.T.; Aggarwal, G.; Anderson, C.B.; Khatri, S.; Flanigan, K.M.; Atkins, J.F. Recoding Elements Located Adjacent to a Subset of Eukaryal Selenocysteine-Specifying UGA Codons. EMBO J. 2005, 24, 1596–1607. [Google Scholar] [CrossRef]
- Hubert, N.; Walczak, R.; Carbon, P.; Krol, A. A Protein Binds the Selenocysteine Insertion Element in the 3′-UTR of Mammalian Selenoprotein MRNAs. Nucleic Acids Res. 1996, 24, 464–469. [Google Scholar] [CrossRef]
- Copeland, P.R.; Driscoll, D.M. Purification, Redox Sensitivity, and RNA Binding Properties of SECIS-Binding Protein 2, a Protein Involved in Selenoprotein Biosynthesis. J. Biol. Chem. 1999, 274, 25447–25454. [Google Scholar] [CrossRef]
- Sturchler, C.; Westhof, E.; Carbon, P.; Krol, A. Unique Secondary and Tertiary Structural Features of the Eucaryotic Selenocysteine TRNA(Sec). Nucleic Acids Res. 1993, 21, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Squires, J.E.; Stoytchev, I.; Forry, E.P.; Berry, M.J. SBP2 Binding Affinity Is a Major Determinant in Differential Selenoprotein MRNA Translation and Sensitivity to Nonsense-Mediated Decay. Mol. Cell. Biol. 2007, 27, 7848–7855. [Google Scholar] [CrossRef]
- Li, S.S.-C. Specificity and Versatility of SH3 and Other Proline-Recognition Domains: Structural Basis and Implications for Cellular Signal Transduction. Biochem. J. 2005, 390, 641–653. [Google Scholar] [CrossRef]
- Castets, P.; Lescure, A.; Guicheney, P.; Allamand, V. Selenoprotein N in Skeletal Muscle: From Diseases to Function. J. Mol. Med. 2012, 90, 1095–1107. [Google Scholar] [CrossRef] [PubMed]
- Addinsall, A.B.; Wright, C.R.; Andrikopoulos, S.; van der Poel, C.; Stupka, N. Emerging Roles of Endoplasmic Reticulum-Resident Selenoproteins in the Regulation of Cellular Stress Responses and the Implications for Metabolic Disease. Biochem. J. 2018, 475, 1037–1057. [Google Scholar] [CrossRef]
- Lescure, A.; Rederstorff, M.; Krol, A.; Guicheney, P.; Allamand, V. Selenoprotein Function and Muscle Disease. Biochim. Biophys. Acta 2009, 1790, 1569–1574. [Google Scholar] [CrossRef]
- Kryukov, G.V.; Castellano, S.; Novoselov, S.V.; Lobanov, A.V.; Zehtab, O.; Guigó, R.; Gladyshev, V.N. Characterization of Mammalian Selenoproteomes. Science 2003, 300, 1439–1443. [Google Scholar] [CrossRef] [PubMed]
- Maiti, B.; Arbogast, S.; Allamand, V.; Moyle, M.W.; Anderson, C.B.; Richard, P.; Guicheney, P.; Ferreiro, A.; Flanigan, K.M.; Howard, M.T. A Mutation in the SEPN1 Selenocysteine Redefinition Element (SRE) Reduces Selenocysteine Incorporation and Leads to SEPN1-Related Myopathy. Hum. Mutat. 2009, 30, 411–416. [Google Scholar] [CrossRef]
- Shi, Z.; Han, Z.; Chen, J.; Zhou, J.-C. Endoplasmic Reticulum-Resident Selenoproteins and Their Roles in Glucose and Lipid Metabolic Disorders. Biochim. Biophys. Acta-Mol. Basis Dis. 2024, 1870, 167246. [Google Scholar] [CrossRef] [PubMed]
- Hetz, C.; Zhang, K.; Kaufman, R.J. Mechanisms, Regulation and Functions of the Unfolded Protein Response. Nat. Rev. Mol. Cell Biol. 2020, 21, 421–438. [Google Scholar] [CrossRef]
- Urano, F.; Wang, X.; Bertolotti, A.; Zhang, Y.; Chung, P.; Harding, H.P.; Ron, D. Coupling of Stress in the ER to Activation of JNK Protein Kinases by Transmembrane Protein Kinase IRE1. Science 2000, 287, 664–666. [Google Scholar] [CrossRef] [PubMed]
