Abstract
Heart failure (HF) marks the culmination of numerous cardiac pathologies, presenting a major medical hurdle in prevention and treatment. In recent years, with the advancements in genomics and metabolomics, research has demonstrated that gut microbiota plays a significant role in the pathogenesis of HF. Trimethylamine N-oxide (TMAO) is a gut microbiota-derived metabolite and primarily sourced from foods abundant in choline, L-carnitine, and betaine. Research has shown that patients with HF exhibit higher levels of TMAO. Accumulating evidence has indicated that TMAO directly or indirectly mediates the occurrence and development of HF through multiple mechanisms. Furthermore, TMAO functions as a crucial prognostic marker in HF. Therefore, TMAO emerges as a potential therapeutic target for HF. This article reviews the generation and metabolic pathways of TMAO, emphasizes its pathophysiological mechanisms in HF, and explores promising therapeutic approaches targeting TMAO, offering novel insights and strategies for HF management.
1. Introduction
Heart failure (HF) represents a complex clinical syndrome, marking the culmination of diverse cardiac pathologies. It is characterized by symptoms and signs arising from structural or functional impairments in ventricular filling or ejection, accompanied by elevated natriuretic peptide levels or objective evidence of pulmonary or systemic congestion [1,2]. Globally, HF affects more than 56 million individuals, constituting over 1% of the world’s population [3,4]. As populations age and cardiovascular disease (CVD) rates rise, the prevalence and mortality of HF continue to increase [2,3]. The primary etiologies include ischemic heart disease (IHD), rheumatic and valvular heart disease, cardiomyopathies, amyloidosis, certain infectious diseases, and endocrine disorders [3]. Based on left ventricular ejection fraction (LVEF), HF is classified into three subtypes: HF with reduced ejection fraction (HFrpEF, LVEF < 40%), HF with mid-range ejection fraction (40% ≤ LVEF < 50%), and HF with preserved ejection fraction (HFpEF, LVEF ≥ 50%) [2,5]. Multiple mechanisms—such as inflammation, oxidative stress, disrupted energy metabolism, and pathological cardiac remodeling—can initiate or worsen HF. Although HF prediction and diagnosis are relatively straightforward, effective prevention and treatment still pose significant challenges [6]. Despite advances in pharmacotherapy and interventions, HF persists as a leading cause of mortality worldwide, underscoring the need for deeper insights into its pathophysiology and innovative therapeutic targets. Recent research has highlighted the role of the gut–heart axis in HF, proposing that gut microbiota dysbiosis may aggravate myocardial remodeling and cardiac dysfunction. This occurs through metabolic disturbances, immune-mediated systemic inflammation, and intestinal barrier damage, leading to translocation of gut-derived toxins [7,8,9,10]. Hence, selective modulation of the gut microbiome emerges as a possible breakthrough in addressing HF.
The gut microbiota can be conceived of as a massive virtual metabolic organ within the human body, encompassing more than 30 trillion microorganisms [11]. Its composition is influenced by host age, sex, diet, antibiotic use, environmental factors, and genetics, among other variables. A balanced gut microbiota is essential for cardiovascular homeostasis, and its disruption could induce atherosclerosis (AS), arrhythmias, and other CVDs [8]. The involvement of gut microbiota in HF has gained increasing attention, with evidence indicating that alterations in microbial communities and their metabolites play a pivotal part in HF occurrence and progression [12,13]. Gut microbiota dysbiosis may worsen HF by impairing metabolic, immune, and barrier functions [14]. Gut microbial metabolites, such as short-chain fatty acids (SCFAs), trimethylamine N-oxide (TMAO), bile acids (BAs), and lipopolysaccharide (LPS; an intrinsic component of the Gram-negative bacterial cell wall that exerts its toxicity through lipid A and is released as endotoxin upon bacterial death and lysis), also exert either beneficial or detrimental effects in HF [15]. For example, SCFAs are generally regarded as advantageous, whereas TMAO and LPS may intensify HF [9].
Further studies reveal the gut microbiota-derived metabolite TMAO as a key mediator connecting HF with intestinal homeostasis, potentially bridging microbial dysbiosis and cardiac pathophysiology. HF patients exhibit elevated TMAO levels [16,17], which may result from intestinal and renal alterations associated with the disease. The gut microbiota composition in HF patients differs significantly from that in healthy individuals, typically showing an increased abundance or proportion of Firmicutes (e.g., Ruminococcus gnavus [18]) and Proteobacteria (e.g., Escherichia, Klebsiella, Campylobacter, and Shigella [19,20]) [21]. These shifts likely promote the production of TMAO and its precursor trimethylamine (TMA). Moreover, HF often coincides with elevated intestinal permeability and impaired renal function, which facilitate TMAO entry into circulation and diminish its excretion. TMAO has also been shown to predict the onset and progression of HF in certain instances [22] and serves as a valuable prognostic marker [21,23,24]. Increasing evidence indicates that TMAO plays an important regulatory role in HF [25,26,27,28].
Although several studies have offered preliminary insights into how TMAO influences HF and into potential TMAO-targeted therapies, direct evidence of causal involvement remains limited, and comprehensive mechanistic and therapeutic investigations are still needed. Accordingly, this review examines the role of TMAO in HF and focuses on potential therapeutic strategies targeting TMAO, thereby offering new targets and perspectives for HF management.
2. The Generation and Metabolism of TMAO
TMAO, a compound bearing the molecular formula (CH3)3NO [29], stands as a gut microbiota-derived metabolite increasingly recognized as a vital contributor to intestinal-origin CVDs [7]. Gut microbiota metabolizes choline, L-carnitine, betaine, and other choline- or TMA-rich compounds into TMA, a transformation catalyzed by specific enzymes notably including choline TMA-lyase CutC and its activator CutD, carnitine monooxygenase CntA/B, betaine reductase, TMAO reductase, and the CntA/B homologs YeaW/X [30,31,32,33]. The resulting TMA permeates the intestinal barrier, enters circulation, and subsequently travels via the portal circulation to the liver, where it is rapidly oxidized by hepatic flavin-containing monooxygenases (FMOs) into TMAO [34]. TMAO then circulates systemically [35]. In healthy individuals, over 90% of TMAO is eliminated by the kidneys and rapidly excreted in urine [36]. The generation and metabolic process of TMAO is detailed in Figure 1.
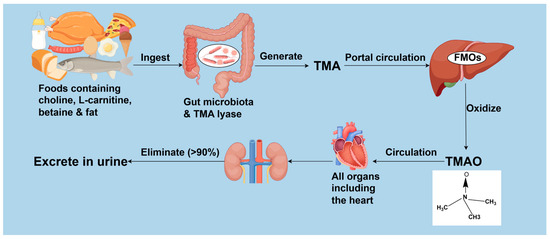
Figure 1.
The generation and metabolism of TMAO. TMA: trimethylamine; FMOs: flavin-containing monooxygenases; TMAO: trimethylamine N-oxide. Created by Fidraw (www.figdraw.com, accessed on 6 August 2025).
Systemic TMAO concentrations are influenced by multiple factors, primarily determined by three key processes: intestinal generation, hepatic conversion, and renal elimination. Dysregulation in any of these processes may elevate TMAO levels. Dietary choline is obtained mainly from meat, eggs, dairy products, fish, grains, and foods derived from them [37]. The primary dietary source of L-carnitine is red meat [38]. Betaine occurs naturally in foods like beets, spinach, grains, and shrimp [39]. Gut microbiota metabolize these dietary precursors into TMA via specific key enzymes. However, the gene clusters encoding these enzymes occur only in certain intestinal bacteria [30]. These clusters are more prevalent among the gut bacteria of carnivores and omnivores than herbivores [30], initiating increased TMAO generation [31]. High-fat diets (HFDs) have also been found to elevate TMAO concentrations [32]. Accordingly, TMAO synthesis is influenced by the composition and function of gut microbiota as well as dietary intake. Concurrently, FMO activity affects TMAO generation; for instance, liver diseases such as non-alcoholic fatty liver disease can upregulate FMO expression [40], promoting TMA oxidation. Among various FMOs that convert TMA to TMAO, FMO3 exhibits the highest activity [41]. Knocking out or silencing of hepatic FMO3 in mice decreases circulating TMAO levels [36,42]. Renal insufficiency may also elevate TMAO due to reduced excretion [43]. A limited number of studies suggested that dietary modulation of TMAO levels may not occur through direct regulation of FMO3 activity [44,45]. However, direct evidence remains scarce that elevated TMAO arises solely from increased TMA formation or impaired renal excretion, independent of FMO-mediated oxidation. Consequently, high TMAO levels likely reflect the combined influence of increased TMA precursors, enhanced FMO conversion efficiency, and impaired renal excretion—three dynamically interacting factors that should be considered together.
Moreover, TMAO concentrations correlate positively with age [46,47]. Genetic factors also influence TMAO production and metabolism. For example, mutations in the FMO3 gene can reduce or abolish enzymatic activity, impairing TMA-to-TMAO conversion and leading to toxic TMA accumulation. This condition, trimethylaminuria (TMAU), is characterized by a fish-like odor in bodily secretions [48] and represents the most direct genetic influence on TMAO metabolism. Host genetics also partially shape gut microbiota composition and function. Individual genetic backgrounds may indirectly modulate TMA production by influencing microbial community structure, thereby contributing to interindividual variation in TMAO levels. A large-scale population study linked variability in TMAO and its precursors to the abundance of Ruminococcaceae genera and the CutC gene in gut microbiota [33]. Certain TMA-producing bacteria, including some Firmicutes, show heritability [49,50]. Additionally, circulating TMAO concentrations vary substantially among healthy adults across geographic regions. In the United States, the median circulating TMAO level in healthy populations was 3.5 μmol/L [51,52], whereas Fang et al. [53] recently documented a lower median of 1.70 μmol/L among healthy Chinese adults. These disparities may arise from genetic, dietary, and ethnic factors.
In conclusion, TMAO levels correlate with various factors, including dietary patterns, gut microbiota structure, activity of TMAO-producing enzymes such as TMA lyase and FMOs, renal function, age, genetics, ethnicity, and geographical region.
3. The Mechanisms by Which TMAO Regulates HF
3.1. Inflammation
Inflammation is closely linked to HF [14]. Higher concentrations of TMAO, such as 600 μmol/L, have been shown to enhance the expression of inflammatory genes and activate proinflammatory cytokines, thus amplifying inflammatory responses [54]. Boini et al. [55] experimentally demonstrated that TMAO stimulated the secretion of inflammatory cytokines, including interleukin (IL)-1β, by activating the nucleotide-binding oligomerization domain-like receptor containing pyrin domain 3 (NLRP3) inflammasome, leading to endothelial inflammation. TMAO also inhibited the deacetylation of superoxide dismutase 2 (SOD2) by suppressing Sirtuin 3 (SIRT3), thereby reducing SOD2 activity. This process promoted mitochondrial reactive oxygen species (mtROS) generation, leading to elevated overall reactive oxygen species (ROS) levels [56,57]. This increased thioredoxin-interacting protein (TXNIP) levels, subsequently activating the NLRP3 inflammasome [57]. Such activation elicited a dose- and time-dependent release of IL-1β and IL-18 [58], culminating in an inflammatory response.
Moreover, TMAO may influence HF by modulating inflammatory pathways. Zhang and colleagues [59] reported that TMAO could possibly exacerbate myocardial inflammation in mice by elevating TNF (tumor necrosis factor)-α and reducing IL-10 levels. Additionally, their study showed that TMAO supplementation (120 mg/kg) in drinking water may counteract the cardioprotective benefits of voluntary exercise [59].
3.2. Abnormal Energy Metabolism
Abnormal energy metabolism plays a pivotal role in the development of HF. In HF, due to the increased dependence of cardiac metabolism on glucose, the primary energy source shifts from fatty acid (FA) oxidation to glucose metabolism [60]. Additionally, hypoxia reduces oxidative phosphorylation (OXPHOS) and enhances glycolysis [61]. These metabolic alterations contribute to an insufficient energy supply, thus worsening the condition of HF.
Elevated TMAO levels are strongly linked to impaired cardiac energy metabolism. HFD-induced TMAO accumulation inhibited mitochondrial activity and consequently compromised energy metabolism [62]. Saaoud et al. [54] revealed that 600 μmol/L TMAO initiated a metabolic switch from OXPHOS to glycolysis. This shift occurred by upregulating mitochondrial regulators such as perilipin 4 (PLIN4), overlapping activity with m-AAA protease 1 (OMA1), and oxoglutarate dehydrogenase L (OGDHL), ultimately disrupting energy homeostasis. Brown adipose tissue (BAT) enhances thermogenesis and supports cardiac function [63,64], but hypoxia induces brown adipocyte apoptosis, resulting in BAT dysfunction [28]. In physiological conditions, BAT absorbs and metabolizes circulating choline, potentially to maintain its cellular membrane integrity. In a murine model of HF, BAT dysfunction led to choline accumulation, which may be partially converted to TMAO via the choline-TMA-TMAO pathway; this increased TMAO synthesis exacerbated HF [28]. This study further revealed that TMAO significantly attenuated creatine phosphate (CP) and adenosine triphosphate (ATP) concentrations in cardiac tissue by suppressing mitochondrial complex IV activity, further impairing cardiac energy metabolism. This led to a vicious cycle of BAT dysfunction and cardiac dysfunction ultimately [28].
In addition, impaired calcium ion (Ca2+) handling in myocardial cells increases the energy demand of the failing heart [65], compromising both systolic and diastolic function and worsening HF. TMAO disrupts Ca2+ regulation in cardiac cells. In endothelial cells, 100 μmol/L TMAO suppressed purinergic response-induced increase in Ca2+ influx and prolongation of Ca2+ signaling, indicating loss of Ca2+ homeostasis [66]. High glucose and high fat could also induce TMAO production, possibly predisposing individuals to Ca2+ overload [67]. Esposito and coworkers [68] proved that 100 μmol/L TMAO transiently suppressed contractile recovery of myocardial cells by elevating intracellular Ca2+ currents.
3.3. Oxidative Stress
Oxidative stress contributes to the pathophysiological HF via multiple mechanisms. Studies have demonstrated that TMAO can mediate oxidative stress. TMAO concentrations were remarkably higher in preeclampsia patients. Transplanting fecal microbiota from these patients into antibiotic-treated mice resulted in pronounced oxidative stress in the recipients [69]. In addition, TMAO can provoke oxidative stress by distinct pathways. A study involving mice and healthy human subjects revealed that TMAO promoted oxidative stress by increasing superoxide levels and impairing endothelial nitric oxide synthase (eNOS) activity [46]. Chen et al. [57] confirmed that TMAO, at concentrations of 150, 300, 600, and 900 μmol/L, augmented ROS generation, particularly mtROS, through modulating the SIRT3-SOD2-mtROS signaling cascade, thus fostering oxidative stress. Moreover, TMAO induced oxidative stress through activation of NADPH oxidase 2 (NOX2) [70].
Elevated TMAO levels have been demonstrated to initiate HF through oxidative stress. Recent research revealed that TMAO induced oxidative stress by downregulating piezo type mechanosensitive ion channel component 1 (PIEZO1), thereby exacerbating cardiac diastolic dysfunction in mice suffering from HFpEF [25]. Therefore, decreasing TMAO concentrations and alleviating oxidative stress appear to be a viable therapeutic approach for HF.
3.4. Myocardial Remodeling
Myocardial remodeling stands as a significant mechanism underlying the occurrence and progression of HF. Its primary features consist of pathological cardiomyocyte hypertrophy and myocardial fibrosis. TMAO directly influences this process. Research in mice indicated that a Western diet (WD) or TMAO supplementation increased TMAO levels, promoted myocardial fibrosis, and impaired cardiac function [59]. Studies demonstrated that TMAO stimulated myocardial remodeling in Sprague-Dawley rats and triggered hypertrophic responses in rat cardiomyocytes, enlarging cell size while upregulating hypertrophy markers such as atrial natriuretic peptide (ANP) and β-myosin heavy chain (β-MHC). This effect might involve activation of the transforming growth factor (TGF)-β1/Smad3 signaling pathway [71]. Another study indicated that transverse aortic constriction-induced HF in mice correlated with elevated TMAO levels, exacerbated myocardial hypertrophy and fibrosis, and compromised cardiac function. The mechanisms could be associated with activation of the TGF-β1/Smad3 and p65 nuclear factor (NF)-κB signaling pathways [27].
