IL-38: A New Player in Inflammatory Autoimmune Disorders
Abstract
1. Introduction
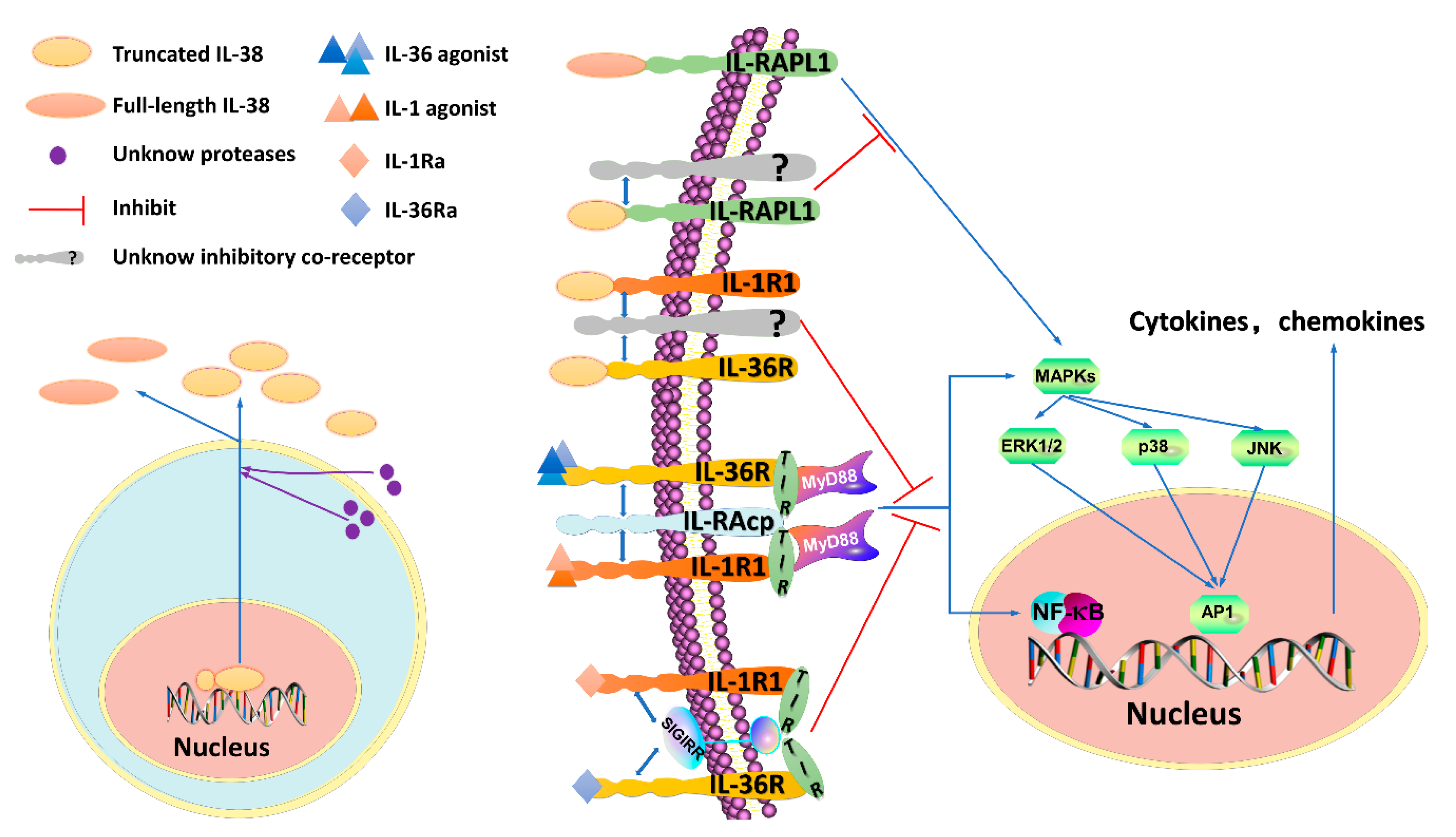
2. Functions of IL-38 and Presumed Signaling
2.1. The IL-38/IL-1R1 Signaling Pathway and Its Functions
2.2. The IL-38/IL-36R Signaling Pathway and Its Functions
2.3. The IL-38/IL-1RAPL1 Signaling Pathway and Its Functions
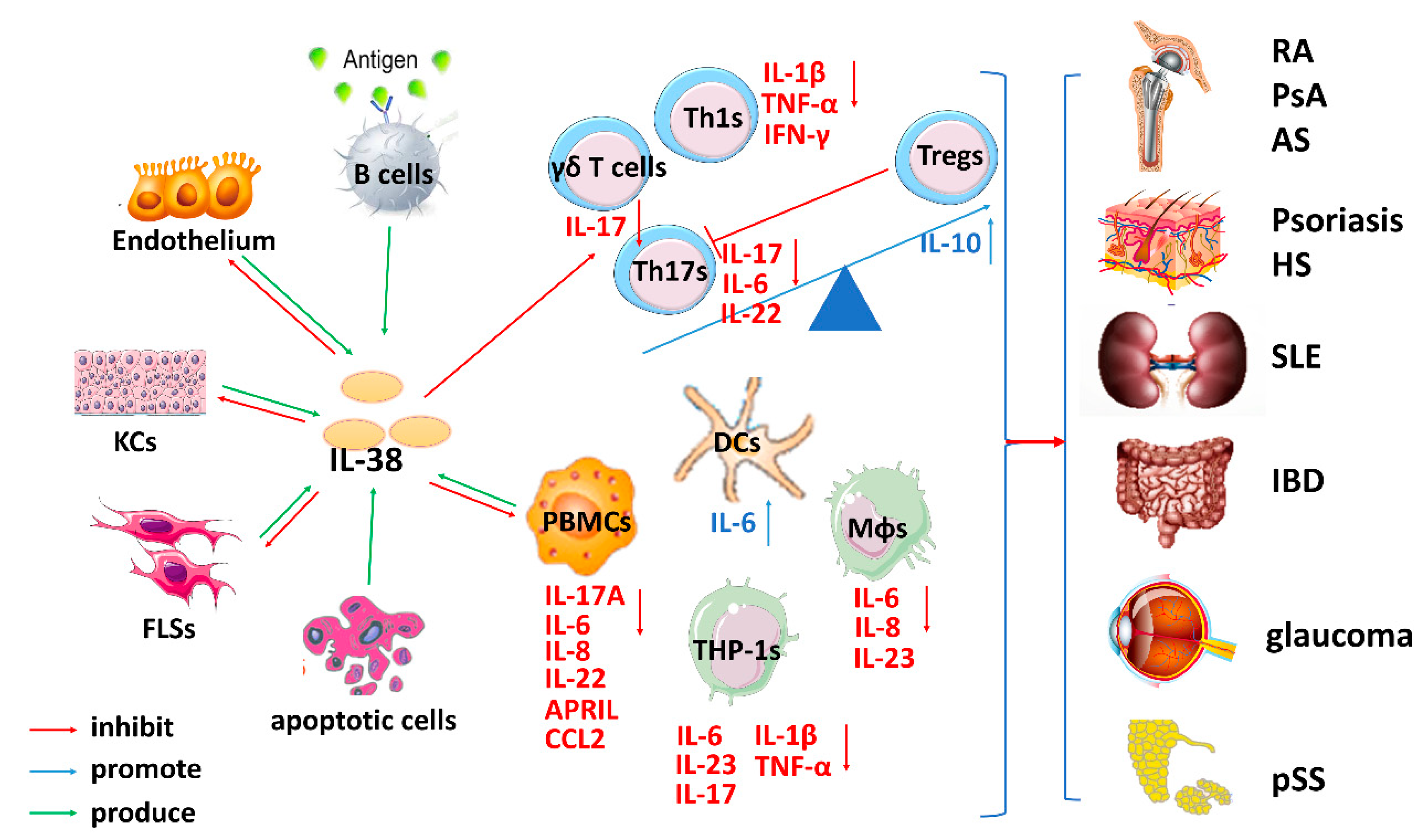
3. The Role of IL-38 in Inflammatory Autoimmune Diseases
3.1. The Role of IL-38 in RA
3.2. The Role of IL-38 in Psoriasis
3.3. The Role of IL-38 in SLE
3.4. The Role of IL-38 in IBD
3.5. The Role of IL-38 in Other Inflammatory Autoimmune Diseases
4. Conclusions
4.1. IL-38 and Inflammatory Autoimmune Diseases
4.2. IL-38 and Immune Cells
4.3. Concentrations/Dose and Forms of IL-38 on Its Biofunction
4.4. Problems to Be Solved Regarding IL-38
Author Contributions
Funding
Conflicts of Interest
References
- Dinarello, C.A. Introduction to the interleukin-1 family of cytokines and receptors: Drivers of innate inflammation and acquired immunity. Immunol. Rev. 2018, 281, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.F.; Leng, R.X.; Li, X.P.; Zheng, S.G.; Ye, D.Q. Targeting T-helper 9 cells and interleukin-9 in autoimmune diseases. Cytokine Growth Factor Rev. 2013, 245, 15–22. [Google Scholar] [CrossRef]
- Tan, Z.; Jiang, R.; Wang, X.; Wang, Y.; Lu, L.; Liu, Q.; Ryffel, B. RORgammat+IL-17+ neutrophils play a critical role in hepatic ischemia-reperfusion injury. J. Mol. Cell Biol. 2013, 51, 43–46. [Google Scholar]
- Gao, Y.; Tang, J.; Chen, W.; Li, Q.; Nie, J.; Lin, F.; Tsun, A. Inflammation negatively regulates FOXP3 and regulatory T-cell function via DBC1. Proc. Natl. Acad. Sci. USA 2015, 112, E3246–E3254. [Google Scholar] [CrossRef] [PubMed]
- Lü, L.; Lan, Q.; Li, Z.; Zhou, X.; Gu, J.; Li, Q.; Wang, J.; Chen, M.; Liu, Y.; Shen, Y.; et al. Critical role of all-trans retinoic acid in stabilizing human natural regulatory T cells under inflammatory conditions. Proc. Natl. Acad. Sci. 2014, 111, E3432–E3440. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lin, F.; Zhuo, C.; Deng, G.; Chen, Z.; Yin, S.; Gao, Z.; Piccioni, M.; Tsun, A.; Cai, S.; et al. PIM1 Kinase Phosphorylates the Human Transcription Factor FOXP3 at Serine 422 to Negatively Regulate Its Activity under Inflammation *. J. Boil. Chem. 2014, 289, 26872–26881. [Google Scholar] [CrossRef]
- Luo, Y.; Zheng, S.G. Hall of Fame among Pro-inflammatory Cytokines: Interleukin-6 Gene and Its Transcriptional Regulation Mechanisms. Front. Immunol. 2016, 7, 503. [Google Scholar] [CrossRef]
- Bensen, J.T.; Dawson, P.A.; Mychaleckyj, J.C.; Bowden, D.W. Bowden. Identification of a Novel Human Cytokine Gene in the Interleukin Gene Cluster on Chromosome 2q12-14. J. Interferon Cytokine Res. 2001, 218, 99–904. [Google Scholar]
- Lin, H.; Ho, A.S.; Haley-Vicente, D.; Zhang, J.; Bernal-Fussell, J.; Pace, A.M.; Hansen, D.; Schweighofer, K.; Mize, N.K.; Ford, J.E. Cloning and Characterization of IL-1HY2, a Novel Interleukin-1 Family Member. J. Boil. Chem. 2001, 276, 20597–20602. [Google Scholar] [CrossRef]
