Roles of Lipid Metabolism in Pulmonary Hypertension: Friend or Foe?
Abstract
1. Introduction
2. The Role of Lipid Metabolic Reprogramming in PH
2.1. Physiological Regulatory Mechanisms of Lipid Metabolism
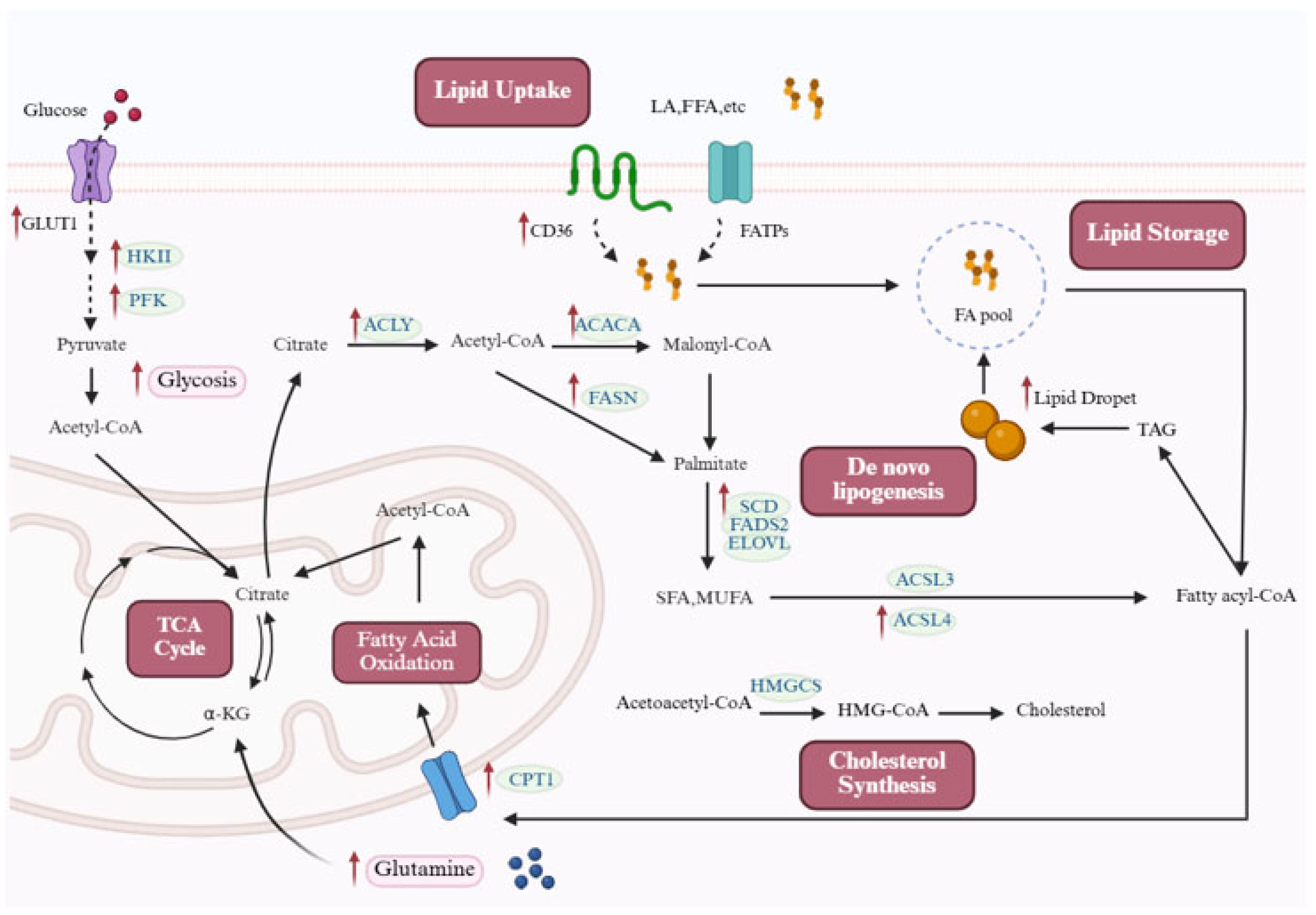
2.2. Enhanced Lipid Uptake and De Novo Synthesis in Pulmonary Hypertension
2.2.1. De Novo Lipid Synthesis
2.2.2. Lipid Uptake
2.3. Dysregulation of FAO
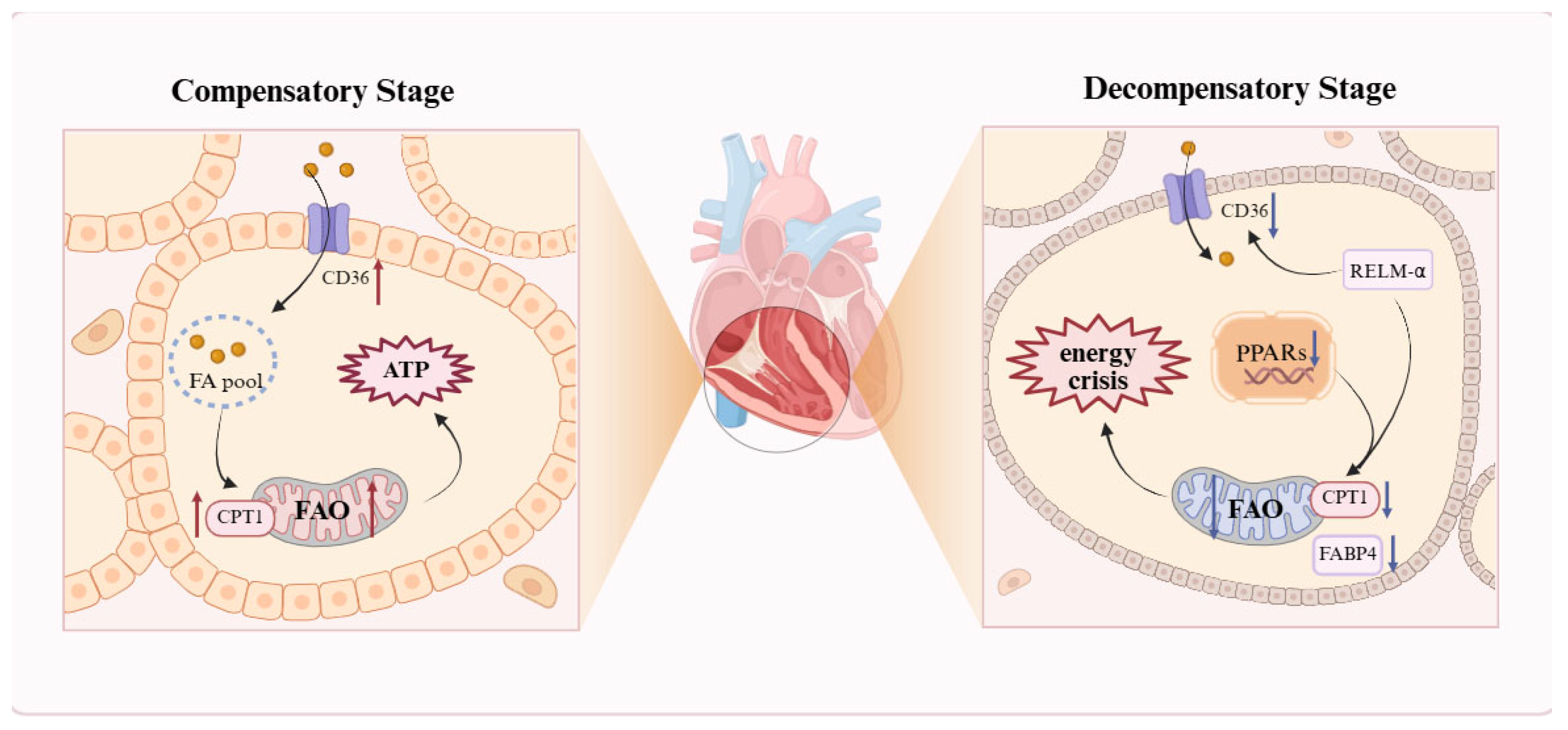
2.3.1. Bidirectional Dysregulation of FAO in Cardiomyocytes
2.3.2. Significant Enhancement of FAO in Pulmonary Vascular Cells
3. Reconstruction of Lipid Signaling Networks
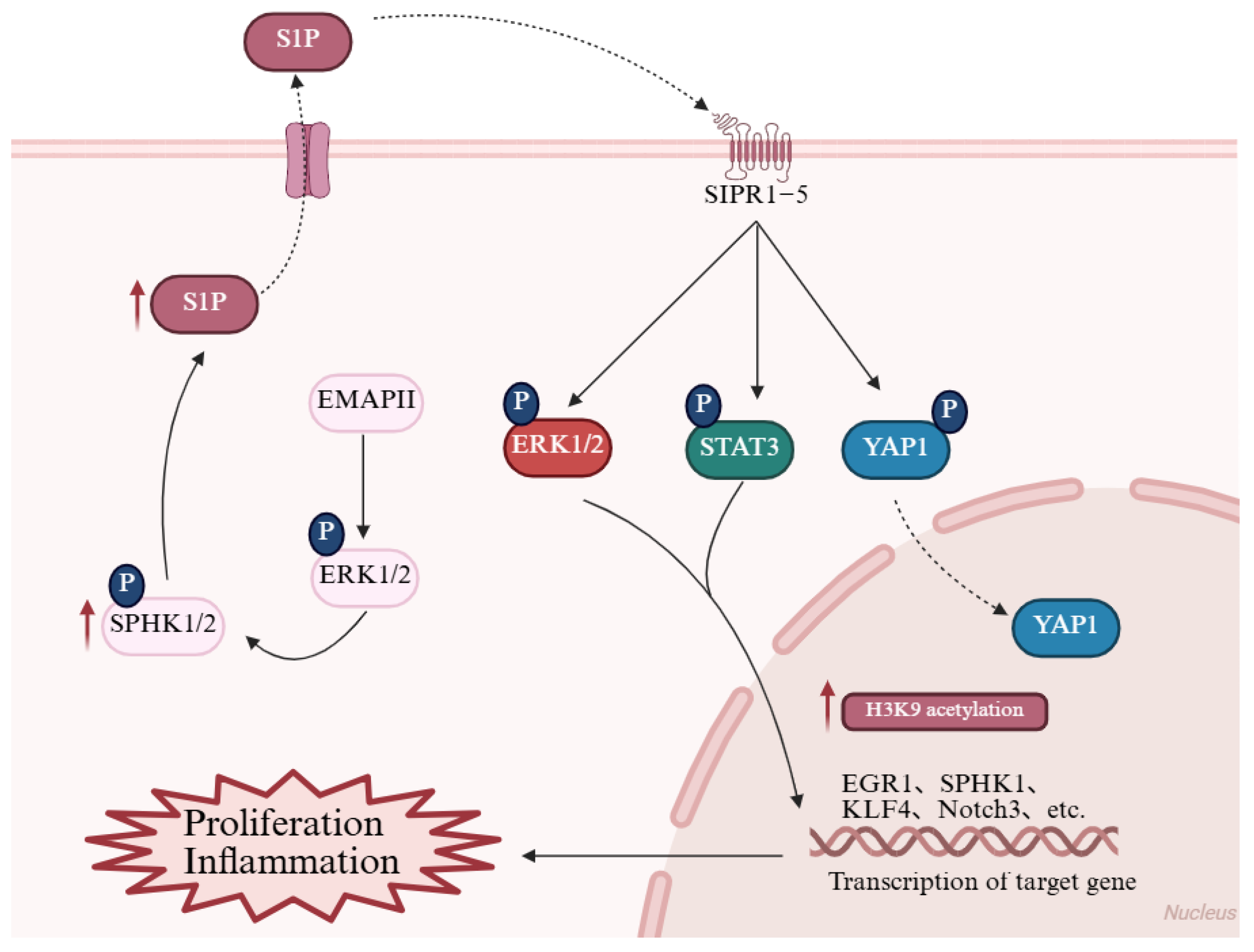
3.1. Membrane Receptor-Mediated Signaling: Proliferation and Inflammation
3.2. Intranuclear Metabolic Signaling: Epigenetic Regulation
4. Cell-Specific Metabolic Phenotypes and Interactions
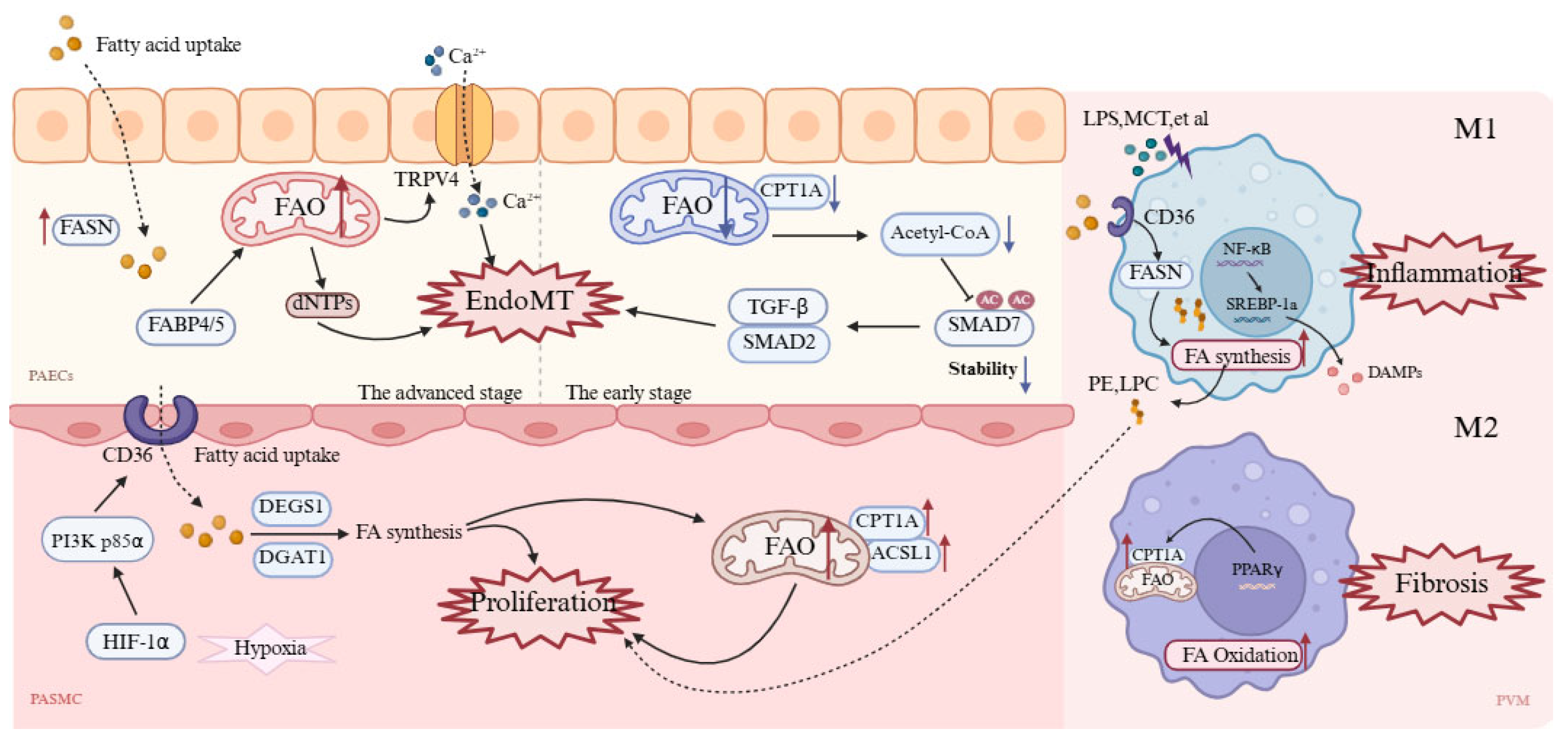
4.1. Pulmonary Artery Endothelial Cells
4.2. Pulmonary Artery Smooth Muscle Cells
4.3. Pulmonary Vascular Macrophages
5. Targeting Lipid Metabolic Reprogramming in PH Therapeutic Strategies
5.1. Targeting Fatty Acid Synthesis
5.2. Targeting Fatty Acid Oxidation
5.3. Therapeutic Potential of Targeting Fatty Acid Transport Proteins
5.4. Prospects of Natural Compounds in PH Treatment
6. Challenges and Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACAT | Acetyl-CoA acetyltransferase |
| ACLY | ATP-citrate lyase |
| ACC/ACACA | Acetyl-CoA carboxylase |
| ACSS | Acetyl-CoA synthetase |
| AMPK | AMP-activated protein kinase |
| BMPR2 | Bone morphogenetic protein receptor type 2 |
| CD36 | Cluster of differentiation 36 |
| CPT1 | Carnitine palmitoyltransferase 1 |
| dNTP | Deoxynucleoside triphosphate |
| DGAT | Diacylglycerol acyltransferase |
| EC | Endothelial cell |
| EMAP II | Endothelial monocyte-activating polypeptide II |
| EndoMT | Endothelial-to-mesenchymal transition |
| ERK | Extracellular signal-regulated kinase |
| ETO | Etomoxir |
| ET-1 | Endothelin-1 |
| FA | Fatty acid |
| FAO | Fatty acid oxidation |
| FAS | Fatty acid synthesis |
| FASN | Fatty acid synthase |
| FABP | Fatty acid-binding protein |
| FADS2 | Fatty acid desaturase 2 |
| FPER-1 | Fluorinated perhexiline derivative 1 |
| GO | Glucose oxidation |
| HIF-1α | Hypoxia-inducible factor-1 alpha |
| HMGCS | 3-hydroxy-3-methylglutaryl-CoA synthase |
| HPH | Hypoxic pulmonary hypertension |
| HPAEC | Human pulmonary artery endothelial cell |
| HPASMC | Human pulmonary artery smooth muscle cell |
| HPMVEC | Human pulmonary microvascular endothelial cell |
| HRMEC | Human retinal microvascular endothelial cell |
| IPAH | Idiopathic pulmonary arterial hypertension |
| LD | Lipid droplet |
| LDHA | Lactate dehydrogenase A |
| LPS | Lipopolysaccharide |
| MCT | Monocrotaline |
| MCD | Malonyl-CoA decarboxylase |
| Mef2 | Myocyte enhancer factor 2 |
| MUFA | Monounsaturated fatty acid |
| PAEC | Pulmonary artery endothelial cell |
| PAH | Pulmonary arterial hypertension |
| PASMC | Pulmonary artery smooth muscle cell |
| PDH | Pyruvate dehydrogenase |
| PH | Pulmonary hypertension |
| RV | Right ventricle |
| RVSP | Right ventricular systolic pressure |
| RVHI | Right ventricular hypertrophy index |
| Sal | Salidroside |
| S1P | Sphingosine-1-phosphate |
| SCD | Stearoyl-CoA desaturase |
| SMAD | Mothers against decapentaplegic homolog |
| SFA | Saturated fatty acid |
| SphK1/2 | Sphingosine kinase 1/2 |
| SREBP | Sterol regulatory element-binding protein |
| SuHx | Sugen/hypoxia model |
| TASK-1 | TWIK-related acid-sensitive K+ channel 1 |
| TAG | Triacylglycerol |
| TCA cycle | Tricarboxylic acid cycle |
| TGF-β | Transforming growth factor-beta |
| TMZ | Trimetazidine |
References
- Kovacs, G.; Bartolome, S.; Denton, C.P.; Gatzoulis, M.A.; Gu, S.; Khanna, D.; Badesch, D.; Montani, D. Definition, Classification and Diagnosis of Pulmonary Hypertension. Eur. Respir. J. 2024, 64, 2401324. [Google Scholar] [CrossRef]
- Balistrieri, A.; Makino, A.; Yuan, J.X.-J. Pathophysiology and Pathogenic Mechanisms of Pulmonary Hypertension: Role of Membrane Receptors, Ion Channels, and Ca2+ Signaling. Physiol. Rev. 2023, 103, 1827–1897. [Google Scholar] [CrossRef]
- Klinger, J.R.; Elliott, C.G.; Levine, D.J.; Bossone, E.; Duvall, L.; Fagan, K.; Frantsve-Hawley, J.; Kawut, S.M.; Ryan, J.J.; Rosenzweig, E.B.; et al. Therapy for Pulmonary Arterial Hypertension in Adults: Update of the CHEST Guideline and Expert Panel Report. Chest 2019, 155, 565–586. [Google Scholar] [CrossRef] [PubMed]
- Farber, H.W.; Miller, D.P.; Poms, A.D.; Badesch, D.B.; Frost, A.E.; Muros-Le Rouzic, E.; Romero, A.J.; Benton, W.W.; Elliott, C.G.; McGoon, M.D.; et al. Five-Year Outcomes of Patients Enrolled in the REVEAL Registry. Chest 2015, 148, 1043–1054. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Li, H.; Li, Y.; Luo, Y.; He, Y.; Shui, X.; Lei, W. Glycolysis Modulation: New Therapeutic Strategies to Improve Pulmonary Hypertension (Review). Int. J. Mol. Med. 2024, 54, 115. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, L.; Zhang, W. Metabolic Reprogramming: A Novel Metabolic Model for Pulmonary Hypertension. Front. Cardiovasc. Med. 2022, 9, 957524. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-Z.; Wu, J.-C.; Lu, G.-F.; Li, H.-B.; Lai, S.-M.; Lin, Y.-C.; Gui, L.-X.; Sham, J.S.K.; Lin, M.-J.; Lin, D.-C. Pulmonary Hypertension Induces Serotonin Hyperreactivity and Metabolic Reprogramming in Coronary Arteries via NOX1/4-TRPM2 Signaling Pathway. Hypertension 2024, 81, 582–594. [Google Scholar] [CrossRef] [PubMed]
- Bordag, N.; Nagy, B.M.; Zügner, E.; Ludwig, H.; Foris, V.; Nagaraj, C.; Biasin, V.; Kovacs, G.; Kneidinger, N.; Bodenhofer, U.; et al. Lipid Ratios for Diagnosis and Prognosis of Pulmonary Hypertension. Am. J. Respir. Crit. Care Med. 2025, 211, 1264–1276. [Google Scholar] [CrossRef]
- Chen, S.; Liang, G.; Qu, Q.; Li, X. Metabolic Alterations Associated With Right Ventricular Dysfunction in Pulmonary Arterial Hypertension: The Modulatory Effects and Improvement Mechanisms of Exercise. Rev. Cardiovasc. Med. 2025, 26, 37460. [Google Scholar] [CrossRef]
- Glatz, J.F.C.; Luiken, J.J.F.P. From Fat to FAT (CD36/SR-B2): Understanding the Regulation of Cellular Fatty Acid Uptake. Biochimie 2017, 136, 21–26. [Google Scholar] [CrossRef]
- Glatz, J.C.; Luiken, J.F. Dynamic Role of the Transmembrane Glycoprotein CD36 (SR-B2) in Cellular Fatty Acid Uptake and Utilization. J. Lipid Res. 2018, 59, 1084–1093. [Google Scholar] [CrossRef]
- Lin, Y.; Li, B.; Shi, X.; Chen, Y.; Pan, S.; Lin, Z.; Gu, Z.; Hailer, F.; Hu, L.; Zhan, X. Homeostasis of Glucose and Lipid Metabolism during Physiological Responses to a Simulated Hypoxic High Altitude Environment. Nat. Commun. 2025, 16, 9406. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.; Tontonoz, P. Nonvesicular Cholesterol Transport in Physiology. J. Clin. Investig. 2025, 135, e188127. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Yu, T.; Wang, X.; Meng, K.; Wang, T.; Wang, B.; Xi, Y.; Wang, C.; Zeng, C.; Hu, S.; et al. Glutamine Deprivation Confers Immunotherapy Resistance by Inhibiting IFN-γ Signaling in Cancer Cells. Pharmacol. Res. 2025, 213, 107643. [Google Scholar] [CrossRef]