TMAO aggravates HF by affecting adverse cardiac remodeling. Organ et al. [72] discovered that diets containing choline (1.2%) or TMAO (0.12%) markedly increased circulating TMAO and brain natriuretic peptide (BNP) levels in HF mice. Concurrently, left ventricular (LV) fractional shortening and ejection fraction were decreased, accompanied by exacerbated LV remodeling, intensified myocardial fibrosis, and deteriorated cardiac dysfunction, ultimately accelerating HF progression. Subsequent experiments showed that discontinuing TMAO intake or inhibiting TMA lyase effectively lowered the HF marker BNP levels, suppressed numerous profibrotic gene expressions, and ultimately mitigated ventricular remodeling and cardiac dysfunction [73].
3.5. Indirect Mechanisms
TMAO indirectly contributes to the emergence and progression of HF by mediating several mechanisms such as endothelial dysfunction [46], renal insufficiency [74], elevated blood pressure [75], enhanced platelet activity and thrombosis [34], and lipid metabolism disorders [9,31].
3.5.1. Endothelial Dysfunction
Endothelial dysfunction plays a crucial part in AS-related CVDs and ensuing HF. Studies uncovered a distinct association between TMAO and endothelial dysfunction biomarkers in patients suffering from HF [9]. Elevated serum TMAO levels disrupt vascular endothelial integrity and exacerbate endothelial dysfunction, promoting vascular dysfunction, inflammation, fibrosis, and AS [9], ultimately increasing CVD risk [9,54].
Multiple studies have explored the mechanisms by which TMAO causes endothelial dysfunction. In both aged mice and middle-aged or elderly human subjects, TMAO levels and the severity of endothelial dysfunction increased significantly with age [46]. Additionally, TMAO exacerbated endothelial dysfunction by promoting inflammation and oxidative stress, suppressing eNOS activity and impairing NO (nitric oxide)-mediated vasodilation. These findings suggested that elevated TMAO levels during aging likely reduce eNOS-derived NO bioavailability through vascular inflammation and oxidative stress, contributing to age-related endothelial dysfunction [46]. Additional research revealed that TMAO induced endothelial dysfunction via the ROS-TXNIP-NLRP3 inflammasome signaling pathway [27]. Evidence indicated that TMAO fostered endothelial dysfunction and vascular calcification through the activation of the NLRP3 inflammasome and NF-κB signaling [76]. Kim et al. [77] revealed that TMAO (50 and 100 μmol/L) mediated chromatin remodeling in coronary artery endothelial cells by augmenting histone methylation, fostering endothelial-to-myofibroblast transformation and vascular fibrosis, thus inducing ischemic HF [78].
3.5.2. Renal Insufficiency
Studies have shown that patients with deficient renal function have higher blood TMAO levels [9,43], and a notable inverse relationship exists between TMAO levels and estimated glomerular filtration rate [79]. Rising TMAO concentrations may worsen renal insufficiency and raise all-cause mortality in chronic kidney disease patients through inducing progressive renal fibrosis and dysfunction [43].
In HF, neurohormonal activation ultimately results in hypoperfusion and dysfunction in multiple organs, the kidneys among them. Likewise, the significance of defective renal function in the advancement of HF is not negligible. TMAO is linked to the onset and development of both HF and renal insufficiency [8]. On one hand, TMAO promotes water and sodium retention by triggering glomerular interstitial fibrosis and renal dysfunction, indirectly deteriorating HF [43,80]. On the other hand, chronic heart failure (CHF) frequently contributes to progressive renal function impairment [74], which decreases TMAO excretion and increases circulating TMAO concentrations. These elevated TMAO levels further exacerbate HF progression. For instance, in a rat model of cardiorenal syndrome featuring both HF and renal insufficiency, increased blood TMAO levels exacerbated cardiac and renal dysfunction [74].
3.5.3. Elevated Blood Pressure
Elevated blood pressure is a primary inducer of HF. Uncontrolled chronic hypertension (HTN) could induce LV remodeling via biochemical and neurohumoral mechanisms, which may subsequently result in hypertensive heart disease and, in its most severe form, progress to HF.
TMAO is closely associated with enhanced blood pressure. A systematic review and meta-analysis revealed an obvious positive dose-dependent correlation between circulating TMAO levels and HTN prevalence [81]. Jiang et al. [75] reported that 1% TMAO enhanced angiotensin (ANG) II-induced vasoconstriction and acute pressor responses by activating protein kinase R-like endoplasmic reticulum kinase-associated pathways, consequently worsening HTN. Another research indicated that higher TMAO concentrations (30 μmol/L) increased systolic blood pressure in both mice and human subjects by inducing advanced glycation end-product accumulation and oxidative stress [82]. These observations suggest that TMAO could function as a potential biomarker for HTN.
3.5.4. Increased Platelet Activity and Thrombosis
Excessive platelet activation may facilitate thrombosis. Research has shown that elevated platelet activity and thrombosis play vital roles in the onset and advancement of HF [83]. Plasma TMAO might trigger excessive platelet activation and thrombosis in humans [42,84]. Clinical studies further indicated that TMAO predicted the likelihood of thrombotic events, like myocardial infarction (MI) and stroke [34,85]. In mice, plasma TMAO concentrations correlated positively with thrombosis rates, and dietary supplementation with choline or TMAO increased platelet activity and accelerated thrombosis [34,84,85]. Similarly, in humans, choline intake resulted in elevated TMAO levels, which enhanced platelet reactivity, ultimately facilitating thrombosis [86]. Zhu and colleagues [34] confirmed that 100 μmol/L TMAO potentiated platelet hyperreactivity by stimulating intracellular Ca2+ release, thereby raising thrombosis risk.
Additionally, TMAO can contribute to CVDs via increased platelet activity and thrombosis [87]. Hence, attenuating TMAO production may serve as a therapeutic target to curb excessive platelet activation and thrombosis, improving clinical outcomes in CVDs, including HF.
3.5.5. Abnormal Lipid Metabolism
Abnormal lipid metabolism emerges as a key pathogenic factor in HF, and correcting lipid metabolism is crucial for its treatment [88]. TMAO accumulation has been confirmed to cause abnormal lipid metabolism [9,89], consequently increasing the risk of diverse CVDs, HF among them. Elevated TMAO disrupts lipid metabolism through mechanisms such as inhibiting reverse cholesterol transport (RCT), promoting macrophage foam-cell formation, and modifying BA synthesis.
First, TMAO suppresses RCT and subsequently disrupts cholesterol metabolism. TMAO and its precursors, choline and carnitine, impede RCT in the body through a gut microbiota-dependent mechanism [31]. Second, TMAO facilitates cholesterol accumulation in macrophages and the formation of foam cells [9,90]. Wang et al. [91] discovered that TMAO enhanced phagocytic activity and cholesterol content in peritoneal macrophages by upregulating scavenger receptor A and CD36, thereby promoting foam-cell formation. Third, TMAO also restricts the production of BA. In apolipoprotein E knockout (ApoE−/−) mice fed a diet containing 0.3% TMAO, TMAO altered the BA profile and suppressed hepatic BA synthesis by specifically inhibiting the classical pathway, leading to abnormal lipid metabolism and remarkably increased blood lipid concentrations [89]. Also, according to He and coworkers [92], TMAO increased hepatic cholesterol accumulation by reducing fecal excretion of acidic sterols, contributing to hyperlipidemia.
In summary, TMAO directly or indirectly modulates HF through multiple pathophysiological mechanisms, including inflammation, abnormal energy metabolism, oxidative stress, and myocardial remodeling [25,28,59,72] (Figure 2). Moreover, these pathways often interact, creating a pernicious cycle that accelerates the advancement of HF even more [93].
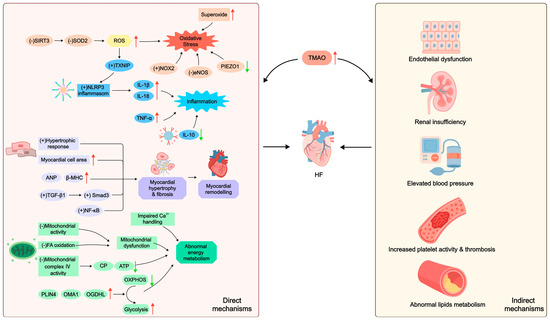
Figure 2.
The mechanisms by which TMAO regulates HF. An upward arrow (↑) indicates an increase. A downward arrow (↓) indicates a decrease. A plus sign (+) indicates promotion. A minus sign (−) indicates inhibition. SIRT3: Sirtuin 3; SOD2: superoxide dismutase 2; ROS: reactive oxygen species; TXNIP: thioredoxin-interacting protein; NOX2: NADPH oxidase 2; eNOS: endothelial nitric oxide synthase; PIEZO1: piezo type mechanosensitive ion channel component 1; NLRP3: nucleotide-binding oligomerization domain-like receptor containing pyrin domain 3; IL: interleukin; TNF: tumor necrosis factor; ANP: atrial natriuretic peptide; β-MHC: β-myosin heavy chain; TGF: transforming growth factor; NF: nuclear factor; Ca2+: calcium ion; FA: fatty acid; CP: creatine phosphate; ATP: adenosine triphosphate; PLIN4: perilipin 4; OMA1: overlapping activity with m-AAA protease 1; OGDHL: oxoglutarate dehydrogenase L; OXPHOS: oxidative phosphorylation; TMAO: trimethylamine N-oxide; HF: heart failure.
4. Potential Therapeutic Approaches
Multiple studies have demonstrated that elevated TMAO levels are independently associated with an increased risk of adverse outcomes in patients with HFpEF [22,94,95] and HFrEF [94,96,97], suggesting its potential as a prognostic biomarker. Targeting TMAO has therefore emerged as a viable therapeutic approach. In HFpEF, strategies to lower TMAO—such as dietary modification, physical activities, inhibitors, or medications like sodium-glucose cotransporter-2 (SGLT2) inhibitors—may reduce systemic inflammation, improve metabolism, and mitigate myocardial remodeling. For HFrEF, interventions including aspirin, angiotensin-converting enzyme inhibitors (ACEIs), or statins can lower TMAO, which may help alleviate vascular inflammation and atherosclerosis, improve myocardial ischemia, and lead to better clinical outcomes.
4.1. Diet Regulation
Current evidence indicates that dietary intervention could effectively lessen circulating TMAO levels, probably aiding in the prevention and treatment of HF [21]. As noted, consuming foods abundant in TMA precursors—including choline, L-carnitine, and betaine—or high-fat foods increases TMAO concentrations in humans. In animal studies, a choline-rich diet significantly increased TMAO and BNP levels, worsened ventricular remodeling, impaired cardiac function, and further aggravated HF [72]. Mo et al. [32] demonstrated that an HFD elevated plasma TMAO concentrations in naturally aging rats. In healthy rats, prolonged HFD intake triggered low-grade systemic inflammation and diminished beneficial SCFA levels, while long-term fructose consumption heightened oxidative stress, elevated blood pressure, and expanded Enterobacter and Escherichia coli populations [98]. The WD, distinguished by high amounts of TMA precursors, fats, and sugars [9,47,62], is strongly associated with increased TMAO concentrations [52]. Consuming WD alters gut microbiota composition, diminishes its diversity, and consequently promotes TMAO production, rising blood TMAO concentrations, even in healthy people [99]. The WD has been proven to elevate circulating TMAO levels, thus inducing cardiac fibrosis and systemic inflammation. These pathological processes can subsequently cause cardiac dysfunction, heightening the risk of developing AS, coronary artery diseases (CADs), and HF [9,21,47,62]. Furthermore, animal experiments demonstrated that a high-salt diet may reduce the ratio of SCFAs to TMAO, lower Lactobacillus abundance, and exacerbate immune and inflammatory responses [100]. Additionally, insufficient dietary fiber could worsen gut microbiota dysbiosis [101]. Hence, it is advisable to steer clear of overindulging in foods rich in TMA precursors, fats, sugars, or salts [99], while augmenting dietary fiber intake.
Studies have shown that consuming red meat raises TMAO concentrations. Decreasing consumption of choline- or L-carnitine-rich foods such as red meat can hinder TMAO synthesis, ultimately reducing HF incidence [7,52]. A plant-based diet primarily consists of fruits, vegetables, seeds, nuts, legumes, whole grains, and herbal plants. It may adhere strictly to a vegetarian diet or incorporate moderate quantities of animal-derived foods like fish, seafood, dairy products, and eggs [102]. This dietary pattern has been demonstrated to modulate gut microbiota, augment the generation of advantageous metabolites such as SCFAs, and diminish TMAO levels [103,104,105]. The Mediterranean diet (MD), a well-established healthy pattern, emphasizes ample fruits, vegetables, nuts, and whole grains, along with moderate meat, eggs, sugar, olive oil, and wine [93]. It is low in saturated FAs, salt, and phosphates, but rich in unsaturated FAs, antioxidants (such as polyphenols, vitamins, and flavonoids), nitrates, and dietary fiber [9,37,67]. Healthy diets like the MD could modulate gut microbiota composition and its metabolites, influencing the occurrence and progression of HF. Studies have indicated that MD mitigates oxidative stress and inflammation while enhancing antioxidant capacity and endothelial function by augmenting the gut microbial diversity and abundance, raising SCFA production, and reducing TMAO concentrations. These effects collectively improve cardiac performance and lower CVD risk [9,106,107]. Adherence to the MD has been linked to decreased HF incidence and mortality [108], and it has exhibited additional benefits such as restoring gut microbial balance and lessening TMAO concentrations [109]. Estruch and colleagues [110] demonstrated that MD consumption, especially when enriched with extra-virgin olive oil, effectively attenuated TMAO levels, thus diminishing major cardiovascular events such as MI and stroke. Kaluza et al. [111] uncovered that certain foods, including vegetables, fruits, and nuts, lessened HF risk in individuals with smoking addictions by modifying gut microbiota, suppressing oxidative stress, and inhibiting cell death. Merques and coworkers [112] revealed that a high-fiber diet regulated gut microbiota, lowered blood pressure, and ameliorated cardiac fibrosis and LV hypertrophy, thereby contributing to the prevention of HF. Furthermore, obese patients following a low-calorie diet may experience decreased TMAO concentrations [113,114]. In summary, healthy eating serves as a practical and economical way to manage HF, mainly by modulating gut microbiota and their metabolites [93].
4.2. Physical Activities
Tailored exercise training can enhance exercise capacity and quality of life in patients with HF, improve survival rates, and lower the likelihood of HF-related hospitalization [115,116]. Additionally, physical activities have been shown to modulate gut microbiota [117], leading to lower TMAO levels. For example, sprint training decreased serum and urinary TMAO concentrations in male participants [118,119]. Physical activity also notably reduced plasma TMAO levels in a study of 16 obese adults [113]. Zhang et al. [120] discovered that voluntary wheel running in mice alleviated gut microbiota dysbiosis and decreased serum levels of TMAO and its precursors, TMA and betaine.
Physical activities confer beneficial effects on the heart by downregulating TMAO concentrations. After being fed a WD, mice that engaged in voluntary exercise demonstrated reduced plasma TMAO levels, diminished myocardial inflammation and fibrosis, and enhanced cardiac function compared with sedentary controls [59]. Brandao and colleagues [121] found that a combined exercise regimen—incorporating both strength and aerobic training—decreased TMAO levels, thereby mitigating cardiovascular risk and strengthening physical function in obese women.
4.3. Probiotics, Prebiotics, and Synbiotics
Probiotics, prebiotics, and synbiotics serve as prevalent therapeutic avenues for preserving gut microbiota homeostasis [122]. Probiotics, live microorganisms capable of adjusting the quantity and composition of gut microbiota, confer health benefits [35]. Prebiotics consist of non-digestible substrates selectively utilized by beneficial microorganisms in the host to promote their growth and/or activity, ultimately improving host health [122,123]. Synbiotics combine live microorganisms with substrates selectively metabolized by host microbiota, effectively integrating the benefits of both probiotics and prebiotics to foster host well-being. The synergistic and complementary effects of live microorganisms and substrates allow synbiotics to effectually influence microbiota composition and immune function [124].
Research has shown that probiotics, prebiotics, or synbiotics can remodel the gut microbiota in animals, thereby reducing gut-derived TMA synthesis, inhibiting or blocking the TMA/TMAO pathway, and ultimately decreasing TMAO levels [7]. Probiotics, including Lactobacillus plantarum ZDY04, Bifidobacterium breve Bb4, Bifidobacterium longum BL1 and BL7, and Lacticaseibacillus rhamnosus L34, may decrease TMAO concentrations by adjusting gut microbiota and restricting TMA generation [44,125,126]. Wang et al. [125] discovered that Bifidobacterium lessened the abundance of TMA-producing Ruminococcaceae UCG-009 and UCG-010, thereby lowering plasma TMAO concentrations. Among lactobacilli strains with robust adherence capability, Lactobacillus amylovorus LAM1345 and Lactiplantibacillus plantarum LP1145 individually reduced serum TMAO in choline-fed mice. Whereas, a multistrain formula (MF) containing these two strains plus Limosilactobacillus fermentum LF33 exhibited the most pronounced reduction, likely due to the additive and synergistic actions in MF [127]. Furthermore, following a 12-week synbiotic intervention decreased serum TMAO and improved metabolic profiles in patients with dyslipidemia [128].
