Trandolapril Attenuates Pro-Arrhythmic Downregulation of Cx43 and Cx40 in Atria of Volume Overloaded Hypertensive and Normotensive Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Serum Biomarker Assessment
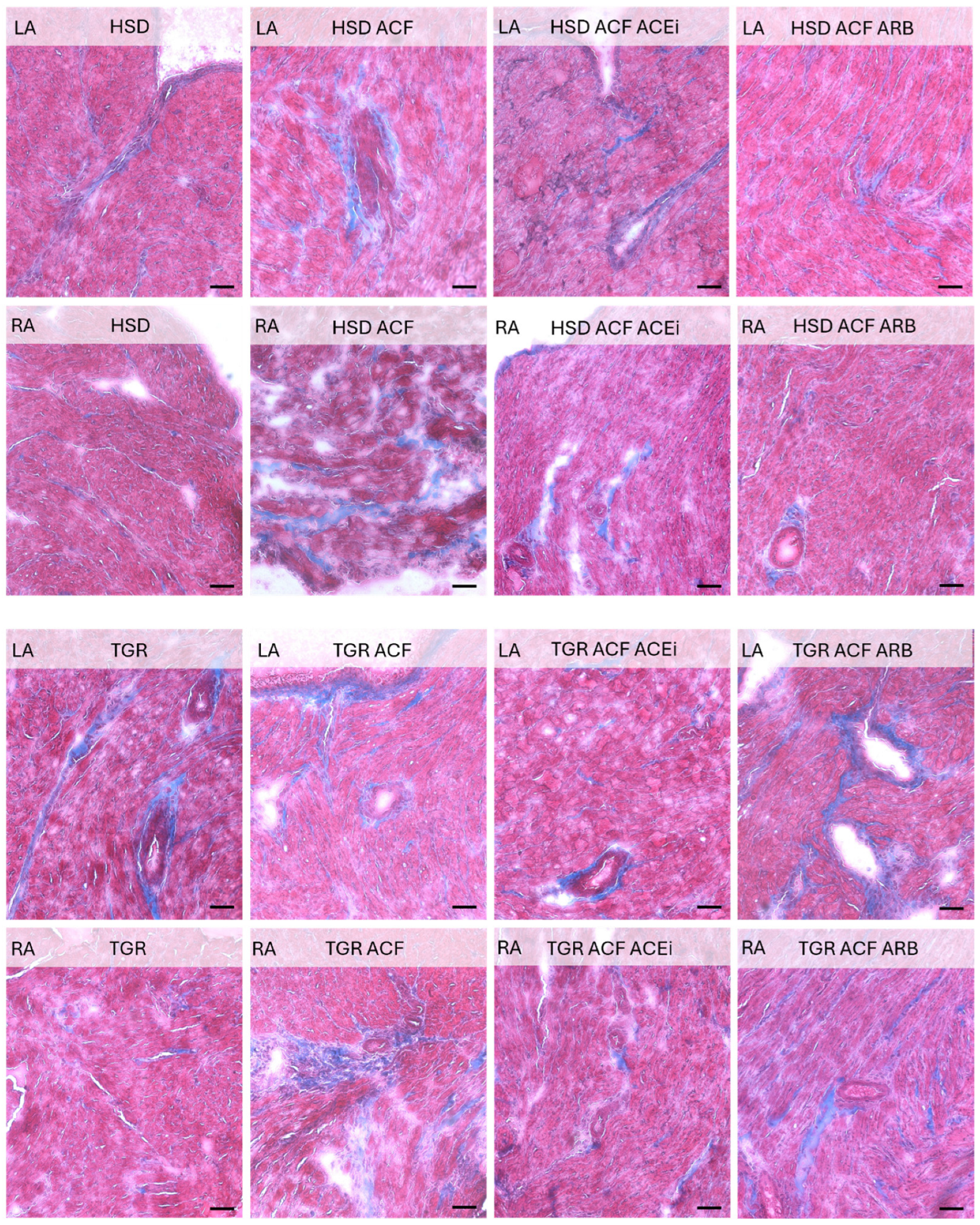
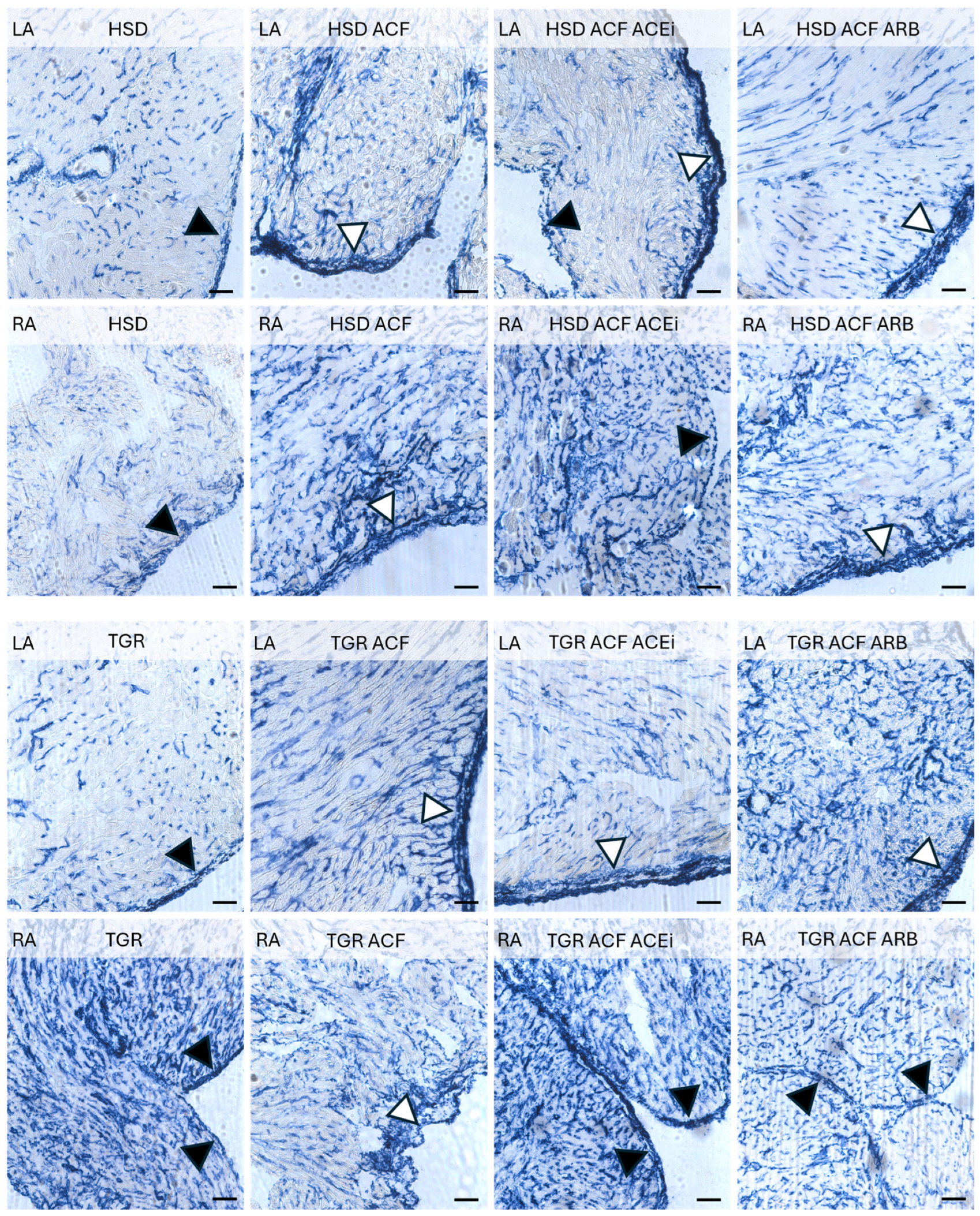
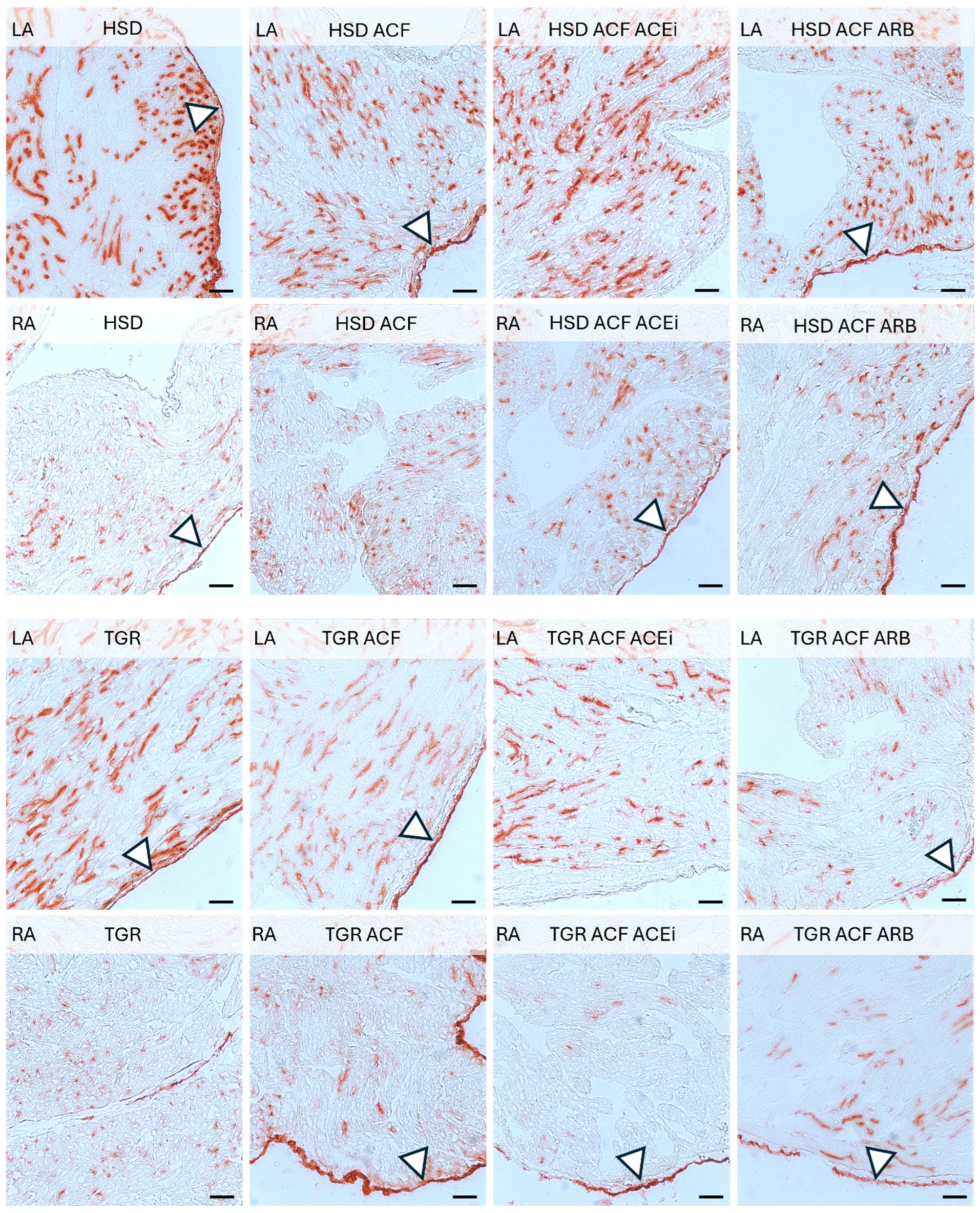
2.3. Microscopic Examination of Left and Right Heart Atrial Tissue
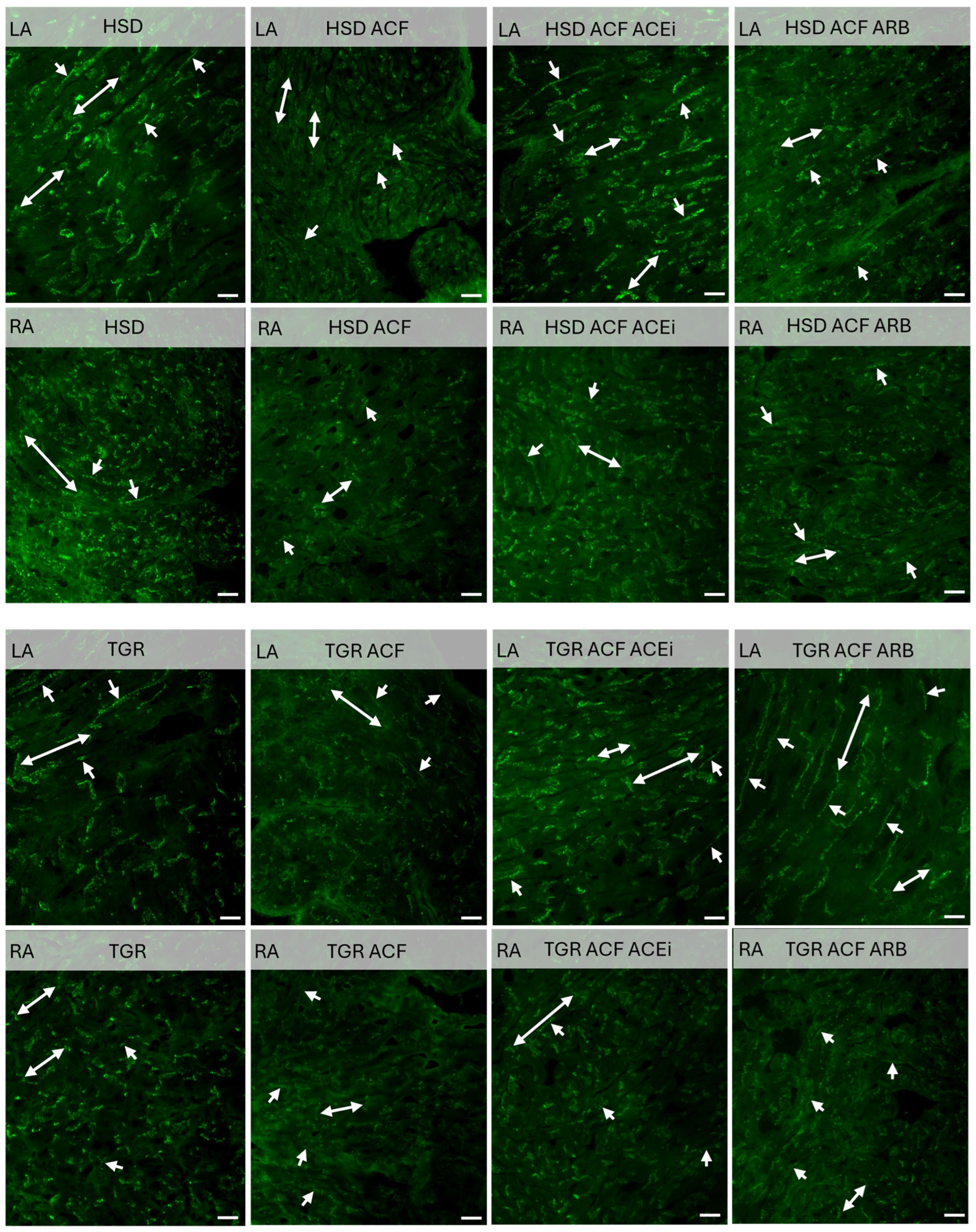
2.4. Western Blot Analysis of Left and Right Atrial Tissue
2.5. Statistical Evaluation
3. Results
3.1. Biometric and Biochemical Parameters
3.2. Microscopic Findings of Left and Right Atrial Tissue of Volume-Overloaded Normotensive and Hypertensive Rats
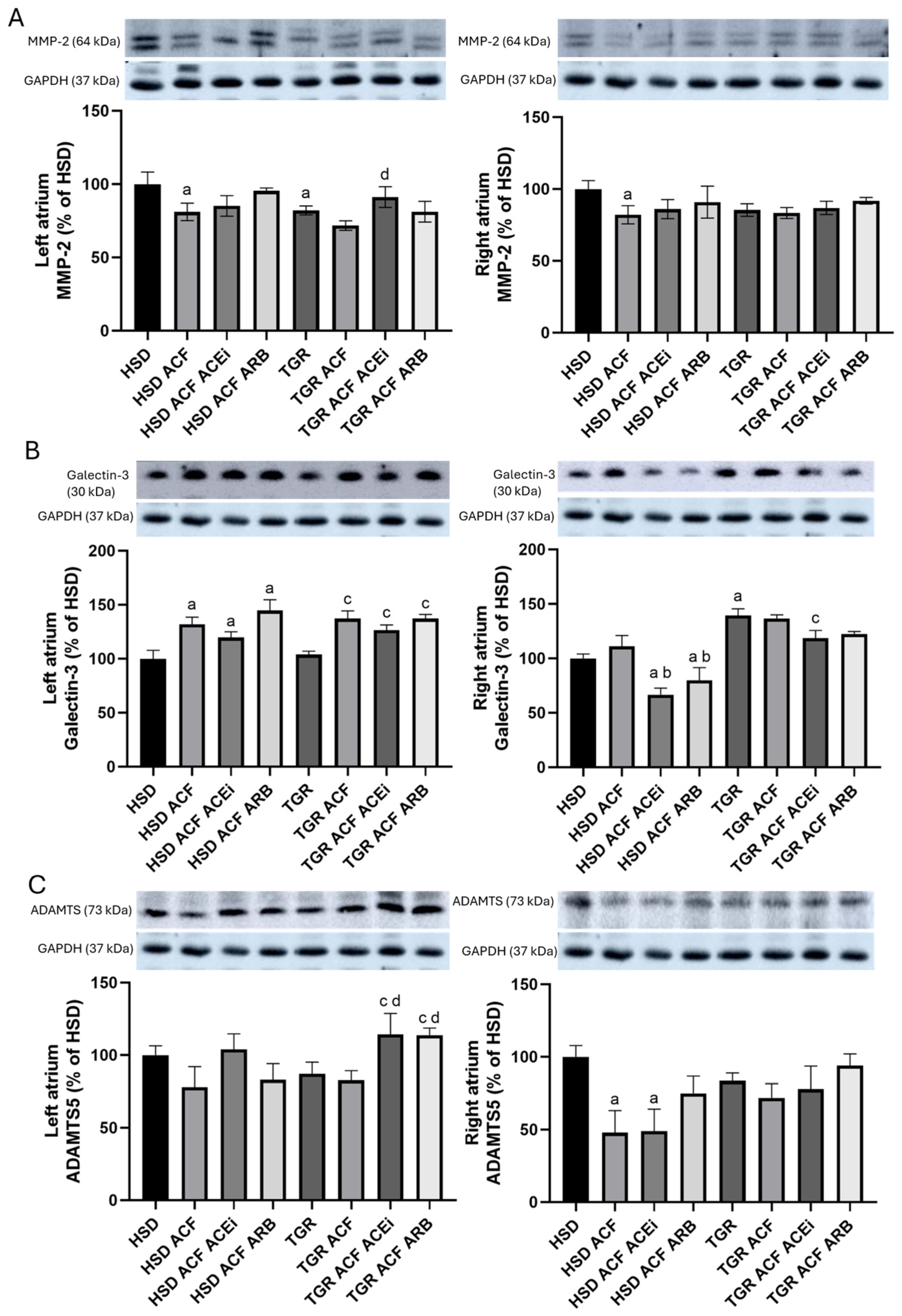
3.3. Findings on Examined Protein Levels in the Left and Right Atria of Volume-Overloaded Normotensive and Hypertensive Rats
4. Discussion
5. Conclusions
Limitations of This Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HTN | Atrial hypertension |
| HF | Heart failure |
| SHR | Spontaneously hypertensive rats |
| TGR | Hypertensive Ren-2 transgenic rats |
| RAAS | Renin–angiotensin–aldosterone system |
| ACF | Aorto-caval fistula |
| AF | Atrial fibrillation |
| Cx43 | Connexin-43 |
| ACEi | Angiotensin-converting enzyme inhibitor |
| ARB | Angiotensin II type 1 receptor blocker |
| AP | Alkaline phosphatase |
| DPP-4 | Dipeptidyl peptidase-4 |
| GP | Glycogen phosphorylase |
| SDH | Succinate dehydrogenase |
| β-HBDH | β-hydroxybutyrate dehydrogenase |
| IOD | Integral optical density |
| BW | Body weight |
| HW | Heart weight |
| LVW | Left ventricular weight |
| RWV | Right ventricular weight |
| ANP | Atrial natriuretic peptide |
| TBARS | Thiobarbituric acid reactive substances |
| TIMP-2 | Tissue inhibitor of metalloproteinases-2 |
| HSD | Hannover Sprague Dawley |
| ACF | Aortocaval fistula, surgical model of volume overload |
| Cx40 | Connexin-40 |
| PKCε | Protein Kinase C epsilon |
| PKCδ | Protein Kinase C delta |
| MMP-2 | Matrix Metalloproteinase-2 |
