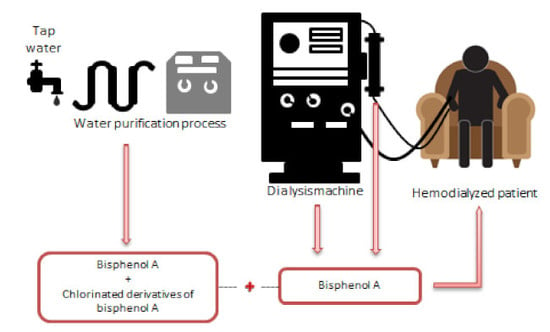
Overexposure to Bisphenol A and Its Chlorinated Derivatives of Patients with End-Stage Renal Disease during Online Hemodiafiltration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Water Purification Process
2.2. Production of Ultrapure Dialysate
2.2.1. Dialysis Concentrates
2.2.2. Ultrafilters
2.2.3. Dialysis Water and Ultrapure Dialysate
2.3. Replacement Fluid
2.4. Tubing
2.5. Dialyzers
2.6. Sample Analysis
2.6.1. Sample Preparation
2.6.2. Online SPE-UPLC-MS/MS
2.6.3. Method Validation
2.6.4. Assigning Values to Non-Detected/No-Quantified Data
2.7. Statistical Analysis
3. Results
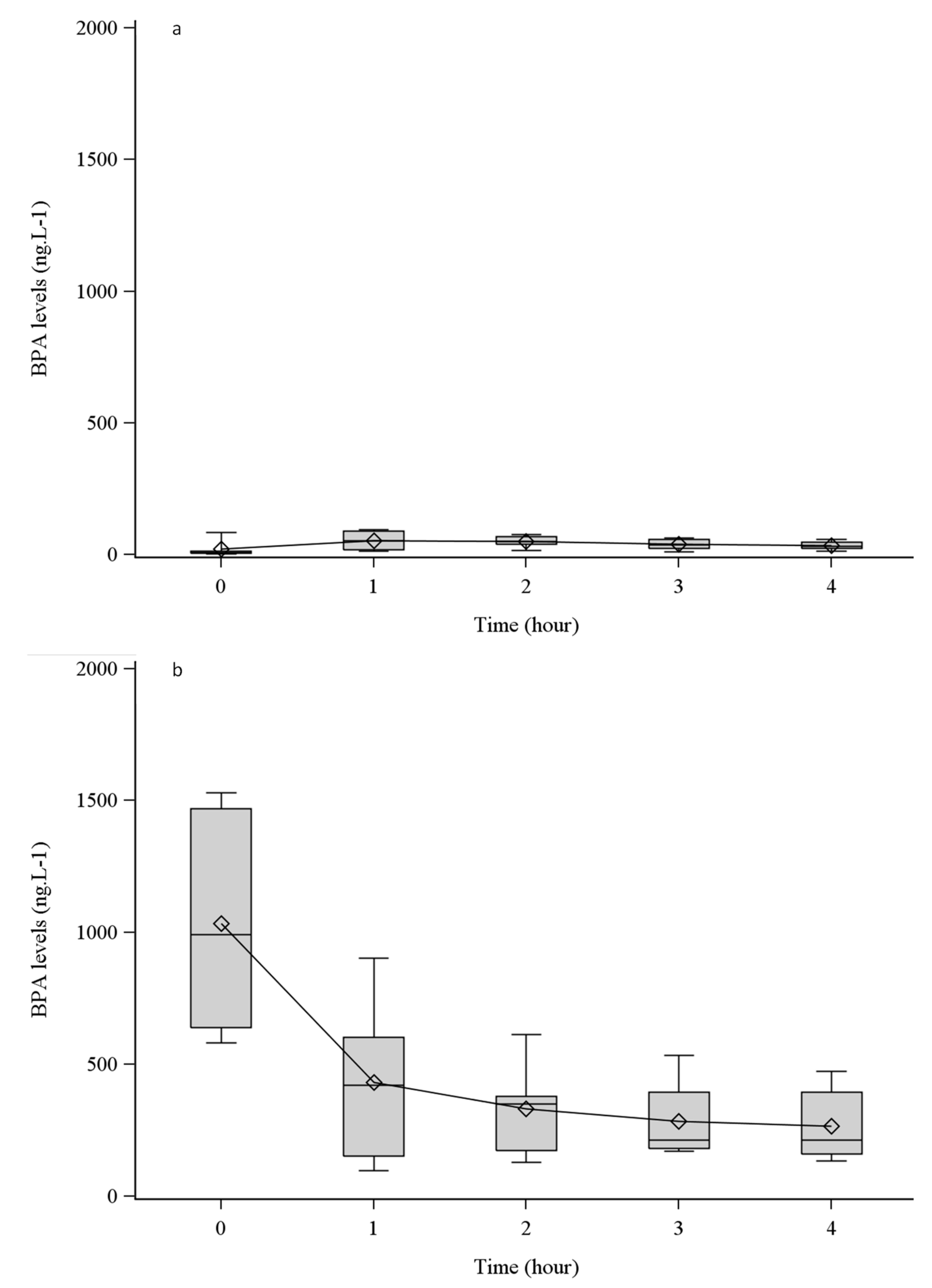
3.1. BPA Leaching from Tubing and HDF Dialyzers
3.2. BPA and ClxBPA Occurrence throughout the Water Purification Process
3.3. BPA and ClxBPA in Ultrapure Dialysate and Replacement Fluid
4. Discussion
4.1. BPA Is Leached from High-Permeability Dialyzers
4.2. BPA and ClxBPA Are Not Eliminated by Water Purification Process
4.3. Dialysate and Replacement Fluid are Sources of Exposure to BPA and ClxBPA
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jha, V.; Garcia-Garcia, G.; Iseki, K.; Li, Z.; Naicker, S.; Plattner, B.; Saran, R.; Wang, A.Y.-M.; Yang, C.-W. Chronic kidney disease: Global dimension and perspectives. Lancet Lond. Engl. 2013, 382, 260–272. [Google Scholar] [CrossRef]
- Krieter, D.H.; Hackl, A.; Rodriguez, A.; Chenine, L.; Moragues, H.L.; Lemke, H.D.; Wanner, C.; Canaud, B. Protein-bound uraemic toxin removal in haemodialysis and post-dilution haemodiafiltration. Nephrol. Dial. Transplant. 2010, 25, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Basile, C.; Libutti, P.; di Turo, A.L.; Casino, F.G.; Vernaglione, L.; Tundo, S.; Maselli, P.; de Nicolò, E.V.; Ceci, E.; Teutonico, A.; et al. Removal of uraemic retention solutes in standard bicarbonate haemodialysis and long-hour slow-flow bicarbonate haemodialysis. Nephrol. Dial. Transplant. 2011, 26, 1296–1303. [Google Scholar] [CrossRef] [PubMed]
- Canaud, B.; Bragg-Gresham, J.; Marshall, M.R.; Desmeules, S.; Gillespie, B.; Depner, T.; Klassen, P.; Port, F. Mortality risk for patients receiving hemodiafiltration versus hemodialysis: European results from the DOPPS. Kidney Int. 2006, 69, 2087–2093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panichi, V.; Rizza, G.M.; Paoletti, S.; Bigazzi, R.; Aloisi, M.; Barsotti, G.; Rindi, P.; Donati, G.; Antonelli, A.; Panicucci, E.; et al. Chronic inflammation and mortality in haemodialysis: Effect of different renal replacement therapies. Results from the RISCAVID study. Nephrol. Dial. Transplant. 2008, 23, 2337–2343. [Google Scholar] [CrossRef] [PubMed]