- Marciniak, S.J.; Yun, C.Y.; Oyadomari, S.; Novoa, I.; Zhang, Y.; Jungreis, R.; Nagata, K.; Harding, H.P.; Ron, D. CHOP Induces Death by Promoting Protein Synthesis and Oxidation in the Stressed Endoplasmic Reticulum. Genes Dev. 2004, 18, 3066–3077. [Google Scholar] [CrossRef]
- Frand, A.R.; Kaiser, C.A. The ERO1 Gene of Yeast Is Required for Oxidation of Protein Dithiols in the Endoplasmic Reticulum. Mol. Cell 1998, 1, 161–170. [Google Scholar] [CrossRef]
- Zito, E. ERO1: A Protein Disulfide Oxidase and H2O2 Producer. Free Radic. Biol. Med. 2015, 83, 299–304. [Google Scholar] [CrossRef]
- Mercuri, E.; Talim, B.; Moghadaszadeh, B.; Petit, N.; Brockington, M.; Counsell, S.; Guicheney, P.; Muntoni, F.; Merlini, L. Clinical and Imaging Findings in Six Cases of Congenital Muscular Dystrophy with Rigid Spine Syndrome Linked to Chromosome 1p (RSMD1). Neuromuscul. Disord. 2002, 12, 631–638. [Google Scholar] [CrossRef]
- Miyake, M.; Nomura, A.; Ogura, A.; Takehana, K.; Kitahara, Y.; Takahara, K.; Tsugawa, K.; Miyamoto, C.; Miura, N.; Sato, R.; et al. Skeletal Muscle-Specific Eukaryotic Translation Initiation Factor 2α Phosphorylation Controls Amino Acid Metabolism and Fibroblast Growth Factor 21-Mediated Non-Cell-Autonomous Energy Metabolism. FASEB J. 2016, 30, 798–812. [Google Scholar] [CrossRef] [PubMed]
- Saheki, Y.; De Camilli, P. Endoplasmic Reticulum-Plasma Membrane Contact Sites. Annu. Rev. Biochem. 2017, 86, 659–684. [Google Scholar] [CrossRef]
- Rowland, A.A.; Voeltz, G.K. Endoplasmic Reticulum–Mitochondria Contacts: Function of the Junction. Nat. Rev. Mol. Cell Biol. 2012, 13, 607–615. [Google Scholar] [CrossRef]
- Giorgi, C.; Missiroli, S.; Patergnani, S.; Duszynski, J.; Wieckowski, M.R.; Pinton, P. Mitochondria-Associated Membranes: Composition, Molecular Mechanisms, and Physiopathological Implications. Antioxid. Redox Signal. 2015, 22, 995–1019. [Google Scholar] [CrossRef]
- Germani, S.; Van Ho, A.T.; Cherubini, A.; Varone, E.; Chernorudskiy, A.; Renna, G.M.; Fumagalli, S.; Gobbi, M.; Lucchetti, J.; Bolis, M.; et al. SEPN1-Related Myopathy Depends on the Oxidoreductase ERO1A and Is Druggable with the Chemical Chaperone TUDCA. Cell Rep. Med. 2024, 5, 101439. [Google Scholar] [CrossRef]
- Ushioda, R.; Miyamoto, A.; Inoue, M.; Watanabe, S.; Okumura, M.; Maegawa, K.; Uegaki, K.; Fujii, S.; Fukuda, Y.; Umitsu, M.; et al. Redox-Assisted Regulation of Ca2+ Homeostasis in the Endoplasmic Reticulum by Disulfide Reductase ERdj5. Proc. Natl. Acad. Sci. USA 2016, 113, E6055–E6063. [Google Scholar] [CrossRef] [PubMed]
- Katona, M.; Bartók, Á.; Nichtova, Z.; Csordás, G.; Berezhnaya, E.; Weaver, D.; Ghosh, A.; Várnai, P.; Yule, D.I.; Hajnóczky, G. Capture at the ER-Mitochondrial Contacts Licenses IP3 Receptors to Stimulate Local Ca2+ Transfer and Oxidative Metabolism. Nat. Commun. 2022, 13, 6779. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.; Stoilova, T.; Giorgi, C.; Bachi, A.; Cattaneo, A.; Auricchio, A.; Pinton, P.; Zito, E. SEPN1, an Endoplasmic Reticulum-Localized Selenoprotein Linked to Skeletal Muscle Pathology, Counteracts Hyperoxidation by Means of Redox-Regulating SERCA2 Pump Activity. Hum. Mol. Genet. 2014, 24, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, E.; Clements, E.; Offiah, A.; Pichiecchio, A.; Vasco, G.; Bianco, F.; Berardinelli, A.; Manzur, A.; Pane, M.; Messina, S.; et al. Muscle Magnetic Resonance Imaging Involvement in Muscular Dystrophies with Rigidity of the Spine. Ann. Neurol. 2010, 67, 201–208. [Google Scholar] [CrossRef]