| ADAMTS | A Disintegrin And Metalloproteinase with Thrombospondin Motifs |
References
- Buckley, L.F.; Baker, W.L.; Van Tassell, B.W.; Cohen, J.B.; Alkhezi, O.; Bress, A.P.; Dixon, D.L. Systolic Blood Pressure Time in Target Range and Major Adverse Kidney and Cardiovascular Events. Hypertension 2023, 80, 305–313. [Google Scholar] [CrossRef]
- Gawrys, O.; Husková, Z.; Škaroupková, P.; Honetschlägerová, Z.; Vaňourková, Z.; Kikerlová, S.; Melenovský, V.; Bačová, B.S.; Sykora, M.; Táborský, M.; et al. The treatment with sGC stimulator improves survival of hypertensive rats in response to volume-overload induced by aorto-caval fistula. Naunyn. Schmiedebergs. Arch. Pharmacol. 2023, 396, 3757–3773. [Google Scholar] [CrossRef] [PubMed]
- Sykora, M.; Andelova, K.; Szeiffova Bacova, B.; Egan Benova, T.; Martiskova, A.; Knezl, V.; Tribulova, N. Hypertension Induces Pro-arrhythmic Cardiac Connexome Disorders: Protective Effects of Treatment. Biomolecules 2023, 13, 330. [Google Scholar] [CrossRef]
- Bueno, H.; Moura, B.; Lancellotti, P.; Bauersachs, J. The year in cardiovascular medicine 2020: Heart failure and cardiomyopathies. Eur. Heart J. 2021, 42, 657–670. [Google Scholar] [CrossRef]
- Kitai, T.; Kohsaka, S.; Kato, T.; Kato, E.; Sato, K.; Teramoto, K.; Yaku, H.; Akiyama, E.; Ando, M.; Izumi, C.; et al. JCS/JHFS 2025 Guideline on Diagnosis and Treatment of Heart Failure. Circ. J. 2025, 89, CJ-25-0002. [Google Scholar] [CrossRef]
- Langheinrich, M.; Lee, M.A.; Böhm, M.; Pinto, Y.M.; Ganten, D.; Paul, M. The hypertensive Ren-2 transgenic rat TGR(mREN2)27 in hypertension research: Characteristics and functional aspects. Am. J. Hypertens. 1996, 9, 506–512. [Google Scholar] [CrossRef]
- Sykora, M.; Kratky, V.; Cervenka, L.; Kopkan, L.; Tribulova, N.; Szeiffova Bacova, B. The treatment with trandolapril and losartan attenuates pressure and volume overload alternations of cardiac connexin-43 and extracellular matrix in Ren-2 transgenic rats. Sci. Rep. 2023, 13, 20923. [Google Scholar] [CrossRef]
