Organometallic Compounds and Metal Complexes in Current and Future Treatments of Inflammatory Bowel Disease and Colorectal Cancer—a Critical Review
Abstract
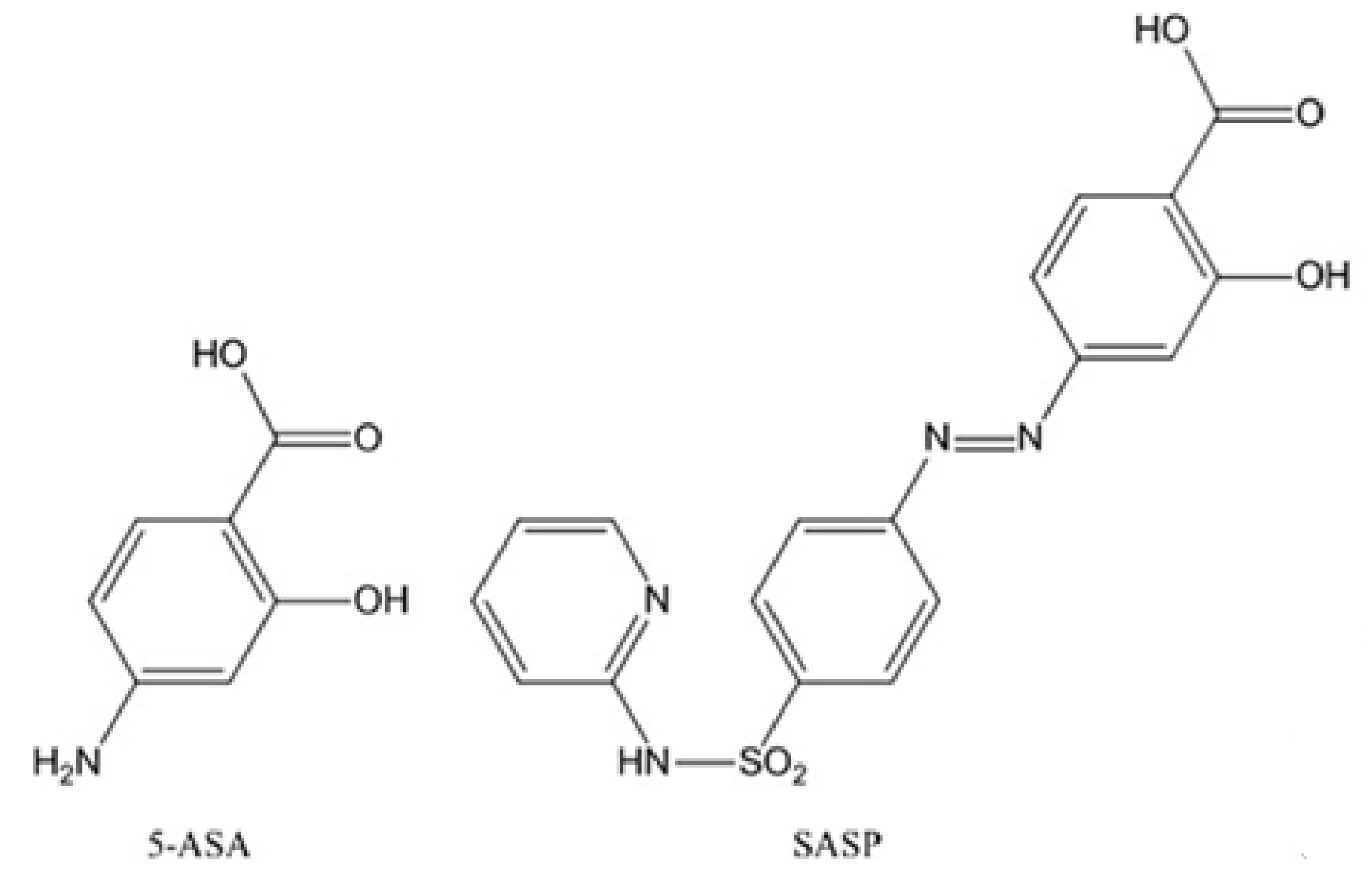
1. Introduction
2. Anti-Inflammatory Properties Related in IBD
2.1. Metal and Metal Complexes
2.1.1. Zinc
2.1.2. Silver
2.1.3. Gold
2.1.4. Rhodium
2.2. Electron Deficient Organometallics
3. Antibacterial Compounds
3.1. Gold
3.2. Zinc
3.3. Triphenyltin Complex (LTP)
3.4. Free Radicals Related in IBD
4. Carbon Monoxide Donors Related in IBD
5. Organometallic Compounds in CRC
5.1. Ruthenium
5.2. Iridium
5.3. Gold Complexes and Anti—Thioredoxin Reductase Activity
5.4. Osmium Complexes
5.5. Platinum
6. Main Problems Associated with Metal Compounds
7. Conclusions and Future Perspective
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 5-ASA | 5-aminosalicylic acid |
| 5-FU | 5-fluorouracil |
| AAS | Atomic absorption spectrometry |
| AgNPs | Silver nanoparticles |
| AzZnSA | 5-(p-sulfophenylazo-)-salicylidenealanine-zinc |
| CD | Crohn’s disease |
| CO | Carbon monoxide |
| CORM2 | Tricarbonyldichlororuthenium (II) dimer |
| CRC | Colorectal cancer |
| DACH | 1,2-diaminocyclohexane ligand |
| EGFR | Epidermal growth factor receptor |
| IBD | Inflammatory bowel diseases |
| IFN-γ | Interferon-gamma |
| IL-1 | Interleukin-1 |
| IL-4 | Interleukin-4 |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| iNOS | Inducible NO synthase |
| LPS | Lipopolysaccharide |
| LTB | Tributyltin complex |
| LTP | Triphenyltin complex |
| NAE | NEDD8-activating enzyme |
| NF-κB | Nuclear factor-κB |
| NO | Nitric oxide |
| OS | Oxidative stress |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| RT | Radiotherapy |
| SASP | Salicylazosulfapyridine |
| SMA | Styrene-maleic acid |
| SOD | Superoxide dismutase |
| STAT3 | Signal transducers and activators of transcription 3 |
| TNF-α | Tumor necrosis factor-alpha |
| TrxR | Thioredoxin reductase |
| TR1 | Thioredoxin reductase 1 |
| UA | Ursolic acid |
| UC | Ulcerative colitis |
References
- Kundu, J.; Surh, Y. Inflammation: Gearing the journey to cancer. Mutat. Res. Mutat. Res. 2008, 659, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Elinav, E.; Nowarski, R.; Thaiss, C.A.; Hu, B.; Jin, C.; Flavell, R.A. Inflammation-induced cancer: Crosstalk between tumours, immune cells and microorganisms. Nat. Rev. Cancer 2013, 13, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Gentschew, L.; Ferguson, L.R. Role of nutrition and microbiota in susceptibility to inflammatory bowel diseases. Mol. Nutr. Food Res. 2012, 56, 524–535. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Pitto-Barry, A.; Shang, L.; Barry, N.P.E. Anti-inflammatory activity of electron-deficient organometallics. R. Soc. Open Sci. 2017, 4, 170786. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T.; Willoughby, D.A.; Gilroy, D.W. Anti-inflammatory lipid mediators and insights into the resolution of inflammation. Nat. Rev. Immunol. 2002, 2, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Halpin, S.J.; Ford, A.C. Prevalence of Symptoms Meeting Criteria for Irritable Bowel Syndrome in Inflammatory Bowel Disease: Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2012, 107, 1474–1482. [Google Scholar] [CrossRef] [PubMed]
- Su, H.-J.; Chiu, Y.-T.; Chiu, C.-T.; Lin, Y.-C.; Wang, C.-Y.; Hsieh, J.-Y.; Wei, S.-C. Inflammatory bowel disease and its treatment in 2018: Global and Taiwanese status updates. J. Formos. Med. Assoc. 2018, 1–10. [Google Scholar] [CrossRef]