- Castets, P.; Bertrand, A.T.; Beuvin, M.; Ferry, A.; Le Grand, F.; Castets, M.; Chazot, G.; Rederstorff, M.; Krol, A.; Lescure, A.; et al. Satellite Cell Loss and Impaired Muscle Regeneration in Selenoprotein N Deficiency. Hum. Mol. Genet. 2011, 20, 694–704. [Google Scholar] [CrossRef]
- Afroze, D.; Kumar, A. ER Stress in Skeletal Muscle Remodeling and Myopathies. FEBS J. 2019, 286, 379–398. [Google Scholar] [CrossRef]
- Abdul-Ghani, M.A.; DeFronzo, R.A. Pathogenesis of Insulin Resistance in Skeletal Muscle. Biomed Res. Int. 2010, 2010, 476279. [Google Scholar] [CrossRef]
- Tubbs, E.; Chanon, S.; Robert, M.; Bendridi, N.; Bidaux, G.; Chauvin, M.-A.; Ji-Cao, J.; Durand, C.; Gauvrit-Ramette, D.; Vidal, H.; et al. Disruption of Mitochondria-Associated Endoplasmic Reticulum Membrane (MAM) Integrity Contributes to Muscle Insulin Resistance in Mice and Humans. Diabetes 2018, 67, 636–650. [Google Scholar] [CrossRef] [PubMed]
- Deniziak, M.; Thisse, C.; Rederstorff, M.; Hindelang, C.; Thisse, B.; Lescure, A. Loss of Selenoprotein N Function Causes Disruption of Muscle Architecture in the Zebrafish Embryo. Exp. Cell Res. 2007, 313, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Xu, Z.; Li, X.; Gao, F.; Guo, E.; Chang, X.; Wei, C.; Zhang, C.; Yu, Q.; Que, C.; et al. Novel SEPN1 Mutations in Exon 1 Are Common in Rigid Spine with Muscular Dystrophy Type 1 in Chinese Patients. Front. Genet. 2022, 13, 825793. [Google Scholar] [CrossRef] [PubMed]
- Clarke, N.F.; Kidson, W.; Quijano-Roy, S.; Estournet, B.; Ferreiro, A.; Guicheney, P.; Manson, J.I.; Kornberg, A.J.; Shield, L.K.; North, K.N. SEPN1: Associated with Congenital Fiber-Type Disproportion and Insulin Resistance. Ann. Neurol. 2006, 59, 546–552. [Google Scholar] [CrossRef]
- Allamand, V.; Richard, P.; Lescure, A.; Ledeuil, C.; Desjardin, D.; Petit, N.; Gartioux, C.; Ferreiro, A.; Krol, A.; Pellegrini, N.; et al. A Single Homozygous Point Mutation in a 3′untranslated Region Motif of Selenoprotein N MRNA Causes SEPN1-Related Myopathy. EMBO Rep. 2006, 7, 450–454. [Google Scholar] [CrossRef]
- Jurynec, M.J.; Xia, R.; Mackrill, J.J.; Gunther, D.; Crawford, T.; Flanigan, K.M.; Abramson, J.J.; Howard, M.T.; Grunwald, D.J. Selenoprotein N Is Required for Ryanodine Receptor Calcium Release Channel Activity in Human and Zebrafish Muscle. Proc. Natl. Acad. Sci. USA 2008, 105, 12485–12490. [Google Scholar] [CrossRef]
- Barraza-Flores, P.; Moghadaszadeh, B.; Lee, W.; Isaac, B.; Sun, L.; Troiano, E.C.; Rockowitz, S.; Sliz, P.; Beggs, A.H. Zebrafish and Cellular Models of SELENON-Related Myopathy Exhibit Novel Embryonic and Metabolic Phenotypes. Skelet. Muscle 2025, 15, 7. [Google Scholar] [CrossRef]
- Castets, P.; Maugenre, S.; Gartioux, C.; Rederstorff, M.; Krol, A.; Lescure, A.; Tajbakhsh, S.; Allamand, V.; Guicheney, P. Selenoprotein N Is Dynamically Expressed during Mouse Development and Detected Early in Muscle Precursors. BMC Dev. Biol. 2009, 9, 46. [Google Scholar] [CrossRef]