- Jarkovská, D.; Miklovič, M.; Švíglerová, J.; Červenka, L.; Škaroupková, P.; Melenovský, V.; Štengl, M. Effects of Trandolapril on Structural, Contractile and Electrophysiological Remodeling in Experimental Volume Overload Heart Failure. Front. Pharmacol. 2021, 12, 729568. [Google Scholar] [CrossRef]
- Stein, M.; Boulaksil, M.; Jansen, J.A.; Herold, E.; Noorman, M.; Joles, J.A.; Van Veen, T.A.B.; Houtman, M.J.C.; Engelen, M.A.; Hauer, R.N.W.; et al. Reduction of fibrosis-related arrhythmias by chronic renin-angiotensin- aldosterone system inhibitors in an aged mouse model. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Tamargo, J.; Agewall, S.; Ambrosio, G.; Borghi, C.; Cerbai, E.; Dan, G.A.; Drexel, H.; Ferdinandy, P.; Grove, E.L.; Klingenberg, R.; et al. New pharmacological agents and novel cardiovascular pharmacotherapy strategies in 2024. Eur. Heart J. Cardiovasc. Pharmacother. 2025, 11, 292–317. [Google Scholar] [CrossRef] [PubMed]
- Kratky, V.; Vanourkova, Z.; Sykora, M.; Bacova, B.S.; Hruskova, Z.; Kikerlova, S.; Huskova, Z.; Kopkan, L. AT1 receptor blocker, but not an ACE inhibitor, prevents kidneys from hypoperfusion during congestive heart failure in normotensive and hypertensive rats. Sci. Rep. 2021, 11, 4271. [Google Scholar] [CrossRef] [PubMed]
- Thiele, A.; Bartolomaeus, H.; Anandakumar, H.; Haase, N.; Heuser, A.; Müller, D.N.; Dechend, R.; Wilck, N. A novel approach to modelling acute cardiac decompensation in rats. Cardiovasc. Res. 2025, 121, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Mansfield, C.; Diakonov, I.; Judina, A.; Delahaye, M.; Bhogal, N.; Sanchez-Alonso, J.L.; Kamp, T.; Gorelik, J. Stretch regulation of β2-Adrenoceptor signalling in cardiomyocytes requires caveolae. Cardiovasc. Res. 2025, 121, 440–453. [Google Scholar] [CrossRef]
- Verdecchia, P.; Angeli, F.; Reboldi, G. Hypertension and atrial fibrillation: Doubts and certainties from basic and clinical studies. Circ. Res. 2018, 122, 352–368. [Google Scholar] [CrossRef] [PubMed]