- Dinarello, C.; Arend, W.; Sims, J.; Smith, D.; Blumberg, H.; O’Neill, L.; Nold, M. IL-1 family nomenclature. Nat. Immunol. 2010, 119, 73. [Google Scholar] [CrossRef]
- Van De Veerdonk, F.L.; Netea, M.G. New Insights in the Immunobiology of IL-1 Family Members. Front. Immunol. 2013, 4, 167. [Google Scholar] [CrossRef]
- Nicklin, M.J.; Barton, J.L.; Nguyen, M.; Fitzgerald, M.G.; Duff, G.W.; Kornman, K. A Sequence-Based Map of the Nine Genes of the Human Interleukin-1 Cluster. Genom. 2002, 79, 718–725. [Google Scholar] [CrossRef]
- Kumar, S.; McDonnell, P.C.; Lehr, R.; Tierney, L.; Tzimas, M.N.; Griswold, D.E.; Capper, E.A.; Tal-Singer, R.; Wells, G.I.; Doyle, M.L.; et al. Identification and Initial Characterization of Four Novel Members of the Interleukin-1 Family. J. Boil. Chem. 2000, 275, 10308–10314. [Google Scholar] [CrossRef]
- Mora, J.; Schlemmer, A.; Wittig, I.; Richter, F.; Putyrski, M.; Frank, A.-C.; Han, Y.; Jung, M.; Ernst, A.; Weigert, A.; et al. Interleukin-38 is released from apoptotic cells to limit inflammatory macrophage responses. J. Mol. Cell Boil. 2016, 8, 426–438. [Google Scholar] [CrossRef]
- Yuan, X.L.; Li, Y.; Pan, X.H.; Zhou, M.; Gao, Q.Y. Production of recombinant human interleukin-38 and its inhibitory effect on the expression of proinflammatory cytokines in THP-1 cells. Mol. Boil. 2016, 50, 405–411. [Google Scholar] [CrossRef]
- Van de Veerdonk, F.L.; Stoeckman, A.K.; Wu, G.; Boeckermann, A.N.; Azam, T.; Netea, M.G.; Dinarello, C.A. IL-38 binds to the IL-36 receptor and has biological effects on immune cells similar to IL-36 receptor antagonist. Proc. Natl. Acad. Sci. USA 2012, 1093, 1–5. [Google Scholar] [CrossRef]
- Zou, Y.; Xu, S.; Xiao, Y.; Qiu, Q.; Shi, M.; Wang, J.; Liang, L.; Zhan, Z.; Yang, X.; Olsen, N.; et al. Long noncoding RNA LERFS negatively regulates rheumatoid synovial aggression and proliferation. J. Clin. Investig. 2018, 128, 4510–4524. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, J.; Li, J.; Li, T.; Chen, Y.; June, R.R.; Zheng, S.G. 1,25-Dihydroxyvitamin D3 Ameliorates Collagen-Induced Arthritis via Suppression of Th17 Cells Through miR-124 Mediated Inhibition of IL-6 Signaling. Front. Immunol. 2019, 10, 178. [Google Scholar] [CrossRef]
- Parks, C.G.; Miller, F.W.; Pollard, K.M.; Selmi, C.; Germolec, D.; Joyce, K.; Rose, N.R.; Humble, M.C. Expert Panel Workshop Consensus Statement on the Role of the Environment in the Development of Autoimmune Disease. Int. J. Mol. Sci. 2014, 15, 14269–14297. [Google Scholar] [CrossRef]
- Cooper, G.S.; Bynum, M.L.; Somers, E.C. Recent Insights in the Epidemiology of Autoimmune Diseases: Improved Prevalence Estimates and Understanding of Clustering of Diseases. J. Autoimmun. 2009, 33, 197–207. [Google Scholar] [CrossRef]
- Zheng, S.G. Regulatory T cells vs. Th17: Differentiation of Th17 versus Treg, are the mutually exclusive? Am. J. Clin. Exp. Immunol. 2013, 2, 94–106. [Google Scholar]