Probiotics, prebiotics, and synbiotics hold potential in treating CVDs, like HF, via various pathophysiological mechanisms [129,130]. The gut microbiota of HF patients showed reduced probiotic abundance and elevated harmful bacteria [131]. Several probiotics, such as Lactobacillus plantarum ZDY04 [44], Lactobacillus rhamnosus GG strain [132], and Bifidobacterium lactis Probio-M8 [133], reduced TMAO concentrations, aid in CVD management, and diminish adverse cardiovascular events, indicating TMAO inhibition contributed to their cardioprotective effects. Sánchez-Quintero et al. [134] transplanted gut microbiota from patients with IHD into mice, elevating TMAO concentrations in recipients. Prebiotic essential oils from parsley (Petroselinum crispum) and rosemary (Rosmarinus officinalis) lessened plasma TMAO levels by reshaping gut microbiota in these mice. Similarly, thyme (Thymus vulgaris) and oregano (Origanum vulgare) essential oils functioned as effective prebiotics and produced comparable effects in gnotobiotic mice colonized with gut microbiota from CAD patients [135]. Synbiotics also reduced NT-proBNP levels and ameliorated the inflammatory status in patients with CHF [130]. As a result, probiotics, prebiotics, synbiotics, and their derivatives may offer safe and effective strategies for HF prevention and treatment through mechanisms including modulating gut microbiota, reducing TMAO levels, and maintaining host intestinal homeostasis [93].
Archaea, ancient single-celled prokaryotes inhabiting extreme environments and morphologically similar to bacteria [136], are also present in the human gastrointestinal tract. Certain archaea partially convert gut-derived TMA into methane, reducing TMA content [137]. Ramezani and coworkers [138] found that intestinal colonization with Methanobrevibacter smithii, a methanogenic archae, reduced plasma TMAO in ApoE−/− mice, consequently attenuating AS progression. Although current research on archaea-based therapies for CVDs is still limited, this approach appears highly promising.
4.4. Inhibitors
As previously mentioned, choline TMA lyase—which metabolizes choline into TMA—and FMOs—which oxidize TMA to produce TMAO—play critical parts in the TMAO synthesis and thus represent crucial targets for impeding TMAO production. Current methods for reducing TMAO generation typically focus on inhibiting TMA lyase or FMOs employing pharmacological inhibitors or genetic modification.
Choline TMA lyase (CutC), encoded by adjacent genes within a gene cluster, is a glycosyl radical enzyme. Both CutC and its activating protein CutD are critical for TMA biosynthesis in the gut microbiota [139]. 3,3-dimethyl-1-butanol (DMB), a choline analog, is the most widely studied inhibitor of this enzyme. It is naturally present in vinegar, extra virgin olive oil, wine, and grape seed oil; notably, extra virgin olive oil and wine are components of the MD [93,140]. Hence, DMB may partly account for the TMAO-lowering effects associated with the MD. Wang and colleagues [140] first showed in vitro and in vivo that DMB can partially inhibit TMA lyase activity in different microorganisms, blocking TMA formation from various substrates, including choline and carnitine, ultimately decreasing plasma TMAO concentrations. Additionally, DMB is non-toxic and non-lethal. Nevertheless, it does not inhibit TMA generation from certain substrates such as γ-butyrobetaine, nor does it affect TMA oxidation by FMO3 [140]. Based on DMB, the Hazen team developed a new generation of TMA lyase inhibitors: fluoromethylcholine (FMC) and iodomethylcholine (IMC). FMC and IMC irreversibly impede CutC/D, thereby effectively and continuously attenuating host TMA and TMAO levels. Furthermore, they exhibit poor absorption by the host, leave commensal bacteria unaffected, demonstrate limited systemic exposure, and are non-toxic and associated with a reduced risk of side effects [85]. Meanwhile, FMO inhibitors lower plasma TMAO by blocking hepatic TMA oxidation [141]. Methimazole, a high-affinity substrate of FMO3, acts as a competitive inhibitor, whereas indoles are non-substrate competitive FMO3 inhibitors. Both have been shown to suppress FMO3 activity and decrease TMAO concentrations [28,142]. However, the impact of methimazole on the thyroid cannot be overlooked. Therefore, inhibiting important TMAO-producing enzymes, specifically TMA lyase and FMO3, offers a plausible approach to lessening TMAO levels and ultimately managing CVDs like HF.
TMA lyase inhibitors have been extensively employed in animal models to hinder TMAO generation and ameliorate cardiovascular impairments. Studies have shown that DMB may attenuate inflammation, cardiomyocyte hypertrophy, and fibrosis, and improve cardiac function in mice with compromised hearts or HF, mainly through TMAO reduction. Furthermore, chronic DMB treatment exhibits no overt toxicity [25,27]. In mice fed WDs, DMB ameliorated excessive TMAO levels, vascular dysfunction, reduced exercise tolerance, and frailty. It also alleviated cardiac inflammation and fibrosis, consequently improving cardiac function and ultimately diminishing CVDs likelihood [143]. IMC, another TMA lyase inhibitor, reduced TMAO and BNP levels in HF mice, downregulated profibrotic gene expression, and mitigated TMAO-induced cardiac remodeling and dysfunction. Importantly, IMC also proved pharmacological safety in relevant tests [73]. FMC and IMC have demonstrated notable curative effects in lowering plasma TMAO levels and suppressing choline-triggered platelet hyperreactivity and thrombosis, without increasing bleeding risk [85]. Although DMB, IMC, and FMC have shown preliminary efficacy and safety in HF models, clinical data remain scarce. Moreover, Yoshida et al. [28] reported that either administering the FMO inhibitor methimazole or depleting the FMO2 gene lessened TMAO levels in HF mice, alleviating cardiac fibrosis and dysfunction. Antisense oligonucleotide-targeted inhibition [84] or knockout [144] of the FMO3 gene effectively lowered TMAO levels and subsequently mitigated TMAO-induced platelet hyperreactivity and thrombosis in mice. These results suggest that pharmacological or genetic inhibition of FMOs to decrease TMAO synthesis may offer a therapeutic avenue for HF. Nevertheless, clinical translation constitutes challenges, considering that FMOs participate in the oxidative metabolism of diverse drugs and chemicals in the body besides TMAO. Inhibition or deficiency of FMOs can cause hepatotoxicity and lead to liver diseases [145]. Additionally, loss of FMO3 function in humans may result in harmful TMA buildup, triggering side effects including TMAU [48]. Therefore, potential adverse effects must be thoroughly evaluated before FMO inhibitors are applied clinically.
In conclusion, targeted inhibition of TMAO-producing enzymes represents a promising tactic for addressing HF, whereas significant gaps remain between experimental studies and clinical application, warranting further comprehensive investigation [35].
4.5. Modern Medicines
4.5.1. Antibiotics
Antibiotics constitute a conventional strategy for correcting gut microbiota imbalance. They could affect diseases driven by gut microbiota, as they modify its abundance, composition, and metabolic products. Furthermore, HF onset and progression are intimately linked to gut microbial dysbiosis. Research has revealed that antibiotics can ameliorate myocardial ischemia and HF by modulating gut microbiota and its metabolites, suppressing inflammation and oxidative stress, mitigating mitochondrial damage, and bolstering cardiac function [146,147]. In addition, antibiotics decreased TMAO levels by reconstructing gut microbiota, inhibiting the proliferation of harmful gut microbiota, and restricting the conversion of dietary precursors such as choline, L-carnitine, and betaine [35]. In both healthy humans [87] and mice [91], broad-spectrum antibiotics significantly suppressed the dietary phosphatidylcholine-induced increase in plasma TMAO by modulating gut microbiota. Li and coworkers [71] found that antibiotics diminished myocardial hypertrophy and fibrosis in rats by impeding TMAO production. Moreover, depleting gut microbiota with antibiotics attenuated TMAO generation, thereby alleviating ANG II-induced HTN [75]. Broad-spectrum antibiotic therapy also lowered TMAO levels, which decreased macrophage cholesterol content and obstructed the progression of dietary choline-induced AS in mice [91].
However, antibiotic treatment is contentious. Human trials demonstrated that plasma TMAO levels declined markedly during antibiotic administration but rebounded after discontinuation [87]. In a mouse model of MI, antibiotic combination treatment depleted gut microbiota, considerably decreased SCFAs, compromised post-infarction repair, impaired cardiac function, and increased mortality [148]. Additionally, long-term antibiotic use may deplete beneficial gut microbiota and promote antibiotic resistance [149]. Given the numerous detrimental impacts, such as gut microbiota dysbiosis, toxicity, and resistance, a careful risk-benefit assessment is essential before prescribing antibiotics [35]. Further studies are needed to evaluate the potential benefits and safety of rational antibiotic use in patients with HF [93].
4.5.2. Aspirin
Aspirin presents a first-line medication for CVDs and exhibits notable therapeutic impacts on HF with AS [150]. Hazen et al. [151] demonstrated in clinical studies that low-dose aspirin significantly blunted the plasma TMAO elevation caused by a high-choline diet, thereby inhibiting thrombosis and attenuating AS progression. These results were corroborated in a subsequent investigation by Zhu and colleagues [86]. The underlying mechanism may involve aspirin’s capacity to adjust gut microbiota and suppress TMA lyase activity [151,152,153].
4.5.3. Antidiabetic Drugs
Type 2 diabetes mellitus (T2DM) constitutes an independent risk factor for HF, and proactive management of T2DM can effectively reduce the likelihood of developing HF. Metformin, a cornerstone medication for T2DM, confers cardioprotective benefits [154]. Studies confirmed that metformin significantly lowered plasma TMAO concentrations and inhibited TMA generation by gut microbiota in a mouse model of T2DM, independently of choline TMA-lyase activity [155]. Su et al. [156] further discovered that metformin reduced excessive serum TMAO levels in mice fed choline by modifying gut microbial communities involved in choline-TMA conversion. Clinical studies have established the cardiovascular benefits of SGLT2 inhibitors [157], which are now guideline-recommended therapies for HF [158]. Mindrescu et al. [159] observed that empagliflozin altered gut microbiota composition in patients with T2DM, promoting beneficial bacteria such as Bifidobacterium and Lactobacillus while suppressing proinflammatory genera like Escherichia and Streptococcus. Experimental studies showed that dapagliflozin attenuated ferroptosis in cardiomyocytes following myocardial ischemia–reperfusion injury in diabetic rats, potentially through gut microbiota modulation and decreased TMAO production [160]. A recent clinical trial found that six-month treatment with either α-glucosidase inhibitor acarbose or the dipeptidyl peptidase-4 inhibitor vildagliptin significantly reduced circulating TMAO levels in overweight or obese patients with T2DM [161]. Nonetheless, no current evidence demonstrates that other antidiabetic agents directly affect TMAO levels.
Therefore, barring any contraindications, antidiabetic drugs could be a viable option to lessen TMAO levels.
4.5.4. Statins
Statins, widely prescribed lipid-lowering agents, exhibit pleiotropic effects such as anti-atherosclerotic, endothelial-improving, anti-inflammatory, antioxidant, antifibrotic, and myocardial energy-enhancing properties [162,163,164]. These multifaceted actions may benefit HF prognosis [94,162,165]. Additionally, Li et al. [166] demonstrated that atorvastatin administration significantly lowered circulating TMAO levels in dyslipidemia patients. Similarly, rosuvastatin therapy in 112 individuals with suspected atherosclerotic cardiovascular disease (ASCVD) improved lipid profiles and notably lessened TMAO concentrations [167].
However, the use of statins in patients with HF remains complex and multifaceted. Although beneficial in many settings, their overall utility in HF patients is not fully established. For instance, different clinical trials in HFpEF populations have not consistently shown significant survival benefits with statin therapy [168,169]. Current guidelines recommend statins for HF patients with concurrent ASCVD, hyperlipidemia, or high cardiovascular risk [2,150]. Patients developing new-onset HF during ongoing statin therapy should maintain treatment unless contraindicated. Clinicians must vigilantly monitor for statin-associated myopathies or hepatotoxicity, necessitating immediate withdrawal upon such adverse events. In hepatic impairment, agents like pravastatin with non-CYP450 metabolism are preferable, whereas high-dose statins should be avoided. Notably, severe liver disease absolutely contraindicates simvastatin, atorvastatin, and lovastatin.
In conclusion, statin use in HF requires careful evaluation of clinical status, HF subtype, and comorbidities. The widespread application of statins in HF still necessitates further research to validate their efficacy and safety. Treatment decisions should therefore be individualized according to patient characteristics and guided by prevailing evidence and guidelines.
4.5.5. ACEIs
ACEIs and angiotensin receptor blockers (ARBs) are highly cost-effective therapies for HF. Studies showed that ACEIs ameliorated host intestinal dysbiosis [170,171]. Konop et al. [172] reported that the ACEI enalapril reduced plasma TMAO levels in rats, suggesting TMAO may serve as a therapeutic target for ACEIs in HF.
Although no direct evidence currently links ARBs to TMAO modulation, these agents are known to influence gut microbiota. Studies showed that ARB treatment reduced blood pressure and alleviated vascular and intestinal injury by altering the diversity, composition, and abundance of gut microbial communities [170,173]. This drug–host–microbiome interaction may extend to angiotensin receptor–neprilysin inhibitors (ARNIs), which incorporate ARB and neprilysin-inhibiting activities. As a foundational HF therapy [174], sacubitril/valsartan belongs to the ARNI class. Research indicated that it ameliorated gut microbiota dysbiosis in murine models of diabetic nephropathy [175].
The above research findings indicate that reducing TMAO levels via modern medicines, including antibiotics, aspirin, antidiabetic drugs, statins, ACEIs, etc., represents a feasible method for managing HF.
4.6. Phytomedicines
Phytomedicines can modulate gut microbiota and their metabolites, reduce TMAO concentrations, and exert certain preventive and therapeutic effects on CVDs such as HF [176]. Phytomedicines, distinguished by multi-component, multi-target, and multi-pathway attributes, could alleviate drug resistance via compensatory mechanisms and reduce adverse drug reactions to some extent [177]. They thus provide underlying new strategies for inhibiting TMAO production and treating CVDs.
Research indicated that berberine (BBR), a bioactive compound derived from Rhizoma coptidis, showed efficacy in managing HF [178]. BBR reduced TMAO generation in animal models and AS patients by remodeling gut microbiota, downregulating TMA-producing enzymes and FMO3 [179], and suppressing the choline-TMA-TMAO pathway [180], thereby curbing TMAO-induced AS. Importantly, BBR exhibited no hepatorenal toxicity [179]. The findings suggest that BBR possesses significant research and development potential. Resveratrol (RES), a natural extract from plants like Polygonum cuspidatum, lowered TMAO levels by decreasing TMA generation via remodeling gut microbiota [181], and was shown to alleviate HF in mice [182]. Garlic (Allium sativum), renowned for its antibacterial properties, contains allicin, which has been proven effective in addressing CVDs, including HF [183]. In vitro and in vivo studies confirmed that allicin and fresh garlic juice rich in allicin suppressed the TMAO synthesis through gut microbiota modulation [184]. Recent investigation has revealed that aged garlic oligosaccharides mitigate AS in ApoE−/− mice fed a high-fat and high-cholesterol diet by regulating gut microbiota and decreasing TMAO levels [185]. Hawthorn (Crataegus) and its extracts also exhibited therapeutic potential in HF [186,187]. He et al. [92] reported that hawthorn fruit extract lowered TMAO concentrations in mice dose-dependently, mediated by anti-inflammatory and antioxidant properties. Gypenosides (GYP), the primary constituent of Gynostemma pentaphyllum, lessened plasma TMAO levels by reshaping gut microbiota and inhibiting TMA lyase activity [188]. Moreover, GYP exerted cardioprotective effects and enhanced cardiac function by promoting mitochondrial autophagy, ultimately ameliorating HF [189]. Ji and coworkers [190] discovered that enemas with rhubarb (Rhei Radix et Rhizoma) modulated gut microbiota, decreasing serum TMAO and TMA concentrations and downregulating inflammatory markers in rats. Additional evidence indicated that the main constituents of rhubarb, emodin [191,192] and rhein [193,194], ameliorated pathological cardiac hypertrophy. These findings indicate that rhubarb and its extracts have promise as an innovative therapeutic agent for lowering TMAO concentrations and aiding in managing HF. Lycium barbarum polysaccharides (LBPs), important ingredients of Fructus lycii, could modulate gut microbiota, reduce intestinal permeability, downregulate inflammatory cytokines, significantly lessen serum TMAO levels, and subsequently improve LV function in mice [195]. Puerarin (PU), derived from Puerariae lobatae Radix, enhanced cardiac function in rats suffering from adriamycin-induced HF [196]. A systematic review and meta-analysis showed that combining PU injection with conventional drug therapy was safer and more effective than conventional drug therapy alone in acute heart failure [197]. Recent investigation has demonstrated that PU attenuates TMAO concentrations by altering gut microbiota composition and hindering TMA synthesis, thus exerting anti-AS effects [198]. Polymethoxyflavones (PMFs) from citrus (Citrus sinensis L.) peel decreased TMAO levels by inhibiting CutC/D and FMO3 activity, ultimately reducing CVD risk [199,200]. In addition, 5-Demethylnobiletin, a natural PMF, ameliorated isoproterenol-induced cardiac injury in mice [201].