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing Incidence and Prevalence of the Inflammatory Bowel Diseases With Time, Based on Systematic Review. Gastroenterology 2012, 142, 46–54. [Google Scholar] [CrossRef]
- Sairenji, T.; Collins, K.L.; Evans, D.V. An Update on Inflammatory Bowel Disease. Prim. Care Clin. Off. Pract. 2017, 44, 673–692. [Google Scholar] [CrossRef]
- Lee, H.S.; Park, S.-K.; Park, D. Il Novel treatments for inflammatory bowel disease. Korean J. Intern. Med. 2018, 33, 20–27. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Li, Y.Y. Inflammatory bowel disease: Pathogenesis. World J. Gastroenterol. 2014, 20, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Bavastrelli, M.; Caivano, L.; Maimone, C.; Navarra, D.; Maimone, M. Cow’s milk proteins allergy which simulates chronic inflammatory bowel disease. Dig. Liver Dis. 2017, 49, e266. [Google Scholar] [CrossRef]
- Hovde, Ø.; Moum, B.A. Epidemiology and clinical course of Crohn’s disease: Results from observational studies. World J. Gastroenterol. 2012, 18, 1723–1731. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.R.; Abar, L.; Chan, D.S.M.; Vingeliene, S.; Polemiti, E.; Stevens, C.; Greenwood, D.; Norat, T. Foods and beverages and colorectal cancer risk: A systematic review and meta-analysis of cohort studies, an update of the evidence of the WCRF-AICR Continuous Update Project. Ann. Oncol. 2017, 28, 1788–1802. [Google Scholar] [CrossRef] [PubMed]
- Long, A.G.; Lundsmith, E.T.; Hamilton, K.E. Inflammation and Colorectal Cancer. Curr. Colorectal Cancer Rep. 2017, 13, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Eluri, S.; Parian, A.M.; Limketkai, B.N.; Ha, C.Y.; Brant, S.R.; Dudley-Brown, S.; Efron, J.E.; Fang, S.G.; Gearhart, S.L.; Marohn, M.R.; et al. Nearly a Third of High-Grade Dysplasia and Colorectal Cancer Is Undetected in Patients with Inflammatory Bowel Disease. Dig. Dis. Sci. 2017, 62, 3586–3593. [Google Scholar] [CrossRef]
- Rutgeerts, P.; Vermeire, S.; Van Assche, G. Biological Therapies for Inflammatory Bowel Diseases. Gastroenterology 2009, 136, 1182–1197. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J.; Adcock, I.M. Glucocorticoid resistance in inflammatory diseases. Lancet 2009, 373, 1905–1917. [Google Scholar] [CrossRef]
- Gasser, G.; Ott, I.; Metzler-Nolte, N. Organometallic anticancer compounds. J. Med. Chem. 2011, 54, 3–25. [Google Scholar] [CrossRef]
- Zhang, P.; Sadler, P.J. Advances in the design of organometallic anticancer complexes. J. Organomet. Chem. 2017, 839, 5–14. [Google Scholar] [CrossRef]
- Liu, Z.; Habtemariam, A.; Pizarro, A.M.; Clarkson, G.J.; Sadler, P.J. Organometallic Iridium(III) Cyclopentadienyl Anticancer Complexes Containing C,N-Chelating Ligands. Organometallics 2011, 30, 4702–4710. [Google Scholar] [CrossRef]
- Betanzos-Lara, S.; Salassa, L.; Habtemariam, A.; Novakova, O.; Pizarro, A.M.; Clarkson, G.J.; Liskova, B.; Brabec, V.; Sadler, P.J. Photoactivatable Organometallic Pyridyl Ruthenium(II) Arene Complexes. Organometallics 2012, 31, 3466–3479. [Google Scholar]
- Hearn, J.M.; Romero-Canelón, I.; Qamar, B.; Liu, Z.; Hands-Portman, I.; Sadler, P.J. Organometallic Iridium(III) Anticancer Complexes with New Mechanisms of Action: NCI-60 Screening, Mitochondrial Targeting, and Apoptosis. ACS Chem. Biol. 2013, 8, 1335–1343. [Google Scholar] [CrossRef] [PubMed]