- Chen, X.; Li, L.; Deng, Y.; Liao, J.; Meng, H.; Liang, L.; Hu, J.; Xie, D.; Liang, G. Inhibition of Glutaminase 1 Reduces M1 Macrophage Polarization to Protect against Monocrotaline-Induced Pulmonary Arterial Hypertension. Immunol. Lett. 2025, 272, 106974. [Google Scholar] [CrossRef]
- Wang, S.; Yan, Y.; Xu, W.-J.; Gong, S.-G.; Zhong, X.-J.; An, Q.-Y.; Zhao, Y.-L.; Liu, J.-M.; Wang, L.; Yuan, P.; et al. The Role of Glutamine and Glutaminase in Pulmonary Hypertension. Front. Cardiovasc. Med. 2022, 9, 838657. [Google Scholar] [CrossRef]
- Durante, W. The Emerging Role of L-Glutamine in Cardiovascular Health and Disease. Nutrients 2019, 11, 2092. [Google Scholar] [CrossRef]
- Le, A.; Lane, A.N.; Hamaker, M.; Bose, S.; Gouw, A.; Barbi, J.; Tsukamoto, T.; Rojas, C.J.; Slusher, B.S.; Zhang, H.; et al. Glucose-Independent Glutamine Metabolism via TCA Cycling for Proliferation and Survival in B Cells. Cell Metab. 2012, 15, 110–121. [Google Scholar] [CrossRef]
- Yoon, H.; Shaw, J.L.; Haigis, M.C.; Greka, A. Lipid Metabolism in Sickness and in Health: Emerging Regulators of Lipotoxicity. Mol. Cell 2021, 81, 3708–3730. [Google Scholar] [CrossRef]
- Pietrocola, F.; Galluzzi, L.; Bravo-San Pedro, J.M.; Madeo, F.; Kroemer, G. Acetyl Coenzyme A: A Central Metabolite and Second Messenger. Cell Metab. 2015, 21, 805–821. [Google Scholar] [CrossRef]
- Geltinger, F.; Schartel, L.; Wiederstein, M.; Tevini, J.; Aigner, E.; Felder, T.K.; Rinnerthaler, M. Friend or Foe: Lipid Droplets as Organelles for Protein and Lipid Storage in Cellular Stress Response, Aging and Disease. Molecules 2020, 25, 5053. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Huang, X. Lipid Metabolism at Membrane Contacts: Dynamics and Functions Beyond Lipid Homeostasis. Front. Cell Dev. Biol. 2020, 8, 615856. [Google Scholar] [CrossRef] [PubMed]
- Benador, I.Y.; Veliova, M.; Liesa, M.; Shirihai, O.S. Mitochondria Bound to Lipid Droplets: Where Mitochondrial Dynamics Regulate Lipid Storage and Utilization. Cell Metab. 2019, 29, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Martin-Perez, M.; Urdiroz-Urricelqui, U.; Bigas, C.; Benitah, S.A. The Role of Lipids in Cancer Progression and Metastasis. Cell Metab. 2022, 34, 1675–1699. [Google Scholar] [CrossRef]
- Jiang, L.; Goncharov, D.A.; Shen, Y.; Lin, D.; Chang, B.; Pena, A.; DeLisser, H.; Goncharova, E.A.; Kudryashova, T.V. Akt-Dependent Glycolysis-Driven Lipogenesis Supports Proliferation and Survival of Human Pulmonary Arterial Smooth Muscle Cells in Pulmonary Hypertension. Front. Med. 2022, 9, 886868. [Google Scholar] [CrossRef]
- Grobs, Y.; Romanet, C.; Lemay, S.-E.; Bourgeois, A.; Voisine, P.; Theberge, C.; Sauvaget, M.; Breuils-Bonnet, S.; Martineau, S.; El Kabbout, R.; et al. ATP Citrate Lyase Drives Vascular Remodeling in Systemic and Pulmonary Vascular Diseases through Metabolic and Epigenetic Changes. Sci. Transl. Med. 2024, 16, eado7824. [Google Scholar] [CrossRef]
- Cohen, E.D.; Yee, M.; Porter, G.A.; Ritzer, E.; McDavid, A.N.; Brookes, P.S.; Pryhuber, G.S.; O’Reilly, M.A. Neonatal Hyperoxia Inhibits Proliferation and Survival of Atrial Cardiomyocytes by Suppressing Fatty Acid Synthesis. JCI Insight 2021, 6, e140785. [Google Scholar] [CrossRef]
- Singh, N.; Manhas, A.; Kaur, G.; Jagavelu, K.; Hanif, K. Inhibition of Fatty Acid Synthase Is Protective in Pulmonary Hypertension. Br. J. Pharmacol. 2016, 173, 2030–2045. [Google Scholar] [CrossRef]
- Singh, N.; Singh, H.; Jagavelu, K.; Wahajuddin, M.; Hanif, K. Fatty Acid Synthase Modulates Proliferation, Metabolic Functions and Angiogenesis in Hypoxic Pulmonary Artery Endothelial Cells. Eur. J. Pharmacol. 2017, 815, 462–469. [Google Scholar] [CrossRef]
- Hou, C.; Chen, J.; Zhao, Y.; Niu, Y.; Lin, S.; Chen, S.; Zong, Y.; Sun, X.; Xie, L.; Xiao, T. The Emerging Role of Fatty Acid Synthase in Hypoxia-Induced Pulmonary Hypertensive Mouse Energy Metabolism. Oxidative Med. Cell. Longev. 2021, 2021, 9990794. [Google Scholar] [CrossRef]
- Chen, C.; Qin, S.; Song, X.; Wen, J.; Huang, W.; Sheng, Z.; Li, X.; Cao, Y. PI3K P85α/HIF-1α Accelerates the Development of Pulmonary Arterial Hypertension by Regulating Fatty Acid Uptake and Mitophagy. Mol. Med. 2024, 30, 208. [Google Scholar] [CrossRef]
- Khan, T.J.; Semenkovich, C.F.; Zayed, M.A. De Novo Lipid Synthesis in Cardiovascular Tissue and Disease. Atherosclerosis 2025, 400, 119066. [Google Scholar] [CrossRef] [PubMed]
- Zuo, H.; Liu, X.; Wang, Y.; Ding, H.; Wan, W.; Zheng, S.; Hou, S.; Hu, K. SREBF1 Facilitates Pathological Retinal Neovascularization by Reprogramming the Fatty Acid Metabolism of Endothelial Cells. Exp. Eye Res. 2025, 252, 110239. [Google Scholar] [CrossRef] [PubMed]
- Dobrzyn, P.; Bednarski, T.; Dobrzyn, A. Metabolic Reprogramming of the Heart through Stearoyl-CoA Desaturase. Prog. Lipid Res. 2015, 57, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tabaczar, S.; Wołosiewicz, M.; Filip, A.; Olichwier, A.; Dobrzyń, P. The Role of Stearoyl-CoA Desaturase in the Regulation of Cardiac Metabolism. Postep. Biochem. 2018, 64, 183–189. [Google Scholar] [CrossRef]
- Gan, A.-M.; Tracz-Gaszewska, Z.; Ellert-Miklaszewska, A.; Navrulin, V.O.; Ntambi, J.M.; Dobrzyn, P. Stearoyl-CoA Desaturase Regulates Angiogenesis and Energy Metabolism in Ischemic Cardiomyocytes. Int. J. Mol. Sci. 2022, 23, 10459. [Google Scholar] [CrossRef]
- Abd Alla, J.; Jamous, Y.F.; Quitterer, U. Stearoyl-CoA Desaturase (SCD) Induces Cardiac Dysfunction with Cardiac Lipid Overload and Angiotensin II AT1 Receptor Protein Up-Regulation. Int. J. Mol. Sci. 2021, 22, 9883. [Google Scholar] [CrossRef]
- Dobrzyn, P.; Sampath, H.; Dobrzyn, A.; Miyazaki, M.; Ntambi, J.M. Loss of Stearoyl-CoA Desaturase 1 Inhibits Fatty Acid Oxidation and Increases Glucose Utilization in the Heart. Am. J. Physiol.-Endocrinol. Metab. 2008, 294, E357–E364. [Google Scholar] [CrossRef]
- Steinhauser, M.L.; Maron, B.A. Viewing Pulmonary Arterial Hypertension Pathogenesis and Opportunities for Disease-Modifying Therapy Through the Lens of Biomass. JACC Basic Transl. Sci. 2024, 9, 1252–1263. [Google Scholar] [CrossRef]
- Sutendra, G.; Michelakis, E.D. The Metabolic Basis of Pulmonary Arterial Hypertension. Cell Metab. 2014, 19, 558–573. [Google Scholar] [CrossRef]
- Bassareo, P.P.; D’Alto, M. Metabolomics in Pulmonary Hypertension-A Useful Tool to Provide Insights into the Dark Side of a Tricky Pathology. Int. J. Mol. Sci. 2023, 24, 13227. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Yang, Y.; Cheng, A.; Xu, G.; He, F. Metabolism of Vascular Smooth Muscle Cells in Vascular Diseases. Am. J. Physiol.-Heart Circ. Physiol. 2020, 319, H613–H631. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kumar, A.; Carmeliet, P. Metabolic Pathways Fueling the Endothelial Cell Drive. Annu. Rev. Physiol. 2019, 81, 483–503. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guan, X.; Zhu, X.; Zhang, L.; Ma, C.; He, S.; Bai, J.; Mei, J.; Li, Q.; Sun, N.; et al. CircNAP1L4 Regulates Pulmonary Artery Smooth Muscle Cell Proliferation via the NAP1L4-Mediated Super-Enhancer-Driven Glycolysis Gene Hexokinase II (HK II) in Pulmonary Hypertension. FASEB J. 2024, 38, e23868. [Google Scholar] [CrossRef]
- Luo, L.; Wu, J.; Lin, T.; Lian, G.; Wang, H.; Gao, G.; Xie, L. Influence of Atorvastatin on Metabolic Pattern of Rats with Pulmonary Hypertension. Aging 2021, 13, 11954–11968. [Google Scholar] [CrossRef]