- Chen, X.; Su, W.; Wan, T.; Yu, J.; Zhu, W.; Tang, F.; Liu, G.; Olsen, N.; Liang, D.; Zheng, S.G. Sodium butyrate regulates Th17/Treg cell balance to ameliorate uveitis via the Nrf2/HO-1 pathway. Biochem. Pharmacol. 2017, 142, 111–119. [Google Scholar] [CrossRef]
- Eisenstein, E.M.; Williams, C.B. The Treg/Th17 Cell Balance: A New Paradigm for Autoimmunity. Pediatr. Res. 2009, 65, 26–31. [Google Scholar] [CrossRef]
- Kong, N.; Lan, Q.; Chen, M.; Wang, J.; Shi, W.; Horwitz, D.A.; Zou, H. Antigen-specific transforming growth factor beta-induced Treg cells, but not natural Treg cells, ameliorate autoimmune arthritis in mice by shifting the Th17/Treg cell balance from Th17 predominance to Treg cell predominance. Arthr. Rheum. 2012, 642, 548–558. [Google Scholar]
- Zheng, S.G.; Wang, J.; Horwitz, D.A. Cutting edge: Foxp3+CD4+CD25+ regulatory T cells induced by IL-2 and TGF-beta are resistant to Th17 conversion by IL-6. J. Immunol. 2008, 1807, 112–116. [Google Scholar] [CrossRef]
- Abdulahad, W.H.; Boots, A.M.H.; Kallenberg, C.G.M. FoxP3+ CD4+ T cells in systemic autoimmune diseases: The delicate balance between true regulatory T cells and effector Th-17 cells. Rheumatology 2011, 506, 46–56. [Google Scholar] [CrossRef]
- Ma, J.; Yu, J.; Tao, X.; Cai, L.; Wang, J.; Zheng, S.G. The imbalance between regulatory and IL-17-secreting CD4+ T cells in lupus patients. Clin. Rheumatol. 2010, 291, 251–258. [Google Scholar] [CrossRef]
- Burgler, S.; Ouaked, N.; Bassin, C.; Basinski, T.M.; Mantel, P.-Y.; Siegmund, K.; Meyer, N.; Akdis, C.A.; Schmidt-Weber, C.B. Differentiation and functional analysis of human TH17 cells. J. Allergy Clin. Immunol. 2009, 123, 588–595.e7. [Google Scholar] [CrossRef]
- Boutet, M.A.; Bart, G.; Penhoat, M.; Amiaud, J.; Brulin, B.; Charrier, C.; Vigne, S. Distinct expression of interleukin (IL)-36alpha, beta and gamma, their antagonist IL-36Ra and IL-38 in psoriasis, rheumatoid arthritis and Crohn’s disease. Clin. Exp. Immunol. 2016, 1841, 59–73. [Google Scholar]
- Rudloff, I.; Godsell, J.; Nold-Petry, C.A.; Hoi, A.; Harris, J.; Morand, E.F.; Nold, M.F. Brief Report: Interleukin-38 Exerts Antiinflammatory Functions and Is Associated with Disease Activity in Systemic Lupus Erythematosus. Arthritis Rheumatol. 2015, 67, 3219–3225. [Google Scholar] [CrossRef]
- Jung, M.; Kang, S.; Kim, S.; Kim, H.-J.; Yun, D.; Yim, S.-V.; Hong, S.; Chung, J.-H. The interleukin-1 family gene polymorphisms in Korean patients with rheumatoid arthritis. Scand. J. Rheumatol. 2010, 39, 190–196. [Google Scholar] [CrossRef]
- Monnet, D.; Kadi, A.; Izac, B.; Lebrun, N.; Letourneur, F.; Zinovieva, E.; Said-Nahal, R.; Chiocchia, G.; Breban, M. Association between the IL-1 family gene cluster and spondyloarthritis. Ann. Rheum. Dis. 2012, 71, 885–890. [Google Scholar] [CrossRef]