- Bergamo, A.; Masi, A.; Peacock, A.F.A.; Habtemariam, A.; Sadler, P.J.; Sava, G. In vivo tumour and metastasis reduction and in vitro effects on invasion assays of the ruthenium RM175 and osmium AFAP51 organometallics in the mammary cancer model. J. Inorg. Biochem. 2010, 104, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Qian, H.; Yiu, S.-M.; Sun, J.; Zhu, G. Multi-targeted organometallic ruthenium(II)–arene anticancer complexes bearing inhibitors of poly(ADP-ribose) polymerase-1: A strategy to improve cytotoxicity. J. Inorg. Biochem. 2014, 131, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Furfaro, F.; Bezzio, C.; Ardizzone, S.; Massari, A.; de Franchis, R.; Maconi, G. Overview of biological therapy in ulcerative colitis: Current and future directions. J. Gastrointest. Liver Dis. 2015, 24, 203–213. [Google Scholar]
- Conner, E.M.; Reglinski, J.; Smith, W.E.; Zeitlin, I.J. Schiff base complexes of copper and zinc as potential anticolitic compounds. Biometals 2017, 30, 423–439. [Google Scholar] [CrossRef][Green Version]
- Itagaki, M.; Saruta, M.; Saijo, H.; Mitobe, J.; Arihiro, S.; Matsuoka, M.; Kato, T.; Ikegami, M.; Tajiri, H. Efficacy of zinc–carnosine chelate compound, Polaprezinc, enemas in patients with ulcerative colitis. Scand. J. Gastroenterol. 2014, 49, 164–172. [Google Scholar] [CrossRef]
- Hansberry, D.R.; Shah, K.; Agarwal, P.; Agarwal, N. Fecal Myeloperoxidase as a Biomarker for Inflammatory Bowel Disease. Cureus 2017, 9, e1004. [Google Scholar] [CrossRef]
- Galli, S.J.; Tsai, M.; Piliponsky, A.M. The development of allergic inflammation. Nature 2008, 454, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Nadworny, P.L.; Wang, J.; Tredget, E.E.; Burrell, R.E. Anti-inflammatory activity of nanocrystalline silver in a porcine contact dermatitis model. Nanomed. Nanotechnol. Biol. Med. 2008, 4, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gao, P.; Du, J.; Zhao, X.; Wong, K.K.Y. Long-term anti-inflammatory efficacy in intestinal anastomosis in mice using silver nanoparticle-coated suture. J. Pediatr. Surg. 2017, 52, 2083–2087. [Google Scholar] [CrossRef] [PubMed]
- Bhol, K.C.; Schechter, P.J. Effects of nanocrystalline silver (NPI 32101) in a rat model of ulcerative colitis. Dig. Dis. Sci. 2007. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, S.; Gilmer, J.F.; Medina, C. Matrix Metalloproteinases in Inflammatory Bowel Disease: An Update. Mediators Inflamm. 2015, 2015, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Siczek, K.; Zatorski, H.; Chmielowiec-Korzeniowska, A.; Kordek, R.; Tymczyna, L.; Fichna, J. Evaluation of anti-inflammatory effect of silver-coated glass beads in mice with experimentally induced colitis as a new type of treatment in inflammatory bowel disease. Pharmacol. Rep. 2017, 69, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Yeo, C.; Ooi, K.; Tiekink, E. Gold-Based Medicine: A Paradigm Shift in Anti-Cancer Therapy? Molecules 2018, 23, 1410. [Google Scholar] [CrossRef]
- Dasari, T.P.S.; Zhang, Y.; Yu, H. Antibacterial Activity and Cytotoxicity of Gold (I) and (III) Ions and Gold Nanoparticles. Biochem. Pharmacol. Open Access 2015, 4. [Google Scholar] [CrossRef]
- Hikisz, P.; Szczupak, Ł.; Koceva-Chyła, A.; Guśpiel, A.; Oehninger, L.; Ott, I.; Therrien, B.; Solecka, J.; Kowalski, K. Anticancer and Antibacterial Activity Studies of Gold(I)-Alkynyl Chromones. Molecules 2015, 20, 19699–19718. [Google Scholar] [CrossRef]