- Aimoto, M.; Yagi, K.; Ezawa, A.; Tsuneoka, Y.; Kumada, K.; Hasegawa, T.; Kuze, T.; Chiba, T.; Nagasawa, Y.; Tanaka, H.; et al. Chronic Volume Overload Caused by Abdominal Aorto-Venocaval Shunt Provides Arrhythmogenic Substrates in the Rat Atrium. Biol. Pharm. Bull. 2022, 45, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Aimoto, M.; Fukumoto, M.; Nagasawa, Y.; Tanaka, H.; Takahara, A. Influence of chronic volume overload-induced atrial remodeling on electrophysiological responses to cholinergic receptor stimulation in the isolated rat atria. J. Pharmacol. Sci. 2018, 136, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Nagasawa, Y.; Zhang, C.; Zhang, H.; Aimoto, M.; Takahara, A. Acehytisine suppresses atrial fibrillation in rats with dilated atria caused by chronic volume overload. J. Pharmacol. Sci. 2020, 142, 34–40. [Google Scholar] [CrossRef]
- Boixel, C.; Fontaine, V.; Rücker-Martin, C.; Milliez, P.; Louedec, L.; Michel, J.B.; Jacob, M.P.; Hatem, S.N. Fibrosis of the left atria during progression of heart failure is associated with increased matrix metalloproteinases in the rat. J. Am. Coll. Cardiol. 2003, 42, 336–344. [Google Scholar] [CrossRef]
- Li, X.; Garcia-Elias, A.; Benito, B.; Nattel, S. The effects of cardiac stretch on atrial fibroblasts: Analysis of the evidence and potential role in atrial fibrillation. Cardiovasc. Res. 2022, 118, 440–460. [Google Scholar] [CrossRef]
- Kléber, A.G.; Rudy, Y. Basic Mechanisms of Cardiac Impulse Propagation and Associated Arrhythmias. Physiol. Rev. 2004, 84, 431–488. [Google Scholar] [CrossRef]
- Li, J.; Solus, J.; Chen, Q.; Rho, Y.H.; Milne, G.; Stein, C.M.; Darbar, D. Role of inflammation and oxidative stress in atrial fibrillation. Heart Rhythm 2010, 7, 438–444. [Google Scholar] [CrossRef]
- Dhein, S.; Salameh, A. Remodeling of cardiac gap junctional cell–cell coupling. Cells 2021, 10, 2422. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, J.; Kong, W. Extracellular matrix in vascular homeostasis and disease. Nat. Rev. Cardiol. 2025, 22, 333–353. [Google Scholar] [CrossRef]