- Canaud, B.; Barbieri, C.; Marcelli, D.; Bellocchio, F.; Bowry, S.; Mari, F.; Amato, C.; Gatti, E. Optimal convection volume for improving patient outcomes in an international incident dialysis cohort treated with online hemodiafiltration. Kidney Int. 2015, 88, 1108–1116. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Wang, H.-S. Impact of Bisphenol A on the Cardiovascular System — Epidemiological and Experimental Evidence and Molecular Mechanisms. Int. J. Environ. Res. Public. Health 2014, 11, 8399–8413. [Google Scholar] [CrossRef] [PubMed]
- Rezg, R.; El-Fazaa, S.; Gharbi, N.; Mornagui, B. Bisphenol A and human chronic diseases: Current evidences, possible mechanisms, and future perspectives. Environ. Int. 2014, 64, 83–90. [Google Scholar] [CrossRef]
- Dupuis, A.; Migeot, V.; Cariot, A.; Albouy-Llaty, M.; Legube, B.; Rabouan, S. Quantification of bisphenol A, 353-nonylphenol and their chlorinated derivatives in drinking water treatment plants. Environ. Sci. Pollut. Res. Int. 2012, 19, 4193–4205. [Google Scholar] [CrossRef]
- Gallard, H.; Leclercq, A.; Croué, J.-P. Chlorination of bisphenol A: Kinetics and by-products formation. Chemosphere 2004, 56, 465–473. [Google Scholar] [CrossRef]
- Fan, Z.; Hu, J.; An, W.; Yang, M. Detection and occurrence of chlorinated byproducts of bisphenol a, nonylphenol, and estrogens in drinking water of china: Comparison to the parent compounds. Environ. Sci. Technol. 2013, 47, 10841–10850. [Google Scholar] [CrossRef] [PubMed]
- Lane, R.F.; Adams, C.D.; Randtke, S.J.; Carter, R.E. Chlorination and chloramination of bisphenol A, bisphenol F, and bisphenol A diglycidyl ether in drinking water. Water Res. 2015, 79, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Haishima, Y.; Hayashi, Y.; Yagami, T.; Nakamura, A. Elution of bisphenol-A from hemodialyzers consisting of polycarbonate and polysulfone resins. J. Biomed. Mater. Res. 2001, 58, 209–215. [Google Scholar] [CrossRef]
- Yamasaki, H.; Nagake, Y.; Makino, H. Determination of Bisphenol A in Effluents of Hemodialyzers. Nephron 2001, 88, 376–378. [Google Scholar] [CrossRef] [PubMed]
- Fukazawa, H.; Watanabe, M.; Shiraishi, F.; Shiraishi, H.; Shiozawa, T.; Matsushita, H.; Terao, Y. Formation of Chlorinated Derivatives of Bisphenol A in Waste Paper Recycling Plants and Their Estrogenic Activities. J. Health Sci. 2002, 48, 242–249. [Google Scholar] [CrossRef]