- Rahman, P.; Sun, S.; Peddle, L.; Snelgrove, T.; Melay, W.; Greenwood, C.; Gladman, D. Association between the interleukin-1 family gene cluster and psoriatic arthritis. Arthritis Rheum. 2006, 54, 2321–2325. [Google Scholar] [CrossRef]
- Chou, C.T.; Timms, A.E.; Wei, J.C.; Tsai, W.C.; Wordsworth, B.P.; Brown, M.A. Replication of association of IL1 gene complex members with ankylosing spondylitis in Taiwanese Chinese. Ann. Rheum. Dis. 2006, 651, 106–109. [Google Scholar] [CrossRef]
- Guo, Z.S.; Li, C.; Lin, Z.M.; Huang, J.X.; Wei, Q.J.; Wang, X.W.; Xie, Y.Y.; Liao, Z.T.; Chao, S.Y.; Gu, J.R. Association of IL-1 gene complex members with ankylosing spondylitis in Chinese Han population. Int. J. Immunogenet. 2010, 37, 33–37. [Google Scholar] [CrossRef]
- Dehghan, A.; Dupuis, J.; Barbalic, M.; Bis, J.C.; Eiriksdottir, G.; Lu, C.; Pellikka, N.; Wallaschofski, H.; Kettunen, J.; Henneman, P.; et al. Meta-analysis of genome-wide association studies in >80,000 subjects identifies multiple loci for C-reactive protein levels. Circulation 2011, 123, 731–738. [Google Scholar] [CrossRef]
- Dorajoo, R.; Li, R.; Ikram, M.K.; Liu, J.; Froguel, P.; Lee, J.; Young, T.L. Are C-Reactive Protein Associated Genetic Variants Associated with Serum Levels and Retinal Markers of Microvascular Pathology in Asian Populations from Singapore? PLoS ONE 2013, 8, e67650. [Google Scholar] [CrossRef]
- Yuan, X.; Peng, X.; Li, Y.; Li, M. Role of IL-38 and Its Related Cytokines in Inflammation. Mediat. Inflamm. 2015, 2015, 1–7. [Google Scholar] [CrossRef]
- Geourjon, C.; Deléage, G. SOPMA: Significant improvements in protein secondary structure prediction by consensus prediction from multiple alignments. Bioinformation 1995, 11, 681–684. [Google Scholar] [CrossRef]
- Boutet, M.-A.; Najm, A.; Bart, G.; Brion, R.; Touchais, S.; Trichet, V.; Layrolle, P.; Gabay, C.; Palmer, G.; Blanchard, F.; et al. IL-38 overexpression induces anti-inflammatory effects in mice arthritis models and in human macrophages in vitro. Ann. Rheum. Dis. 2017, 76, 1304–1312. [Google Scholar] [CrossRef]
- Weigert, A.; Johann, A.M.; Von Knethen, A.; Schmidt, H.; Geisslinger, G.; Brüne, B. Apoptotic cells promote macrophage survival by releasing the antiapoptotic mediator sphingosine-1-phosphate. Blood 2006, 108, 1635–1642. [Google Scholar] [CrossRef]
- Nold-Petry, C.A.; Lo, C.Y.; Rudloff, I.; Elgass, K.D.; Li, S.; Gantier, M.P.; Lotz-Havla, A.S.; Gersting, S.W.; Cho, S.X.; Lao, J.C.; et al. IL-37 requires the receptors IL-18Rα and IL-1R8 (SIGIRR) to carry out its multifaceted anti-inflammatory program upon innate signal transduction. Nat. Immunol. 2015, 16, 354–365. [Google Scholar] [CrossRef]
- Boutet, M.A.; Blanchard, F.; Le Goff, B. Response to: Does IL-38 act on macrophages and/or dendritic cells in arthritis? Ann. Rheum. Dis. 2018, 77, e13. [Google Scholar] [CrossRef]
- Palomo, J.; Troccaz, S.; Talabot-Ayer, D.; Rodriguez, E.; Palmer, G. The severity of imiquimod-induced mouse skin inflammation is independent of endogenous IL-38 expression. PLoS ONE 2018, 13, e0194667. [Google Scholar] [CrossRef]