- Leung, C.-H.; Lin, S.; Zhong, H.-J.; Ma, D.-L. Metal complexes as potential modulators of inflammatory and autoimmune responses. Chem. Sci. 2015, 6, 871–884. [Google Scholar] [CrossRef]
- Khanna, P.; Chua, P.J.; Bay, B.H.; Baeg, G.H. The JAK/STAT signaling cascade in gastric carcinoma (Review). Int. J. Oncol. 2015, 47, 1617–1626. [Google Scholar] [CrossRef] [PubMed]
- Musso, A.; Dentelli, P.; Carlino, A.; Chiusa, L.; Repici, A.; Sturm, A.; Fiocchi, C.; Rizzetto, M.; Pegoraro, L.; Sategna-Guidetti, C.; et al. Signal transducers and activators of transcription 3 signaling pathway: An essential mediator of inflammatory bowel disease and other forms of intestinal inflammation. Inflamm. Bowel Dis. 2005, 11, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Kupcewicz, B.; Sobiesiak, K.; Malinowska, K.; Koprowska, K.; Czyz, M.; Keppler, B.; Budzisz, E. Copper(II) complexes with derivatives of pyrazole as potential antioxidant enzyme mimics. Med. Chem. Res. 2013, 22, 2395–2402. [Google Scholar] [CrossRef] [PubMed]
- Roder, C.; Thomson, M.J. Auranofin: Repurposing an old drug for a golden new age. Drugs R D 2015, 15, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Trávníček, Z.; Štarha, P.; Vančo, J.; Šilha, T.; Hošek, J.; Suchý, P.; Pražanová, G. Anti-inflammatory Active Gold(I) Complexes Involving 6-Substituted-Purine Derivatives. J. Med. Chem. 2012, 55, 4568–4579. [Google Scholar] [CrossRef] [PubMed]
- Vančo, J.; Gáliková, J.; Hošek, J.; Dvořák, Z.; Paráková, L.; Trávníček, Z. Gold(I) Complexes of 9-Deazahypoxanthine as Selective Antitumor and Anti-Inflammatory Agents. PLoS ONE 2014, 9, e109901. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.-J.; Wang, W.; Kang, T.-S.; Yan, H.; Yang, Y.; Xu, L.; Wang, Y.; Ma, D.-L.; Leung, C.-H. A Rhodium(III) Complex as an Inhibitor of Neural Precursor Cell Expressed, Developmentally Down-Regulated 8-Activating Enzyme with in Vivo Activity against Inflammatory Bowel Disease. J. Med. Chem. 2017, 60, 497–503. [Google Scholar] [CrossRef]
- Wang, F.; Chen, H.; Parsons, S.; Oswald, I.D.H.; Davidson, J.E.; Sadler, P.J. Kinetics of Aquation and Anation of Ruthenium(II) Arene Anticancer Complexes, Acidity and X-ray Structures of Aqua Adducts. Chem. A Eur. J. 2003, 9, 5810–5820. [Google Scholar] [CrossRef]
- Süss-Fink, G. Areneruthenium complexes as anticancer agents. Dalt. Trans. 2010, 39, 1673–1688. [Google Scholar] [CrossRef]
- Cross, R.K.; Wilson, K.T. Nitric oxide in inflammatory bowel disease. Inflamm. Bowel Dis. 2003, 9, 179–189. [Google Scholar] [CrossRef]
- Matsuoka, K.; Kanai, T. The gut microbiota and inflammatory bowel disease. Semin. Immunopathol. 2015, 37, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, S.; Steed, H.; Macfarlane, G.T. Intestinal bacteria and inflammatory bowel disease. Crit. Rev. Clin. Lab. Sci. 2009, 46, 25–54. [Google Scholar] [CrossRef] [PubMed]
- Conte, M.P.; Schippa, S.; Zamboni, I.; Penta, M.; Chiarini, F.; Seganti, L.; Osborn, J.; Falconieri, P.; Borrelli, O.; Cucchiara, S. Gut-associated bacterial microbiota in paediatric patients with inflammatory bowel disease. Gut 2006, 55, 1760–1767. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Medina, M.; Garcia-Gil, L.J. Escherichia coli in chronic inflammatory bowel diseases: An update on adherent invasive Escherichia coli pathogenicity. World J. Gastrointest. Pathophysiol. 2014, 5, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Palmela, C.; Chevarin, C.; Xu, Z.; Torres, J.; Sevrin, G.; Hirten, R.; Barnich, N.; Ng, S.C.; Colombel, J.-F. Adherent-invasive Escherichia coli in inflammatory bowel disease. Gut 2018, 67, 574–587. [Google Scholar] [CrossRef] [PubMed]