- Mutou, Y.; Ibuki, Y.; Terao, Y.; Kojima, S.; Goto, R. Change of estrogenic activity and release of chloride ion in chlorinated bisphenol a after exposure to ultraviolet B. Biol. Pharm. Bull. 2006, 29, 2116–2119. [Google Scholar] [CrossRef] [PubMed]
- Rivas, A.; Lacroix, M.; Olea-Serrano, F.; Laíos, I.; Leclercq, G.; Olea, N. Estrogenic effect of a series of bisphenol analogues on gene and protein expression in MCF-7 breast cancer cells. J. Steroid Biochem. Mol. Biol. 2002, 82, 45–53. [Google Scholar] [CrossRef]
- Takemura, H.; Ma, J.; Sayama, K.; Terao, Y.; Zhu, B.T.; Shimoi, K. In vitro and in vivo estrogenic activity of chlorinated derivatives of bisphenol A. Toxicology 2005, 207, 215–221. [Google Scholar] [CrossRef]
- Riu, A.; Le Maire, A.; Grimaldi, M.; Audebert, M.; Hillenweck, A.; Bourguet, W.; Balaguer, P.; Zalko, D. Characterization of novel ligands of ERα, Erβ, and PPARγ: The case of halogenated bisphenol A and their conjugated metabolites. Toxicol. Sci. Off. J. Soc. Toxicol. 2011, 122, 372–382. [Google Scholar] [CrossRef]
- Andra, S.S.; Kalyvas, H.; Andrianou, X.D.; Charisiadis, P.; Christophi, C.A.; Makris, K.C. Preliminary evidence of the association between monochlorinated bisphenol A exposure and type II diabetes mellitus: A pilot study. J. Environ. Sci. Health Part A 2015, 50, 243–259. [Google Scholar] [CrossRef]
- Terasaki, M.; Kosaka, K.; Kunikane, S.; Makino, M.; Shiraishi, F. Assessment of thyroid hormone activity of halogenated bisphenol A using a yeast two-hybrid assay. Chemosphere 2011, 84, 1527–1530. [Google Scholar] [CrossRef] [PubMed]
- Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR). Opinion on the Safety of the Use of Bisphenol A in Medical Devices; Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR): Warwick, UK, 2015. [Google Scholar]
- Bacle, A.; Thevenot, S.; Grignon, C.; Belmouaz, M.; Bauwens, M.; Teychene, B.; Venisse, N.; Migeot, V.; Dupuis, A. Determination of bisphenol A in water and the medical devices used in hemodialysis treatment. Int. J. Pharm. 2016, 505, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Hoenich, N.A.; Thompson, J.; McCabe, J.; Appleton, D.R. Particle release from haemodialysers. Int. J. Artif. Organs 1990, 13, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Cariot, A.; Dupuis, A.; Albouy-Llaty, M.; Legube, B.; Rabouan, S.; Migeot, V. Reliable quantification of bisphenol A and its chlorinated derivatives in human breast milk using UPLC-MS/MS method. Talanta 2012, 100, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Diao, C.