Asiatic Acid from Centella asiatica as a Potent EGFR Tyrosine Kinase Inhibitor with Anticancer Activity in NSCLC Cells Harboring Wild-Type and T790M-Mutated EGFR
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction and Isolation of Asiaticoside and Asiatic Acid
2.2. EGFR Inhibitory Activity of Asiaticoside and Asiatic Acid
2.3. Preparation of Structure and Molecular Docking
2.4. Cell Lines and Cell Culture
2.5. Cell Viability Assay
2.6. Cell Apoptosis Assay
2.7. Western Blot Analysis
2.8. Statistic Analysis
3. Results
3.1. Inhibitory Effect of Asiaticoside and Asiatic Acid on the EGFR Tyrosine Kinase Activity
3.2. Molecular Docking of Asiatic Acid into the Wild-Type and Double-Mutant Forms of EGFR
3.3. Effect of Asiatic Acid on the Cell Viability
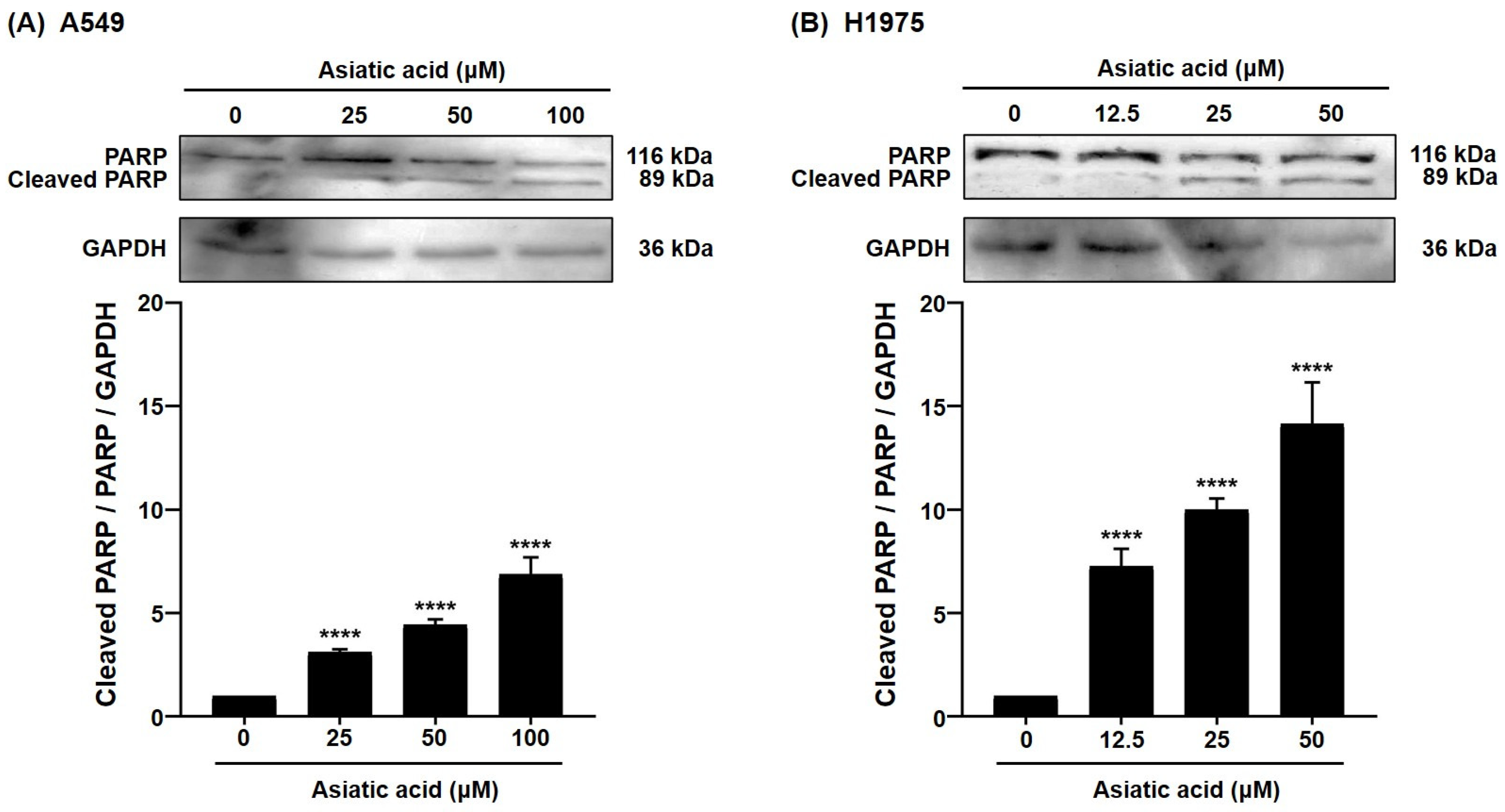
3.4. Effect of Asiatic Acid on the A549 and H1975 Cell Apoptosis
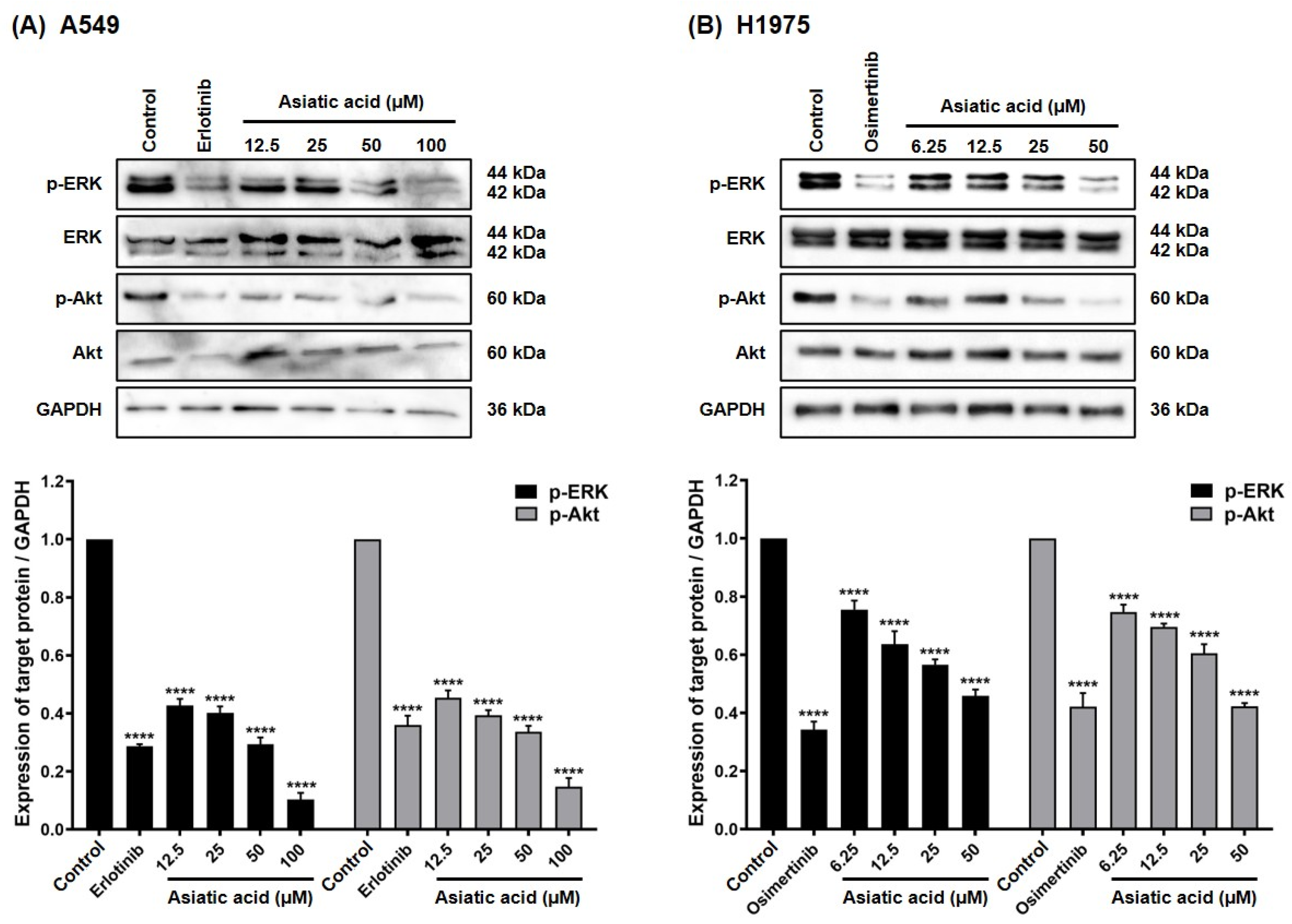
3.5. Effect of Asiatic Acid on EGFR Downstream Signaling Pathways
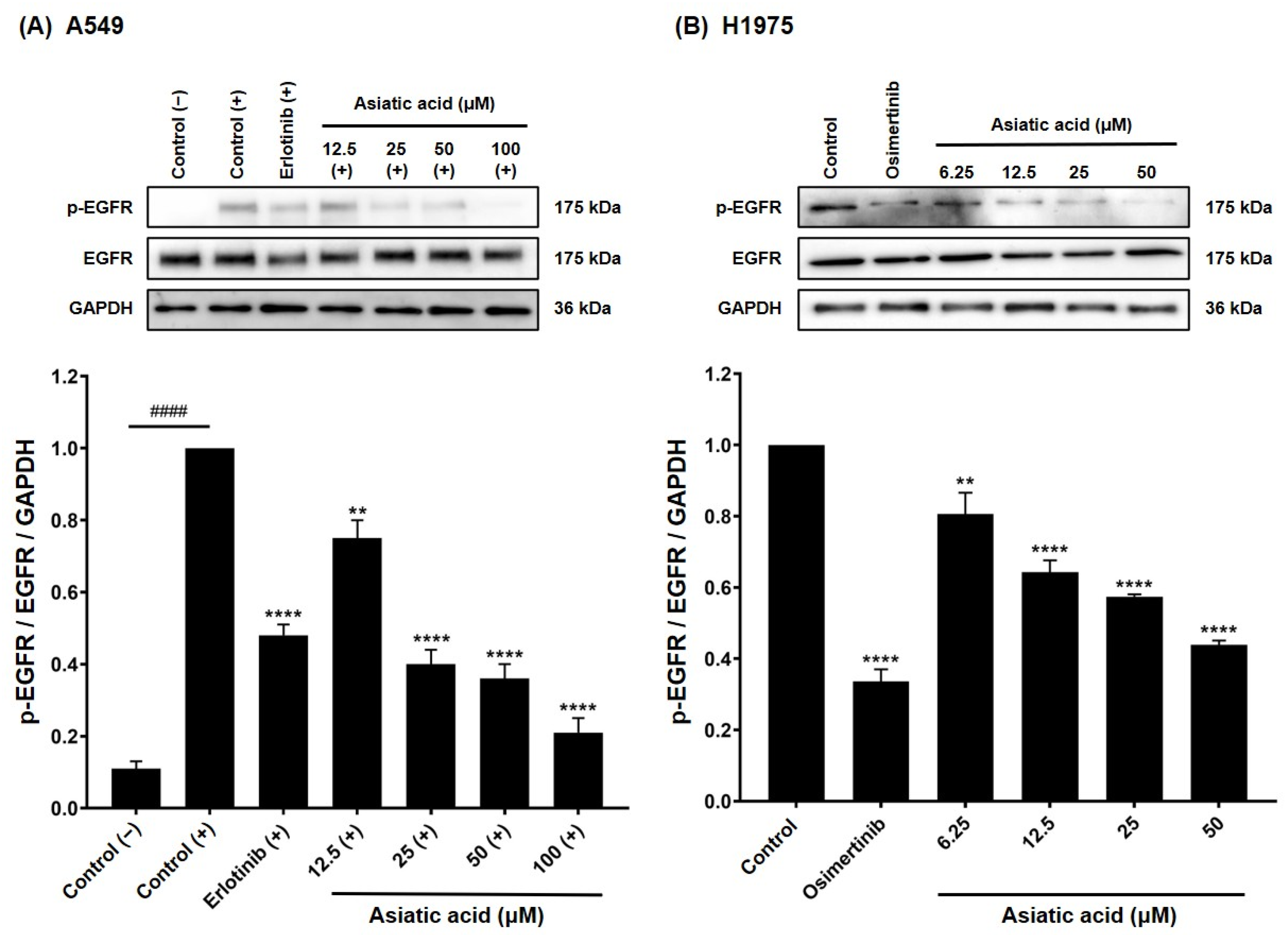
3.6. Effect of Asiatic Acid on EGFR Activation in A549 and H1975 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Din, S.R.U.; Saeed, S.; Khan, S.U.; Arbi, F.M.; Xuefang, G.; Zhong, M. Bacteria-driven cancer therapy: Exploring advancements and challenges. Crit. Rev. Oncol. Hematol. 2023, 191, 104141. [Google Scholar] [CrossRef]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer chemotherapy and beyond: Current status, drug candidates, associated risks and progress in targeted therapeutics. Genes. Dis. 2023, 10, 1367–1401. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, S.; Zhang, M.; Liu, M. Dual roles of methylglyoxal in cancer. Front. Oncol. 2025, 15, 1557162. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wang, Z.; Chen, Y.; Yin, G.; Liu, J.; Chen, W.; Zhu, L.; Xu, W.; Li, X. The predictive role of immune related subgroup classification in immune checkpoint blockade therapy for lung adenocarcinoma. Front. Genet. 