- Le, N.N.; Tran, T.Q.B.; Mcclure, J.; Gill, D.; Padmanabhan, S. Emerging antihypertensive therapies and cardiovascular, kidney, and metabolic outcomes: A Mendelian randomization study. Eur. Heart J. Cardiovasc. Pharmacother. 2025, 11, 264–274. [Google Scholar] [CrossRef]
- Shlafer, M.; Shepard, B.M. A method to reduce interference by sucrose in the detection of thiobarbituric acid-reactive substances. Anal. Biochem. 1984, 137, 269–276. [Google Scholar] [CrossRef]
- Lojda, Z.; Gossrau, R.; Schiebler, T.H. Enzymhistochemische Methoden; Springer: Berlin/Heidelberg, Germany, 1976. [Google Scholar]
- Koyama, T.; Xie, Z.; Gao, M.; Suzuki, J.; Batra, S. Adaptive changes in the capillary network in the left ventricle of rat heart. Jpn. J. Physiol. 1998, 48, 229–241. [Google Scholar] [CrossRef]
- Andelova, K.; Szeiffova Bacova, B.; Sykora, M.; Pavelka, S.; Rauchova, H.; Tribulova, N. Cardiac Cx43 Signaling Is Enhanced and TGF-β1/SMAD2/3 Suppressed in Response to Cold Acclimation and Modulated by Thyroid Status in Hairless SHRM. Biomedicines 2022, 10, 1707. [Google Scholar] [CrossRef]
- Sykora, M.; Kratky, V.; Kopkan, L.; Tribulova, N.; Szeiffova Bacova, B. Anti-Fibrotic Potential of Angiotensin (1-7) in Hemodynamically Overloaded Rat Heart. Int. J. Mol. Sci. 2023, 24, 3490. [Google Scholar] [CrossRef]
- Lippi, G.; Sanchis-Gomar, F.; Cervellin, G. Global epidemiology of atrial fibrillation: An increasing epidemic and public health challenge. Int. J. Stroke 2021, 16, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Ben Morrison, T.; Jared Bunch, T.; Gersh, B.J. Pathophysiology of concomitant atrial fibrillation and heart failure: Implications for management. Nat. Clin. Pract. Cardiovasc. Med. 2009, 6, 46–56. [Google Scholar] [CrossRef]
- Havlenova, T.; Skaroupkova, P.; Miklovic, M.; Behounek, M.; Chmel, M.; Jarkovska, D.; Sviglerova, J.; Stengl, M.; Kolar, M.; Novotny, J.; et al. Right versus left ventricular remodeling in heart failure due to chronic volume overload. Sci. Rep. 2021, 11, 17136. [Google Scholar] [CrossRef]