- Dumas, S.J.; Bru-Mercier, G.; Courboulin, A.; Quatredeniers, M.; Rücker-Martin, C.; Antigny, F.; Nakhleh, M.K.; Ranchoux, B.; Gouadon, E.; Vinhas, M.-C.; et al. NMDA-Type Glutamate Receptor Activation Promotes Vascular Remodeling and Pulmonary Arterial Hypertension. Circulation 2018, 137, 2371–2389. [Google Scholar] [CrossRef]
- Chen, S.; Lin, S.; Liu, W.; Lin, Q.; Yang, Y.; Qiu, Q.; Zong, Y.; Xiao, T.; Hou, C.; Xie, L. Serum Metabolomic Profile in Hypoxia-Induced Pulmonary Hypertension Mice after C75 Treatment. Front. Biosci. 2023, 28, 251. [Google Scholar] [CrossRef]
- Egnatchik, R.A.; Brittain, E.L.; Shah, A.T.; Fares, W.H.; Ford, H.J.; Monahan, K.; Kang, C.J.; Kocurek, E.G.; Zhu, S.; Luong, T.; et al. Dysfunctional BMPR2 Signaling Drives an Abnormal Endothelial Requirement for Glutamine in Pulmonary Arterial Hypertension. Pulm. Circ. 2017, 7, 186–199. [Google Scholar] [CrossRef]
- Samovski, D.; Jacome-Sosa, M.; Abumrad, N.A. Fatty Acid Transport and Signaling: Mechanisms and Physiological Implications. Annu. Rev. Physiol. 2023, 85, 317–337. [Google Scholar] [CrossRef]
- Adu-Amankwaah, J.; You, Q.; Liu, X.; Jiang, J.; Yang, D.; Liu, K.; Yuan, J.; Wang, Y.; Hu, Q.; Tan, R. Pulmonary Hypertension: Molecular Mechanisms and Clinical Studies. MedComm 2025, 6, e70134. [Google Scholar] [CrossRef]
- Wei, Y.; Zhao, H.; Kalionis, B.; Huai, X.; Hu, X.; Wu, W.; Jiang, R.; Gong, S.; Wang, L.; Liu, J.; et al. The Impact of Abnormal Lipid Metabolism on the Occurrence Risk of Idiopathic Pulmonary Arterial Hypertension. Int. J. Mol. Sci. 2023, 24, 14280. [Google Scholar] [CrossRef]
- Talati, M.H.; Brittain, E.L.; Fessel, J.P.; Penner, N.; Atkinson, J.; Funke, M.; Grueter, C.; Jerome, W.G.; Freeman, M.; Newman, J.H.; et al. Mechanisms of Lipid Accumulation in the Bone Morphogenetic Protein Receptor Type 2 Mutant Right Ventricle. Am. J. Respir. Crit. Care Med. 2016, 194, 719–728. [Google Scholar] [CrossRef]
- Mistry, J.J.; Hellmich, C.; Moore, J.A.; Jibril, A.; Macaulay, I.; Moreno-Gonzalez, M.; Di Palma, F.; Beraza, N.; Bowles, K.M.; Rushworth, S.A. Free Fatty-Acid Transport via CD36 Drives β-Oxidation-Mediated Hematopoietic Stem Cell Response to Infection. Nat. Commun. 2021, 12, 7130. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Wu, F.; Chen, M.; Li, Y.; You, M.; Zhang, Y.; Yang, P.; Wei, L.; Ruan, X.Z.; Zhao, L.; et al. Inhibition of Fatty Acid Translocase (FAT/CD36) Palmitoylation Enhances Hepatic Fatty Acid β-Oxidation by Increasing Its Localization to Mitochondria and Interaction with Long-Chain Acyl-CoA Synthetase 1. Antioxid. Redox Signal. 2022, 36, 1081–1100. [Google Scholar] [CrossRef] [PubMed]
- Papathanasiou, A.E.; Spyropoulos, F.; Michael, Z.; Joung, K.E.; Briana, D.D.; Malamitsi-Puchner, A.; Mantzoros, C.S.; Christou, H. Adipokines and Metabolic Regulators in Human and Experimental Pulmonary Arterial Hypertension. Int. J. Mol. Sci. 2021, 22, 1435. [Google Scholar] [CrossRef] [PubMed]
- Talati, M.; Hemnes, A. Fatty Acid Metabolism in Pulmonary Arterial Hypertension: Role in Right Ventricular Dysfunction and Hypertrophy. Pulm. Circ. 2015, 5, 269–278. [Google Scholar] [CrossRef]
- Brittain, E.L.; Talati, M.; Fessel, J.P.; Zhu, H.; Penner, N.; Calcutt, M.W.; West, J.D.; Funke, M.; Lewis, G.D.; Gerszten, R.E.; et al. Fatty Acid Metabolic Defects and Right Ventricular Lipotoxicity in Human Pulmonary Arterial Hypertension. Circulation 2016, 133, 1936–1944. [Google Scholar] [CrossRef]
- Mansoor, M.; Ibrahim, A.F. Emerging Mechanistic Insights and Therapeutic Strategies for Pulmonary Arterial Hypertension: A Focus on Right Ventricular Dysfunction and Novel Treatment Pathways. Biomedicines 2025, 13, 600. [Google Scholar] [CrossRef]
- Caro, P.; Kishan, A.U.; Norberg, E.; Stanley, I.A.; Chapuy, B.; Ficarro, S.B.; Polak, K.; Tondera, D.; Gounarides, J.; Yin, H.; et al. Metabolic Signatures Uncover Distinct Targets in Molecular Subsets of Diffuse Large B Cell Lymphoma. Cancer Cell 2012, 22, 547–560. [Google Scholar] [CrossRef]
- Hemnes, A.R.; Fessel, J.P.; Chen, X.; Zhu, S.; Fortune, N.L.; Jetter, C.; Freeman, M.; Newman, J.H.; West, J.D.; Talati, M.H. BMPR2 Dysfunction Impairs Insulin Signaling and Glucose Homeostasis in Cardiomyocytes. Am. J. Physiol. Lung Cell Mol. Physiol. 2020, 318, L429–L441. [Google Scholar] [CrossRef]
- Kazmirczak, F.; Vogel, N.T.; Prisco, S.Z.; Patterson, M.T.; Annis, J.; Moon, R.T.; Hartweck, L.M.; Mendelson, J.B.; Kim, M.; Mancipe, N.C.; et al. Ferroptosis Integrates Mitochondrial Derangements and Pathological Inflammation to Promote Pulmonary Hypertension. bioRxiv 2024. [Google Scholar] [CrossRef]
- Piao, L.; Fang, Y.-H.; Parikh, K.; Ryan, J.J.; Toth, P.T.; Archer, S.L. Cardiac Glutaminolysis: A Maladaptive Cancer Metabolism Pathway in the Right Ventricle in Pulmonary Hypertension. J. Mol. Med. 2013, 91, 1185–1197. [Google Scholar] [CrossRef] [PubMed]
- Drake, J.I.; Bogaard, H.J.; Mizuno, S.; Clifton, B.; Xie, B.; Gao, Y.; Dumur, C.I.; Fawcett, P.; Voelkel, N.F.; Natarajan, R. Molecular Signature of a Right Heart Failure Program in Chronic Severe Pulmonary Hypertension. Am. J. Respir. Cell Mol. Biol. 2011, 45, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Piao, L.; Fang, Y.-H.; Cadete, V.J.J.; Wietholt, C.; Urboniene, D.; Toth, P.T.; Marsboom, G.; Zhang, H.J.; Haber, I.; Rehman, J.; et al. The Inhibition of Pyruvate Dehydrogenase Kinase Improves Impaired Cardiac Function and Electrical Remodeling in Two Models of Right Ventricular Hypertrophy: Resuscitating the Hibernating Right Ventricle. J. Mol. Med. 2010, 88, 47–60. [Google Scholar] [CrossRef]
- Sutendra, G.; Dromparis, P.; Paulin, R.; Zervopoulos, S.; Haromy, A.; Nagendran, J.; Michelakis, E.D. A Metabolic Remodeling in Right Ventricular Hypertrophy Is Associated with Decreased Angiogenesis and a Transition from a Compensated to a Decompensated State in Pulmonary Hypertension. J. Mol. Med. 2013, 91, 1315–1327. [Google Scholar] [CrossRef]
- Paulin, R.; Sutendra, G.; Gurtu, V.; Dromparis, P.; Haromy, A.; Provencher, S.; Bonnet, S.; Michelakis, E.D. A miR-208-Mef2 Axis Drives the Decompensation of Right Ventricular Function in Pulmonary Hypertension. Circ. Res. 2015, 116, 56–69. [Google Scholar] [CrossRef]
- Qiu, H.; Chen, J.; Mei, Z.; Chen, W.; Jun, L.; Chen, Y.; Tan, Y.; Wang, T.; Chen, Y.; Li, J. Dysregulated Fatty Acid Metabolism in Pericardiac Adipose Tissue of Pulmonary Hypertension Due to Left Heart Disease Mice. FASEB J. 2025, 39, e70355. [Google Scholar] [CrossRef]
- Ohira, H.; deKemp, R.; Pena, E.; Davies, R.A.; Stewart, D.J.; Chandy, G.; Contreras-Dominguez, V.; Dennie, C.; Mc Ardle, B.; Mc Klein, R.; et al. Shifts in Myocardial Fatty Acid and Glucose Metabolism in Pulmonary Arterial Hypertension: A Potential Mechanism for a Maladaptive Right Ventricular Response. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1424–1431. [Google Scholar] [CrossRef]
- Nagaya, N.; Goto, Y.; Satoh, T.; Uematsu, M.; Hamada, S.; Kuribayashi, S.; Okano, Y.; Kyotani, S.; Shimotsu, Y.; Fukuchi, K.; et al. Impaired Regional Fatty Acid Uptake and Systolic Dysfunction in Hypertrophied Right Ventricle. J. Nucl. Med. 1998, 39, 1676–1680. [Google Scholar]