In summary, TMAO stands out as a potential novel therapeutic target for HF, as illustrated in Figure 3.
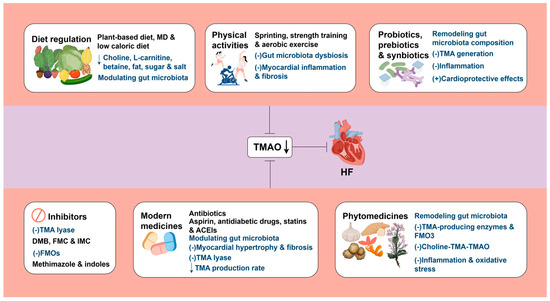
Figure 3.
TMAO represents a promising therapeutic target for HF. A downward arrow (↓) indicates a decrease. A plus sign (+) indicates promotion. A minus sign (−) indicates inhibition. MD: Mediterranean diet; TMA: trimethylamine; TMAO: trimethylamine N-oxide; HF: heart failure; DMB: 3,3-dimethyl-1-butanol; FMC: fluoromethylcholine; IMC: iodomethylcholine; FMOs: flavin-containing monooxygenases; ACEIs: Angiotensin-converting enzyme inhibitors. Created by Fidraw (www.figdraw.com, accessed on 10 October 2025).
5. Conclusions and Prospects
HF, a potentially fatal syndrome, poses major obstacles in terms of management, thereby imposing a considerable burden on healthcare systems and affected families. Research has revealed complex relationships between HF pathogenesis and dysregulation of the gut–heart axis. Specifically, patients with HF exhibit gut microbiota dysbiosis with concomitant elevation of the microbial metabolite TMAO. Furthermore, elevated TMAO levels are strongly linked to the occurrence, progression, and prognosis of HF. Therefore, the disparity between TMAO generation and metabolism could constitute a significant mechanism underlying HF. TMAO regulates the advancement of HF via multiple pathways, including inflammation, abnormal energy metabolism, oxidative stress, myocardial remodeling, and so on. Studies have confirmed that decreasing TMAO levels is advantageous in managing HF. Hence, TMAO emerges as a viable target for preventing and treating HF. Several interventions—including healthy diets, regular physical activities, probiotics, prebiotics, synbiotics, inhibitors, antibiotics, aspirin, antidiabetic drugs, statins, ACEIs, and phytomedicines—have shown promising effects in lowering TMAO concentrations and mitigating HF.
However, current research exploring the precise role of TMAO in HF pathogenesis remains limited, particularly in human studies. Many intervention trials often face constraints such as limited sample sizes and insufficient generalizability, necessitating further validation. Furthermore, current research into the mechanisms and treatment of heart failure lacks investigations across distinct phenotypes such as HFpEF and HFrEF, which obscures the phenotype-specific effects of TMAO. Dietary studies have largely been conducted in developed countries, frequently neglecting regional, ethnic, cultural, and customary disparities. Exercise protocols often include sprinting or strength training, which may be unsuitable for HF patients; more research is needed to identify safe and effective activities for those with early-stage HF or at a high risk of developing HF. Clinical trials on other preventive or therapeutic tactics are few, leaving mechanisms and safety in humans incompletely characterized. Interventions using probiotics, prebiotics, and synbiotics encounter limitations, including restricted therapeutic targets and uncertain efficacy, while microbial diversity and environmental complexity complicate the identification of beneficial strains. Although TMA lyase inhibitors have shown preliminary promise, their clinical safety and efficacy require further evaluation. Considering that mutations in the human FMO gene may result in conditions including hepatitis and TMAU, utmost prudence is required during the clinical research and development of FMO inhibitors. Antibiotic use is controversial because of potential toxic side effects and the risk of promoting antimicrobial resistance. Therefore, further clinical studies are urgently needed to fully assess the benefits, risks, and prognostic impact of antibiotic therapy. Initial studies have demonstrated that aspirin, antidiabetic drugs, statins, and ACEIs can reduce TMAO levels and HF risk. Phytomedicines possess advantages, including multi-target effects and a lower propensity for drug resistance compared to conventional drugs. Nevertheless, robust clinical data are lacking to fully substantiate the efficacy and underlying mechanisms of these agents. Moreover, the effects of commonly used HF medications—including ARBs, sacubitril/valsartan, and β-adrenergic antagonists—on TMAO levels remain poorly understood.
Furthermore, although cardiac transplantation effectively treats end-stage HF, patients receiving this procedure and its accompanying immunosuppressive therapy often exhibit elevated TMAO levels [202,203]. Several mechanisms may explain this phenomenon. Elevated TMAO precursors were associated with acute rejection and greater atherosclerotic burden [203]. And higher TMAO levels correlated with increased risk of adverse outcomes, including those post-transplantation [97,204], implying that TMAO may already be raised prior to surgery. Immunosuppressed patients are also more vulnerable to infections, and perioperative management often requires broad-spectrum antibiotics. Additionally, heart transplant recipients commonly have multiple comorbidities necessitating polypharmacy. These medications can induce intestinal dysbiosis, which may elevate circulating levels of gut microbiota-derived TMAO [205,206]. Consequently, improved hemodynamics after heart transplantation do not lower TMAO concentrations. In summary, heart transplantation does not mitigate TMAO as a pathological factor in HF. Future studies should further examine the clinical implications of elevated TMAO levels in patients receiving cardiac transplantation and associated immunosuppressive therapy.
Despite these shortcomings and limitations, targeting TMAO remains a promising innovative strategy for preventing and treating HF. More systematic clinical studies are warranted, particularly large-scale, multicenter, randomized controlled trials with sufficient observation durations, clear phenotypes, unambiguous interventions, and precise endpoints. Such efforts would help clarify the mechanisms, efficacy, and safety of TMAO-targeted intervention, providing fresh insights into HF pathogenesis and a scientific foundation for lowering TMAO as a novel strategy in its management.
Author Contributions
Conceptualization, Z.D., Y.Y. and Z.W.; methodology, J.W.; software, Z.D.; investigation, Z.D.; resources, Y.L.; writing—original draft preparation, Z.D.; writing—review and editing, Y.Y., J.W. and R.L.; visualization, Z.D.; supervision, Z.G. and Y.L.; project administration, Z.G.; funding acquisition, Z.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (82174343), the Natural Science Foundation of Hunan Province (2025JJ50658), Key Research and Development Plan of Hunan Province (2022SK2012), Key Subject of Scientific Research Projects of Traditional Chinese Medicine in Hunan Province (A2023017), Hunan Provincial Health Commission Research Project (D202303017861), Innovation Project for Graduate Students at Hunan University of Traditional Chinese Medicine (2024CX019), the Postdoctoral Fellowship Program of CPSF (GZC20252636), the China Postdoctoral Science Foundation (2025M773968 and 2025T181082), and National Natural Science Foundation of China for Young Scientists (82405372).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The graphical abstract, Figure 1 and Figure 3 were created by Figdraw (www.figdraw.com, accessed on 6 August 2025 and 10 October 2025).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| HF | Heart failure |
| CVD | Cardiovascular disease |
| IHD | Ischemic heart disease |
| LVEF | Left ventricular ejection fraction |
| HFrpEF | HF with reduced ejection fraction |
| HFpEF | HF with preserved ejection fraction |
| AS | Atherosclerosis |
| SCFAs | Short-chain fatty acids |
| TMAO | Trimethylamine N-oxide |
| BAs | Bile acids |
| LPS | Lipopolysaccharide |
| TMA | Trimethylamine |
| FMOs | Flavin-containing monooxygenases |
| TMAU | Trimethylaminuria |
| HFD | High-fat diet |
| IL | Interleukin |
| NLRP3 | Nucleotide-binding oligomerization domain-like receptor containing pyrin domain 3 |
| SIRT3 | Sirtuin 3 |
| SOD2 | Superoxide dismutase 2 |
| mtROS | Mitochondrial reactive oxygen species |
| ROS | Reactive oxygen species |
| TXNIP | Thioredoxin-interacting protein |
| TNF | Tumor necrosis factor |
| FA | Fatty acid |
| OXPHOS | Oxidative phosphorylation |
| PLIN4 | Perilipin 4 |
| OMA1 | Overlapping activity with m-AAA protease 1 |
| OGDHL | Oxoglutarate dehydrogenase L |
| BAT | Brown adipose tissue |
| CP | Creatine phosphate |
| ATP | Adenosine triphosphate |
| Ca2+ | Calcium ion |
| eNOS | Endothelial nitric oxide synthase |
| NOX2 | NADPH oxidase 2 |
| PIEZO1 | Piezo type mechanosensitive ion channel component 1 |
| WD | Western diet |
| ANP | atrial natriuretic peptide |
| β-MHC | β-myosin heavy chain |
| TGF | Transforming growth factor |
| NF | Nuclear factor |
| BNP | Brain natriuretic peptide |
| LV | Left ventricular |
| NO | Nitric oxide |
| CHF | Chronic heart failure |
| HTN | Hypertension |
| ANG | Angiotensin |
| MI | Myocardial infarction |
| RCT | Reverse cholesterol transport |
| ApoE−/− | Apolipoprotein E knockout |
| CADs | Coronary artery diseases |
| SGLT2 | Sodium-glucose cotransporter-2 |
| ACEIs | Angiotensin-converting enzyme inhibitors |
| MD | Mediterranean diet |
| MF | Multistrain formula |
| DMB | 3,3-dimethyl-1-butanol |
| FMC | Fluoromethylcholine |
| IMC | Iodomethylcholine |
| T2DM | Type 2 diabetes mellitus |
| ASCVD | Atherosclerotic cardiovascular disease |
| ARBs | Angiotensin receptor blockers |
| ARNIs | Angiotensin receptor–neprilysin inhibitors |
| BBR | Berberine |
| RES | Resveratrol |
| GYP | Gypenosides |
| LBPs | Lycium barbarum polysaccharides |
| PU | Puerarin |
| PMFs | Polymethoxyflavones |
References
- Bozkurt, B.; Coats, A.J.S.; Tsutsui, H.; Abdelhamid, C.M.; Adamopoulos, S.; Albert, N.; Anker, S.D.; Atherton, J.; Böhm, M.; Butler, J.; et al. Universal Definition and Classification of Heart Failure: A Report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: Endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur. J. Heart Fail. 2021, 23, 352–380. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef]
- Khan, M.S.; Shahid, I.; Bennis, A.; Rakisheva, A.; Metra, M.; Butler, J. Global Epidemiology of Heart Failure. Nat. Rev. Cardiol. 2024, 21, 717–734. [Google Scholar] [CrossRef]
- Yan, T.; Zhu, S.; Yin, X.; Xie, C.; Xue, J.; Zhu, M.; Weng, F.; Zhu, S.; Xiang, B.; Zhou, X.; et al. Burden, Trends, and Inequalities of Heart Failure Globally, 1990 to 2019: A Secondary Analysis Based on the Global Burden of Disease 2019 Study. J. Am. Heart Assoc. 2023, 12, e027852. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. Heart. J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Whellan, D.J.; Lindenfeld, J. Easy to Predict, Difficult to Prevent. JACC Heart Fail. 2017, 5, 226–228. [Google Scholar] [CrossRef]
- Karmazyn, M.; Gan, X.T. Probiotics as Potential Treatments to Reduce Myocardial Remodelling and Heart Failure via the Gut-Heart Axis: State-of-the-Art Review. Mol. Cell Biochem. 2023, 478, 2539–2551. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Bäckhed, F.; Landmesser, U.; Hazen, S.L. Intestinal Microbiota in Cardiovascular Health and Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 2089–2105. [Google Scholar] [CrossRef] [PubMed]
- Hemmati, M.; Kashanipoor, S.; Mazaheri, P.; Alibabaei, F.; Babaeizad, A.; Asli, S.; Mohammadi, S.; Gorgin, A.H.; Ghods, K.; Yousefi, B.; et al. Importance of Gut Microbiota Metabolites in the Development of Cardiovascular Diseases (CVD). Life Sci. 2023, 329, 121947. [Google Scholar] [CrossRef] [PubMed]
- Salzano, A.; Cassambai, S.; Yazaki, Y.; Israr, M.Z.; Bernieh, D.; Wong, M.; Suzuki, T. The Gut Axis Involvement in Heart Failure: Focus on Trimethylamine N-Oxide. Cardiol. Clin. 2022, 40, 161–169. [Google Scholar] [CrossRef]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef]
- Kaye, D.M.; Shihata, W.A.; Jama, H.A.; Tsyganov, K.; Ziemann, M.; Kiriazis, H.; Horlock, D.; Vijay, A.; Giam, B.; Vinh, A.; et al. Deficiency of Prebiotic Fiber and Insufficient Signaling Through Gut Metabolite-Sensing Receptors Leads to Cardiovascular Disease. Circulation 2020, 141, 1393–1403. [Google Scholar] [CrossRef] [PubMed]
- Trøseid, M.; Andersen, G.Ø.; Broch, K.; Hov, J.R. The Gut Microbiome in Coronary Artery Disease and Heart Failure: Current Knowledge and Future Directions. eBioMedicine 2020, 52, 102649. [Google Scholar] [CrossRef]
- Mann, D.L. Innate Immunity and the Failing Heart: The Cytokine Hypothesis Revisited. Circ. Res. 2015, 116, 1254–1268. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Huda, M.N.; Bennett, B.J. Sequence Meets Function-Microbiota and Cardiovascular Disease. Cardiovasc. Res. 2022, 118, 399–412. [Google Scholar] [CrossRef]