2021, 12, 771830. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, C.; Xiang, Q.; Fan, S.; Xiao, T.; Chen, Y.; Zheng, D. Transient receptor potential cation channel subfamily V member 1 expression promotes chemoresistance in non-small-cell lung cancer. Front. Oncol. 2022, 12, 773654. [Google Scholar] [CrossRef]
- Bai, Y.; Yang, W.; Käsmann, L.; Sorich, M.J.; Tao, H.; Hu, Y. Immunotherapy for advanced non-small cell lung cancer with negative programmed death-ligand 1 expression: A literature review. Transl. Lung Cancer Res. 2024, 13, 398–422. [Google Scholar] [CrossRef]
- Gutierrez, E.; Cahatol, I.; Bailey, C.A.R.; Lafargue, A.; Zhang, N.; Song, Y.; Tian, H.; Zhang, Y.; Chan, R.; Gu, K.; et al. Regulation of RhoB gene expression during tumorigenesis and aging process and its potential applications in these processes. Cancers 2019, 11, 818. [Google Scholar] [CrossRef]
- Haraldsdottir, S.; Bekaii-Saab, T. Integrating anti-EGFR therapies in metastatic colorectal cancer. J. Gastrointest. Oncol. 2013, 4, 285–298. [Google Scholar] [CrossRef]
- Wiest, N.; Majeed, U.; Seegobin, K.; Zhao, Y.; Lou, Y.; Manochakian, R. Role of immune checkpoint inhibitor therapy in advanced EGFR-mutant non-small cell lung cancer. Front. Oncol. 2021, 11, 751209. [Google Scholar] [CrossRef]
- Šutić, M.; Vukić, A.; Baranašić, J.; Försti, A.; Džubur, F.; Samaržija, M.; Jakopović, M.; Brčić, L.; Knežević, J. Diagnostic, predictive, and prognostic biomarkers in non-small cell lung cancer (NSCLC) management. J. Pers. Med. 2021, 11, 1102. [Google Scholar] [CrossRef]
- Imamura, F.; Uchida, J.; Kukita, Y.; Kumagai, T.; Nishino, K.; Inoue, T.; Kimura, M.; Oba, S.; Kato, K. Monitoring of treatment responses and clonal evolution of tumor cells by circulating tumor DNA of heterogeneous mutant EGFR genes in lung cancer. Lung Cancer 2016, 94, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Wiciński, M.; Fajkiel-Madajczyk, A.; Kurant, Z.; Gajewska, S.; Kurant, D.; Kurant, M.; Sousak, M. Can asiatic acid from Centella asiatica be a potential remedy in cancer therapy?—A review. Cancers 2024, 16, 1317. [Google Scholar] [CrossRef] [PubMed]