- Sakao, S.; Miyauchi, H.; Voelkel, N.F.; Sugiura, T.; Tanabe, N.; Kobayashi, Y.; Tatsumi, K. Increased Right Ventricular Fatty Acid Accumulation in Chronic Thromboembolic Pulmonary Hypertension. Ann. Am. Thorac. Soc. 2015, 12, 1465–1472. [Google Scholar] [CrossRef]
- Salagre, D.; Bajit, H.; Fernández-Vázquez, G.; Dwairy, M.; Garzón, I.; Haro-López, R.; Agil, A. Melatonin Induces Fiber Switching by Improvement of Mitochondrial Oxidative Capacity and Function via NRF2/RCAN/MEF2 in the Vastus Lateralis Muscle from Both Sex Zücker Diabetic Fatty Rats. Free Radic. Biol. Med. 2025, 227, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Niu, Y.; Liu, X.; Fu, L. Exercise Increases the Binding of MEF2A to the Cpt1b Promoter in Mouse Skeletal Muscle. Acta Physiol. 2014, 212, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Hong, J.; Umar, S. Comparative Analysis of Right Ventricular Metabolic Reprogramming in Pre-Clinical Rat Models of Severe Pulmonary Hypertension-Induced Right Ventricular Failure. Front. Cardiovasc. Med. 2022, 9, 935423. [Google Scholar] [CrossRef] [PubMed]
- Mendelson, J.B.; Sternbach, J.D.; Doyle, M.J.; Mills, L.; Hartweck, L.M.; Tollison, W.; Carney, J.P.; Lahti, M.T.; Bianco, R.W.; Kalra, R.; et al. Multi-Omic and Multispecies Analysis of Right Ventricular Dysfunction. J. Heart Lung Transplant. 2024, 43, 303–313. [Google Scholar] [CrossRef]
- Khassafi, F.; Chelladurai, P.; Valasarajan, C.; Nayakanti, S.R.; Martineau, S.; Sommer, N.; Yokokawa, T.; Boucherat, O.; Kamal, A.; Kiely, D.G.; et al. Transcriptional Profiling Unveils Molecular Subgroups of Adaptive and Maladaptive Right Ventricular Remodeling in Pulmonary Hypertension. Nat. Cardiovasc. Res. 2023, 2, 917–936. [Google Scholar] [CrossRef]
- Luptak, I.; Balschi, J.A.; Xing, Y.; Leone, T.C.; Kelly, D.P.; Tian, R. Decreased Contractile and Metabolic Reserve in Peroxisome Proliferator-Activated Receptor-Alpha-Null Hearts Can Be Rescued by Increasing Glucose Transport and Utilization. Circulation 2005, 112, 2339–2346. [Google Scholar] [CrossRef]
- Liu, J.; Wang, P.; He, L.; Li, Y.; Luo, J.; Cheng, L.; Qin, Q.; Brako, L.A.; Lo, W.-K.; Lewis, W.; et al. Cardiomyocyte-Restricted Deletion of PPARβ/δ in PPARα-Null Mice Causes Impaired Mitochondrial Biogenesis and Defense, but No Further Depression of Myocardial Fatty Acid Oxidation. PPAR Res. 2011, 2011, 372854. [Google Scholar] [CrossRef]
- Legchenko, E.; Chouvarine, P.; Borchert, P.; Fernandez-Gonzalez, A.; Snay, E.; Meier, M.; Maegel, L.; Mitsialis, S.A.; Rog-Zielinska, E.A.; Kourembanas, S.; et al. PPARγ Agonist Pioglitazone Reverses Pulmonary Hypertension and Prevents Right Heart Failure via Fatty Acid Oxidation. Sci. Transl. Med. 2018, 10, eaao0303. [Google Scholar] [CrossRef]
- Tao, B.; Kumar, S.; Gomez-Arroyo, J.; Fan, C.; Zhang, A.; Skinner, J.; Hunter, E.; Yamaji-Kegan, K.; Samad, I.; Hillel, A.T.; et al. Resistin-Like Molecule α Dysregulates Cardiac Bioenergetics in Neonatal Rat Cardiomyocytes. Front. Cardiovasc. Med. 2021, 8, 574708. [Google Scholar] [CrossRef]
- Hemnes, A.R.; Brittain, E.L.; Trammell, A.W.; Fessel, J.P.; Austin, E.D.; Penner, N.; Maynard, K.B.; Gleaves, L.; Talati, M.; Absi, T.; et al. Evidence for Right Ventricular Lipotoxicity in Heritable Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 2014, 189, 325–334. [Google Scholar] [CrossRef]
- Graham, B.B.; Kumar, R.; Mickael, C.; Sanders, L.; Gebreab, L.; Huber, K.M.; Perez, M.; Smith-Jones, P.; Serkova, N.J.; Tuder, R.M. Severe Pulmonary Hypertension Is Associated with Altered Right Ventricle Metabolic Substrate Uptake. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2015, 309, L435–L440. [Google Scholar] [CrossRef]
- Prisco, S.Z.; Thenappan, T.; Prins, K.W. Treatment Targets for Right Ventricular Dysfunction in Pulmonary Arterial Hypertension. JACC Basic Transl. Sci. 2020, 5, 1244–1260. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, V.; Hemnes, A.R.; Shelburne, N.J.; Fortune, N.; Fuentes, J.L.; Colvin, D.; Calcutt, M.W.; Talati, M.; Poovey, E.; West, J.D.; et al. L-Carnitine Therapy Improves Right Heart Dysfunction through Cpt1-Dependent Fatty Acid Oxidation. Pulm. Circ. 2022, 12, e12107. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, K.; Grand, R.J.; Horan, I.; Certo, M.; Keeler, R.C.; Mauro, C.; Tseng, C.-C.; Greig, I.; Morrell, N.W.; Zanda, M.; et al. Fluorinated Perhexiline Derivative Attenuates Vascular Proliferation in Pulmonary Arterial Hypertension Smooth Muscle Cells. Vasc. Pharmacol. 2024, 156, 107399. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, W.; Lian, G.; Huang, B.; Du, A.; Gong, J.; Xiao, G.; Xu, C.; Wang, H.; Xie, L. CPT1 Regulates the Proliferation of Pulmonary Artery Smooth Muscle Cells through the AMPK-P53-P21 Pathway in Pulmonary Arterial Hypertension. Mol. Cell Biochem. 2019, 455, 169–183. [Google Scholar] [CrossRef]
- Sutendra, G.; Bonnet, S.; Rochefort, G.; Haromy, A.; Folmes, K.D.; Lopaschuk, G.D.; Dyck, J.R.B.; Michelakis, E.D. Fatty Acid Oxidation and Malonyl-CoA Decarboxylase in the Vascular Remodeling of Pulmonary Hypertension. Sci. Transl. Med. 2010, 2, 44ra58. [Google Scholar] [CrossRef]
- Lee, M.H.; Sanders, L.; Kumar, R.; Hernandez-Saavedra, D.; Yun, X.; Ford, J.A.; Perez, M.J.; Mickael, C.; Gandjeva, A.; Koyanagi, D.E.; et al. Contribution of Fatty Acid Oxidation to the Pathogenesis of Pulmonary Hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 2022, 323, L355–L371. [Google Scholar] [CrossRef]
- Randle, P.J.; Garland, P.B.; Hales, C.N.; Newsholme, E.A. The Glucose Fatty-Acid Cycle. Its Role in Insulin Sensitivity and the Metabolic Disturbances of Diabetes Mellitus. Lancet 1963, 1, 785–789. [Google Scholar] [CrossRef]
- Fang, Y.-H.; Piao, L.; Hong, Z.; Toth, P.T.; Marsboom, G.; Bache-Wiig, P.; Rehman, J.; Archer, S.L. Therapeutic Inhibition of Fatty Acid Oxidation in Right Ventricular Hypertrophy: Exploiting Randle’s Cycle. J. Mol. Med. 2012, 90, 31–43. [Google Scholar] [CrossRef]
- Han, Y.; Forfia, P.; Vaidya, A.; Mazurek, J.A.; Park, M.H.; Ramani, G.; Chan, S.Y.; Waxman, A.B. Ranolazine Improves Right Ventricular Function in Patients With Precapillary Pulmonary Hypertension: Results From a Double-Blind, Randomized, Placebo-Controlled Trial. J. Card. Fail. 2021, 27, 253–257. [Google Scholar] [CrossRef]
- Yanagisawa, A.; Kim, J.-D.; Naito, A.; Kobayashi, T.; Misawa, T.; Sakao, S.; Jujo-Sanada, T.; Kawasaki, T.; Muroi, S.; Sasaki, S.-I.; et al. Deciphering the Inhibitory Effects of Trimetazidine on Pulmonary Hypertension Development via Decreasing Fatty Acid Oxidation and Promoting Glucose Oxidation. Sci. Rep. 2024, 14, 27069. [Google Scholar] [CrossRef] [PubMed]
- Parra, V.; Bravo-Sagua, R.; Norambuena-Soto, I.; Hernández-Fuentes, C.P.; Gómez-Contreras, A.G.; Verdejo, H.E.; Mellado, R.; Chiong, M.; Lavandero, S.; Castro, P.F. Inhibition of Mitochondrial Fission Prevents Hypoxia-Induced Metabolic Shift and Cellular Proliferation of Pulmonary Arterial Smooth Muscle Cells. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 2891–2903. [Google Scholar] [CrossRef] [PubMed]
- Jamil, M.; Cowart, L.A. Sphingolipids in Mitochondria—From Function to Disease. Front. Cell Dev. Biol. 2023, 11, 1302472. [Google Scholar] [CrossRef]