-P.; Zhao, R.-S. Rapid determination of bisphenol A in drinking water using dispersive liquid-phase microextraction with in situ derivatization prior to GC-MS. J. Sep. Sci. 2009, 32, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Zoffoli, H.J.O.; Varella, C.A.A.; do Amaral-Sobrinho, N.M.B.; Zonta, E.; Tolón-Becerra, A. Method of median semi-variance for the analysis of left-censored data: Comparison with other techniques using environmental data. Chemosphere 2013, 93, 1701–1709. [Google Scholar] [CrossRef] [PubMed]
- Krieter, D.H.; Canaud, B.; Lemke, H.D.; Rodriguez, A.; Morgenroth, A.; von Appen, K.; Dragoun, G.P.; Wanner, C. Bisphenol A in chronic kidney disease. Artif. Organs 2013, 37, 283–290. [Google Scholar] [CrossRef]
- Murakami, K.; Ohashi, A.; Hori, H.; Hibiya, M.; Shoji, Y.; Kunisaki, M.; Akita, M.; Yagi, A.; Sugiyama, K.; Shimozato, S.; et al. Accumulation of bisphenol A in hemodialysis patients. Blood Purif. 2007, 25, 290–294. [Google Scholar] [CrossRef]
- Bosch-Panadero, E.; Mas, S.; Sanchez-Ospina, D.; Camarero, V.; Pérez-Gómez, M.V.; Saez-Calero, I.; Abaigar, P.; Ortiz, A.; Egido, J.; González-Parra, E. The Choice of Hemodialysis Membrane Affects Bisphenol A Levels in Blood. J. Am. Soc. Nephrol. JASN 2016, 27, 1566–1574. [Google Scholar] [CrossRef]
- Fürhacker, M.; Scharf, S.; Weber, H. Bisphenol A: Emissions from point sources. Chemosphere 2000, 41, 751–756. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Anastopoulos, I. Adsorptive removal of bisphenol A (BPA) from aqueous solution: A review. Chemosphere 2017, 168, 885–902. [Google Scholar] [CrossRef] [PubMed]
- Yüksel, S.; Kabay, N.; Yüksel, M. Removal of bisphenol A (BPA) from water by various nanofiltration (NF) and reverse osmosis (RO) membranes. J. Hazard. Mater. 2013, 263 Pt 2, 307–310. [Google Scholar] [CrossRef]
- Dekant, W.; Völkel, W. Human exposure to bisphenol A by biomonitoring: Methods, results and assessment of environmental exposures. Toxicol. Appl. Pharmacol. 2008, 228, 114–134. [Google Scholar] [CrossRef] [PubMed]
- Hengstler, J.G.; Foth, H.; Gebel, T.; Kramer, P.-J.; Lilienblum, W.; Schweinfurth, H.; Völkel, W.; Wollin, K.-M.; Gundert-Remy, U. Critical evaluation of key evidence on the human health hazards of exposure to bisphenol A. Crit. Rev. Toxicol. 2011, 41, 263–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okur, R.; Turgut, F.; Sungur, S.; Yaprak, M.; Ozsan, M.; Ustun, I.; Gokce, C. Higher Serum Bisphenol A Levels in Diabetic Hemodialysis Patients. Blood Purif. 2016, 42, 77–82. [Google Scholar]