- Gohil, K.J.; Patel, J.A.; Gajjar, A.K. Pharmacological review on Centella asiatica: A potential herbal cure-all. Indian J. Pharm. Sci. 2010, 72, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Arribas-López, E.; Zand, N.; Ojo, O.; Snowden, M.J.; Kochhar, T. A systematic review of the effect of Centella asiatica on wound healing. Int. J. Environ. Res. Public Health 2022, 19, 3266. [Google Scholar] [CrossRef]
- Rashid, M.H.-O.; Akter, M.M.; Uddin, J.; Islam, S.; Rahman, M.; Jahan, K.; Sarker, M.M.R.; Sadik, G. Antioxidant, cytotoxic, antibacterial and thrombolytic activities of Centella asiatica L.: Possible role of phenolics and flavonoids. Clin. Phytoscience 2023, 9, 1. [Google Scholar] [CrossRef]
- Shin, H.Y.; Kim, Y.S.; Ha, E.J.; Koo, J.P.; Jeong, W.B.; Joung, M.Y.; Shin, K.-S.; Yu, K.-W. Anti-inflammatory action and associated intracellular signaling of Centella asiatica extract on lipopolysaccharide-stimulated RAW 264.7 macrophage. Food Biosci. 2024, 61, 104614. [Google Scholar] [CrossRef]
- Ferah Okkay, I.; Okkay, U.; Aydin, I.C.; Bayram, C.; Ertugrul, M.S.; Mendil, A.S.; Hacimuftuoglu, A. Centella asiatica extract protects against cisplatin-induced hepatotoxicity via targeting oxidative stress, inflammation, and apoptosis. Environ. Sci. Pollut. Res. Int. 2022, 29, 33774–33784. [Google Scholar] [CrossRef]
- Singh, L.S.; Singh, W.S. Centella asiatica and its bioactive compounds: A comprehensive approach to managing hyperglycemia and associated disorders. Discov. Plants 2024, 1, 54. [Google Scholar] [CrossRef]
- Al-Saeedi, F.J. Study of the cytotoxicity of asiaticoside on rats and tumour cells. BMC Cancer 2014, 14, 220. [Google Scholar] [CrossRef]