- Gluschke, H.; Siegert, E.; Minich, W.B.; Hackler, J.; Riemekasten, G.; Kuebler, W.M.; Simmons, S.; Schomburg, L. Autoimmunity to Sphingosine-1-Phosphate-Receptors in Systemic Sclerosis and Pulmonary Arterial Hypertension. Front. Immunol. 2022, 13, 935787. [Google Scholar] [CrossRef]
- Ebenezer, D.L.; Fu, P.; Natarajan, V. Targeting Sphingosine-1-Phosphate Signaling in Lung Diseases. Pharmacol. Ther. 2016, 168, 143–157. [Google Scholar] [CrossRef]
- Yan, X.; Wang, J.; Zhu, Y.; Feng, W.; Zhai, C.; Liu, L.; Shi, W.; Wang, Q.; Zhang, Q.; Chai, L.; et al. S1P Induces Pulmonary Artery Smooth Muscle Cell Proliferation by Activating Calcineurin/NFAT/OPN Signaling Pathway. Biochem. Biophys. Res. Commun. 2019, 516, 921–927. [Google Scholar] [CrossRef]
- Chen, J.; Tang, H.; Sysol, J.R.; Moreno-Vinasco, L.; Shioura, K.M.; Chen, T.; Gorshkova, I.; Wang, L.; Huang, L.S.; Usatyuk, P.V.; et al. The Sphingosine Kinase 1/Sphingosine-1-Phosphate Pathway in Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 2014, 190, 1032–1043. [Google Scholar] [CrossRef]
- Ranasinghe, A.D.C.U.; Lee, D.D.; Schwarz, M.A. Mechanistic Regulation of SPHK1 Expression and Translocation by EMAP II in Pulmonary Smooth Muscle Cells. Biochim. Biophys. Acta BBA-Mol. Cell Biol. Lipids 2020, 1865, 158789. [Google Scholar] [CrossRef]
- Chen, J.; Lockett, A.; Zhao, S.; Huang, L.S.; Wang, Y.; Wu, W.; Tang, M.; Haider, S.; Velez Rendon, D.; Khan, R.; et al. Sphingosine Kinase 1 Deficiency in Smooth Muscle Cells Protects against Hypoxia-Mediated Pulmonary Hypertension via YAP1 Signaling. Int. J. Mol. Sci. 2022, 23, 14516. [Google Scholar] [CrossRef]
- Shi, W.; Wang, Q.; Wang, J.; Yan, X.; Feng, W.; Zhang, Q.; Zhai, C.; Chai, L.; Li, S.; Xie, X.; et al. Activation of Yes-Associated Protein Mediates Sphingosine-1-Phosphate-Induced Proliferation and Migration of Pulmonary Artery Smooth Muscle Cells and Its Potential Mechanisms. J. Cell Physiol. 2021, 236, 4694–4708. [Google Scholar] [CrossRef]
- Lee, D.D.; Hochstetler, A.; Murphy, C.; Lowe, C.-W.; Schwarz, M.A. A Distinct Transcriptional Profile in Response to Endothelial Monocyte Activating Polypeptide II Is Partially Mediated by JAK-STAT3 in Murine Macrophages. Am. J. Physiol. Cell Physiol. 2019, 317, C449–C456. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, D.; Zhang, L.; Ye, F.; Li, M.; Wen, K. Role of JAK-STAT Pathway in Reducing Cardiomyocytes Hypoxia/Reoxygenation Injury Induced by S1P Postconditioning. Eur. J. Pharmacol. 2016, 784, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhou, C.; Zhao, K.; Duan, Y.; Yue, J.; Liu, X.; Wu, J.; Deng, S. Lymphatic Endothelial Sphingosine 1-Phosphate Receptor 1 Enhances Macrophage Clearance via Lymphatic System Following Myocardial Infarction. Front. Cardiovasc. Med. 2022, 9, 872102. [Google Scholar] [CrossRef]
- Mohammed, S.; Harikumar, K.B. Sphingosine 1-Phosphate: A Novel Target for Lung Disorders. Front. Immunol. 2017, 8, 296. [Google Scholar] [CrossRef]
- Zhai, C.; Feng, W.; Shi, W.; Wang, J.; Zhang, Q.; Yan, X.; Wang, Q.; Li, S.; Liu, L.; Pan, Y.; et al. Sphingosine-1-Phosphate Promotes Pulmonary Artery Smooth Muscle Cells Proliferation by Stimulating Autophagy-Mediated E-Cadherin/CDH1 down-Regulation. Eur. J. Pharmacol. 2020, 884, 173302. [Google Scholar] [CrossRef]
- Gairhe, S.; Joshi, S.R.; Bastola, M.M.; McLendon, J.M.; Oka, M.; Fagan, K.A.; McMurtry, I.F. Sphingosine-1-Phosphate Is Involved in the Occlusive Arteriopathy of Pulmonary Arterial Hypertension. Pulm. Circ. 2016, 6, 369–380. [Google Scholar] [CrossRef]
- Mitra, A.; Yi, D.; Dai, Z.; de Jesus Perez, V. Unraveling the Role of HIF and Epigenetic Regulation in Pulmonary Arterial Hypertension: Implications for Clinical Research and Its Therapeutic Approach. Front. Med. 2024, 11, 1460376. [Google Scholar] [CrossRef]
- Ranasinghe, A.D.C.U.; Schwarz, M.A. Integrating Epigenetics and Metabolomics to Advance Treatments for Pulmonary Arterial Hypertension. Biochem. Pharmacol. 2022, 204, 115245. [Google Scholar] [CrossRef]
- Rafikov, R.; de Jesus Perez, V.; Dekan, A.; Kudryashova, T.V.; Rafikova, O. Deciphering the Complexities of Pulmonary Hypertension: The Emergent Role of Single-Cell Omics. Am. J. Respir. Cell Mol. Biol. 2024, 72, 32–40. [Google Scholar] [CrossRef]
- Ranasinghe, A.D.C.U.; Holohan, M.; Borger, K.M.; Donahue, D.L.; Kuc, R.D.; Gerig, M.; Kim, A.; Ploplis, V.A.; Castellino, F.J.; Schwarz, M.A. Altered Smooth Muscle Cell Histone Acetylome by the SPHK2/S1P Axis Promotes Pulmonary Hypertension. Circ. Res. 2023, 133, 704–719. [Google Scholar] [CrossRef]
- Ranasinghe, A.D.C.U.; Tennakoon, T.M.P.B.; Schwarz, M.A. Emerging Epigenetic Targets and Their Molecular Impact on Vascular Remodeling in Pulmonary Hypertension. Cells 2024, 13, 244. [Google Scholar] [CrossRef]
- Jiang, P.; Huang, H.; Xie, M.; Liu, Z.; Jiang, L.; Shi, H.; Wu, X.; Hao, S.; Li, S. Single-Cell Characterization of the Immune Heterogeneity of Pulmonary Hypertension Identifies Novel Targets for Immunotherapy. BMC Immunol. 2025, 26, 5. [Google Scholar] [CrossRef] [PubMed]
- Ranchoux, B.; Antigny, F.; Rucker-Martin, C.; Hautefort, A.; Péchoux, C.; Bogaard, H.J.; Dorfmüller, P.; Remy, S.; Lecerf, F.; Planté, S.; et al. Endothelial-to-Mesenchymal Transition in Pulmonary Hypertension. Circulation 2015, 131, 1006–1018. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Gong, J.; Dennery, P.A.; Yao, H. Endothelial-to-Mesenchymal Transition: Pathogenesis and Therapeutic Targets for Chronic Pulmonary and Vascular Diseases. Biochem. Pharmacol. 2019, 168, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Sakao, S.; Taraseviciene-Stewart, L.; Lee, J.D.; Wood, K.; Cool, C.D.; Voelkel, N.F. Initial Apoptosis Is Followed by Increased Proliferation of Apoptosis-Resistant Endothelial Cells. FASEB J. 2005, 19, 1178–1180. [Google Scholar] [CrossRef]
- Liu, B.; Yi, D.; Li, S.; Ramirez, K.; Xia, X.; Cao, Y.; Zhao, H.; Tripathi, A.; Qiu, S.; Kala, M.; et al. Single-Cell and Spatial Transcriptomics Identified Fatty Acid-Binding Proteins Controlling Endothelial Glycolytic and Arterial Programming in Pulmonary Hypertension. bioRxiv 2024. [Google Scholar] [CrossRef]
- Philip, N.; Yun, X.; Pi, H.; Murray, S.; Hill, Z.; Fonticella, J.; Perez, P.; Zhang, C.; Pathmasiri, W.; Sumner, S.; et al. Fatty Acid Metabolism Promotes TRPV4 Activity in Lung Microvascular Endothelial Cells in Pulmonary Arterial Hypertension. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2024, 326, L252–L265. [Google Scholar] [CrossRef]
- Fessel, J.P.; Hamid, R.; Wittmann, B.M.; Robinson, L.J.; Blackwell, T.; Tada, Y.; Tanabe, N.; Tatsumi, K.; Hemnes, A.R.; West, J.D. Metabolomic Analysis of Bone Morphogenetic Protein Receptor Type 2 Mutations in Human Pulmonary Endothelium Reveals Widespread Metabolic Reprogramming. Pulm. Circ. 2012, 2, 201–213. [Google Scholar] [CrossRef]
- Hernandez-Saavedra, D.; Sanders, L.; Freeman, S.; Reisz, J.A.; Lee, M.H.; Mickael, C.; Kumar, R.; Kassa, B.; Gu, S.; Alessandro, A.D.; et al. Stable Isotope Metabolomics of Pulmonary Artery Smooth Muscle and Endothelial Cells in Pulmonary Hypertension and with TGF-Beta Treatment. Sci. Rep. 2020, 10, 413. [Google Scholar] [CrossRef]
- Xiong, J.; Kawagishi, H.; Yan, Y.; Liu, J.; Wells, Q.; Edmunds, L.R.; Fergusson, M.M.; Yu, Z.-X.; Rovira, I.I.; Brittain, E.; et al. A Metabolic Basis for Endothelial-to-Mesenchymal Transition. Mol. Cell 2018, 69, 689–698.e7. [Google Scholar] [CrossRef]