- Csanády, G.; Oberste-Frielinghaus, H.; Semder, B.; Baur, C.; Schneider, K.; Filser, J. Distribution and unspecific protein binding of the xenoestrogens bisphenol A and daidzein. Arch. Toxicol. 2002, 76, 299–305. [Google Scholar]
- Shankar, A.; Teppala, S.; Sabanayagam, C. Bisphenol A and peripheral arterial disease: Results from the NHANES. Environ. Health Perspect. 2012, 120, 1297–1300. [Google Scholar] [CrossRef] [PubMed]
- Saura, M.; Márquez, S.; Reventun, P.; Olea-Herrero, N.; Arenas, M.I.; Moreno-Gomez-Toledano, R.; Gomez-Parrizas, M.; Muñóz-Moreno, C.; Gonzalez-Santander, M.; Zaragoza, C.; et al. Oral administration of bisphenol A induces high blood pressure through angiotensin II/CaMKII-dependent uncoupling of eNOS. FASEB J. 2014, 28, 4719–4728. [Google Scholar] [CrossRef] [PubMed]
- Neri, M.; Virzì, G.M.; Brocca, A.; Garzotto, F.; Kim, J.C.; Ramponi, F.; de Cal, M.; Lorenzin, A.; Brendolan, A.; Nalesso, F.; et al. In vitro Cytotoxicity of Bisphenol A in Monocytes Cell Line. Blood Purif. 2015, 40, 180–186. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Opinion on BPA; European Food Safety Authority (EFSA): Parma, Italy, 2015. [Google Scholar]
- Quiroga, B.; Bosch, R.J.; Fiallos, R.A.; Sánchez-Heras, M.; Olea-Herrero, N.; López-Aparicio, P.; Muñóz-Moreno, C.; Pérez-Alvarsan, M.A.; De Arriba, G. Online Hemodiafiltration Reduces Bisphenol A Levels. Ther. Apher. Dial. 2017, 21, 96–101. [Google Scholar] [CrossRef]
- Mas, S.; Bosch-Panadero, E.; Abaigar, P.; Camarero, V.; Mahillo, I.; Civantos, E.; Sanchez-Ospina, D.; Ruiz-Priego, A.; Egido, J.; Ortiz, A.; et al. Influence of dialysis membrane composition on plasma bisphenol A levels during online hemodiafiltration. PLoS ONE 2018, 13, e0193288. [Google Scholar] [CrossRef] [PubMed]
- Kuruto-Niwa, R.; Tateoka, Y.; Usuki, Y.; Nozawa, R. Measurement of bisphenol A concentrations in human colostrum. Chemosphere 2007, 66, 1160–1164. [Google Scholar] [CrossRef] [PubMed]
- Leusch, F.D.; Neale, P.A.; Busetti, F.; Card, M.; Humpage, A.; Orbell, J.D.; Ridgway, H.F.; Stewart, M.B.; Van De Merwe, J.P.; Escher, B.I. Transformation of endocrine disrupting chemicals, pharmaceutical and personal care products during drinking water disinfection. Sci. Total Environ. 2019, 657, 1480–1490. [Google Scholar] [CrossRef] [PubMed]
 : SP).