- Mylonaki, M.; Rayment, N.B.; Rampton, D.S.; Hudspith, B.N.; Brostoff, J. Molecular Characterization of Rectal Mucosa-Associated Bacterial Flora in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2005, 11, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Tarafdar, A.; Sinha, A.; Bhunia, A.; Harms, K.; Nayek, H.P. Mononuclear metal (II) complexes of a Bis(organoamido)phosphate ligand with antimicrobial activities against Escherichia coli. Appl. Organomet. Chem. 2017, 31, e3821. [Google Scholar] [CrossRef]
- Özdemir, İ.; Denizci, A.; Öztürk, H.T.; Çetinkaya, B. Synthetic and antimicrobial studies on new gold(I) complexes of imidazolidin-2-ylidenes. Appl. Organomet. Chem. 2004, 18, 318–322. [Google Scholar] [CrossRef]
- Tapiero, H.; Townsend, D.M.; Tew, K.D. The antioxidant role of selenium and seleno-compounds. Biomed. Pharmacother. 2003, 57, 134–144. [Google Scholar] [CrossRef]
- Abu Ali, H.; Fares, H.; Darawsheh, M.; Rappocciolo, E.; Akkawi, M.; Jaber, S. Synthesis, characterization and biological activity of new mixed ligand complexes of Zn(II) naproxen with nitrogen based ligands. Eur. J. Med. Chem. 2015, 89, 67–76. [Google Scholar] [CrossRef]
- Chiba, M.; Hoshina, S.; Kono, M.; Tobita, M.; Fukushima, T.; Iizuka, M.; Watanabe, S. Staphylococcus aureus in inflammatory bowel disease. Scand. J. Gastroenterol. 2001, 36, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, M.; Patra, M.; Senges, C.H.R.; Ott, I.; Stepanek, J.J.; Pinto, A.; Prochnow, P.; Vuong, C.; Langklotz, S.; Metzler-Nolte, N.; et al. Analysis of the Mechanism of Action of Potent Antibacterial Hetero-tri-organometallic Compounds: A Structurally New Class of Antibiotics. ACS Chem. Biol. 2013, 8, 1442–1450. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, M.; Ahmad, S.; Shahid, K.; Sadiq, A.; Rashid, U. Ursolic Acid Hydrazide Based Organometallic Complexes: Synthesis, Characterization, Antibacterial, Antioxidant, and Docking Studies. Front. Chem. 2018, 6, 55. [Google Scholar] [CrossRef] [PubMed]
- Holani, R.; Marin, M.S.; Kastelic, J.P.; Cobo, E.R. Host Defense Peptides as Innate Immunomodulators in the Pathogenesis of Colitis. In Antimicrobial Peptides in Gastrointestinal Diseases; Academic Press: Cambridge, MA, USA, 2018; pp. 133–164. [Google Scholar]
- Alvarez-Suarez, J.M.; Giampieri, F.; Battino, M. Honey as a source of dietary antioxidants: Structures, bioavailability and evidence of protective effects against human chronic diseases. Curr. Med. Chem. 2013, 20, 621–638. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Achitei, D.; Ciobica, A.; Balan, G.; Gologan, E.; Stanciu, C.; Stefanescu, G. Different Profile of Peripheral Antioxidant Enzymes and Lipid Peroxidation in Active and Non-active Inflammatory Bowel Disease Patients. Dig. Dis. Sci. 2013, 58, 1244–1249. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Lam, Y.T.; Tsoi, K.K.F.; Chan, F.K.L.; Sung, J.J.Y.; Wu, J.C.Y. Systematic review: The efficacy of herbal therapy in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2013, 38, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.; Lan, S. Implications of Antioxidant Systems in Inflammatory Bowel Disease. Biomed Res. Int. 2018, 2018, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bencini, A.; Failli, P.; Valtancoli, B.; Bani, D. Low Molecular Weight Compounds with Transition Metals as Free Radical Scavengers and Novel Therapeutic Agents. Cardiovasc. Hematol. Agents Med. Chem. 2010, 8, 128–146. [Google Scholar] [CrossRef]