- Hao, Y.; Huang, J.; Ma, Y.; Chen, W.; Fan, Q.; Sun, X.; Shao, M.; Cai, H. Asiatic acid inhibits proliferation, migration and induces apoptosis by regulating Pdcd4 via the PI3K/Akt/mTOR/p70S6K signaling pathway in human colon carcinoma cells. Oncol. Lett. 2018, 15, 8223–8230. [Google Scholar] [CrossRef]
- Hsu, Y.-L.; Kuo, P.-L.; Lin, L.-T.; Lin, C.-C. Asiatic acid, a triterpene, induces apoptosis and cell cycle arrest through activation of extracellular signal-regulated kinase and p38 mitogen-activated protein kinase pathways in human breast cancer cells. J. Pharmacol. Exp. Ther. 2005, 313, 333–344. [Google Scholar] [CrossRef]
- Lee, Y.S.; Jin, D.-Q.; Kwon, E.J.; Park, S.H.; Lee, E.-S.; Jeong, T.C.; Nam, D.H.; Huh, K.; Kim, J.-A. Asiatic acid, a triterpene, induces apoptosis through intracellular Ca2+ release and enhanced expression of p53 in HepG2 human hepatoma cells. Cancer Lett. 2002, 186, 83–91. [Google Scholar] [CrossRef]
- Li, Z.; You, K.; Li, J.; Wang, Y.; Xu, H.; Gao, B.; Wang, J. Madecassoside suppresses proliferation and invasiveness of HGF-induced human hepatocellular carcinoma cells via PKC-cMET-ERK1/2-COX-2-PGE2 pathway. Int. Immunopharmacol. 2016, 33, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Valdeira, A.S.C.; Darvishi, E.; Woldemichael, G.M.; Beutler, J.A.; Gustafson, K.R.; Salvador, J.A.R. Madecassic acid derivatives as potential anticancer agents: Synthesis and cytotoxic evaluation. J. Nat. Prod. 2019, 82, 2094–2105. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Wang, Z. Triterpenoid saponins and phenylethanoid glycosides from stem of Akebia trifoliata var. australis. Phytochemistry 2006, 67, 2697–2705. [Google Scholar] [CrossRef] [PubMed]
- Acebey-Castellon, I.L.; Voutquenne-Nazabadioko, L.; Doan Thi Mai, H.; Roseau, N.; Bouthagane, N.; Muhammad, D.; Le Magrex Debar, E.; Gangloff, S.C.; Litaudon, M.; Sevenet, T.; et al. Triterpenoid saponins from Symplocos lancifolia. J. Nat. Prod. 2011, 74, 163–168. [Google Scholar] [CrossRef]