- Garlanda, C.; Dinarello, C.A.; Mantovani, A. The interleukin-1 family: Back to the future. Immunology 2013, 39, 1003–1018. [Google Scholar] [CrossRef]
- Boraschi, D.; Tagliabue, A. The interleukin-1 receptor family. Semin. Immunol. 2013, 253, 94–407. [Google Scholar] [CrossRef]
- Vigne, S.; Palmer, G.; Lamacchia, C.; Martin, P.; Talabot-Ayer, D.; Rodriguez, E.; Ronchi, F.; Sallusto, F.; Dinh, H.; Sims, J.E.; et al. CS16-5. IL-36R Ligands are Potent Regulators of Dendritic and T Cells. Cytokine 2011, 56, 105–106. [Google Scholar] [CrossRef]
- Mutamba, S.; Allison, A.; Mahida, Y.; Barrow, P.; Foster, N. Expression of IL-1Rrp2 by human myelomonocytic cells is unique to DCs and facilitates DC maturation by IL-1F8 and IL-1F9. Eur. J. Immunol. 2012, 42, 607–617. [Google Scholar] [CrossRef]
- Towne, J.E.; Renshaw, B.R.; Douangpanya, J.; Lipsky, B.P.; Shen, M.; Gabel, C.A.; Sims, J.E. Interleukin-36 (IL-36) ligands require processing for full agonist (IL-36α, IL-36β and IL-36γ) or antagonist (IL-36Ra) activity. J. Biol. Chem. 2011, 286, 42594–42602. [Google Scholar] [CrossRef]
- Hidaka, K.; Kanda, T.; Kitoh, K.; Nishida, A.; Imaeda, H.; Shioya, M.; Inatomi, O.; Bamba, S.; Sugimoto, M.; Andoh, A. Increased Expression of Interleukin-36, a Member of the Interleukin-1 Cytokine Family, in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2016, 22, 303–314. [Google Scholar]
- Carrier, Y.; Ma, H.-L.; Ramon, H.E.; Napierata, L.; Small, C.; O’Toole, M.; Young, D.A.; Fouser, L.A.; Nickerson-Nutter, C.; Collins, M.; et al. Inter-Regulation of Th17 Cytokines and the IL-36 Cytokines In Vitro and In Vivo: Implications in Psoriasis Pathogenesis. J. Investig. Dermatol. 2011, 131, 2428–2437. [Google Scholar] [CrossRef]
- Chi, H.-H.; Hua, K.-F.; Lin, Y.-C.; Chu, C.-L.; Hsieh, C.-Y.; Hsu, Y.-J.; Ka, S.-M.; Tsai, Y.-L.; Liu, F.-C.; Chen, A. IL-36 Signaling Facilitates Activation of the NLRP3 Inflammasome and IL-23/IL-17 Axis in Renal Inflammation and Fibrosis. J. Am. Soc. Nephrol. 2017, 28, 2022–2037. [Google Scholar] [CrossRef]
- Riva, F.; Bonavita, E.; Barbati, E.; Muzio, M.; Mantovani, A.; Garlanda, C. TIR8/SIGIRR is an Interleukin-1 Receptor/Toll Like Receptor Family Member with Regulatory Functions in Inflammation and Immunity. Front. Immunol. 2012, 3, 322. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Seki, T.; Kobayashi, N.; Sano, K.; Shigemura, T.; Shimojo, H.; Matsumoto, K.; Agematsu, K. Analysis of serum IL-38 in juvenile-onset systemic lupus erythematosus. Mod. Rheumatol. 2018, 28, 1069–1072. [Google Scholar] [CrossRef]
- Lea, W.-I.; Lee, Y.H. The associations between interleukin-1 polymorphisms and susceptibility to ankylosing spondylitis: A meta-analysis. Jt. Bone Spine 2012, 79, 370–374. [Google Scholar] [CrossRef]