- Guivala, S.J.; Bode, K.A.; Okun, J.G.; Kartal, E.; Schwedhelm, E.; Pohl, L.V.; Werner, S.; Erbs, S.; Thiele, H.; Büttner, P. Interactions between the Gut Microbiome, Associated Metabolites and the Manifestation and Progression of Heart Failure with Preserved Ejection Fraction in ZSF1 Rats. Cardiovasc. Diabetol. 2024, 23, 299. [Google Scholar] [CrossRef]
- Kozhevnikova, M.V.; Kakotkina, A.V.; Korobkova, E.O.; Kuznetsov, I.V.; Shestakova, K.M.; Moskaleva, N.E.; Appolonova, S.A.; Belenkov, Y.N. Metabolomic Panel for the Diagnosis of Heart Failure with Preserved Ejection Fraction. Int. J. Mol. Sci. 2025, 26, 2102. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A Human Gut Microbial Gene Catalogue Established by Metagenomic Sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Pasini, E.; Aquilani, R.; Testa, C.; Baiardi, P.; Angioletti, S.; Boschi, F.; Verri, M.; Dioguardi, F. Pathogenic Gut Flora in Patients with Chronic Heart Failure. JACC Heart Fail. 2016, 4, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Emoto, T.; Hayashi, T.; Tabata, T.; Yamashita, T.; Watanabe, H.; Takahashi, T.; Gotoh, Y.; Kami, K.; Yoshida, N.; Saito, Y.; et al. Metagenomic Analysis of Gut Microbiota Reveals Its Role in Trimethylamine Metabolism in Heart Failure. Int. J. Cardiol. 2021, 338, 138–142. [Google Scholar] [CrossRef]
- Suzuki, T.; Yazaki, Y.; Voors, A.A.; Jones, D.J.L.; Chan, D.C.S.; Anker, S.D.; Cleland, J.G.; Dickstein, K.; Filippatos, G.; Hillege, H.L.; et al. Association with Outcomes and Response to Treatment of Trimethylamine N-Oxide in Heart Failure: Results from BIOSTAT-CHF. Eur. J. Heart Fail. 2019, 21, 877–886. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Lemaitre, R.N.; Jensen, P.N.; Wang, M.; Wang, Z.; Li, X.S.; Nemet, I.; Lee, Y.; Lai, H.T.M.; de Oliveira Otto, M.C.; et al. Trimethylamine N-Oxide and Related Gut Microbe-Derived Metabolites and Incident Heart Failure Development in Community-Based Populations. Circ. Heart Fail. 2024, 17, e011569. [Google Scholar] [CrossRef] [PubMed]
- Schuett, K.; Kleber, M.E.; Scharnagl, H.; Lorkowski, S.; März, W.; Niessner, A.; Marx, N.; Meinitzer, A. Trimethylamine-N-Oxide and Heart Failure with Reduced Versus Preserved Ejection Fraction. J. Am. Coll. Cardiol. 2017, 70, 3202–3204. [Google Scholar] [CrossRef]
- Salzano, A.; Israr, M.Z.; Yazaki, Y.; Heaney, L.M.; Kanagala, P.; Singh, A.; Arnold, J.R.; Gulsin, G.S.; Squire, I.B.; McCann, G.P.; et al. Combined Use of Trimethylamine N-Oxide with BNP for Risk Stratification in Heart Failure with Preserved Ejection Fraction: Findings from the DIAMONDHFpEF Study. Eur. J. Prev. Cardiol. 2020, 27, 2159–2162. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, H.; Chen, Y.; Peng, Y.; Yao, Y.; Xue, H.; Guo, Q.; Tian, D.; Xiao, L.; Teng, X.; et al. Trimethylamine N-Oxide Induces Cardiac Diastolic Dysfunction by down-Regulating Piezo1 in Mice with Heart Failure with Preserved Ejection Fraction. Life Sci. 2025, 369, 123554. [Google Scholar] [CrossRef]
- Chen, K.; Zheng, X.; Feng, M.; Li, D.; Zhang, H. Gut Microbiota-Dependent Metabolite Trimethylamine N-Oxide Contributes to Cardiac Dysfunction in Western Diet-Induced Obese Mice. Front. Physiol. 2017, 8, 139. [Google Scholar] [CrossRef]
- Wang, G.; Kong, B.; Shuai, W.; Fu, H.; Jiang, X.; Huang, H. 3,3-Dimethyl-1-Butanol Attenuates Cardiac Remodeling in Pressure-Overload-Induced Heart Failure Mice. J. Nutr. Biochem. 2020, 78, 108341. [Google Scholar] [CrossRef]
- Yoshida, Y.; Shimizu, I.; Shimada, A.; Nakahara, K.; Yanagisawa, S.; Kubo, M.; Fukuda, S.; Ishii, C.; Yamamoto, H.; Ishikawa, T.; et al. Brown Adipose Tissue Dysfunction Promotes Heart Failure via a Trimethylamine N-Oxide-Dependent Mechanism. Sci. Rep. 2022, 12, 14883. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Jiang, J.; Cao, S.; Xu, X.; Zhou, J.; Zhang, R.; Huang, B.; Lu, P.; Peng, L.; Liu, M. The Role of Gut Microbiota-Derived Trimethylamine N-Oxide in the Pathogenesis and Treatment of Mild Cognitive Impairment. Int. J. Mol. Sci. 2025, 26, 1373. [Google Scholar] [CrossRef]
- Rath, S.; Rud, T.; Pieper, D.H.; Vital, M. Potential TMA-Producing Bacteria Are Ubiquitously Found in Mammalia. Front. Microbiol. 2019, 10, 2966. [Google Scholar] [CrossRef] [PubMed]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal Microbiota Metabolism of L-Carnitine, a Nutrient in Red Meat, Promotes Atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.; Cheng, R.; Shen, L.; Sun, Y.; Wang, P.; Jiang, G.; Wen, L.; Li, X.; Peng, X.; Liao, Y.; et al. High-Fat Diet Induces Sarcopenic Obesity in Natural Aging Rats through the Gut-Trimethylamine N-Oxide-Muscle Axis. J. Adv. Res. 2025, 70, 405–422. [Google Scholar] [CrossRef] [PubMed]
- Andreu-Sánchez, S.; Ahmad, S.; Kurilshikov, A.; Beekman, M.; Ghanbari, M.; van Faassen, M.; van den Munckhof, I.C.L.; Steur, M.; Harms, A.; Hankemeier, T.; et al. Unraveling Interindividual Variation of Trimethylamine N-Oxide and Its Precursors at the Population Level. iMeta 2024, 3, e183. [Google Scholar] [CrossRef]
- Zhu, W.; Gregory, J.C.; Org, E.; Buffa, J.A.; Gupta, N.; Wang, Z.; Li, L.; Fu, X.; Wu, Y.; Mehrabian, M.; et al. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell 2016, 165, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Wang, Y.; Zhang, W.; Shang, H. Trimethylamine Oxide: A Potential Target for Heart Failure Therapy. Heart 2022, 108, 917–922. [Google Scholar] [CrossRef]
- Bennett, B.J.; de Aguiar Vallim, T.Q.; Wang, Z.; Shih, D.M.; Meng, Y.; Gregory, J.; Allayee, H.; Lee, R.; Graham, M.; Crooke, R.; et al. Trimethylamine-N-Oxide, a Metabolite Associated with Atherosclerosis, Exhibits Complex Genetic and Dietary Regulation. Cell Metab. 2013, 17, 49–60. [Google Scholar] [CrossRef]
- Wiedeman, A.M.; Barr, S.I.; Green, T.J.; Xu, Z.; Innis, S.M.; Kitts, D.D. Dietary Choline Intake: Current State of Knowledge Across the Life Cycle. Nutrients 2018, 10, 1513. [Google Scholar] [CrossRef]
- Koeth, R.A.; Lam-Galvez, B.R.; Kirsop, J.; Wang, Z.; Levison, B.S.; Gu, X.; Copeland, M.F.; Bartlett, D.; Cody, D.B.; Dai, H.J.; et al. L-Carnitine in Omnivorous Diets Induces an Atherogenic Gut Microbial Pathway in Humans. J. Clin. Investig. 2019, 129, 373–387. [Google Scholar] [CrossRef]
- Nikrandt, G.; Chmurzynska, A. Decoding Betaine: A Critical Analysis of Therapeutic Potential Compared with Marketing Hype-A Narrative Review. J. Nutr. 2024, 154, 3167–3176. [Google Scholar] [CrossRef]
- Jiang, H.; Hu, Y.; Zhang, Z.; Chen, X.; Gao, J. Identification of Metabolic Biomarkers Associated with Nonalcoholic Fatty Liver Disease. Lipids Health Dis. 2023, 22, 150. [Google Scholar] [CrossRef]
- Zhen, J.; Zhou, Z.; He, M.; Han, H.-X.; Lv, E.-H.; Wen, P.-B.; Liu, X.; Wang, Y.-T.; Cai, X.-C.; Tian, J.-Q.; et al. The Gut Microbial Metabolite Trimethylamine N-Oxide and Cardiovascular Diseases. Front. Endocrinol. 2023, 14, 1085041. [Google Scholar] [CrossRef] [PubMed]
- Duttaroy, A.K. Role of Gut Microbiota and Their Metabolites on Atherosclerosis, Hypertension and Human Blood Platelet Function: A Review. Nutrients 2021, 13, 144. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.W.; Wang, Z.; Kennedy, D.J.; Wu, Y.; Buffa, J.A.; Agatisa-Boyle, B.; Li, X.S.; Levison, B.S.; Hazen, S.L. Gut Microbiota-Dependent Trimethylamine N-Oxide (TMAO) Pathway Contributes to Both Development of Renal Insufficiency and Mortality Risk in Chronic Kidney Disease. Circ. Res. 2015, 116, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Tao, X.; Xiong, H.; Yu, J.; Wei, H. Lactobacillus Plantarum ZDY04 Exhibits a Strain-Specific Property of Lowering TMAO via the Modulation of Gut Microbiota in Mice. Food Funct. 2018, 9, 4299–4309. [Google Scholar] [CrossRef]
- Iglesias-Carres, L.; Chadwick-Corbin, S.A.; Sweet, M.G.; Neilson, A.P. Dietary Phenolics and Their Microbial Metabolites Are Poor Inhibitors of Trimethylamine Oxidation to Trimethylamine N-Oxide by Hepatic Flavin Monooxygenase 3. J. Nutr. Biochem. 2023, 120, 109428. [Google Scholar] [CrossRef]
- Brunt, V.E.; Gioscia-Ryan, R.A.; Casso, A.G.; VanDongen, N.S.; Ziemba, B.P.; Sapinsley, Z.J.; Richey, J.J.; Zigler, M.C.; Neilson, A.P.; Davy, K.P.; et al. Trimethylamine-N-Oxide Promotes Age-Related Vascular Oxidative Stress and Endothelial Dysfunction in Mice and Healthy Humans. Hypertension 2020, 76, 101–112. [Google Scholar] [CrossRef]
- Rath, S.; Rox, K.; Kleine Bardenhorst, S.; Schminke, U.; Dörr, M.; Mayerle, J.; Frost, F.; Lerch, M.M.; Karch, A.; Brönstrup, M.; et al. Higher Trimethylamine-N-Oxide Plasma Levels with Increasing Age Are Mediated by Diet and Trimethylamine-Forming Bacteria. mSystems 2021, 6, e0094521. [Google Scholar] [CrossRef]
- Flaherty, C.C.; Phillips, I.R.; Janmohamed, A.; Shephard, E.A. Living with Trimethylaminuria and Body and Breath Malodour: Personal Perspectives. BMC Public. Health 2024, 24, 222. [Google Scholar] [CrossRef]
- Hall, A.B.; Tolonen, A.C.; Xavier, R.J. Human Genetic Variation and the Gut Microbiome in Disease. Nat. Rev. Genet. 2017, 18, 690–699. [Google Scholar] [CrossRef]
- Kurilshikov, A.; Medina-Gomez, C.; Bacigalupe, R.; Radjabzadeh, D.; Wang, J.; Demirkan, A.; Le Roy, C.I.; Raygoza Garay, J.A.; Finnicum, C.T.; Liu, X.; et al. Large-Scale Association Analyses Identify Host Factors Influencing Human Gut Microbiome Composition. Nat. Genet. 2021, 53, 156–165. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Wang, Z.; Fan, Y.; Levison, B.; Hazen, J.E.; Donahue, L.M.; Wu, Y.; Hazen, S.L. Prognostic Value of Elevated Levels of Intestinal Microbe-Generated Metabolite Trimethylamine-N-Oxide in Patients with Heart Failure: Refining the Gut Hypothesis. J. Am. Coll. Cardiol. 2014, 64, 1908–1914. [Google Scholar] [CrossRef]
- Wang, Z.; Bergeron, N.; Levison, B.S.; Li, X.S.; Chiu, S.; Jia, X.; Koeth, R.A.; Li, L.; Wu, Y.; Tang, W.H.W.; et al. Impact of Chronic Dietary Red Meat, White Meat, or Non-Meat Protein on Trimethylamine N-Oxide Metabolism and Renal Excretion in Healthy Men and Women. Eur. Heart J. 2019, 40, 583–594. [Google Scholar] [CrossRef]
- Fang, Q.; Lei, Y.; Wu, H.; Li, C.; Jiang, J.; Wang, S.; Wu, Y.; Chen, L.; Ouyang, D.; Li, X.; et al. Plasma Reference Interval of Trimethylamine-N-Oxide in Healthy Adults: A Multicenter Study Using Trimethylamine-N-Oxide Assay Kit for Analysis and Validation. Clin. Chim. Acta 2025, 571, 120223. [Google Scholar] [CrossRef] [PubMed]
- Saaoud, F.; Liu, L.; Xu, K.; Cueto, R.; Shao, Y.; Lu, Y.; Sun, Y.; Snyder, N.W.; Wu, S.; Yang, L.; et al. Aorta- and Liver-Generated TMAO Enhances Trained Immunity for Increased Inflammation via ER Stress/Mitochondrial ROS/Glycolysis Pathways. JCI Insight 2023, 8, e158183. [Google Scholar] [CrossRef]
- Boini, K.M.; Puchchakayala, G.; Zhang, Y.; Koka, S. TMAO Activates Carotid Endothelial Inflammasomes Leading to Enhanced Neointimal Formation in Nlrp3 Mice. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Lin, Y.; Lei, Q.; Guan, K.-L.; Zhao, S.; Xiong, Y. Tumour Suppressor SIRT3 Deacetylates and Activates Manganese Superoxide Dismutase to Scavenge ROS. EMBO Rep. 2011, 12, 534–541. [Google Scholar] [CrossRef]
- Chen, M.-L.; Zhu, X.-H.; Ran, L.; Lang, H.-D.; Yi, L.; Mi, M.-T. Trimethylamine-N-Oxide Induces Vascular Inflammation by Activating the NLRP3 Inflammasome Through the SIRT3-SOD2-mtROS Signaling Pathway. J. Am. Heart Assoc. 2017, 6, e006347. [Google Scholar] [CrossRef]
- Abderrazak, A.; Syrovets, T.; Couchie, D.; El Hadri, K.; Friguet, B.; Simmet, T.; Rouis, M. NLRP3 Inflammasome: From a Danger Signal Sensor to a Regulatory Node of Oxidative Stress and Inflammatory Diseases. Redox Biol. 2015, 4, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Meng, J.; Yu, H. Trimethylamine N-Oxide Supplementation Abolishes the Cardioprotective Effects of Voluntary Exercise in Mice Fed a Western Diet. Front. Physiol. 2017, 8, 944. [Google Scholar] [CrossRef]
- Kolwicz, S.C.; Purohit, S.; Tian, R. Cardiac Metabolism and Its Interactions with Contraction, Growth, and Survival of Cardiomyocytes. Circ. Res. 2013, 113, 603–616. [Google Scholar] [CrossRef]
- Bedi, K.C.; Snyder, N.W.; Brandimarto, J.; Aziz, M.; Mesaros, C.; Worth, A.J.; Wang, L.L.; Javaheri, A.; Blair, I.A.; Margulies, K.B.; et al. Evidence for Intramyocardial Disruption of Lipid Metabolism and Increased Myocardial Ketone Utilization in Advanced Human Heart Failure. Circulation 2016, 133, 706–716. [Google Scholar] [CrossRef]
- Yoo, W.; Zieba, J.K.; Foegeding, N.J.; Torres, T.P.; Shelton, C.D.; Shealy, N.G.; Byndloss, A.J.; Cevallos, S.A.; Gertz, E.; Tiffany, C.R.; et al. High-Fat Diet-Induced Colonocyte Dysfunction Escalates Microbiota-Derived Trimethylamine N-Oxide. Science 2021, 373, 813–818. [Google Scholar] [CrossRef]
- Ikeda, K.; Maretich, P.; Kajimura, S. The Common and Distinct Features of Brown and Beige Adipocytes. Trends Endocrinol. Metab. 2018, 29, 191–200. [Google Scholar] [CrossRef]
- Tahara, A.; Tahara, N.; Maeda-Ogata, S.; Bekki, M.; Sugiyama, Y.; Honda, A.; Abe, T.; Yamagishi, S.-I.; Fukumoto, Y. Brown Adipose Tissue Activation in Severe Heart Failure. Eur. Heart J. 2020, 41, 2415. [Google Scholar] [CrossRef] [PubMed]