- Ferrian, S.; Cao, A.; McCaffrey, E.F.; Saito, T.; Greenwald, N.F.; Nicolls, M.R.; Bruce, T.; Zamanian, R.T.; Del Rosario, P.; Rabinovitch, M.; et al. Single-Cell Imaging Maps Inflammatory Cell Subsets to Pulmonary Arterial Hypertension Vasculopathy. Am. J. Respir. Crit. Care Med. 2024, 209, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Cober, N.D.; McCourt, E.; Godoy, R.S.; Deng, Y.; Schlosser, K.; Qamsari, E.S.; Azami, J.; Salehisiavashani, E.; Cook, D.P.; Lemay, S.-E.; et al. Mapping Disease-Specific Vascular Cell Populations Responsible for Obliterative Arterial Remodelling during the Development of Pulmonary Arterial Hypertension. Cardiovasc. Res. 2025, 121, 2095–2112. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Cao, Y.; Qin, J.; Chen, Z.; Hu, G.; Li, Q. Pulmonary Artery Smooth Muscle Cell Phenotypic Switching: A Key Event in the Early Stage of Pulmonary Artery Hypertension. Drug Discov. Today 2023, 28, 103559. [Google Scholar] [CrossRef] [PubMed]
- Sunaga, H.; Matsui, H.; Anjo, S.; Syamsunarno, M.R.A.A.; Koitabashi, N.; Iso, T.; Matsuzaka, T.; Shimano, H.; Yokoyama, T.; Kurabayashi, M. Elongation of Long-Chain Fatty Acid Family Member 6 (Elovl6)-Driven Fatty Acid Metabolism Regulates Vascular Smooth Muscle Cell Phenotype Through AMP-Activated Protein Kinase/Krüppel-Like Factor 4 (AMPK/KLF4) Signaling. J. Am. Heart Assoc. 2016, 5, e004014. [Google Scholar] [CrossRef]
- Cao, K.; Zhang, T.; Li, Z.; Song, M.; Li, A.; Yan, J.; Guo, S.; Wang, L.; Huang, S.; Li, Z.; et al. Glycolysis and de Novo Fatty Acid Synthesis Cooperatively Regulate Pathological Vascular Smooth Muscle Cell Phenotypic Switching and Neointimal Hyperplasia. J. Pathol. 2023, 259, 388–401. [Google Scholar] [CrossRef]
- James, J.; Zemskova, M.; Eccles, C.A.; Varghese, M.V.; Niihori, M.; Barker, N.K.; Luo, M.; Mandarino, L.J.; Langlais, P.R.; Rafikova, O.; et al. Single Mutation in the NFU1 Gene Metabolically Reprograms Pulmonary Artery Smooth Muscle Cells. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 734–754. [Google Scholar] [CrossRef]
- Viola, A.; Munari, F.; Sánchez-Rodríguez, R.; Scolaro, T.; Castegna, A. The Metabolic Signature of Macrophage Responses. Front. Immunol. 2019, 10, 1462. [Google Scholar] [CrossRef]
- Kazmirczak, F.; Vogel, N.T.; Prisco, S.Z.; Patterson, M.T.; Annis, J.; Moon, R.T.; Hartweck, L.M.; Mendelson, J.B.; Kim, M.; Calixto Mancipe, N.; et al. Ferroptosis-Mediated Inflammation Promotes Pulmonary Hypertension. Circ. Res. 2024, 135, 1067–1083. [Google Scholar] [CrossRef]
- Posokhova, E.N.; Khoshchenko, O.M.; Chasovskikh, M.I.; Pivovarova, E.N.; Dushkin, M.I. Lipid Synthesis in Macrophages during Inflammation in Vivo: Effect of Agonists of Peroxisome Proliferator Activated Receptors Alpha and Gamma and of Retinoid X Receptors. Biochemistry 2008, 73, 296–304. [Google Scholar] [CrossRef]
- Jha, A.K.; Huang, S.C.-C.; Sergushichev, A.; Lampropoulou, V.; Ivanova, Y.; Loginicheva, E.; Chmielewski, K.; Stewart, K.M.; Ashall, J.; Everts, B.; et al. Network Integration of Parallel Metabolic and Transcriptional Data Reveals Metabolic Modules That Regulate Macrophage Polarization. Immunity 2015, 42, 419–430. [Google Scholar] [CrossRef]
- Odegaard, J.I.; Ricardo-Gonzalez, R.R.; Goforth, M.H.; Morel, C.R.; Subramanian, V.; Mukundan, L.; Red Eagle, A.; Vats, D.; Brombacher, F.; Ferrante, A.W.; et al. Macrophage-Specific PPARgamma Controls Alternative Activation and Improves Insulin Resistance. Nature 2007, 447, 1116–1120. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Zhang, Y.; Li, Z.; Jiang, P.; Guo, S.; He, Y.; Guo, Y. Donepezil Ameliorates Pulmonary Arterial Hypertension by Inhibiting M2-Macrophage Activation. Front. Cardiovasc. Med. 2021, 8, 639541. [Google Scholar] [CrossRef] [PubMed]
- Marsh, L.M.; Jandl, K.; Grünig, G.; Foris, V.; Bashir, M.; Ghanim, B.; Klepetko, W.; Olschewski, H.; Olschewski, A.; Kwapiszewska, G. The Inflammatory Cell Landscape in the Lungs of Patients with Idiopathic Pulmonary Arterial Hypertension. Eur. Respir. J. 2018, 51, 1701214. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Shafiq, M.; Jagavelu, K.; Hanif, K. Involvement of Fatty Acid Synthase in Right Ventricle Dysfunction in Pulmonary Hypertension. Exp. Cell Res. 2019, 383, 111569. [Google Scholar] [CrossRef]
- Brittain, E.L.; Niswender, K.; Agrawal, V.; Chen, X.; Fan, R.; Pugh, M.E.; Rice, T.W.; Robbins, I.M.; Song, H.; Thompson, C.; et al. Mechanistic Phase II Clinical Trial of Metformin in Pulmonary Arterial Hypertension. J. Am. Heart Assoc. 2020, 9, e018349. [Google Scholar] [CrossRef]
- Noordali, H.; Loudon, B.L.; Frenneaux, M.P.; Madhani, M. Cardiac Metabolism—A Promising Therapeutic Target for Heart Failure. Pharmacol. Ther. 2018, 182, 95–114. [Google Scholar] [CrossRef]
- Sharma, A.; Roy, T.; Bhattacharya, P.; Agarwal, D.; Kumar, R.; Naqvi, S.M.H.; Mittal, R. Stable Angina Pectoris: A Review of Pathophysiology, Diagnosis, and Its Management. J. Assoc. Physicians India 2024, 72, 92–97. [Google Scholar] [CrossRef]
- Wu, S.; Chang, G.; Gao, L.; Jiang, D.; Wang, L.; Li, G.; Luo, X.; Qin, S.; Guo, X.; Zhang, D. Trimetazidine Protects against Myocardial Ischemia/Reperfusion Injury by Inhibiting Excessive Autophagy. J. Mol. Med. 2018, 96, 791–806. [Google Scholar] [CrossRef]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the Diagnosis and Management of Chronic Coronary Syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef]
- Shu, H.; Peng, Y.; Hang, W.; Zhou, N.; Wang, D.W. Trimetazidine in Heart Failure. Front. Pharmacol. 2020, 11, 569132. [Google Scholar] [CrossRef]
- Verdejo, H.E.; Rojas, A.; López-Crisosto, C.; Baraona, F.; Gabrielli, L.; Maracaja-Coutinho, V.; Chiong, M.; Lavandero, S.; Castro, P.F. Effects of Trimetazidine on Right Ventricular Function and Ventricular Remodeling in Patients with Pulmonary Artery Hypertension: A Randomised Controlled Trial. J. Clin. Med. 2023, 12, 1571. [Google Scholar] [CrossRef] [PubMed]
- Rocchetti, M.; Sala, L.; Rizzetto, R.; Staszewsky, L.I.; Alemanni, M.; Zambelli, V.; Russo, I.; Barile, L.; Cornaghi, L.; Altomare, C.; et al. Ranolazine Prevents INaL Enhancement and Blunts Myocardial Remodelling in a Model of Pulmonary Hypertension. Cardiovasc. Res. 2014, 104, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Teixeira-Fonseca, J.L.; de Lima Conceição, M.R.; Leal-Silva, P.; Roman-Campos, D. Ranolazine Exerts Atrial Antiarrhythmic Effects in a Rat Model of Monocrotaline-Induced Pulmonary Hypertension. Basic Clin. Pharmacol. Toxicol. 2023, 132, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.S.; Cuttica, M.J.; Beussink-Nelson, L.; Kozyleva, A.; Sanchez, C.; Mkrdichian, H.; Selvaraj, S.; Dematte, J.E.; Lee, D.C.; Shah, S.J. Effects of Ranolazine on Exercise Capacity, Right Ventricular Indices, and Hemodynamic Characteristics in Pulmonary Arterial Hypertension: A Pilot Study. Pulm. Circ. 2015, 5, 547–556. [Google Scholar] [CrossRef]
- Gomberg-Maitland, M.; Schilz, R.; Mediratta, A.; Addetia, K.; Coslet, S.; Thomeas, V.; Gillies, H.; Oudiz, R.J. Phase I Safety Study of Ranolazine in Pulmonary Arterial Hypertension. Pulm. Circ. 2015, 5, 691–700. [Google Scholar] [CrossRef]