- Reddy, S.; Bernstein, D.; Alto, P. Molecular Mechanisms of RV failure. Circulation 2016, 132, 1734–1742. [Google Scholar] [CrossRef]
- Díez, J.; De Boer, R.A. Management of cardiac fibrosis is the largest unmet medical need in heart failure. Cardiovasc. Res. 2022, 118, E20–E22. [Google Scholar] [CrossRef]
- Gonçalves, P.R.; Nascimento, L.D.; Gerlach, R.F.; Rodrigues, K.E.; Prado, A.F. Matrix Metalloproteinase 2 as a Pharmacological Target in Heart Failure. Pharmaceuticals 2022, 15, 920. [Google Scholar] [CrossRef]
- Keefe, J.A.; Wang, J.; Song, J.; Ni, L.; Wehrens, X.H.T. Immune cells and arrhythmias. Cardiovasc. Res. 2025, 121, 382–395. [Google Scholar] [CrossRef]
- Andelova, K.; Sykora, M.; Farkasova, V.; Stankovicova, T.; Szeiffova Bacova, B.; Knezl, V.; Egan Benova, T.; Pravenec, M.; Tribulova, N. Acclimation of Hairless Spontaneously Hypertensive Rat to Ambient Temperature Attenuates Hypertension-Induced Pro-Arrhythmic Downregulation of Cx43 in the Left Heart Ventricle of Males. Biomolecules 2024, 14, 1509. [Google Scholar] [CrossRef]
- Lee, S.Y.; Wu, S.T.; Du, C.X.; Ku, H.C. Potential Role of Dipeptidyl Peptidase−4 in Regulating Mitochondria and Oxidative Stress in Cardiomyocytes. Cardiovasc. Toxicol. 2024, 24, 1090–1104. [Google Scholar] [CrossRef]
- Lendeckel, U.; Arndt, M.; Wrenger, S.; Nepple, K.; Huth, C.; Ansorge, S.; Klein, H.U.; Goette, A. Expression and activity of ectopeptidases in fibrillating human atria. J. Mol. Cell. Cardiol. 2001, 33, 1273–1281. [Google Scholar] [CrossRef]
- La Vecchia, G.; Fumarulo, I.; Caffè, A.; Chiatto, M.; Montone, R.A.; Aspromonte, N. Microvascular Dysfunction across the Spectrum of Heart Failure Pathology: Pathophysiology, Clinical Features and Therapeutic Implications. Int. J. Mol. Sci. 2024, 25, 7628. [Google Scholar] [CrossRef]