- Gorski, P.A.; Ceholski, D.K.; Hajjar, R.J. Altered Myocardial Calcium Cycling and Energetics in Heart Failure--a Rational Approach for Disease Treatment. Cell Metab. 2015, 21, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Querio, G.; Antoniotti, S.; Geddo, F.; Levi, R.; Gallo, M.P. Trimethylamine N-Oxide (TMAO) Impairs Purinergic Induced Intracellular Calcium Increase and Nitric Oxide Release in Endothelial Cells. Int. J. Mol. Sci. 2022, 23, 3982. [Google Scholar] [CrossRef]
- Singh, R.B.; Fedacko, J.; Pella, D.; Fatima, G.; Elkilany, G.; Moshiri, M.; Hristova, K.; Jakabcin, P.; Vaňova, N. High Exogenous Antioxidant, Restorative Treatment (Heart) for Prevention of the Six Stages of Heart Failure: The Heart Diet. Antioxidants 2022, 11, 1464. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; McGuinness, L.R.; Sharma, P.; Chadwick, A.E.; Rainbow, R.D. Trimethylamine N-Oxide (TMAO) Acutely Alters Ionic Currents but Does Not Increase Cardiac Cell Death. Front. Physiol. 2025, 16, 1505813. [Google Scholar] [CrossRef]
- Wang, J.; Gao, Y.; Ren, S.; Li, J.; Chen, S.; Feng, J.; He, B.; Zhou, Y.; Xuan, R. Gut Microbiota-Derived Trimethylamine N-Oxide: A Novel Target for the Treatment of Preeclampsia. Gut Microbes 2024, 16, 2311888. [Google Scholar] [CrossRef]
- Lee, J.; Lee, J.; Kim, K.; Lee, J.; Jung, Y.; Hyeon, J.S.; Seo, A.; Jin, W.; Weon, B.; Shin, N.; et al. Antibiotic-Induced Intestinal Microbiota Depletion Can Attenuate the Acute Kidney Injury to Chronic Kidney Disease Transition via NADPH Oxidase 2 and Trimethylamine-N-Oxide Inhibition. Kidney Int. 2024, 105, 1239–1253. [Google Scholar] [CrossRef]
- Li, Z.; Wu, Z.; Yan, J.; Liu, H.; Liu, Q.; Deng, Y.; Ou, C.; Chen, M. Gut Microbe-Derived Metabolite Trimethylamine N-Oxide Induces Cardiac Hypertrophy and Fibrosis. Lab. Investig. 2019, 99, 346–357. [Google Scholar] [CrossRef]
- Organ, C.L.; Otsuka, H.; Bhushan, S.; Wang, Z.; Bradley, J.; Trivedi, R.; Polhemus, D.J.; Tang, W.H.W.; Wu, Y.; Hazen, S.L.; et al. Choline Diet and Its Gut Microbe-Derived Metabolite, Trimethylamine N-Oxide, Exacerbate Pressure Overload-Induced Heart Failure. Circ. Heart Fail. 2016, 9, e002314. [Google Scholar] [CrossRef]
- Organ, C.L.; Li, Z.; Sharp, T.E.; Polhemus, D.J.; Gupta, N.; Goodchild, T.T.; Tang, W.H.W.; Hazen, S.L.; Lefer, D.J. Nonlethal Inhibition of Gut Microbial Trimethylamine N-Oxide Production Improves Cardiac Function and Remodeling in a Murine Model of Heart Failure. J. Am. Heart Assoc. 2020, 9, e016223. [Google Scholar] [CrossRef]
- Zou, D.; Li, Y.; Sun, G. Attenuation of Circulating Trimethylamine N-Oxide Prevents the Progression of Cardiac and Renal Dysfunction in a Rat Model of Chronic Cardiorenal Syndrome. Front. Pharmacol. 2021, 12, 751380. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Shui, Y.; Cui, Y.; Tang, C.; Wang, X.; Qiu, X.; Hu, W.; Fei, L.; Li, Y.; Zhang, S.; et al. Gut Microbiota Dependent Trimethylamine N-Oxide Aggravates Angiotensin II-Induced Hypertension. Redox Biol. 2021, 46, 102115. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Yang, P.; Liu, X.; Lu, L.; Chen, Y.; Zhong, X.; Li, Z.; Liu, H.; Ou, C.; et al. Trimethylamine-N-Oxide Promotes Vascular Calcification Through Activation of NLRP3 (Nucleotide-Binding Domain, Leucine-Rich-Containing Family, Pyrin Domain-Containing-3) Inflammasome and NF-κB (Nuclear Factor κB) Signals. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 751–765. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Bale, S.; Verma, P.; Wan, Q.; Ma, F.; Gudjonsson, J.E.; Hazen, S.L.; Harms, P.W.; Tsou, P.-S.; Khanna, D.; et al. Gut Microbe-Derived Metabolite Trimethylamine N-Oxide Activates PERK to Drive Fibrogenic Mesenchymal Differentiation. iScience 2022, 25, 104669. [Google Scholar] [CrossRef] [PubMed]
- Hatamnejad, M.R.; Medzikovic, L.; Dehghanitafti, A.; Rahman, B.; Vadgama, A.; Eghbali, M. Role of Gut Microbial Metabolites in Ischemic and Non-Ischemic Heart Failure. Int. J. Mol. Sci. 2025, 26, 2242. [Google Scholar] [CrossRef] [PubMed]
- Cheng, E.; Hung, S.-C.; Lin, T.-Y. Association of Trimethylamine N-Oxide and Metabolites with Kidney Function Decline in Patients with Chronic Kidney Disease. Clin. Nutr. 2025, 44, 239–247. [Google Scholar] [CrossRef]
- Dannenberg, L.; Zikeli, D.; Benkhoff, M.; Ahlbrecht, S.; Kelm, M.; Levkau, B.; Polzin, A. Targeting the Human Microbiome and Its Metabolite TMAO in Cardiovascular Prevention and Therapy. Pharmacol. Ther. 2020, 213, 107584. [Google Scholar] [CrossRef]
- Ge, X.; Zheng, L.; Zhuang, R.; Yu, P.; Xu, Z.; Liu, G.; Xi, X.; Zhou, X.; Fan, H. The Gut Microbial Metabolite Trimethylamine N-Oxide and Hypertension Risk: A Systematic Review and Dose-Response Meta-Analysis. Adv. Nutr. 2020, 11, 66–76. [Google Scholar] [CrossRef]
- Brunt, V.E.; Casso, A.G.; Gioscia-Ryan, R.A.; Sapinsley, Z.J.; Ziemba, B.P.; Clayton, Z.S.; Bazzoni, A.E.; VanDongen, N.S.; Richey, J.J.; Hutton, D.A.; et al. Gut Microbiome-Derived Metabolite Trimethylamine N-Oxide Induces Aortic Stiffening and Increases Systolic Blood Pressure with Aging in Mice and Humans. Hypertension 2021, 78, 499–511. [Google Scholar] [CrossRef]
- Tang, X.; Wang, P.; Zhang, R.; Watanabe, I.; Chang, E.; Vinayachandran, V.; Nayak, L.; Lapping, S.; Liao, S.; Madera, A.; et al. KLF2 Regulates Neutrophil Activation and Thrombosis in Cardiac Hypertrophy and Heart Failure Progression. J. Clin. Investig. 2022, 132, e147191. [Google Scholar] [CrossRef]
- Zhu, W.; Buffa, J.A.; Wang, Z.; Warrier, M.; Schugar, R.; Shih, D.M.; Gupta, N.; Gregory, J.C.; Org, E.; Fu, X.; et al. Flavin Monooxygenase 3, the Host Hepatic Enzyme in the Metaorganismal Trimethylamine N-Oxide-Generating Pathway, Modulates Platelet Responsiveness and Thrombosis Risk. J. Thromb. Haemost. 2018, 16, 1857–1872. [Google Scholar] [CrossRef]
- Roberts, A.B.; Gu, X.; Buffa, J.A.; Hurd, A.G.; Wang, Z.; Zhu, W.; Gupta, N.; Skye, S.M.; Cody, D.B.; Levison, B.S.; et al. Development of a Gut Microbe-Targeted Nonlethal Therapeutic to Inhibit Thrombosis Potential. Nat. Med. 2018, 24, 1407–1417. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Wang, Z.; Tang, W.H.W.; Hazen, S.L. Gut Microbe-Generated Trimethylamine N-Oxide From Dietary Choline Is Prothrombotic in Subjects. Circulation 2017, 135, 1671–1673. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.W.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal Microbial Metabolism of Phosphatidylcholine and Cardiovascular Risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Angelini, A.; Li, S.; Wang, G.; Li, L.; Patterson, C.; Pi, X.; Xie, L. CRAT Links Cholesterol Metabolism to Innate Immune Responses in the Heart. Nat. Metab. 2023, 5, 1382–1394. [Google Scholar] [CrossRef]
- Ding, L.; Chang, M.; Guo, Y.; Zhang, L.; Xue, C.; Yanagita, T.; Zhang, T.; Wang, Y. Trimethylamine-N-Oxide (TMAO)-Induced Atherosclerosis Is Associated with Bile Acid Metabolism. Lipids Health Dis. 2018, 17, 286. [Google Scholar] [CrossRef]
- Komaroff, A.L. The Microbiome and Risk for Atherosclerosis. JAMA 2018, 319, 2381–2382. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.-M.; et al. Gut Flora Metabolism of Phosphatidylcholine Promotes Cardiovascular Disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef]
- He, Z.; Kwek, E.; Hao, W.; Zhu, H.; Liu, J.; Ma, K.Y.; Chen, Z.-Y. Hawthorn Fruit Extract Reduced Trimethylamine-N-Oxide (TMAO)-Exacerbated Atherogenesis in Mice via Anti-Inflammation and Anti-Oxidation. Nutr. Metab. 2021, 18, 6. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Ke, B.; Du, J. TMAO: How Gut Microbiota Contributes to Heart Failure. Transl. Res. 2021, 228, 109–125. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Hernández, S.; González-Sosa, S.; Conde-Martel, A.; Trullàs, J.C.; Llàcer, P.; Pérez-Silvestre, J.; Arévalo-Lorido, J.C.; Casado, J.; Formiga, F.; Manzano, L.; et al. Prognostic Impact of Statins in Heart Failure with Preserved Ejection Fraction. J. Clin. Med. 2024, 13, 5844. [Google Scholar] [CrossRef] [PubMed]
- Kinugasa, Y.; Nakamura, K.; Kamitani, H.; Hirai, M.; Yanagihara, K.; Kato, M.; Yamamoto, K. Trimethylamine N-Oxide and Outcomes in Patients Hospitalized with Acute Heart Failure and Preserved Ejection Fraction. ESC Heart Fail. 2021, 8, 2103–2110. [Google Scholar] [CrossRef]
- Gui, X.Y.; Rabkin, S.W. C-Reactive Protein, Interleukin-6, Trimethylamine-N-Oxide, Syndecan-1, Nitric Oxide, and Tumor Necrosis Factor Receptor-1 in Heart Failure with Preserved Versus Reduced Ejection Fraction: A Meta-Analysis. Curr. Heart Fail. Rep. 2023, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Zhao, M.; Huang, M.; Li, C.; Gao, J.; Yu, T.; Zhang, Q.; Shen, X.; Ji, L.; Ni, L.; et al. FMO3-TMAO Axis Modulates the Clinical Outcome in Chronic Heart-Failure Patients with Reduced Ejection Fraction: Evidence from an Asian Population. Front. Med. 2022, 16, 295–305. [Google Scholar] [CrossRef]
- Ramos-Romero, S.; Hereu, M.; Atienza, L.; Casas, J.; Jáuregui, O.; Amézqueta, S.; Dasilva, G.; Medina, I.; Nogués, M.R.; Romeu, M.; et al. Mechanistically Different Effects of Fat and Sugar on Insulin Resistance, Hypertension, and Gut Microbiota in Rats. Am. J. Physiol. Endocrinol. Metab. 2018, 314, E552–E563. [Google Scholar] [CrossRef]
- Matsiras, D.; Bezati, S.; Ventoulis, I.; Verras, C.; Parissis, J.; Polyzogopoulou, E. Gut Failure: A Review of the Pathophysiology and Therapeutic Potentials in the Gut-Heart Axis. J. Clin. Med. 2023, 12, 2567. [Google Scholar] [CrossRef]
- Miranda, P.M.; De Palma, G.; Serkis, V.; Lu, J.; Louis-Auguste, M.P.; McCarville, J.L.; Verdu, E.F.; Collins, S.M.; Bercik, P. High Salt Diet Exacerbates Colitis in Mice by Decreasing Lactobacillus Levels and Butyrate Production. Microbiome 2018, 6, 57. [Google Scholar] [CrossRef]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell 2016, 167, 1339–1353.e21. [Google Scholar] [CrossRef]
- Hargreaves, S.M.; Raposo, A.; Saraiva, A.; Zandonadi, R.P. Vegetarian Diet: An Overview through the Perspective of Quality of Life Domains. Int. J. Environ. Res. Public. Health 2021, 18, 4067. [Google Scholar] [CrossRef]
- Shen, X.; Tilves, C.; Kim, H.; Tanaka, T.; Spira, A.P.; Chia, C.W.; Talegawkar, S.A.; Ferrucci, L.; Mueller, N.T. Plant-Based Diets and the Gut Microbiome: Findings from the Baltimore Longitudinal Study of Aging. Am. J. Clin. Nutr. 2024, 119, 628–638. [Google Scholar] [CrossRef]
- Soldán, M.; Argalášová, Ľ.; Hadvinová, L.; Galileo, B.; Babjaková, J. The Effect of Dietary Types on Gut Microbiota Composition and Development of Non-Communicable Diseases: A Narrative Review. Nutrients 2024, 16, 3134. [Google Scholar] [CrossRef] [PubMed]
- Alzughayyar, D.-K.N.; Weber, R.-M.; Husain, S.; Schoch, N.; Englert, H. Impact of the Healthy Lifestyle Community Program (HLCP-3) on Trimethylamine N-Oxide (TMAO) and Risk Profile Parameters Related to Lifestyle Diseases During the Six Months Following an Intervention Study. Nutrients 2025, 17, 298. [Google Scholar] [CrossRef]
- Merra, G.; Noce, A.; Marrone, G.; Cintoni, M.; Tarsitano, M.G.; Capacci, A.; De Lorenzo, A. Influence of Mediterranean Diet on Human Gut Microbiota. Nutrients 2020, 13, 7. [Google Scholar] [CrossRef]
- Ghosh, T.S.; Rampelli, S.; Jeffery, I.B.; Santoro, A.; Neto, M.; Capri, M.; Giampieri, E.; Jennings, A.; Candela, M.; Turroni, S.; et al. Mediterranean Diet Intervention Alters the Gut Microbiome in Older People Reducing Frailty and Improving Health Status: The NU-AGE 1-Year Dietary Intervention across Five European Countries. Gut 2020, 69, 1218–1228. [Google Scholar] [CrossRef]
- Tektonidis, T.G.; Åkesson, A.; Gigante, B.; Wolk, A.; Larsson, S.C. Adherence to a Mediterranean Diet Is Associated with Reduced Risk of Heart Failure in Men. Eur. J. Heart Fail. 2016, 18, 253–259. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. High-Level Adherence to a Mediterranean Diet Beneficially Impacts the Gut Microbiota and Associated Metabolome. Gut 2016, 65, 1812–1821. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- Kaluza, J.; Levitan, E.B.; Michaëlsson, K.; Wolk, A. Anti-Inflammatory Diet and Risk of Heart Failure: Two Prospective Cohort Studies. Eur. J. Heart Fail. 2020, 22, 676–682. [Google Scholar] [CrossRef]
- Marques, F.Z.; Nelson, E.; Chu, P.-Y.; Horlock, D.; Fiedler, A.; Ziemann, M.; Tan, J.K.; Kuruppu, S.; Rajapakse, N.W.; El-Osta, A.; et al. High-Fiber Diet and Acetate Supplementation Change the Gut Microbiota and Prevent the Development of Hypertension and Heart Failure in Hypertensive Mice. Circulation 2017, 135, 964–977. [Google Scholar] [CrossRef]
- Erickson, M.L.; Malin, S.K.; Wang, Z.; Brown, J.M.; Hazen, S.L.; Kirwan, J.P. Effects of Lifestyle Intervention on Plasma Trimethylamine N-Oxide in Obese Adults. Nutrients 2019, 11, 179. [Google Scholar] [CrossRef]
- Battillo, D.J.; Malin, S.K. Impact of Caloric Restriction and Exercise on Trimethylamine N-Oxide Metabolism in Women with Obesity. Nutrients 2023, 15, 1455. [Google Scholar] [CrossRef]