- Li, Q.; Browne, W.R.; Roelfes, G. DNA Cleavage Activity of Fe(II)N4Py under Photo Irradiation in the Presence of 1,8-Naphthalimide and 9-Aminoacridine: Unexpected Effects of Reactive Oxygen Species Scavengers. Inorg. Chem. 2011, 50, 8318–8325. [Google Scholar] [CrossRef]
- Serena, C.; Calvo, E.; Clares, M.P.; Diaz, M.L.; Chicote, J.U.; Beltrán-Debon, R.; Fontova, R.; Rodriguez, A.; García-España, E.; García-España, A. Significant In Vivo Anti-Inflammatory Activity of Pytren4Q-Mn a Superoxide Dismutase 2 (SOD2) Mimetic Scorpiand-Like Mn (II) Complex. PLoS ONE 2015, 10, e0119102. [Google Scholar] [CrossRef] [PubMed]
- Checker, R.; Sandur, S.K.; Sharma, D.; Patwardhan, R.S.; Jayakumar, S.; Kohli, V.; Sethi, G.; Aggarwal, B.B.; Sainis, K.B. Potent Anti-Inflammatory Activity of Ursolic Acid, a Triterpenoid Antioxidant, Is Mediated through Suppression of NF-κB, AP-1 and NF-AT. PLoS ONE 2012, 7, e31318. [Google Scholar] [CrossRef] [PubMed]
- Shishodia, S.; Majumdar, S.; Banerjee, S.; Aggarwal, B.B. Ursolic acid inhibits nuclear factor-kappaB activation induced by carcinogenic agents through suppression of IkappaBalpha kinase and p65 phosphorylation: Correlation with down-regulation of cyclooxygenase 2, matrix metalloproteinase 9, and cyclin D1. Cancer Res. 2003, 63, 4375–4383. [Google Scholar] [PubMed]
- Zeng, G.; Chen, J.; Liang, Q.; You, W.; Wu, H.; Xiong, X. Ursolic acid inhibits T-cell activation through modulating nuclear factor-κ B signaling. Chin. J. Integr. Med. 2012, 18, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Fang, J.; Liao, L.; Nakamura, H.; Maeda, H. Styrene-maleic acid copolymer-encapsulated CORM2, a water-soluble carbon monoxide (CO) donor with a constant CO-releasing property, exhibits therapeutic potential for inflammatory bowel disease. J. Control. Release 2014, 187, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Muggia, F. Platinum compounds 30 years after the introduction of cisplatin: Implications for the treatment of ovarian cancer. Gynecol. Oncol. 2009, 112, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Massai, L.; Fernández-Gallardo, J.; Guerri, A.; Arcangeli, A.; Pillozzi, S.; Contel, M.; Messori, L. Design, synthesis and characterisation of new chimeric ruthenium( ii )–gold( i ) complexes as improved cytotoxic agents. Dalt. Trans. 2015, 44, 11067–11076. [Google Scholar] [CrossRef]
- Althumairi, A.A.; Lazarev, M.G.; Gearhart, S.L. Inflammatory bowel disease associated neoplasia: A surgeon’s perspective. World J. Gastroenterol. 2016, 22, 961–973. [Google Scholar] [CrossRef]
- Zhiqin, W.; Palaniappan, S.; Raja Ali, R.A. Inflammatory Bowel Disease-related Colorectal Cancer in the Asia-Pacific Region: Past, Present, and Future. Intest. Res. 2014, 12, 194–204. [Google Scholar] [CrossRef]
- Turker, N.S.; Heidari, P.; Kucherlapati, R.; Kucherlapati, M.; Mahmood, U. An EGFR Targeted PET Imaging Probe for the Detection of Colonic Adenocarcinomas in the Setting of Colitis. Theranostics 2014, 4, 893–903. [Google Scholar] [CrossRef]