- Tolls, J.; van Dijk, J.; Verbruggen, E.J.M.; Hermens, J.L.M.; Loeprecht, B.; Schüürmann, G. Aqueous solubility−molecular size relationships: A mechanistic case study using C10- to C19-alkanes. J. Phys. Chem. 2002, 106, 2760–2765. [Google Scholar] [CrossRef]
- Aiebchun, T.; Mahalapbutr, P.; Auepattanapong, A.; Khaikate, O.; Seetaha, S.; Tabtimmai, L.; Kuhakarn, C.; Choowongkomon, K.; Rungrotmongkol, T. Identification of vinyl sulfone derivatives as EGFR tyrosine kinase inhibitor: In vitro and in silico studies. Molecules 2021, 26, 2211. [Google Scholar] [CrossRef]
- Lin, G.B.; Chen, W.T.; Kuo, Y.Y.; Liu, H.H.; Chen, Y.M.; Leu, S.J.; Chao, C.Y. Thermal cycling—hyperthermia sensitizes non—small cell lung cancer A549 cells to EGFR tyrosine kinase inhibitor erlotinib. Oncol. Rep. 2025, 53, 5. [Google Scholar] [CrossRef]
- Alhazzani, K.; Alsahli, M.; Alanazi, A.Z.; Algahtani, M.; Alenezi, A.A.; Alhoshani, A.; Alqinyah, M.; Alhamed, A.S.; Alhosaini, K. Augmented antitumor effects of erlotinib and cabozantinib on A549 non-small cell lung cancer: In vitro and in vivo studies. Saudi Pharm. J. 2023, 31, 101756. [Google Scholar] [CrossRef]
- Jaramillo, M.L.; Banville, M.; Collins, C.; Paul-Roc, B.; Bourget, L.; O’Connor-McCourt, M. Differential sensitivity of A549 non-small lung carcinoma cell responses to epidermal growth factor receptor pathway inhibitors. Cancer Biol. Ther. 2008, 7, 557–568. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tomshine, J.C.; Severson, S.R.; Wigle, D.A.; Sun, Z.; Beleford, D.A.; Shridhar, V.; Horazdovsky, B.F. Cell proliferation and epidermal growth factor signaling in non-small cell lung adenocarcinoma cell lines are dependent on Rin1. J. Biol. Chem. 2009, 284, 26329–26331. [Google Scholar] [CrossRef] [PubMed]