- Li, J.; Liu, L.; Rui, W.; Li, X.; Xuan, D.; Zheng, S.; Yu, Y.; Zhang, J.; Kong, N.; Zhu, X.; et al. New Interleukins in Psoriasis and Psoriatic Arthritis Patients: The Possible Roles of Interleukin-33 to Interleukin-38 in Disease Activities and Bone Erosions. Dermatology 2017, 233, 37–46. [Google Scholar] [CrossRef]
- Ciccia, F.; Accardo-Palumbo, A.; Alessandro, R.; Alessandri, C.; Priori, R.; Guggino, G.; Raimondo, S.; Carubbi, F.; Valesini, G.; Giacomelli, R.; et al. Interleukin-36α axis is modulated in patients with primary Sjögren’s syndrome. Clin. Exp. Immunol. 2015, 181, 230–238. [Google Scholar] [CrossRef]
- Chu, M.; Chu, I.M.; Yung, E.C.; Lam, C.W.; Leung, T.F.; Wong, G.W.; Wong, C.K. Aberrant Expression of Novel Cytokine IL-38 and Regulatory T Lymphocytes in Childhood Asthma. Molecules 2016, 21, 933. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, R.; Chen, J.; Jin, J.; Yu, Y.; Tian, Y.; Li, W.; Wang, W.; Zhou, H.; Su, S.B. The Effect of Interleukin 38 on Angiogenesis in a Model of Oxygen-induced Retinopathy. Sci. Rep. 2017, 7, 2756. [Google Scholar] [CrossRef]
- Wang, H.-J.; Jiang, Y.-F.; Wang, X.-R.; Zhang, M.-L.; Gao, P.-J. Elevated serum interleukin-38 level at baseline predicts virological response in telbivudine-treated patients with chronic hepatitis B. World J. Gastroenterol. 2016, 22, 4529–4537. [Google Scholar] [CrossRef]
- Takada, K.; Okamoto, T.; Tominaga, M.; Teraishi, K.; Akamine, T.; Takamori, S.; Katsura, M.; Toyokawa, G.; Shoji, F.; Okamoto, M.; et al. Clinical implications of the novel cytokine IL-38 expressed in lung adenocarcinoma: Possible association with PD-L1 expression. PLoS ONE 2017, 12, e0181598. [Google Scholar] [CrossRef]
- Zhong, Y.; Yu, K.; Wang, X.; Wang, X.; Ji, Q.; Zeng, Q. Elevated Plasma IL-38 Concentrations in Patients with Acute ST-Segment Elevation Myocardial Infarction and Their Dynamics after Reperfusion Treatment. Mediat. Inflamm. 2015, 2015, 1–10. [Google Scholar] [CrossRef]
- Derer, A.; Groetsch, B.; Harre, U.; Bohm, C.; Towne, J.; Schett, G.; Frey, S.; Hueber, A.J. Blockade of IL-36 Receptor Signaling Does Not Prevent from TNF-Induced Arthritis. PLoS ONE 2014, 9, 101954. [Google Scholar] [CrossRef]
- Lamacchia, C.; Palmer, G.; Rodriguez, E.; Martin, P.; Vigne, S.; Seemayer, C.A.; Talabot-Ayer, D.; E Towne, J.; Gabay, C. The severity of experimental arthritis is independent of IL-36 receptor signaling. Arthritis Res. Ther. 2013, 15, R38. [Google Scholar] [CrossRef]
- Jiang, K.; Guo, Y.; Xiao, L.; Pan, W.; Ma, J. Dendritic cells should not be overlooked when studying the effect of IL-38 administration in arthritis. Ann. Rheum. Dis. 2018, 77, e12. [Google Scholar] [CrossRef]
- Carrié, A.; Jun, L.; Bienvenu, T.; Vinet, M.-C.; McDonell, N.; Couvert, P.; Zemni, R.; Cardona, A.; Van Buggenhout, G.; Frints, S.; et al. A new member of the IL-1 receptor family highly expressed in hippocampus and involved in X-linked mental retardation. Nat. Genet. 1999, 23, 25–31. [Google Scholar] [CrossRef]