: SP).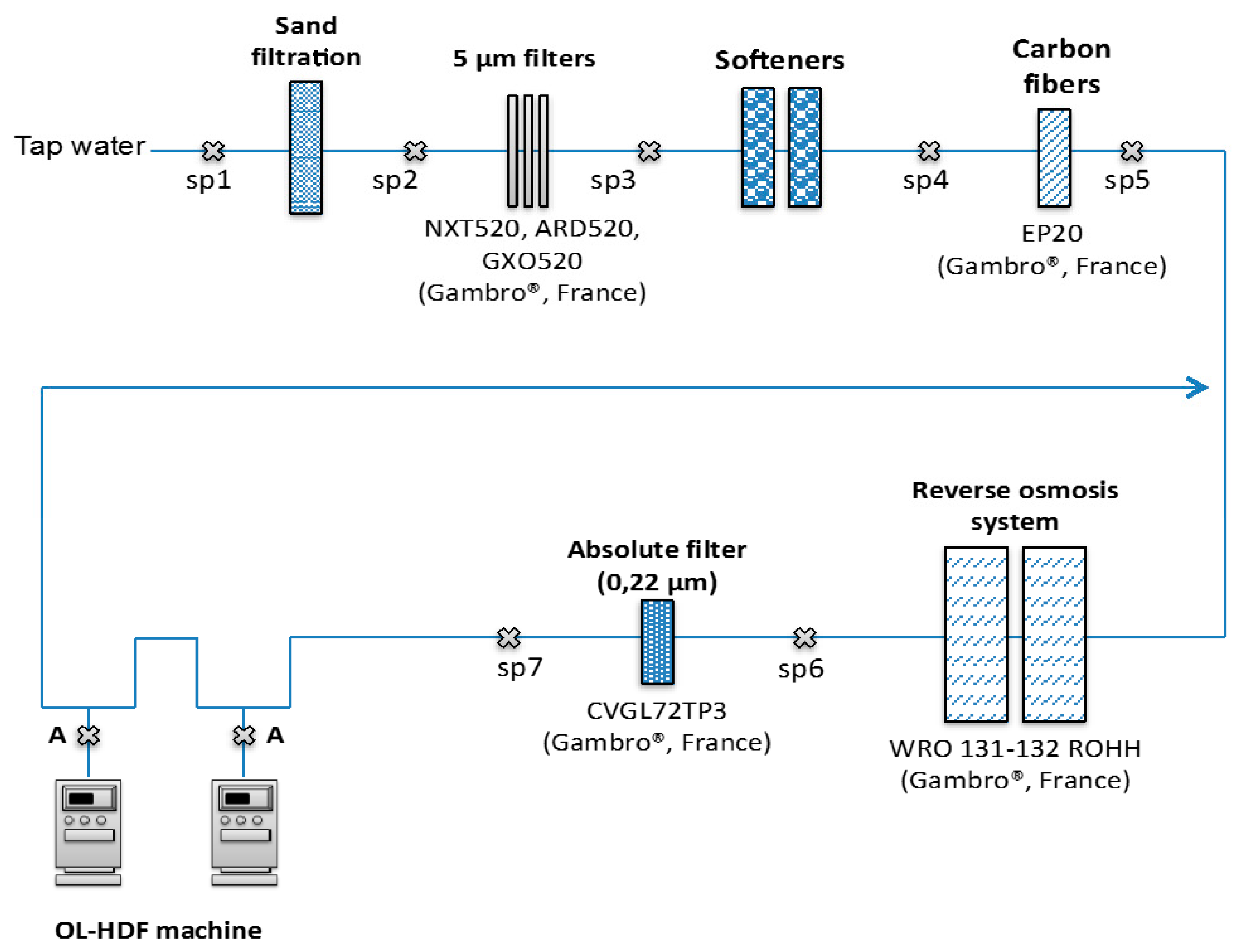
 : SP) for ultrapure water, ultrapure dialysate and replacement fluid.