- Reguart, N.; Cardona, A.F.; Rosell, R. Role of erlotinib in first-line and maintenance treatment of advanced non-small-cell lung cancer. Cancer Manag. Res. 2010, 2, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Mao, T.; Wang, J.; Zheng, H.; Hu, Z.; Cao, P.; Yang, S.; Zhu, L.; Guo, S.; Zhao, X.; et al. Toward the next generation EGFR inhibitors: An overview of osimertinib resistance mediated by EGFR mutations in non-small cell lung cancer. Cell Commun. Signal. 2023, 21, 71. [Google Scholar] [CrossRef]
- Onodera, K.; Sakurada, A.; Notsuda, H.; Watanabe, T.; Matsuda, Y.; Noda, M.; Endo, C.; Okada, Y. Growth inhibition of KRAS—and EGFR—mutant lung adenocarcinoma by cosuppression of STAT3 and the SRC/ARHGAP35 axis. Oncol. Rep. 2018, 40, 1761–1768. [Google Scholar] [CrossRef]
- Landi, L.; Cappuzzo, F. Front-line therapy in lung cancer with mutations in EGFR. Nat. Rev. Clin. Oncol. 2011, 8, 571–573. [Google Scholar] [CrossRef]
- Blagosklonny, M.V. Selective protection of normal cells from chemotherapy, while killing drug-resistant cancer cells. Oncotarget 2023, 14, 193–206. [Google Scholar] [CrossRef]
- Wang, L.; Xu, J.; Zhao, C.; Zhao, L.; Feng, B. Antiproliferative, cell-cycle dysregulation effects of novel asiatic acid derivatives on human non-small cell lung cancer cells. Chem. Pharm. Bull. 2013, 61, 1015–1023. [Google Scholar] [CrossRef]
- Singh, J.; Hussain, Y.; Meena, A.; Sinha, R.A.; Luqman, S. Asiatic acid impedes NSCLC progression by inhibiting COX-2 and modulating PI3K signaling. FEBS Lett. 2024, 598, 3036–3052. [Google Scholar] [CrossRef]
- Wu, T.; Geng, J.; Guo, W.; Gao, J.; Zhu, X. Asiatic acid inhibits lung cancer cell growth in vitro and in vivo by destroying mitochondria. Acta Pharm. Sin. B 2017, 7, 65–72. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, W.; Wang, Y. PARP-1 and its associated nucleases in DNA damage response. DNA Repair 2019, 81, 102651. [Google Scholar] [CrossRef] [PubMed]