- Born, T.L.; Smith, D.E.; Garka, K.E.; Renshaw, B.R.; Bertles, J.S.; Sims, J.E. Identification and Characterization of Two Members of a Novel Class of the Interleukin-1 Receptor (IL-1R) Family: Delineation of a new class of IL-1R-related proteins based on signaling. J. Boil. Chem. 2000, 275, 29946–29954. [Google Scholar] [CrossRef]
- Pavlowsky, A.; Zanchi, A.; Pallotto, M.; Giustetto, M.; Chelly, J.; Sala, C.; Billuart, P. Neuronal JNK pathway activation by IL-1 is mediated through IL1RAPL1, a protein required for development of cognitive functions. Commun. Integr. Boil. 2010, 3, 245–247. [Google Scholar] [CrossRef]
- Mo, B.Y.; Guo, X.H.; Yang, M.R.; Liu, F.; Bi, X.; Liu, Y.; Fang, L.K.; Luo, X.Q.; Wang, J.; Bellanti, J.A.; et al. Long Non-Coding RNA GAPLINC Promotes Tumor-Like Biologic Behaviors of Fibroblast-Like Synoviocytes as MicroRNA Sponging in Rheumatoid Arthritis Patients. Front. Immunol. 2018, 9, 702. [Google Scholar] [CrossRef]
- Magyari, L.; Varszegi, D.; Kovesdi, E.; Sarlos, P.; Farago, B.; Javorhazy, A.; Sumegi, K.; Banfai, Z.; Melegh, B. Interleukins and interleukin receptors in rheumatoid arthritis: Research, diagnostics and clinical implications. World J. Orthop. 2014, 5, 516–536. [Google Scholar] [CrossRef]
- Boissier, M.-C.; Assier, E.; Falgarone, G.; Bessis, N. Shifting the imbalance from Th1/Th2 to Th17/treg: The changing rheumatoid arthritis paradigm. Jt. Bone Spine 2008, 75, 373–375. [Google Scholar] [CrossRef]
- Boissier, M.-C. Cell and cytokine imbalances in rheumatoid synovitis. Jt. Bone Spine 2011, 78, 230–234. [Google Scholar] [CrossRef]
- Leipe, J.; Grunke, M.; DeChant, C.; Reindl, C.; Kerzendorf, U.; Skapenko, A.; Schulze-Koops, H.; Schulze-Koops, H. Role of Th17 cells in human autoimmune arthritis. Arthritis Rheum. 2010, 62, 2876–2885. [Google Scholar] [CrossRef]
- Harris, E.D., Jr. Rheumatoid arthritis: Pathophysiology and implications for therapy. N. Engl. J. Med. 1990, 3221, 277–289. [Google Scholar]
- Zhe, H.; Juan, N.; Huan, W. Mechanism of IL-38 inhibits LPS/TLR4 induced inflammation in patients with rheumatoid arthritis. Chin. J. Immunol. 2017, 1, 647–651. [Google Scholar]
- Takenaka, S.-I.; Kaieda, S.; Kawayama, T.; Matsuoka, M.; Kaku, Y.; Kinoshita, T.; Sakazaki, Y.; Okamoto, M.; Tominaga, M.; Kanesaki, K.; et al. IL-38: A new factor in rheumatoid arthritis. Biochem. Biophys. Rep. 2015, 4, 386–391. [Google Scholar] [CrossRef]
- Wang, M.; Wang, B.; Ma, Z.; Sun, X.; Tang, Y.; Li, X.; Wu, X. Detection of the novel IL-1 family cytokines by QAH-IL1F-1 assay in rheumatoid arthritis. Cell. Mol. Boil. 2016, 62, 31–34. [Google Scholar]
- Xu, W.-D.; Su, L.-C.; He, C.-S.; Huang, A.-F. Plasma interleukin-38 in patients with rheumatoid arthritis. Int. Immunopharmacol. 2018, 65, 1–7. [Google Scholar] [CrossRef]
- Magne, D.; Palmer, G.; Barton, J.L.; Mézin, F.; Talabot-Ayer, D.; Bas, S.; Duffy, T.; Noger, M.; Guerne, P.-A.; Nicklin, M.J.H.; et al. The new IL-1 family member IL-1F8 stimulates production of inflammatory mediators by synovial fibroblasts and articular chondrocytes. Arthritis Res. Ther. 2006, 8, R80. [Google Scholar] [CrossRef]