- Florindo, P.R.; Pereira, D.M.; Borralho, P.M.; Rodrigues, C.M.P.; Piedade, M.F.M.; Fernandes, A.C. Cyclopentadienyl–Ruthenium(II) and Iron(II) Organometallic Compounds with Carbohydrate Derivative Ligands as Good Colorectal Anticancer Agents. J. Med. Chem. 2015, 58, 4339–4347. [Google Scholar] [CrossRef] [PubMed]
- Carter, R.; Westhorpe, A.; Romero, M.; Habtemariam, A.; Gallevo, C.; Bark, Y.; Menezes, N.; Sadler, P.; Sharma, R. Radiosensitisation of human colorectal cancer cells by ruthenium(II) arene anticancer complexes. Sci. Rep. 2016, 6, 20596. [Google Scholar] [CrossRef] [PubMed]
- Dougan, S.J.; Habtemariam, A.; McHale, S.E.; Parsons, S.; Sadler, P.J. Catalytic organometallic anticancer complexes. Proc. Natl. Acad. Sci. USA 2008, 105, 11628–11633. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, P.-S.; Pichler, V.; Roller, A.; Hejl, M.; Jakupec, M.A.; Kandioller, W.; Keppler, B.K. Improved reaction conditions for the synthesis of new NKP-1339 derivatives and preliminary investigations on their anticancer potential. Dalt. Trans. 2015, 44, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Lentz, F.; Drescher, A.; Lindauer, A.; Henke, M.; Hilger, R.A.; Hartinger, C.G.; Scheulen, M.E.; Dittrich, C.; Keppler, B.K.; Jaehde, U.; et al. Pharmacokinetics of a novel anticancer ruthenium complex (KP1019, FFC14A) in a phase I dose-escalation study. Anticancer. Drugs 2009, 20, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Novakova, O.; Kasparkova, J.; Bursova, V.; Hofr, C.; Vojtiskova, M.; Chen, H.; Sadler, P.J.; Brabec, V. Conformation of DNA Modified by Monofunctional Ru(II) Arene Complexes: Recognition by DNA Binding Proteins and Repair. Relationship to Cytotoxicity. Chem. Biol. 2005, 12, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.; Bonvin, D.; Berndsen, R.H.; Scherrer, E.; Wong, T.J.; Dyson, P.J.; Griffioen, A.W.; Nowak-Sliwinska, P. Angiostatic treatment prior to chemo- or photodynamic therapy improves anti-tumor efficacy. Sci. Rep. 2015, 5, 8990. [Google Scholar] [CrossRef] [PubMed]
- Lord, R.M.; Zegke, M.; Henderson, I.R.; Pask, C.M.; Shepherd, H.J.; McGowan, P.C. β-Ketoiminato Iridium(III) Organometallic Complexes: Selective Cytotoxicity towards Colorectal Cancer Cells HCT116 p53 -/-. Chem. A Eur. J. 2019, 25, 495–500. [Google Scholar] [CrossRef]
- Hatfield, D.L.; Tsuji, P.A.; Carlson, B.A.; Gladyshev, V.N. Selenium and selenocysteine: Roles in cancer, health, and development. Trends Biochem. Sci. 2014, 39, 112–120. [Google Scholar] [CrossRef]
- Yoo, M.-H.; Xu, X.-M.; Carlson, B.A.; Patterson, A.D.; Gladyshev, V.N.; Hatfield, D.L. Targeting Thioredoxin Reductase 1 Reduction in Cancer Cells Inhibits Self-Sufficient Growth and DNA Replication. PLoS ONE 2007, 2, e1112. [Google Scholar] [CrossRef]
- van Rijt, S.H.; Peacock, A.F.A.; Johnstone, R.D.L.; Parsons, S.; Sadler, P.J. Organometallic Osmium(II) Arene Anticancer Complexes Containing Picolinate Derivatives. Inorg. Chem. 2009, 48, 1753–1762. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Habtemariam, A.; Pizarro, A.M.; van Rijt, S.H.; Healey, D.J.; Cooper, P.A.; Shnyder, S.D.; Clarkson, G.J.; Sadler, P.J. Organometallic Osmium Arene Complexes with Potent Cancer Cell Cytotoxicity. J. Med. Chem. 2010, 53, 8192–8196. [Google Scholar] [CrossRef] [PubMed]