- Mashimo, M.; Onishi, M.; Uno, A.; Tanimichi, A.; Nobeyama, A.; Mori, M.; Yamada, S.; Negi, S.; Bu, X.; Kato, J.; et al. The 89-kDa PARP1 cleavage fragment serves as a cytoplasmic PAR carrier to induce AIF-mediated apoptosis. J. Biol. Chem. 2021, 296, 100046. [Google Scholar] [CrossRef] [PubMed]
- Ciardiello, F.; Tortora, G. Anti-epidermal growth factor receptor drugs in cancer therapy. Expert. Opin. Investig. Drugs 2002, 11, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, F.R.; Varella-Garcia, M.; Bunn, P.A., Jr.; Di Maria, M.V.; Veve, R.; Bremmes, R.M.; Barón, A.E.; Zeng, C.; Franklin, W.A. Epidermal growth factor receptor in non-small-cell lung carcinomas: Correlation between gene copy number and protein expression and impact on prognosis. J. Clin. Oncol. 2003, 21, 3798–3807. [Google Scholar] [CrossRef]
- Montor, W.R.; Salas, A.R.O.S.E.; de Melo, F.H.M. Receptor tyrosine kinases and downstream pathways as druggable targets for cancer treatment: The current arsenal of inhibitors. Mol. Cancer 2018, 17, 55. [Google Scholar] [CrossRef]
- Tito, C.; Masciarelli, S.; Colotti, G.; Fazi, F. EGF receptor in organ development, tissue homeostasis and regeneration. J. Biomed. Sci. 2025, 32, 24. [Google Scholar] [CrossRef]
- Muta, Y.; Matsuda, M.; Imajo, M. Divergent dynamics and functions of ERK MAP kinase signaling in development, homeostasis and cancer: Lessons from fluorescent bioimaging. Cancers 2019, 11, 513. [Google Scholar] [CrossRef]
- Martini, M.; Chiara, D.S.M.; Laura, B.; Federico, G.; Hirsch, E. PI3K/AKT signaling pathway and cancer: An updated review. Ann. Med. 2014, 46, 372–383. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, W.; Zhang, M.; Liu, W.; Feng, W.; Zhang, Y. Asiatic acid in anticancer effects: Emerging roles and mechanisms. Front. Pharmacol. 2025, 16, 1545654. [Google Scholar] [CrossRef]