- Bozkurt, B.; Fonarow, G.C.; Goldberg, L.R.; Guglin, M.; Josephson, R.A.; Forman, D.E.; Lin, G.; Lindenfeld, J.; O’Connor, C.; Panjrath, G.; et al. Cardiac Rehabilitation for Patients with Heart Failure: JACC Expert Panel. J. Am. Coll. Cardiol. 2021, 77, 1454–1469. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.S.; Dalal, H.M.; Zwisler, A.-D. Cardiac Rehabilitation for Heart Failure: “Cinderella” or Evidence-Based Pillar of Care? Eur. Heart J. 2023, 44, 1511–1518. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.M.; Mailing, L.J.; Niemiro, G.M.; Moore, R.; Cook, M.D.; White, B.A.; Holscher, H.D.; Woods, J.A. Exercise Alters Gut Microbiota Composition and Function in Lean and Obese Humans. Med. Sci. Sports Exerc. 2018, 50, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Pechlivanis, A.; Papaioannou, K.G.; Tsalis, G.; Saraslanidis, P.; Mougios, V.; Theodoridis, G.A. Monitoring the Response of the Human Urinary Metabolome to Brief Maximal Exercise by a Combination of RP-UPLC-MS and (1)H NMR Spectroscopy. J. Proteome Res. 2015, 14, 4610–4622. [Google Scholar] [CrossRef] [PubMed]
- Pechlivanis, A.; Kostidis, S.; Saraslanidis, P.; Petridou, A.; Tsalis, G.; Veselkov, K.; Mikros, E.; Mougios, V.; Theodoridis, G.A. 1H NMR Study on the Short- and Long-Term Impact of Two Training Programs of Sprint Running on the Metabolic Fingerprint of Human Serum. J. Proteome Res. 2013, 12, 470–480. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, G.; Li, R.; Liu, R.; Yu, Z.; Zhang, Z.; Wan, Z. Trimethylamine N-Oxide Aggravated Cognitive Impairment from APP/PS1 Mice and Protective Roles of Voluntary Exercise. Neurochem. Int. 2023, 162, 105459. [Google Scholar] [CrossRef]
- Brandao, C.F.C.; Krempf, M.; Giolo de Carvalho, F.; Aguesse, A.; Junqueira-Franco, M.V.M.; Batitucci, G.; de Freitas, E.C.; Noronha, N.Y.; Rodrigues, G.d.S.; Junqueira, G.P.; et al. Sphingolipid and Trimethylamine-N-Oxide (TMAO) Levels in Women with Obesity after Combined Physical Training. Metabolites 2024, 14, 398. [Google Scholar] [CrossRef]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and Prebiotics in Intestinal Health and Disease: From Biology to the Clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar] [CrossRef]
- Hiel, S.; Gianfrancesco, M.A.; Rodriguez, J.; Portheault, D.; Leyrolle, Q.; Bindels, L.B.; Gomes da Silveira Cauduro, C.; Mulders, M.D.G.H.; Zamariola, G.; Azzi, A.-S.; et al. Link between Gut Microbiota and Health Outcomes in Inulin -Treated Obese Patients: Lessons from the Food4Gut Multicenter Randomized Placebo-Controlled Trial. Clin. Nutr. 2020, 39, 3618–3628. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Guo, M.; Liu, Y.; Xu, M.; Shi, L.; Li, X.; Zhao, J.; Zhang, H.; Wang, G.; Chen, W. Bifidobacterium Breve and Bifidobacterium Longum Attenuate Choline-Induced Plasma Trimethylamine N-Oxide Production by Modulating Gut Microbiota in Mice. Nutrients 2022, 14, 1222. [Google Scholar] [CrossRef]
- Tungsanga, S.; Panpetch, W.; Bhunyakarnjanarat, T.; Udompornpitak, K.; Katavetin, P.; Chancharoenthana, W.; Chatthanathon, P.; Somboonna, N.; Tungsanga, K.; Tumwasorn, S.; et al. Uremia-Induced Gut Barrier Defect in 5/6 Nephrectomized Mice Is Worsened by Candida Administration through a Synergy of Uremic Toxin, Lipopolysaccharide, and (1→3)-β-D-Glucan, but Is Attenuated by Lacticaseibacillus Rhamnosus L34. Int. J. Mol. Sci. 2022, 23, 2511. [Google Scholar] [CrossRef]
- Ramireddy, L.; Tsen, H.-Y.; Chiang, Y.-C.; Hung, C.-Y.; Wu, S.-R.; Young, S.-L.; Lin, J.-S.; Huang, C.-H.; Chiu, S.-H.; Chen, C.-C.; et al. Molecular Identification and Selection of Probiotic Strains Able to Reduce the Serum TMAO Level in Mice Challenged with Choline. Foods 2021, 10, 2931. [Google Scholar] [CrossRef] [PubMed]
- Salamat, S.; Jahan-Mihan, A.; Tabandeh, M.R.; Mansoori, A. Randomized Clinical Trial Evaluating the Efficacy of Synbiotic Supplementation on Serum Endotoxin and Trimethylamine N-Oxide Levels in Patients with Dyslipidaemia. Arch. Med. Sci. Atheroscler. Dis. 2024, 9, e18–e25. [Google Scholar] [CrossRef]
- Pourrajab, B.; Naderi, N.; Janani, L.; Mofid, V.; Hajahmadi, M.; Dehnad, A.; Shidfar, F. Comparison of Probiotic Yogurt and Ordinary Yogurt Consumption on Serum Pentraxin3, NT-proBNP, oxLDL, and ApoB100 in Patients with Chronic Heart Failure: A Randomized, Triple-Blind, Controlled Trial. Food Funct. 2020, 11, 10000–10010. [Google Scholar] [CrossRef]
- Shoaei Matin, S.; Shidfar, F.; Naderi, N.; Amin, A.; Hosseini-Baharanchi, F.S.; Dehnad, A. The Effect of Synbiotic Consumption on Serum NTproBNP, hsCRP and Blood Pressure in Patients with Chronic Heart Failure: A Randomized, Triple-Blind, Controlled Trial. Front. Nutr. 2021, 8, 822498. [Google Scholar] [CrossRef]
- Cui, H.; Han, S.; Dai, Y.; Xie, W.; Zheng, R.; Sun, Y.; Xia, X.; Deng, X.; Cao, Y.; Zhang, M.; et al. Gut Microbiota and Integrative Traditional Chinese and Western Medicine in Prevention and Treatment of Heart Failure. Phytomedicine 2023, 117, 154885. [Google Scholar] [CrossRef]
- Liu, J.; Li, T.; Wu, H.; Shi, H.; Bai, J.; Zhao, W.; Jiang, D.; Jiang, X. Lactobacillus Rhamnosus GG Strain Mitigated the Development of Obstructive Sleep Apnea-Induced Hypertension in a High Salt Diet via Regulating TMAO Level and CD4+ T Cell Induced-Type I Inflammation. Biomed. Pharmacother. 2019, 112, 108580. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Ma, T.; Li, Y.; Yang, N.; Li, B.; Zhou, X.; Guo, S.; Zhang, S.; Kwok, L.-Y.; Sun, Z.; et al. Bifidobacterium Lactis Probio-M8 Adjuvant Treatment Confers Added Benefits to Patients with Coronary Artery Disease via Target Modulation of the Gut-Heart/-Brain Axes. mSystems 2022, 7, e0010022. [Google Scholar] [CrossRef]
- Sánchez-Quintero, M.J.; Delgado, J.; Medina-Vera, D.; Becerra-Muñoz, V.M.; Queipo-Ortuño, M.I.; Estévez, M.; Plaza-Andrades, I.; Rodríguez-Capitán, J.; Sánchez, P.L.; Crespo-Leiro, M.G.; et al. Beneficial Effects of Essential Oils from the Mediterranean Diet on Gut Microbiota and Their Metabolites in Ischemic Heart Disease and Type-2 Diabetes Mellitus. Nutrients 2022, 14, 4650. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Quintero, M.J.; Delgado, J.; Martín Chaves, L.; Medina-Vera, D.; Murri, M.; Becerra-Muñoz, V.M.; Estévez, M.; Crespo-Leiro, M.G.; Paz López, G.; González-Jiménez, A.; et al. Multi-Omics Approach Reveals Prebiotic and Potential Antioxidant Effects of Essential Oils from the Mediterranean Diet on Cardiometabolic Disorder Using Humanized Gnotobiotic Mice. Antioxidants 2023, 12, 1643. [Google Scholar] [CrossRef]
- Eme, L.; Doolittle, W.F. Archaea. Curr. Biol. 2015, 25, R851–R855. [Google Scholar] [CrossRef]
- Hoegenauer, C.; Hammer, H.F.; Mahnert, A.; Moissl-Eichinger, C. Methanogenic Archaea in the Human Gastrointestinal Tract. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 805–813. [Google Scholar] [CrossRef]
- Ramezani, A.; Nolin, T.D.; Barrows, I.R.; Serrano, M.G.; Buck, G.A.; Regunathan-Shenk, R.; West, R.E.; Latham, P.S.; Amdur, R.; Raj, D.S. Gut Colonization with Methanogenic Archaea Lowers Plasma Trimethylamine N-Oxide Concentrations in Apolipoprotein e−/− Mice. Sci. Rep. 2018, 8, 14752. [Google Scholar] [CrossRef] [PubMed]
- Craciun, S.; Balskus, E.P. Microbial Conversion of Choline to Trimethylamine Requires a Glycyl Radical Enzyme. Proc. Natl. Acad. Sci. USA 2012, 109, 21307–21312. [Google Scholar] [CrossRef]
- Wang, Z.; Roberts, A.B.; Buffa, J.A.; Levison, B.S.; Zhu, W.; Org, E.; Gu, X.; Huang, Y.; Zamanian-Daryoush, M.; Culley, M.K.; et al. Non-Lethal Inhibition of Gut Microbial Trimethylamine Production for the Treatment of Atherosclerosis. Cell 2015, 163, 1585–1595. [Google Scholar] [CrossRef]
- Zixin, Y.; Lulu, C.; Xiangchang, Z.; Qing, F.; Binjie, Z.; Chunyang, L.; Tai, R.; Dongsheng, O. TMAO as a Potential Biomarker and Therapeutic Target for Chronic Kidney Disease: A Review. Front. Pharmacol. 2022, 13, 929262. [Google Scholar] [CrossRef]
- Chen, S.; Henderson, A.; Petriello, M.C.; Romano, K.A.; Gearing, M.; Miao, J.; Schell, M.; Sandoval-Espinola, W.J.; Tao, J.; Sha, B.; et al. Trimethylamine N-Oxide Binds and Activates PERK to Promote Metabolic Dysfunction. Cell Metab. 2019, 30, 1141–1151.e5. [Google Scholar] [CrossRef]
- Brunt, V.E.; Greenberg, N.T.; Sapinsley, Z.J.; Casso, A.G.; Richey, J.J.; VanDongen, N.S.; Gioscia-Ryan, R.A.; Ziemba, B.P.; Neilson, A.P.; Davy, K.P.; et al. Suppression of Trimethylamine N-Oxide with DMB Mitigates Vascular Dysfunction, Exercise Intolerance, and Frailty Associated with a Western-Style Diet in Mice. J. Appl. Physiol. 2022, 133, 798–813. [Google Scholar] [CrossRef]
- Shih, D.M.; Zhu, W.; Schugar, R.C.; Meng, Y.; Jia, X.; Miikeda, A.; Wang, Z.; Zieger, M.; Lee, R.; Graham, M.; et al. Genetic Deficiency of Flavin-Containing Monooxygenase 3 (Fmo3) Protects Against Thrombosis but Has Only a Minor Effect on Plasma Lipid Levels-Brief Report. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1045–1054. [Google Scholar] [CrossRef]
- Agarwal, M.; Roth, K.; Yang, Z.; Sharma, R.; Maddipati, K.; Westrick, J.; Petriello, M.C. Loss of Flavin-Containing Monooxygenase 3 Modulates Dioxin-like Polychlorinated Biphenyl 126-Induced Oxidative Stress and Hepatotoxicity. Environ. Res. 2024, 250, 118492. [Google Scholar] [CrossRef] [PubMed]
- Riba, A.; Deres, L.; Eros, K.; Szabo, A.; Magyar, K.; Sumegi, B.; Toth, K.; Halmosi, R.; Szabados, E. Doxycycline Protects against ROS-Induced Mitochondrial Fragmentation and ISO-Induced Heart Failure. PLoS ONE 2017, 12, e0175195. [Google Scholar] [CrossRef] [PubMed]
- Lam, V.; Su, J.; Hsu, A.; Gross, G.J.; Salzman, N.H.; Baker, J.E. Intestinal Microbial Metabolites Are Linked to Severity of Myocardial Infarction in Rats. PLoS ONE 2016, 11, e0160840. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.W.H.; Chen, H.-C.; Chen, C.-Y.; Yen, C.Y.T.; Lin, C.-J.; Prajnamitra, R.P.; Chen, L.-L.; Ruan, S.-C.; Lin, J.-H.; Lin, P.-J.; et al. Loss of Gut Microbiota Alters Immune System Composition and Cripples Postinfarction Cardiac Repair. Circulation 2019, 139, 647–659. [Google Scholar] [CrossRef]
- Peng, J.; Xiao, X.; Hu, M.; Zhang, X. Interaction between Gut Microbiome and Cardiovascular Disease. Life Sci. 2018, 214, 153–157. [Google Scholar] [CrossRef]
- Virani, S.S.; Newby, L.K.; Arnold, S.V.; Bittner, V.; Brewer, L.C.; Demeter, S.H.; Dixon, D.L.; Fearon, W.F.; Hess, B.; Johnson, H.M.; et al. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA Guideline for the Management of Patients with Chronic Coronary Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2023, 148, e9–e119. [Google Scholar] [CrossRef]
- Hazen, S.L. Treating and Preventing Disease with TMA and TMAO Lowering Agents 2016. US9694020B2, 4 July 2017. [Google Scholar]
- Zhao, W.; Li, Z.; Yu, M.L.; Liu, Y.; Liu, C.C.; Jia, X.J.; Liu, M.Q.; Li, Y.G. Aspirin Inhibits Rotavirus Replication and Alters Rat Gut Microbial Composition. Virol. J. 2023, 20, 237. [Google Scholar] [CrossRef]
- Li, T.; Ding, N.; Guo, H.; Hua, R.; Lin, Z.; Tian, H.; Yu, Y.; Fan, D.; Yuan, Z.; Gonzalez, F.J.; et al. A Gut Microbiota-Bile Acid Axis Promotes Intestinal Homeostasis upon Aspirin-Mediated Damage. Cell Host Microbe 2024, 32, 191–208.e9. [Google Scholar] [CrossRef]
- Xu, Z.; Pan, Z.; Jin, Y.; Gao, Z.; Jiang, F.; Fu, H.; Chen, X.; Zhang, X.; Yan, H.; Yang, X.; et al. Inhibition of PRKAA/AMPK (Ser485/491) Phosphorylation by Crizotinib Induces Cardiotoxicity via Perturbing Autophagosome-Lysosome Fusion. Autophagy 2024, 20, 416–436. [Google Scholar] [CrossRef]
- Kuka, J.; Videja, M.; Makrecka-Kuka, M.; Liepins, J.; Grinberga, S.; Sevostjanovs, E.; Vilks, K.; Liepinsh, E.; Dambrova, M. Metformin Decreases Bacterial Trimethylamine Production and Trimethylamine N-Oxide Levels in Db/Db Mice. Sci. Rep. 2020, 10, 14555. [Google Scholar] [CrossRef]
- Su, C.; Li, X.; Yang, Y.; Du, Y.; Zhang, X.; Wang, L.; Hong, B. Metformin Alleviates Choline Diet-Induced TMAO Elevation in C57BL/6J Mice by Influencing Gut-Microbiota Composition and Functionality. Nutr. Diabetes 2021, 11, 27. [Google Scholar] [CrossRef]
- Piperis, C.; Marathonitis, A.; Anastasiou, A.; Theofilis, P.; Mourouzis, K.; Giannakodimos, A.; Tryfou, E.; Oikonomou, E.; Siasos, G.; Tousoulis, D. Multifaceted Impact of SGLT2 Inhibitors in Heart Failure Patients: Exploring Diverse Mechanisms of Action. Biomedicines 2024, 12, 2314. [Google Scholar] [CrossRef]
- Beghini, A.; Sammartino, A.M.; Papp, Z.; von Haehling, S.; Biegus, J.; Ponikowski, P.; Adamo, M.; Falco, L.; Lombardi, C.M.; Pagnesi, M.; et al. 2024 Update in Heart Failure. ESC Heart Fail 2025, 12, 8–42. [Google Scholar] [CrossRef]