- Tribulova, N.; Egan Benova, T.; Szeiffova Bacova, B.; Viczenczova, C.; Barancik, M. New aspects of pathogenesis of atrial fibrillation: Remodeling of intercalated discs. J. Physiol. Pharmacol. 2015, 66, 625–634. [Google Scholar]
- Sanders, P.; Morton, J.B.; Davidson, N.C.; Spence, S.J.; Vohra, J.K.; Sparks, P.B.; Kalman, J.M. Electrical remodeling of the atria in congestive heart failure: Electrophysiological and electroanatomic mapping in humans. Circulation 2003, 108, 1461–1468. [Google Scholar] [CrossRef]
- Cruciani, V.; Mikalsen, S.O. Connexins, gap junctional intercellular communication and kinases. Biol. Cell 2002, 94, 433–443. [Google Scholar] [CrossRef]
- Seropian, I.M.; El-Diasty, M.; El-Sherbini, A.H.; González, G.E.; Rabinovich, G.A. Central role of Galectin-3 at the cross-roads of cardiac inflammation and fibrosis: Implications for heart failure and transplantation. Cytokine Growth Factor Rev. 2024, 80, 47–58. [Google Scholar] [CrossRef]
- Barallobre-Barreiro, J.; Radovits, T.; Fava, M.; Mayr, U.; Lin, W.Y.; Ermolaeva, E.; Martínez-López, D.; Lindberg, E.L.; Duregotti, E.; Daróczi, L.; et al. Extracellular Matrix in Heart Failure: Role of ADAMTS5 in Proteoglycan Remodeling. Circulation 2021, 144, 2021–2034. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Qiao, X.; Zhang, L.; Li, X.; Liu, Q. Apelin-13 Regulates Angiotensin II-Induced Cx43 Downregulation and Autophagy via the AMPK/mTOR Signaling Pathway in HL-1 Cells. Physiol. Res. 2020, 69, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Ruan, L.; Wang, Y.; Chen, J. Effects of angiotensin II on connexin 43 of VSMCs in arteriosclerosis. J. Zhejiang Univ. Sci. B 2006, 7, 648–653. [Google Scholar] [CrossRef] [PubMed][Green Version]



| Parameters | HSD | HSD ACF | HSD ACF ACEi | HSD ACF ARB | TGR | TGR ACF | TGR ACF ACEi | TGR ACF ARB |
|---|---|---|---|---|---|---|---|---|
| BW (g) | 543 ± 37 | 548 ± 45 | 537 ± 31 | 575 ± 56 | 652 ± 46 a | 483 ± 46 c | 606 ± 53 d | 643 ± 38 d |
| HW (mg/mm) | 40.5 ± 3.3 | 71.2 ± 5.2 a | 59.2 ± 4.7 ab | 63.8 ± 4.4 a | 52.0 ± 4.1 a | 76.2 ± 3.0 c | 51.5 ± 8.7 d | 65.2 ± 6.7 cd |