- Moghadaszadeh, B.; Rider, B.E.; Lawlor, M.W.; Childers, M.K.; Grange, R.W.; Gupta, K.; Boukedes, S.S.; Owen, C.A.; Beggs, A.H. Selenoprotein N Deficiency in Mice Is Associated with Abnormal Lung Development. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2013, 27, 1585–1599. [Google Scholar] [CrossRef]
- Hofmann, A.F. The Continuing Importance of Bile Acids in Liver and Intestinal Disease. Arch. Intern. Med. 1999, 159, 2647–2658. [Google Scholar] [CrossRef] [PubMed]
- Retini, M.; Cherubini, A.; Marrazza, A.; Varone, E.; Germani, S.; Renna, G.M.; Recchia, A.; Guidarelli, A.; Fumagalli, S.; Grasselli, C.; et al. Pyrazolone-based ERO1 inhibitors in ERO1-driven Triple-Negative Breast Cancer and SEPN1-Related Myopathy: Structure-activity relationship and therapeutic potential. Pharmacol. Res. 2025, 222, 108037. [Google Scholar] [CrossRef] [PubMed]
- Arbogast, S.; Dill, C.; Ramahefasolo, C.; Piemonte, F.; Serreri, C.; Lescure, A.; Ferry, A.; Bonay, M.; Bertini, E.; Ferreiro, A. G.P.209: N-Acetylcysteine as an Effective Treatment In Vivo and Identification of Biomarkers in SEPN1-Related Myopathy: A First Preclinical Trial. Neuromuscul. Disord. 2014, 24, 879–880. [Google Scholar] [CrossRef]
- Dill, C.; Prigent, H.; Behin, A.; Piemonte, F.; Bertini, E.; Orlikowski, D.; Estournet, B.; Ferreiro, A. Launching the First Clinical Trial in SEPN1-Related Myopathy: The SELNAC Study. Neuromuscul. Disord. 2015, 25, S270. [Google Scholar] [CrossRef]
| Zebrafish | ||
| Techniques applied | Features | References |
| Antisense morpholino oligonucleotides |
| [58,62] |
| CRISPR/Cas9 |
| [63] |
| Murines | ||
| Techniques applied | Features | References |
| SelN knockout mouse |
| [14,64,65] |
| Hela Cells | ||
| Techniques applied | Features | References |
| CRISPR/Cas9 |
| [7] |
| C2C12 Murine Myoblasts | ||
| Techniques applied | Features | References |
| CRISPR/Cas9 |
| [7,63] |
| Patient-Derived Fibroblasts and Myoblasts | ||
| Techniques applied | Features | References |
| Patient mutation |
| [8,22,49] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Lanza, M.; Zito, E.; Dinoi, G.; Buono, A.V.; De Luca, A.; Imbrici, P.; Liantonio, A.; Conte, E. Selenoprotein N and SEPN1-Related Myopathies: Mechanisms, Models, and Therapeutic Perspectives. Biomolecules 2026, 16, 125. https://doi.org/10.3390/biom16010125
Lanza M, Zito E, Dinoi G, Buono AV, De Luca A, Imbrici P, Liantonio A, Conte E. Selenoprotein N and SEPN1-Related Myopathies: Mechanisms, Models, and Therapeutic Perspectives. Biomolecules. 2026; 16(1):125. https://doi.org/10.3390/biom16010125
Chicago/Turabian StyleLanza, Martina, Ester Zito, Giorgia Dinoi, Antonio Vittorio Buono, Annamaria De Luca, Paola Imbrici, Antonella Liantonio, and Elena Conte. 2026. "Selenoprotein N and SEPN1-Related Myopathies: Mechanisms, Models, and Therapeutic Perspectives" Biomolecules 16, no. 1: 125. https://doi.org/10.3390/biom16010125
APA StyleLanza, M., Zito, E., Dinoi, G., Buono, A. V., De Luca, A., Imbrici, P., Liantonio, A., & Conte, E. (2026). Selenoprotein N and SEPN1-Related Myopathies: Mechanisms, Models, and Therapeutic Perspectives. Biomolecules, 16(1), 125. https://doi.org/10.3390/biom16010125