: SP) for ultrapure water, ultrapure dialysate and replacement fluid.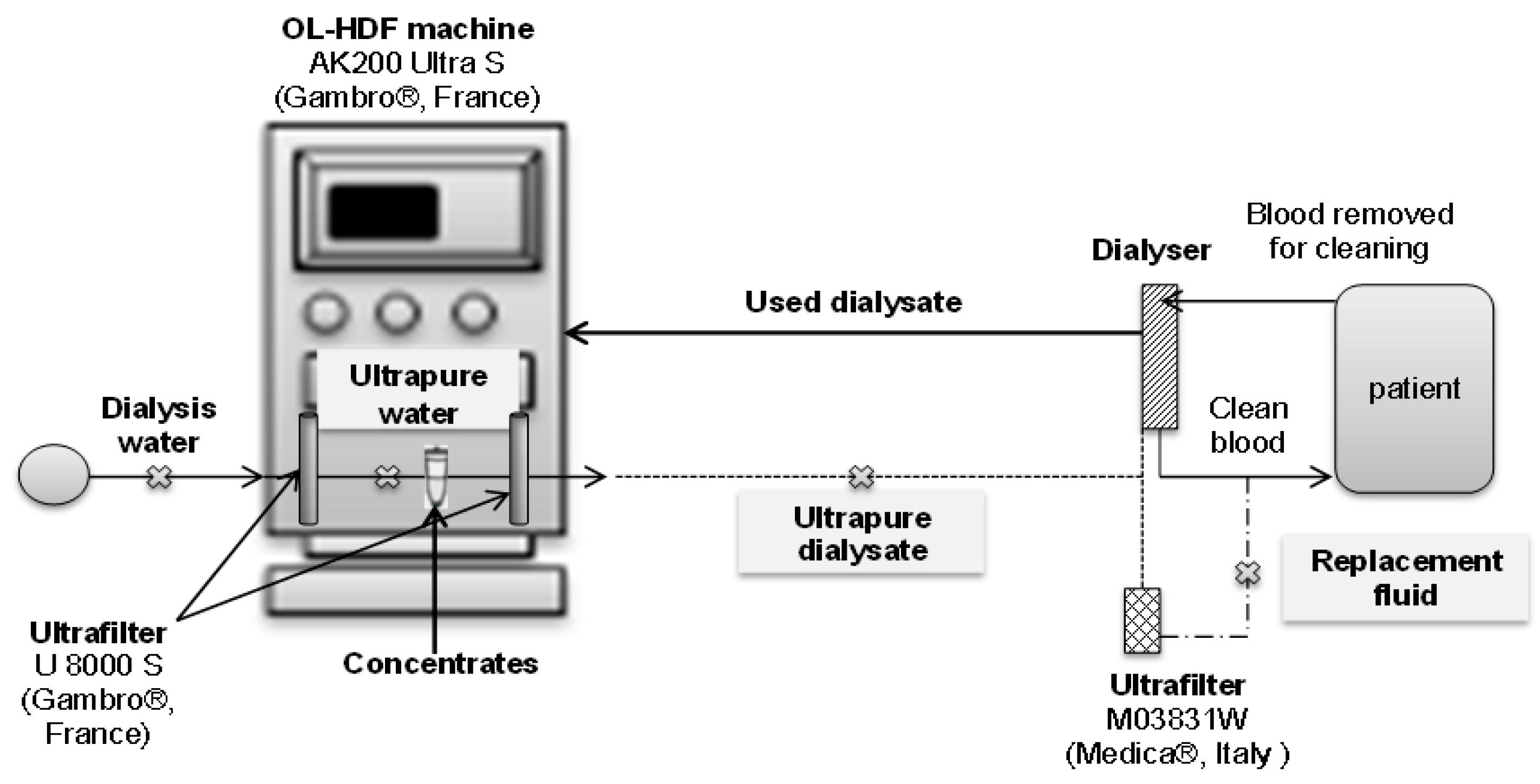
| Dialyzers | TS-2.1SL | Vie-21A | Elisio-21H | Polyflux 210H |
|---|---|---|---|---|
| Manufacturers | Toray (Tokyo, Japan) | AsahiKASEI (Tokyo, Japan) | Nipro EUROPE (St.Beauzire, France) | Gambro (Colombes, France) |
| Housing material | Polycarbonate | Polycarbonate | Polypropylene | Polycarbonate |
| Fiber material | Polysulfone | Polysulfone | Polyethersulfone | Polyarylethersulfone, Polyvinylpyrrolidone, Polyamide blend |
| [Inner diameter (µm) | 200 | 185 | 200 | 215 |
| Membrane thickness (µm) | 40 | 45 | 40 | 50 |
| Effective surface area (m²) | 2.1 | 2.1 | 2.1 | 2.1 |
| Potting material | Polyurethane | Polyurethane | Polyurethane | Polyurethane |
| Sterilization | Gamma-ray irradiation | Gamma-ray irradiation | Dry gamma | Steam |
| Dialyzers | Compartment | BPA (ng/dialyzer) |
|---|---|---|
| TS-2.1 SL | Rinsing solution | 365.9 ± 266.1 |
| Simulating blood | 0.6 ± 0.5 | |
| Dialysate | 0.9 ± 0.1 | |
| Polyflux 210H | Rinsing solution | 299.0 ± 129.7 |
| Simulating blood | 1.4 ± 0.2 | |
| Dialysate | 3.2 ± 3.5 | |
| Vie-21A | Rinsing solution | 440.4 ± 188.3 |
| Simulating blood | 38.5 ± 41.7 | |
| Dialysate | 16.2 ± 5.3 | |
| Elisio-21H | Rinsing solution | 111.7 ± 48.4 |
| Simulating blood | 3.7 ± 0.5 | |
| Dialysate | 3 ± 0.4 |
| BPA (ng·L−1) | MCBPA (ng·L−1) | DCBPA (ng·L−1) | TCBPA (ng·L−1) | TTCBPA (ng·L−1) | |
|---|---|---|---|---|---|
| Water intake (SP1) | 2.8 ± 2.0 | 0.7 ± 1.3 | 14.4 ± 14.3 | <LOQ | 1.9 ± 2.3 |
| Sand filter (SP2) | 0.8 ± 0.5 | <LOQ | 8.9 ± 6.6 | <LOQ | 0.6 ± 0.4 |
| 5 µm filter (SP3) | 2.0 ± 2.4 | <LOQ | 15.3 ± 13.0 | <LOQ | 1.0 ± 1.2 |
| Softener (SP4) | 3.2 ± 4.4 | <LOQ | 9.7 ± 4.0 | <LOQ | 0.6 ± 0.6 |
| Carbon filter (SP5) | 4.6 ± 7.6 | <LOQ | 12.9 ± 9.0 | ND | 0.9 ± 0.8 |