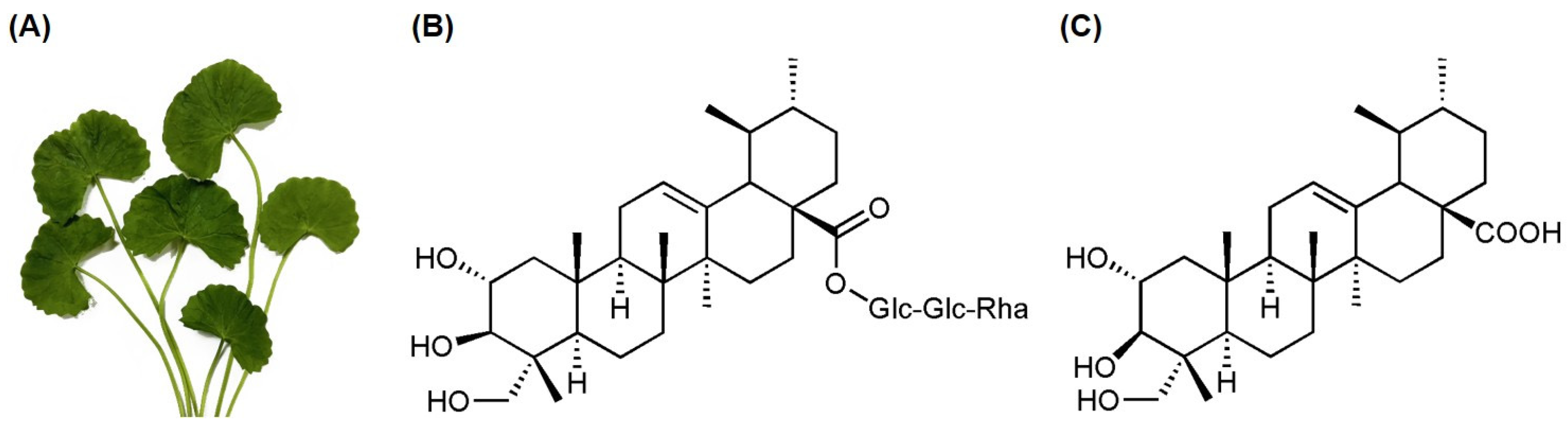

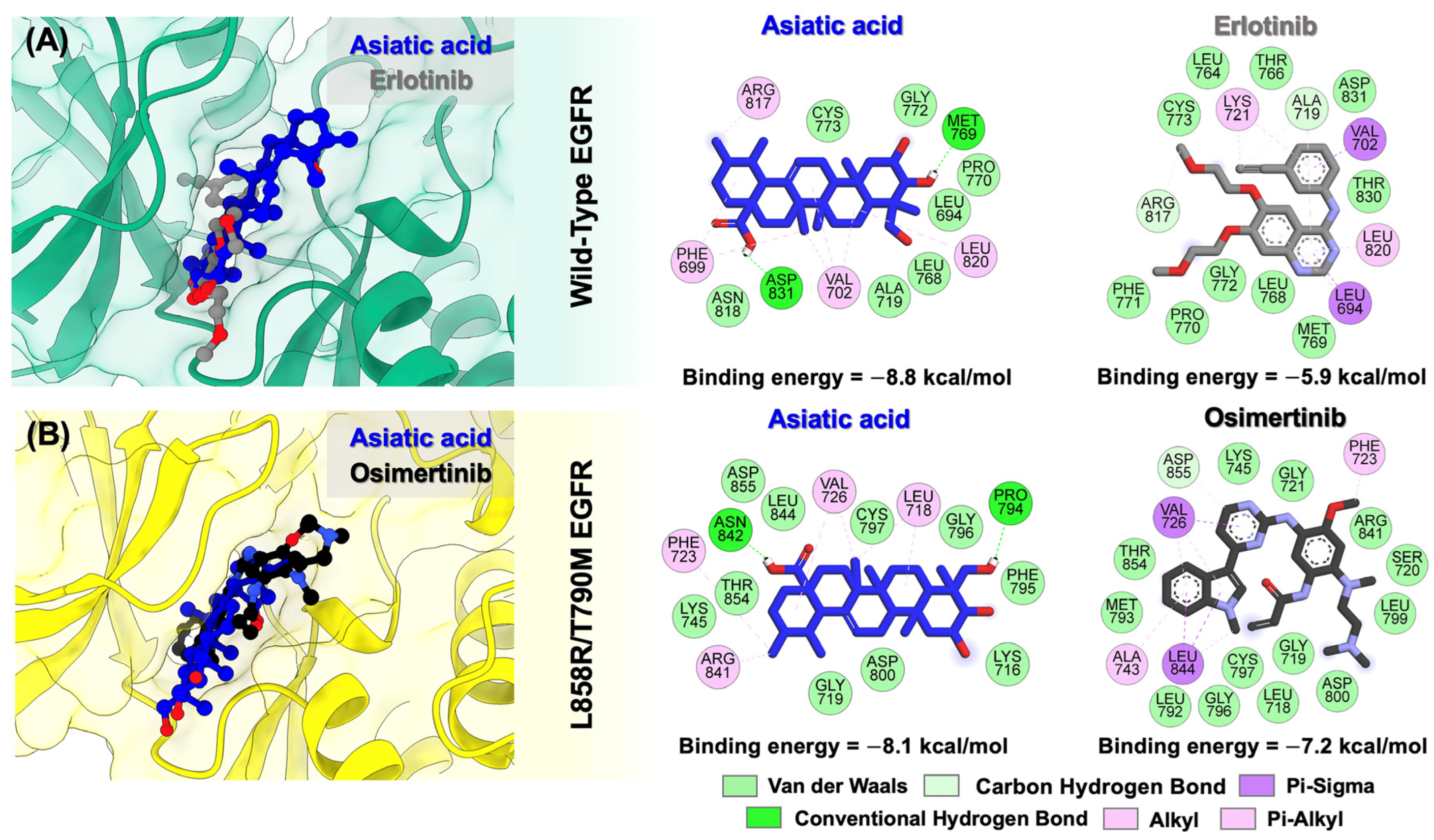
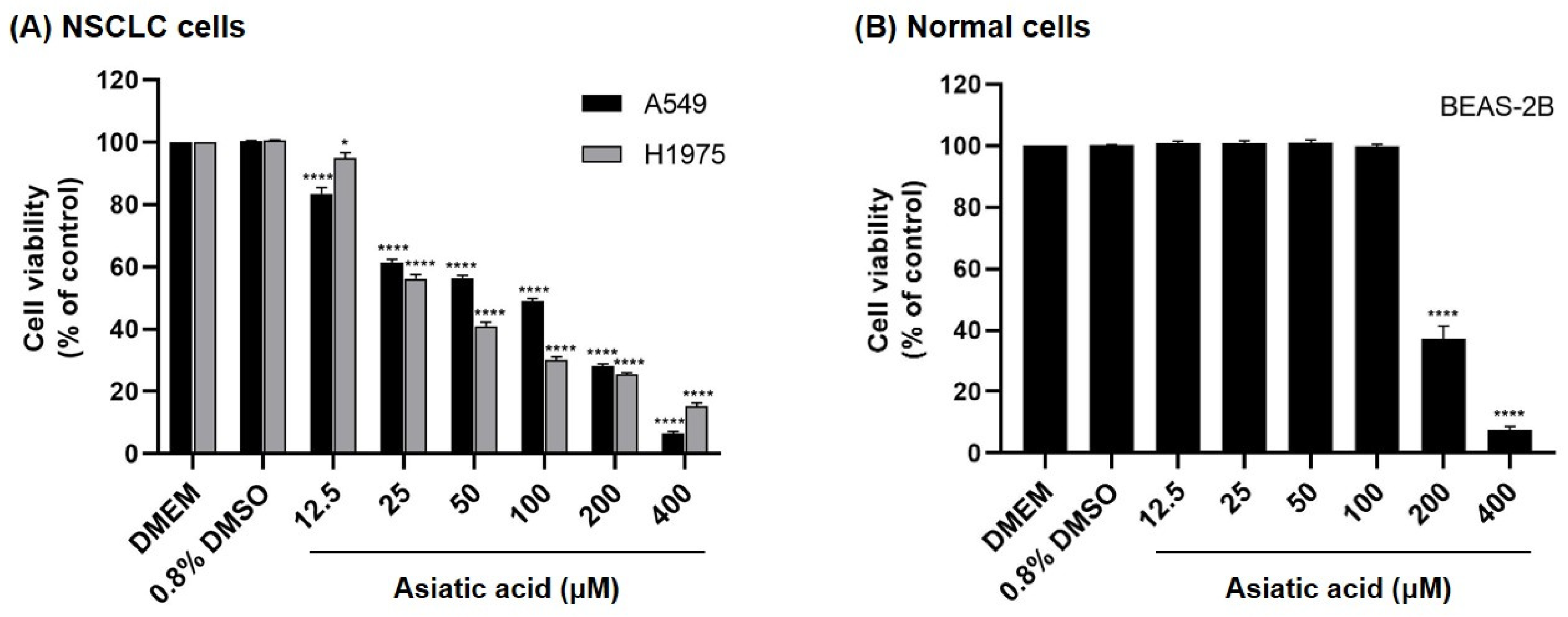
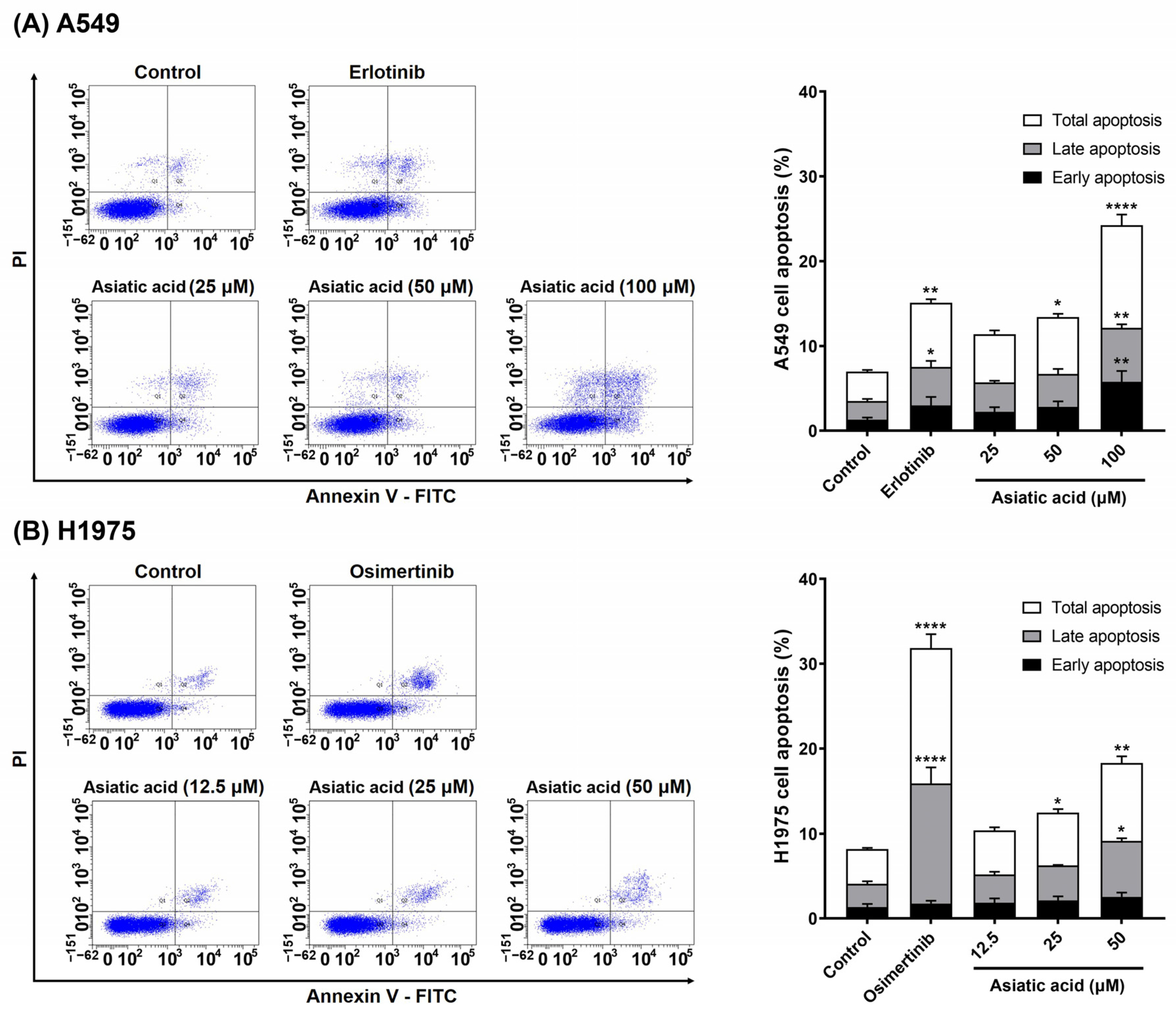
| Compounds | IC50 (nM) a | |
|---|---|---|
| Wild-Type EGFR | L858R/T790M EGFR | |
| Asiaticoside | >80 | >80 |
| Asiatic acid | 0.035 ± 0.002 | 0.348 ± 0.055 |
| Erlotinib | 0.062 ± 0.009 | - |
| Osimertinib | - | 0.116 ± 0.038 |
| Cell Lines | IC50 (μM) a |
|---|---|
| A549 | 64.52 ± 2.49 |
| H1975 | 36.55 ± 0.86 |
| BEAS-2B | 184.10 ± 5.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monmai, C.; Sabuakham, S.; Pabuprapap, W.; Chaichompoo, W.; Suksamrarn, A.; Mahalapbutr, P. Asiatic Acid from Centella asiatica as a Potent EGFR Tyrosine Kinase Inhibitor with Anticancer Activity in NSCLC Cells Harboring Wild-Type and T790M-Mutated EGFR. Biomolecules 2025, 15, 1410. https://doi.org/10.3390/biom15101410
Monmai C, Sabuakham S, Pabuprapap W, Chaichompoo W, Suksamrarn A, Mahalapbutr P. Asiatic Acid from Centella asiatica as a Potent EGFR Tyrosine Kinase Inhibitor with Anticancer Activity in NSCLC Cells Harboring Wild-Type and T790M-Mutated EGFR. Biomolecules. 2025; 15(10):1410. https://doi.org/10.3390/biom15101410
Chicago/Turabian StyleMonmai, Chaiwat, Sahachai Sabuakham, Wachirachai Pabuprapap, Waraluck Chaichompoo, Apichart Suksamrarn, and Panupong Mahalapbutr. 2025. "Asiatic Acid from Centella asiatica as a Potent EGFR Tyrosine Kinase Inhibitor with Anticancer Activity in NSCLC Cells Harboring Wild-Type and T790M-Mutated EGFR" Biomolecules 15, no. 10: 1410. https://doi.org/10.3390/biom15101410
APA StyleMonmai, C., Sabuakham, S., Pabuprapap, W., Chaichompoo, W., Suksamrarn, A., & Mahalapbutr, P. (2025). Asiatic Acid from Centella asiatica as a Potent EGFR Tyrosine Kinase Inhibitor with Anticancer Activity in NSCLC Cells Harboring Wild-Type and T790M-Mutated EGFR. Biomolecules, 15(10), 1410. https://doi.org/10.3390/biom15101410