- Mindrescu, N.M.; Guja, C.; Jinga, V.; Ispas, S.; Curici, A.; Danciulescu Miulescu, R.E.; Nelson Twakor, A.; Pantea Stoian, A.M. SGLT-2 Inhibitors and Metabolic Outcomes: A Primary Data Study Exploring the Microbiota-Diabetes Connection. Metabolites 2025, 15, 411. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Y.; Xu, H.; Li, W. Effect of Dapagliflozin on Ferroptosis through the Gut Microbiota Metabolite TMAO during Myocardial Ischemia-Reperfusion Injury in Diabetes Mellitus Rats. Sci. Rep. 2024, 14, 13851. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, X.; Sun, C.; Zhao, C.; Kong, X.; Zhao, M.; Ji, L.; Li, Y. Effect of Acarbose and Vildagliptin on Plasma Trimethylamine N-Oxide Levels in Patients with Type 2 Diabetes Mellitus: A 6-Month, Two-Arm Randomized Controlled Trial. Front. Endocrinol. 2025, 16, 1575087. [Google Scholar] [CrossRef] [PubMed]
- Ovchinnikov, A.; Potekhina, A.; Arefieva, T.; Filatova, A.; Ageev, F.; Belyavskiy, E. Use of Statins in Heart Failure with Preserved Ejection Fraction: Current Evidence and Perspectives. Int. J. Mol. Sci. 2024, 25, 4958. [Google Scholar] [CrossRef]
- Birnbaum, Y.; Ye, Y. Pleiotropic Effects of Statins: The Role of Eicosanoid Production. Curr. Atheroscler. Rep. 2012, 14, 135–139. [Google Scholar] [CrossRef]
- Cc, H.; Cy, L.; Ch, H.; Hl, C.; Yh, C.; Yp, L.; Yr, L.; Hf, K.; Pl, L. Mitochondrial Protection by Simvastatin against Angiotensin II-Mediated Heart Failure. Br. J. Pharmacol. 2019, 176, 3791–3804. [Google Scholar] [CrossRef]
- Zheng, X.; Tan, L.; Zhang, Y. The Impact of Statin Use on Short-Term and Long-Term Mortality in Patients with Heart Failure. Front. Pharmacol. 2024, 15, 1397763. [Google Scholar] [CrossRef]
- Li, D.Y.; Li, X.S.; Chaikijurajai, T.; Li, L.; Wang, Z.; Hazen, S.L.; Tang, W.H.W. Relation of Statin Use to Gut Microbial Trimethylamine N-Oxide and Cardiovascular Risk. Am. J. Cardiol. 2022, 178, 26–34. [Google Scholar] [CrossRef]
- Xiong, X.; Zhou, J.; Fu, Q.; Xu, X.; Wei, S.; Yang, S.; Chen, B. The Associations between TMAO-Related Metabolites and Blood Lipids and the Potential Impact of Rosuvastatin Therapy. Lipids Health Dis. 2022, 21, 60. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, T.; Kajio, H. Favorable Effects of Statins in the Treatment of Heart Failure with Preserved Ejection Fraction in Patients without Ischemic Heart Disease. Int. J. Cardiol. 2018, 255, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Alehagen, U.; Benson, L.; Edner, M.; Dahlström, U.; Lund, L.H. Association Between Use of Statins and Mortality in Patients with Heart Failure and Ejection Fraction of ≥50. Circ. Heart Fail. 2015, 8, 862–870. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, P.; Jiao, J.; Yang, X.; Chen, M.; Li, J. Antihypertensive Therapy by ACEI/ARB Is Associated with Intestinal Flora Alterations and Metabolomic Profiles in Hypertensive Patients. Front. Cell Dev. Biol. 2022, 10, 861829. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Jiang, P.; Li, S.; Sun, J.; Qi, C. ACE Inhibitory Casein Peptide Lowers Blood Pressure and Reshapes Gut Microbiota in a Randomized Double Blind Placebo Controlled Trial. Sci. Rep. 2025, 15, 13840. [Google Scholar] [CrossRef]
- Konop, M.; Radkowski, M.; Grochowska, M.; Perlejewski, K.; Samborowska, E.; Ufnal, M. Enalapril Decreases Rat Plasma Concentration of TMAO, a Gut Bacteria-Derived Cardiovascular Marker. Biomarkers 2018, 23, 380–385. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.-Y.; Yan, K.-X.; Wang, P.; Jiao, J.; Wang, Y.-D.; Chen, M.-L.; Dong, Y.; Zhong, J.-C. Intestinal Microbiota by Angiotensin Receptor Blocker Therapy Exerts Protective Effects against Hypertensive Damages. iMeta 2024, 3, e222. [Google Scholar] [CrossRef]
- Drazner, M.H. Angiotensin Receptor-Neprilysin Inhibition (ARNI) Therapy and Reverse Remodeling in Heart Failure with Reduced Ejection Fraction. JAMA 2019, 322, 1051–1053. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Guo, R.; Bai, X.; Cui, W.; Zhang, Y.; Li, H.; Shang, J.; Zhao, Z. Sacubitril/Valsartan Contributes to Improving the Diabetic Kidney Disease and Regulating the Gut Microbiota in Mice. Front. Endocrinol. 2022, 13, 1034818. [Google Scholar] [CrossRef]
- Jia, Q.; Wang, L.; Zhang, X.; Ding, Y.; Li, H.; Yang, Y.; Zhang, A.; Li, Y.; Lv, S.; Zhang, J. Prevention and Treatment of Chronic Heart Failure through Traditional Chinese Medicine: Role of the Gut Microbiota. Pharmacol. Res. 2020, 151, 104552. [Google Scholar] [CrossRef] [PubMed]
- Hao, P.-P.; Jiang, F.; Chen, Y.-G.; Yang, J.; Zhang, K.; Zhang, M.-X.; Zhang, C.; Zhao, Y.-X.; Zhang, Y. Traditional Chinese Medication for Cardiovascular Disease. Nat. Rev. Cardiol. 2015, 12, 115–122. [Google Scholar] [CrossRef]
- Cai, Y.; Xin, Q.; Lu, J.; Miao, Y.; Lin, Q.; Cong, W.; Chen, K. A New Therapeutic Candidate for Cardiovascular Diseases: Berberine. Front. Pharmacol. 2021, 12, 631100. [Google Scholar] [CrossRef]
- Li, X.; Su, C.; Jiang, Z.; Yang, Y.; Zhang, Y.; Yang, M.; Zhang, X.; Du, Y.; Zhang, J.; Wang, L.; et al. Berberine Attenuates Choline-Induced Atherosclerosis by Inhibiting Trimethylamine and Trimethylamine-N-Oxide Production via Manipulating the Gut Microbiome. NPJ Biofilms Microbiomes 2021, 7, 36. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.-R.; Tong, Q.; Lin, Y.; Pan, L.-B.; Fu, J.; Peng, R.; Zhang, X.-F.; Zhao, Z.-X.; Li, Y.; Yu, J.-B.; et al. Berberine Treats Atherosclerosis via a Vitamine-like Effect down-Regulating Choline-TMA-TMAO Production Pathway in Gut Microbiota. Signal Transduct. Target. Ther. 2022, 7, 207. [Google Scholar] [CrossRef]
- Chen, M.; Yi, L.; Zhang, Y.; Zhou, X.; Ran, L.; Yang, J.; Zhu, J.; Zhang, Q.; Mi, M. Resveratrol Attenuates Trimethylamine-N-Oxide (TMAO)-Induced Atherosclerosis by Regulating TMAO Synthesis and Bile Acid Metabolism via Remodeling of the Gut Microbiota. mBio 2016, 7, e02210–e02215. [Google Scholar] [CrossRef]
- Zhang, W.; Qian, S.; Tang, B.; Kang, P.; Zhang, H.; Shi, C. Resveratrol Inhibits Ferroptosis and Decelerates Heart Failure Progression via Sirt1/P53 Pathway Activation. J. Cell Mol. Med. 2023, 27, 3075–3089. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, B.; Qin, G.; Liang, S.; Yin, J.; Jiang, H.; Liu, M.; Li, X. Therapeutic Potentials of Allicin in Cardiovascular Disease: Advances and Future Directions. Chin. Med. 2024, 19, 93. [Google Scholar] [CrossRef]
- Panyod, S.; Wu, W.-K.; Chen, P.-C.; Chong, K.-V.; Yang, Y.-T.; Chuang, H.-L.; Chen, C.-C.; Chen, R.-A.; Liu, P.-Y.; Chung, C.-H.; et al. Atherosclerosis Amelioration by Allicin in Raw Garlic through Gut Microbiota and Trimethylamine-N-Oxide Modulation. NPJ Biofilms Microbiomes 2022, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cui, J.; Gu, Z.; Guo, L.; Liu, R.; Guo, Y.; Qin, N.; Yang, Y. Aged Garlic Oligosaccharides Modulate Host Metabolism and Gut Microbiota to Alleviate High-Fat and High-Cholesterol Diet-Induced Atherosclerosis in ApoE−/− Mice. Food Chem. 2025, 463, 141409. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-T.; Lin, H.-R.; Yang, C.-S.; Liaw, C.-C.; Sung, P.-J.; Kuo, Y.-H.; Cheng, M.-J.; Chen, J.-J. Antioxidant and Anti-α-Glucosidase Activities of Various Solvent Extracts and Major Bioactive Components from the Fruits of Crataegus Pinnatifida. Antioxidants 2022, 11, 320. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Jang, E.; Lee, J.-H. Potential Roles and Key Mechanisms of Hawthorn Extract against Various Liver Diseases. Nutrients 2022, 14, 867. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Guo, X.; Xie, C.; Cao, Z.; Wang, X.; Liu, L.; Yang, P. Unraveling the Metabolic Pathway of Choline-TMA-TMAO: Effects of Gypenosides and Implications for the Therapy of TMAO Related Diseases. Pharmacol. Res. 2021, 173, 105884. [Google Scholar] [CrossRef]
- Zheng, Y.; Wei, W.; Wang, Y.; Li, T.; Wei, Y.; Gao, S. Gypenosides Exert Cardioprotective Effects by Promoting Mitophagy and Activating PI3K/Akt/GSK-3β/Mcl-1 Signaling. PeerJ 2024, 12, e17538. [Google Scholar] [CrossRef]
- Ji, C.; Li, Y.; Mo, Y.; Lu, Z.; Lu, F.; Lin, Q.; Liu, X.; Zou, C.; Wu, Y. Rhubarb Enema Decreases Circulating Trimethylamine N-Oxide Level and Improves Renal Fibrosis Accompanied with Gut Microbiota Change in Chronic Kidney Disease Rats. Front. Pharmacol. 2021, 12, 780924. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, K.; Wang, Y.; Guo, R.; Liu, H.; Jia, C.; Sun, X.; Wu, C.; Wang, W.; Du, J.; et al. A Machine Learning-Driven Study Indicates Emodin Improves Cardiac Hypertrophy by Modulation of Mitochondrial SIRT3 Signaling. Pharmacol. Res. 2020, 155, 104739. [Google Scholar] [CrossRef]
- Evans, L.W.; Bender, A.; Burnett, L.; Godoy, L.; Shen, Y.; Staten, D.; Zhou, T.; Angermann, J.E.; Ferguson, B.S. Emodin and Emodin-Rich Rhubarb Inhibits Histone Deacetylase (HDAC) Activity and Cardiac Myocyte Hypertrophy. J. Nutr. Biochem. 2020, 79, 108339. [Google Scholar] [CrossRef]
- Lu, W.; Zhu, H.; Wu, J.; Liao, S.; Cheng, G.; Li, X. Rhein Attenuates Angiotensin II-Induced Cardiac Remodeling by Modulating AMPK-FGF23 Signaling. J. Transl. Med. 2022, 20, 305. [Google Scholar] [CrossRef]
- Li, R.-J.; Xu, J.-J.; Zhang, Z.-H.; Chen, M.-W.; Liu, S.-X.; Yang, C.; Li, Y.-L.; Luo, P.; Liu, Y.-J.; Tang, R.; et al. Rhein Ameliorates Transverse Aortic Constriction-Induced Cardiac Hypertrophy via Regulating STAT3 and P38 MAPK Signaling Pathways. Front. Pharmacol. 2022, 13, 940574. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, H.; Yu, B.; Tao, H.; Li, J.; Wu, Z.; Liu, G.; Yuan, C.; Guo, L.; Cui, B. Lycium barbarum Polysaccharide Attenuates Myocardial Injury in High-Fat Diet-Fed Mice through Manipulating the Gut Microbiome and Fecal Metabolome. Food Res. Int. 2020, 138, 109778. [Google Scholar] [CrossRef]
- Pan, G.; Cui, B.; Han, M.; Lin, L.; Li, Y.; Wang, L.; Guo, S.; Yin, Y.; Zhan, H.; Li, P. Puerarin Inhibits NHE1 Activity by Interfering with the P38 Pathway and Attenuates Mitochondrial Damage Induced by Myocardial Calcium Overload in Heart Failure Rats. Acta Biochim. Biophys. Sin. 2024, 56, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Fan, Y.; Huang, C.; Liu, Q.; Huang, M.; Chen, B.; Peng, Z.; Zhu, W.; Ding, B. Efficacy and Safety of Puerarin Injection on Acute Heart Failure: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2022, 9, 934598. [Google Scholar] [CrossRef]
- Li, Z.H.; Weng, J.; Yan, J.; Zeng, Y.H.; Hao, Q.Y.; Sheng, H.F. Puerarin Alleviates Atherosclerosis via the Inhibition of Prevotella copri and Its Trimethylamine Production. Gut 2024, 73, 1934–1943. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Liu, X.; An, J.-P.; Wang, Y. Identification of Polymethoxyflavones (PMFs) from Orange Peel and Their Inhibitory Effects on the Formation of Trimethylamine (TMA) and Trimethylamine-N-Oxide (TMAO) Using cntA/B and cutC/D Enzymes and Molecular Docking. J. Agric. Food Chem. 2023, 71, 16114–16124. [Google Scholar] [CrossRef]
- Chen, P.-Y.; Li, S.; Koh, Y.-C.; Wu, J.-C.; Yang, M.-J.; Ho, C.-T.; Pan, M.-H. Oolong Tea Extract and Citrus Peel Polymethoxyflavones Reduce Transformation of L-Carnitine to Trimethylamine-N-Oxide and Decrease Vascular Inflammation in l-Carnitine Feeding Mice. J. Agric. Food Chem. 2019, 67, 7869–7879. [Google Scholar] [CrossRef]
- Du, H.; Huangfu, W.; Liu, Z.; Jia, G.; Zhao, F.; Cheng, W. 5-Demethylnobiletin Ameliorates Isoproterenol-Induced Cardiac Fibrosis and Apoptosis by Repressing the Sirt1/FOXO3a/NF-κB and Wnt/β-Catenin Pathways. Biol. Pharm. Bull. 2024, 47, 1774–1785. [Google Scholar] [CrossRef] [PubMed]
- Yuzefpolskaya, M.; Bohn, B.; Javaid, A.; Mondellini, G.M.; Braghieri, L.; Pinsino, A.; Onat, D.; Cagliostro, B.; Kim, A.; Takeda, K.; et al. Levels of Trimethylamine N-Oxide Remain Elevated Long Term After Left Ventricular Assist Device and Heart Transplantation and Are Independent from Measures of Inflammation and Gut Dysbiosis. Circ. Heart Fail. 2021, 14, e007909. [Google Scholar] [CrossRef] [PubMed]
- Trøseid, M.; Mayerhofer, C.C.K.; Broch, K.; Arora, S.; Svardal, A.; Hov, J.R.; Andreassen, A.K.; Gude, E.; Karason, K.; Dellgren, G.; et al. The Carnitine-Butyrobetaine-TMAO Pathway after Cardiac Transplant: Impact on Cardiac Allograft Vasculopathy and Acute Rejection. J. Heart Lung Transplant. 2019, 38, 1097–1103. [Google Scholar] [CrossRef]
- Trøseid, M.; Ueland, T.; Hov, J.R.; Svardal, A.; Gregersen, I.; Dahl, C.P.; Aakhus, S.; Gude, E.; Bjørndal, B.; Halvorsen, B.; et al. Microbiota-Dependent Metabolite Trimethylamine-N-Oxide Is Associated with Disease Severity and Survival of Patients with Chronic Heart Failure. J. Intern. Med. 2015, 277, 717–726. [Google Scholar] [CrossRef]
- Yuzefpolskaya, M.; Bohn, B.; Nasiri, M.; Zuver, A.M.; Onat, D.D.; Royzman, E.A.; Nwokocha, J.; Mabasa, M.; Pinsino, A.; Brunjes, D.; et al. Gut Microbiota, Endotoxemia, Inflammation, and Oxidative Stress in Patients with Heart Failure, Left Ventricular Assist Device, and Transplant. J. Heart Lung Transplant. 2020, 39, 880–890. [Google Scholar] [CrossRef] [PubMed]
- Maier, L.; Pruteanu, M.; Kuhn, M.; Zeller, G.; Telzerow, A.; Anderson, E.E.; Brochado, A.R.; Fernandez, K.C.; Dose, H.; Mori, H.; et al. Extensive Impact of Non-Antibiotic Drugs on Human Gut Bacteria. Nature 2018, 555, 623–628. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).