- Shnyder, S.D.; Fu, Y.; Habtemariam, A.; van Rijt, S.H.; Cooper, P.A.; Loadman, P.M.; Sadler, P.J. Anti-colorectal cancer activity of an organometallic osmium arene azopyridine complex. Medchemcomm 2011, 2, 666. [Google Scholar] [CrossRef]
- Jungwirth, U.; Kowol, C.R.; Keppler, B.K.; Hartinger, C.G.; Berger, W.; Heffeter, P. Anticancer Activity of Metal Complexes: Involvement of Redox Processes. Antioxid. Redox Signal. 2011, 15, 1085–1127. [Google Scholar] [CrossRef] [PubMed]
- Lebwohl, D.; Canetta, R. Clinical development of platinum complexes in cancer therapy: An historical perspective and an update. Eur. J. Cancer 1998, 34, 1522–1534. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Faivre, S.; Chan, D.; Salinas, R.; Woynarowska, B.; Woynarowski, J.M. DNA strand breaks and apoptosis induced by oxaliplatin in cancer cells. Biochem. Pharmacol. 2003, 66, 225–237. [Google Scholar] [CrossRef]
- Chaney, S.G.; Campbell, S.L.; Bassett, E.; Wu, Y. Recognition and processing of cisplatin- and oxaliplatin-DNA adducts. Crit. Rev. Oncol. Hematol. 2005, 53, 3–11. [Google Scholar] [CrossRef]
- Palermo, G.; Magistrato, A.; Riedel, T.; von Erlach, T.; Davey, C.A.; Dyson, P.J.; Rothlisberger, U. Fighting Cancer with Transition Metal Complexes: From Naked DNA to Protein and Chromatin Targeting Strategies. ChemMedChem 2016, 11, 1199–1210. [Google Scholar] [CrossRef]
- Mostafa El Ashry, G.; El Melegy, K.M. Synthesis, Characterization and Effectiveness of Chelated Mineral as Aflatoxin Absorbents. J. Chem. Biol. Ther. 2016, 1, 1–5. [Google Scholar] [CrossRef]
- Tőkés, L. The allure of mass spectrometry: From an earlyday chemist’s perspective. Mass Spectrom. Rev. 2017, 36, 520–542. [Google Scholar]














| Organometallic Compound | Application in Medicine/Mechanism of Action | Ref. |
|---|---|---|
| Na2[Fe(CN)(NO)] | Anti-hypertension | [21] |
| Irridium (III) Cyclopentadienyl containing C, N-chelating ligands Pyridyl Ruthenium (II) arene | Anti-tumor properties Aqua-adduct formation | [22] [23] |
| Irridium (III) cyclopentadienyl containing C,N or N,N–chelating ligands | DNA fragmentation Inhibition of protein synthesis | [24] |
| Osmium(II) arene AFAP51 Multi-targeted organometallic ruthenium (II)-arene | Anti-tumor properties | [25] [26] |
| Iridium compounds [Ir (η5-pentamethylcyclopentadien) (1,2-dicarb-klozo-dodecarboran-1,2-ditiolato)] | Anti-inflammatory action | [4] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szczepaniak, A.; Fichna, J. Organometallic Compounds and Metal Complexes in Current and Future Treatments of Inflammatory Bowel Disease and Colorectal Cancer—a Critical Review. Biomolecules 2019, 9, 398. https://doi.org/10.3390/biom9090398
Szczepaniak A, Fichna J. Organometallic Compounds and Metal Complexes in Current and Future Treatments of Inflammatory Bowel Disease and Colorectal Cancer—a Critical Review. Biomolecules. 2019; 9(9):398. https://doi.org/10.3390/biom9090398
Chicago/Turabian StyleSzczepaniak, Adrian, and Jakub Fichna. 2019. "Organometallic Compounds and Metal Complexes in Current and Future Treatments of Inflammatory Bowel Disease and Colorectal Cancer—a Critical Review" Biomolecules 9, no. 9: 398. https://doi.org/10.3390/biom9090398
APA StyleSzczepaniak, A., & Fichna, J. (2019). Organometallic Compounds and Metal Complexes in Current and Future Treatments of Inflammatory Bowel Disease and Colorectal Cancer—a Critical Review. Biomolecules, 9(9), 398. https://doi.org/10.3390/biom9090398