| LVW (mg/mm) | 18.8 ± 1.9 | 27.0 ± 2.8 a | 23.6 ± 3.2 a | 24. 7 ± 1.3 a | 22.2 ± 2.7 | 33.5 ± 3.0 c | 17.1 ± 3.5 d | 22.4 ± 1.8 d |
| RVW (mg/mm) | 5.2 ± 0.4 | 12.9 ± 1.9 a | 12. ± 1.2 a | 13.4 ± 1.34 a | 8.04 ± 1.3 a | 12.4 ± 0.4 c | 10.4 ± 1.6 | 13.5 ± 2.68 c |
| Serum ANP (pg/mL) | 2.7 ± 0.3 | 3.4 ± 0.3 a | 2.9 ± 0.5 | 2.3 ± 0.1 b | 2.4 ± 0.2 | 4.0 ± 0.7 c | 3.3 ± 0.2 d | 3.3 ± 0.9 d |
| Serum TBARS (µmol/µg) | 18.7 ± 0.8 | 25.0 ± 0.8 a | 19.9 ± 1.9 b | 20.7 ± 1.2 b | 21.3 ± 0.8 | 24.5 ± 1.1 c | 20.1 ± 0.9 d | 21.9 ± 2.1 |
| Serum MMP-2 (%) | 100 ± 8 | 95 ± 3 | 83 ± 12 | 96 ± 4 | 80 ± 7 | 65 ± 5 | 60 ± 12 c | 78 ± 4 |
| Serum TIMP-2 (pg/mL) | 245 ± 39 | 212 ± 2 | 341 ± 17 ab | 211 ± 20 | 171 ± 21 | 144 ± 20 | 226 ± 43 d | 134 ± 13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sýkora, M.; Ondreják Andelová, K.; Mrvová, A.; Szeiffová Bačová, B.; Tribulová, N. Trandolapril Attenuates Pro-Arrhythmic Downregulation of Cx43 and Cx40 in Atria of Volume Overloaded Hypertensive and Normotensive Rats. Biomolecules 2025, 15, 1457. https://doi.org/10.3390/biom15101457
Sýkora M, Ondreják Andelová K, Mrvová A, Szeiffová Bačová B, Tribulová N. Trandolapril Attenuates Pro-Arrhythmic Downregulation of Cx43 and Cx40 in Atria of Volume Overloaded Hypertensive and Normotensive Rats. Biomolecules. 2025; 15(10):1457. https://doi.org/10.3390/biom15101457
Chicago/Turabian StyleSýkora, Matúš, Katarína Ondreják Andelová, Alexandra Mrvová, Barbara Szeiffová Bačová, and Narcis Tribulová. 2025. "Trandolapril Attenuates Pro-Arrhythmic Downregulation of Cx43 and Cx40 in Atria of Volume Overloaded Hypertensive and Normotensive Rats" Biomolecules 15, no. 10: 1457. https://doi.org/10.3390/biom15101457
APA StyleSýkora, M., Ondreják Andelová, K., Mrvová, A., Szeiffová Bačová, B., & Tribulová, N. (2025). Trandolapril Attenuates Pro-Arrhythmic Downregulation of Cx43 and Cx40 in Atria of Volume Overloaded Hypertensive and Normotensive Rats. Biomolecules, 15(10), 1457. https://doi.org/10.3390/biom15101457