| Reverse osmosis (SP6) | 6.1 ± 10.0 | <LOQ | 14.5 ± 11.5 | ND | 1.0 ± 1.2 |
| Absolute filter (SP7) | 3.1 ± 2.8 | <LOQ | 8.4 ± 9.6 | ND | 0.6 ± 0.9 |
| Ultrapure Water (ng·L−1) | Ultrapure Dialysate (ng·L−1) | Replacement Fluid (ng·L−1) | |
|---|---|---|---|
| MCBPA | 0.4 ± 0.9 | 1.6 ± 1.7 | 3.4 ± 3.2 |
| DCBPA | 6.5 ± 1.2 | 12.8 ± 5.9 | 42.5 ± 22.1 |
| TCBPA | 1.8 ± 1.3 | 1.3 ± 0.7 | 1.3 ± 0.6 |
| TTBPA | 1.4 ± 0.9 | 0.7 ± 0.6 | 0.5 ± 0.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bacle, A.; Dupuis, A.; Belmouaz, M.; Bauwens, M.; Cambien, G.; Venisse, N.; Pierre-Eugene, P.; Potin, S.; Migeot, V.; Ayraud-Thevenot, S. Overexposure to Bisphenol A and Its Chlorinated Derivatives of Patients with End-Stage Renal Disease during Online Hemodiafiltration. Biomolecules 2019, 9, 403. https://doi.org/10.3390/biom9090403
Bacle A, Dupuis A, Belmouaz M, Bauwens M, Cambien G, Venisse N, Pierre-Eugene P, Potin S, Migeot V, Ayraud-Thevenot S. Overexposure to Bisphenol A and Its Chlorinated Derivatives of Patients with End-Stage Renal Disease during Online Hemodiafiltration. Biomolecules. 2019; 9(9):403. https://doi.org/10.3390/biom9090403
Chicago/Turabian StyleBacle, Astrid, Antoine Dupuis, Mohamed Belmouaz, Marc Bauwens, Guillaume Cambien, Nicolas Venisse, Pascale Pierre-Eugene, Sophie Potin, Virginie Migeot, and Sarah Ayraud-Thevenot. 2019. "Overexposure to Bisphenol A and Its Chlorinated Derivatives of Patients with End-Stage Renal Disease during Online Hemodiafiltration" Biomolecules 9, no. 9: 403. https://doi.org/10.3390/biom9090403
APA StyleBacle, A., Dupuis, A., Belmouaz, M., Bauwens, M., Cambien, G., Venisse, N., Pierre-Eugene, P., Potin, S., Migeot, V., & Ayraud-Thevenot, S. (2019). Overexposure to Bisphenol A and Its Chlorinated Derivatives of Patients with End-Stage Renal Disease during Online Hemodiafiltration. Biomolecules, 9(9), 403. https://doi.org/10.3390/biom9090403