- Han, Y.; Forfia, P.R.; Vaidya, A.; Mazurek, J.A.; Park, M.H.; Ramani, G.; Chan, S.Y.; Waxman, A.B. Rationale and Design of the Ranolazine PH-RV Study: A Multicentred Randomised and Placebo-Controlled Study of Ranolazine to Improve RV Function in Patients with Non-Group 2 Pulmonary Hypertension. Open Heart 2018, 5, e000736. [Google Scholar] [CrossRef]
- Calvier, L.; Boucher, P.; Herz, J.; Hansmann, G. LRP1 Deficiency in Vascular SMC Leads to Pulmonary Arterial Hypertension That Is Reversed by PPARγ Activation. Circ. Res. 2019, 124, 1778–1785. [Google Scholar] [CrossRef]
- Calvier, L.; Chouvarine, P.; Legchenko, E.; Hoffmann, N.; Geldner, J.; Borchert, P.; Jonigk, D.; Mozes, M.M.; Hansmann, G. PPARγ Links BMP2 and TGFβ1 Pathways in Vascular Smooth Muscle Cells, Regulating Cell Proliferation and Glucose Metabolism. Cell Metab. 2017, 25, 1118–1134.e7. [Google Scholar] [CrossRef]
- Zhang, Z.; Liang, Y.; Mo, S.; Zhao, M.; Li, Y.; Zhang, C.; Shan, X.; Liu, S.; Liao, J.; Luo, X.; et al. Oral Administration of Pioglitazone Inhibits Pulmonary Hypertension by Regulating the Gut Microbiome and Plasma Metabolome in Male Rats. Physiol. Rep. 2024, 13, e70174. [Google Scholar] [CrossRef]
- Hansmann, G.; Calvier, L.; Risbano, M.G.; Chan, S.Y. Activation of the Metabolic Master Regulator PPARγ: A Potential PIOneering Therapy for Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 2020, 62, 143–156. [Google Scholar] [CrossRef]
- Harbaum, L.; Klose, H.; Wilkins, M.R. From Blood to Vessel: Lipid Ratios in Pulmonary Hypertension. Am. J. Respir. Crit. Care Med. 2025, 211, 1124–1126. [Google Scholar] [CrossRef]
- Sommer, N.; Ghofrani, H.A.; Pak, O.; Bonnet, S.; Provencher, S.; Sitbon, O.; Rosenkranz, S.; Hoeper, M.M.; Kiely, D.G. Current and Future Treatments of Pulmonary Arterial Hypertension. Br. J. Pharmacol. 2021, 178, 6–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, R.; Li, Y.; Huang, J.; Liu, Y.; Wang, J.; Xian, P.; Zhang, Y.; Yang, Y.; Zhang, H.; et al. Lipolysis Engages CD36 to Promote ZBP1-Mediated Necroptosis-Impairing Lung Regeneration in COPD. Cell Rep. Med. 2024, 5, 101732. [Google Scholar] [CrossRef] [PubMed]
- Pascual, G.; Avgustinova, A.; Mejetta, S.; Martín, M.; Castellanos, A.; Attolini, C.S.-O.; Berenguer, A.; Prats, N.; Toll, A.; Hueto, J.A.; et al. Targeting Metastasis-Initiating Cells through the Fatty Acid Receptor CD36. Nature 2017, 541, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Song, P.; Tang, G.; Wei, J.; Rao, L.; Ma, L.; Jiang, M.; Huang, J.; Xu, Q.; Wu, J.; et al. Osthole Attenuates Macrophage Activation in Experimental Asthma by Inhibitingthe NF-ĸB/MIF Signaling Pathway. Front. Pharmacol. 2021, 12, 572463. [Google Scholar] [CrossRef]
- Tsai, Y.-F.; Yu, H.-P.; Chung, P.-J.; Leu, Y.-L.; Kuo, L.-M.; Chen, C.-Y.; Hwang, T.-L. Osthol Attenuates Neutrophilic Oxidative Stress and Hemorrhagic Shock-Induced Lung Injury via Inhibition of Phosphodiesterase 4. Free Radic. Biol. Med. 2015, 89, 387–400. [Google Scholar] [CrossRef]
- Wang, S.; Xie, Y.; Huo, Y.-W.; Li, Y.; Abel, P.W.; Jiang, H.; Zou, X.; Jiao, H.-Z.; Kuang, X.; Wolff, D.W.; et al. Airway Relaxation Mechanisms and Structural Basis of Osthole for Improving Lung Function in Asthma. Sci. Signal. 2020, 13, eaax0273. [Google Scholar] [CrossRef]
- Yue, Y.; Li, Y.-Q.; Fu, S.; Wu, Y.-T.; Zhu, L.; Hua, L.; Lv, J.-Y.; Li, Y.-L.; Yang, D.-L. Osthole Inhibits Cell Proliferation by Regulating the TGF-Β1/Smad/P38 Signaling Pathways in Pulmonary Arterial Smooth Muscle Cells. Biomed. Pharmacother. 2020, 121, 109640. [Google Scholar] [CrossRef]
- Zhu, L.; Li, Y.-L.; Qian, Z.-Q.; Hua, L.; Yue, Y.; Yang, D.-L. Osthole Improves Pulmonary Artery Hypertension by Inducing Apoptosis in Pulmonary Artery Smooth Muscle Cells. J. Pharm. Pharmacol. 2021, 73, 1109–1117. [Google Scholar] [CrossRef]
- Niu, Z.; Fu, M.; Li, Y.; Ren, H.; Zhang, X.; Yao, L. Osthole Alleviates Pulmonary Vascular Remodeling by Modulating microRNA-22-3p Mediated Lipid Metabolic Reprogramming. Phytomedicine 2022, 96, 153840. [Google Scholar] [CrossRef]
- Yao, L.; Yang, Y.-X.; Cao, H.; Ren, H.-H.; Niu, Z.; Shi, L. Osthole Attenuates Pulmonary Arterial Hypertension by the Regulation of Sphingosine 1-Phosphate in Rats. Chin. J. Nat. Med. 2020, 18, 308–320. [Google Scholar] [CrossRef] [PubMed]
- Xie, N.; Fan, F.; Jiang, S.; Hou, Y.; Zhang, Y.; Cairang, N.; Wang, X.; Meng, X. Rhodiola Crenulate Alleviates Hypobaric Hypoxia-Induced Brain Injury via Adjusting NF-κB/NLRP3-Mediated Inflammation. Phytomedicine 2022, 103, 154240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, X.; Hu, X.; Chu, X.; Li, X.; Han, F. Neuroprotective Effects of a Rhodiola Crenulata Extract on Amyloid-β Peptides (Aβ1-42) -Induced Cognitive Deficits in Rat Models of Alzheimer’s Disease. Phytomedicine 2019, 57, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-J.; Chung, H.-H.; Cheng, Y.-Z.; Lin, H.J.; Cheng, J.-T. Rhodiola-Water Extract Induces β-Endorphin Secretion to Lower Blood Pressure in Spontaneously Hypertensive Rats. Phytother. Res. 2013, 27, 1543–1547. [Google Scholar] [CrossRef]
- Sun, Z.-Y.; Lu, G.-Q.; Sun, H.-Y.; Jiang, W.-D.; Wang, L.; Wang, Y.-H.; Liu, L.-Q.; Wang, H.-J.; Tang, B.; Gao, Q.; et al. Salidroside Ameliorates Hypoxic Pulmonary Hypertension by Regulating the Two-Pore Domain Potassium TASK-1 Channel. Phytomedicine 2024, 135, 156206. [Google Scholar] [CrossRef]
- Yuan, C.; Jin, Y.; Yao, L.; Liu, L.; Li, J.; Li, H.; Lai, Y.; Chen, Z.; Pan, Z.; Han, T.; et al. Rhodiola Crenulata Root Extract Ameliorates Fructose-Induced Hepatic Steatosis in Rats: Association with Activating Autophagy. Biomed. Pharmacother. 2020, 125, 109836. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Lai, F.-Y.; Shi, L.-S.; Chou, Y.-C.; Yen, I.-C.; Chang, T.-C. Rhodiola Crenulata Extract Suppresses Hepatic Gluconeogenesis via Activation of the AMPK Pathway. Phytomedicine 2015, 22, 477–486. [Google Scholar] [CrossRef]
- Ren, H.-H.; Niu, Z.; Guo, R.; Fu, M.; Li, H.-R.; Zhang, X.-Y.; Yao, L. Rhodiola Crenulata Extract Decreases Fatty Acid Oxidation and Autophagy to Ameliorate Pulmonary Arterial Hypertension by Targeting Inhibiton of Acylcarnitine in Rats. Chin. J. Nat. Med. 2021, 19, 120–133. [Google Scholar] [CrossRef]
- Harvey, L.D.; Alotaibi, M.; Tai, Y.-Y.; Tang, Y.; Kim, H.-J.J.; Kelly, N.J.; Sun, W.; Woodcock, C.-S.C.; Arshad, S.; Culley, M.K.; et al. Lysosomal Dysfunction and Inflammatory Sterol Metabolism in Pulmonary Arterial Hypertension. Science 2025, 387, eadn7277. [Google Scholar] [CrossRef]
- Zhao, L.; Ashek, A.; Wang, L.; Fang, W.; Dabral, S.; Dubois, O.; Cupitt, J.; Pullamsetti, S.S.; Cotroneo, E.; Jones, H.; et al. Heterogeneity in Lung (18)FDG Uptake in Pulmonary Arterial Hypertension: Potential of Dynamic (18)FDG Positron Emission Tomography with Kinetic Analysis as a Bridging Biomarker for Pulmonary Vascular Remodeling Targeted Treatments. Circulation 2013, 128, 1214–1224. [Google Scholar] [CrossRef]
- Lundgrin, E.L.; Park, M.M.; Sharp, J.; Tang, W.H.W.; Thomas, J.D.; Asosingh, K.; Comhair, S.A.; DiFilippo, F.P.; Neumann, D.R.; Davis, L.; et al. Fasting 2-Deoxy-2-[18F]Fluoro-d-Glucose Positron Emission Tomography to Detect Metabolic Changes in Pulmonary Arterial Hypertension Hearts over 1 Year. Ann. Am. Thorac. Soc. 2013, 10, 1–9. [Google Scholar] [CrossRef]
- Taylor, M.; Wallhaus, T.R.; DeGrado, T.R.; Russell, D.C.; Stanko, P.; Nickles, R.J.; Stone, C.K. An Evaluation of Myocardial Fatty Acid and Glucose Uptake Using PET with [18F]Fluoro-6-Thia-Heptadecanoic Acid and [18F]FDG in Patients with Congestive Heart Failure. J. Nucl. Med. 2001, 42, 55–62. [Google Scholar] [PubMed]
- Nogales, P.; Velasco, C.; González-Cintado, L.; Sharysh, D.; Mota-Cobián, A.; Izquierdo-Serrano, R.; Torroja, C.; Del Rio-Aledo, D.; Morales-Cano, D.; Mota, R.A.; et al. Atherosclerotic Disease Activity Is Associated with Glycolytic Enzyme Expression across Multiple Cell Types and Is Trackable by FDG-PET. Sci. Transl. Med. 2025, 17, eado6467. [Google Scholar] [CrossRef] [PubMed]
- Marsboom, G.; Wietholt, C.; Haney, C.R.; Toth, P.T.; Ryan, J.J.; Morrow, E.; Thenappan, T.; Bache-Wiig, P.; Piao, L.; Paul, J.; et al. Lung 18F-Fluorodeoxyglucose Positron Emission Tomography for Diagnosis and Monitoring of Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 2012, 185, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Wang, W.; Che, W.; Xu, Y.; Jin, C.; Dong, L.; Xia, Q. Nanomedicines Targeting Metabolic Pathways in the Tumor Microenvironment: Future Perspectives and the Role of AI. Metabolites 2025, 15, 201. [Google Scholar] [CrossRef]
- Li, B.; Teng, C.; Yu, H.; Jiang, X.; Xing, X.; Jiang, Q.; Lin, C.; Zhao, Z.; Zhang, R.; He, W. Alleviating Experimental Pulmonary Hypertension via Co-Delivering FoxO1 Stimulus and Apoptosis Activator to Hyperproliferating Pulmonary Arteries. Acta Pharm. Sin. B 2023, 13, 2369–2382. [Google Scholar] [CrossRef]
- Deng, Z.; Gao, W.; Kohram, F.; Li, E.; Kalin, T.V.; Shi, D.; Kalinichenko, V.V. Fluorinated Amphiphilic Poly(β-Amino Ester) Nanoparticle for Highly Efficient and Specific Delivery of Nucleic Acids to the Lung Capillary Endothelium. Bioact. Mater. 2024, 31, 1–17. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, C.; Tang, X.; Sun, S.; Liu, S.; Yang, L.; Chen, Y.; Yang, Q.; Wei, T.-Y.W.; Wu, X.; et al. Endothelium-Specific SIRT7 Targeting Ameliorates Pulmonary Hypertension through Krüpple-like Factor 4 Deacetylation. Cardiovasc. Res. 2024, 120, 403–416. [Google Scholar] [CrossRef]
- Klingenberg, R.; Leiherer, A.; Dobrev, D.; Kaski, J.C.; Levkau, B.; März, W.; Sossalla, S.; von Eckardstein, A.; Drexel, H. Ceramides in Cardiovascular Disease: Emerging Role as Independent Risk Predictors and Novel Therapeutic Targets. Cardiovasc. Res. 2025, 121, 1345–1358. [Google Scholar] [CrossRef]
- Dan-Zeng, Z.-G.; Bai-Ma, Y.-J.; Huang, J.; Suo-Lang, W.-J.; Ge-Sang, Q.-Z.; Suo-Na, Y.-Z.; Wang, Y.-S.; Baima, Z.-G.; Ge-Sang, L.-B. Integrative Proteomics and Metabolomics Reveal Important Pathways and Potential Biomarkers in High-Altitude Pulmonary Hypertension. Sci. Rep. 2025, 15, 24999. [Google Scholar] [CrossRef]

| Cell Type | Key Metabolic Features | Functional Consequences | Key Enzymes/Proteins | Pathways |
|---|---|---|---|---|
| Right Ventricular Cardiomyocytes | Bidirectional FAO Dysregulation: upregulation in compensatory stage; Decompensated stage: FAO downregulation, lipid deposition. |
| compensatory stage: ↑CD36, CPT1b, Mef2,PGC-1α Decompensated stage: ↓PPARγ, CPT1b, FABP4 | AMPK, WNK1 |
| Pulmonary artery endothelial cells |
|
| ↑FASN, FABP4/5 ↓CPT1a | AMPK/mTOR, TGF-β/SMAD2 |
| Pulmonary artery smooth muscle cells | Markedly enhanced fatty acid uptake and FAO, providing energy and biomass for proliferation. | Aberrant proliferation, migration, and apoptosis resistance, driving vascular remodeling. | ↑CD36, CPT1A, ACSL1 | HIF-1α/PI3K, PPARγ/CPT1 |
| Pulmonary Vascular Macrophages | M1 (Pro-inflammatory): Increased fatty acid synthesis and lipid droplet storage. M2 (Anti-inflammatory): Enhanced FAO supporting oxidative phosphorylation. | M1: Drives inflammation, promotes PASMC proliferation. M2: Anti-inflammatory, tissue repair, but excessive activation exacerbates fibrosis | M1: ↑CD36, FASN M2: ↑CPT1A | NF-κB/SREBP-1a |
| Drug | Pathways | Target | Function | Research Subjects | Clinical Trials |
|---|---|---|---|---|---|
| Trimetazidine | FAO | Long-chain 3-ketoacyl-CoA thiolase | Reduces FAO, promotes glucose oxidation and improves RV function and remodeling | PAH | NCT02102672 |
| Ranolazine | FAO | Late sodium channel and FAO pathway | Inhibition of FAO, increased glucose utilization, reduced ROS, reversal of RV lipotoxicity and inflammation | PAH | NCT01174173, NCT01757808, NCT01839110, NCT02829034, NCT02133352 |
| Pioglitazone | FAO | PPARγ | Activation of PPARγ, promotion of balanced FAO, reduction of inflammation and remodeling, restoration of mitochondrial morphology and function | PH due to Chronic Lung Disease (CLD) | Currently recruiting clinical trial, NCT06336798 |
| Oxfenicine | FAO | CPT-1 | Block fatty acid entry into mitochondria, shift to glucose pathway, reduce lipotoxicity | SuHx rats; Schistosoma and hypoxia-induced PH mice | Preclinical |
| Etomoxir | FAO | CPT-1 | Inhibit FAO, promote glucose utilization, reduce lipid accumulation | MCT rats | Preclinical |
| Perhexiline | FAO | CPT-1 | Inhibit CPT-1, reduce lipid oxidation, promote glucose oxidation, reverse PH cell proliferation | Human PASMCs from PAH doners | Preclinical |
| FPER-1 | FAO | CPT-1,PDH | Fluorinated perhexiline derivative, inhibits FAO, reduces vascular smooth muscle cell proliferation | Human PASMCs from PAH doners | Preclinical |
| Metformin | FAS | AMPK and fatty acid synthesis pathway | Activate AMPK, inhibit lipid synthesis, reduce lipid accumulation, improve endothelial function and vascular remodeling | PAH | NCT01884051 |
| C75 | FAS | FAS | Inhibit fatty acid synthase while increasing FAO, reduce lipid accumulation | Hypoxia-induced PH mice; MCT rats | Preclinical |
| BMS-303141 | FAS | ACLY | Inhibit ACLY, reduce lipid synthesis, reverse vascular remodeling | SuHx rats and mice | Preclinical |
| Osthole | NA | miRNA-22-3p and lipid metabolism–related enzymes | Regulate miRNA-22-3p, inhibit CD36, FAS, and CPT1A activities, restore lipid homeostasis, and reduce PASMC proliferation and vascular remodeling | MCT rats | Preclinical |
| Rhodiola crenulata extract | NA | lipid metabolism–related enzymes | Downregulate CPT1A mRNA and protein expression to inhibit FAO; reduce autophagy via PPARγ and LKB1-AMPK signaling pathways | MCT rats | Preclinical |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.; Zheng, R.; Gong, L.; Zhang, Y.; Tan, J.; Cao, X.; Song, L.; Dai, A. Roles of Lipid Metabolism in Pulmonary Hypertension: Friend or Foe? Biomolecules 2025, 15, 1679. https://doi.org/10.3390/biom15121679
Huang W, Zheng R, Gong L, Zhang Y, Tan J, Cao X, Song L, Dai A. Roles of Lipid Metabolism in Pulmonary Hypertension: Friend or Foe? Biomolecules. 2025; 15(12):1679. https://doi.org/10.3390/biom15121679
Chicago/Turabian StyleHuang, Wei, Runxiu Zheng, Lijun Gong, Yu Zhang, Junlan Tan, Xianya Cao, Lan Song, and Aiguo Dai. 2025. "Roles of Lipid Metabolism in Pulmonary Hypertension: Friend or Foe?" Biomolecules 15, no. 12: 1679. https://doi.org/10.3390/biom15121679
APA StyleHuang, W., Zheng, R., Gong, L., Zhang, Y., Tan, J., Cao, X., Song, L., & Dai, A. (2025). Roles of Lipid Metabolism in Pulmonary Hypertension: Friend or Foe? Biomolecules, 15(12), 1679. https://doi.org/10.3390/biom15121679