- Frey, S.; Derer, A.; Messbacher, M.E.; Baeten, D.L.; Bugatti, S.; Montecucco, C.; Hueber, A.J. The novel cytokine interleukin-36α is expressed in psoriatic and rheumatoid arthritis synovium. Ann. Rheum. Dis. 2013, 721, 569–574. [Google Scholar] [CrossRef]
- Zhou, X.; Kong, N.; Wang, J.; Fan, H.; Zou, H.; Horwitz, D.; Brand, D.; Liu, Z.; Zheng, S.G. Cutting Edge: All-Trans Retinoic Acid Sustains the Stability and Function of Natural Regulatory T Cells in an Inflammatory Milieu. J. Immunol. 2010, 185, 2675–2679. [Google Scholar] [CrossRef]
- Horai, R.; Tanioka, H.; Nakae, S.; Okahara, A.; Ikuse, T.; Iwakura, Y.; Saijo, S.; Sudo, K.; Asano, M. Development of Chronic Inflammatory Arthropathy Resembling Rheumatoid Arthritis in Interleukin 1 Receptor Antagonist-Deficient Mice. J. Exp. Med. 2000, 191, 313–320. [Google Scholar] [CrossRef]
- Fujimoto, M.; Serada, S.; Mihara, M.; Uchiyama, Y.; Yoshida, H.; Koike, N.; Ohsugi, Y.; Nishikawa, T.; Ripley, B.; Kimura, A.; et al. Interleukin-6 blockade suppresses autoimmune arthritis in mice by the inhibition of inflammatory Th17 responses. Arthritis Rheum. 2008, 58, 3710–3719. [Google Scholar] [CrossRef]
- Koenders, M.I.; Devesa, I.; Marijnissen, R.J.; Boots, A.M.H.; Walgreen, B.; Di Padova, F.E.; Nicklin, M.J.H.; Joosten, L.A.B.; Berg, W.B.V.D.; Abdollahi-Roodsaz, S.; et al. Interleukin-1 drives pathogenic Th17 cells during spontaneous arthritis in interleukin-1 receptor antagonist-deficient mice. Arthritis Rheum. 2008, 58, 3461–3470. [Google Scholar] [CrossRef]
- Abramson, S.B.; Amin, A. Blocking the effects of IL-1 in rheumatoid arthritis protects bone and cartilage. Rheumatology 2002, 41, 972–980. [Google Scholar] [CrossRef]
- Lamacchia, C.; Palmer, G.; Seemayer, C.A.; Talabot-Ayer, D.; Lamacchia, C.; Gabay, C. Enhanced Th1 and Th17 responses and arthritis severity in mice with a deficiency of myeloid cell–specific interleukin-1 receptor antagonist. Arthritis Rheum. 2010, 62, 52–62. [Google Scholar]
- Korganow, A.-S.; Ji, H.; Mangialaio, S.; Duchatelle, V.; Pelanda, R.; Martin, T.; Degott, C.; Kikutani, H.; Rajewsky, K.; Pasquali, J.-L.; et al. From Systemic T Cell Self-Reactivity to Organ-Specific Autoimmune Disease via Immunoglobulins. Immunity 1999, 10, 451–461. [Google Scholar] [CrossRef]
- Bush, K.A.; Farmer, K.M.; Walker, J.S.; Kirkham, B.W. Reduction of joint inflammation and bone erosion in rat adjuvant arthritis by treatment with interleukin-17 receptor IgG1 Fc fusion protein. Arthritis Rheum. 2002, 46, 802–805. [Google Scholar] [CrossRef]
- Wooley, P.H.; Whalen, J.D.; Chapman, D.L.; Berger, A.E.; Richard, K.A.; Aspar, D.G.; Staite, N.D. The effect of an interleukin-1 receptor antagonist protein on type ii collagen–induced arthritis and antigen-induced arthritis in mice. Arthritis Rheum. 1993, 36, 1305–1314. [Google Scholar] [CrossRef]
- Towne, J.E.; Sims, J.E. IL-36 in psoriasis. Curr. Opin. Pharm. 2012, 124, 86–90. [Google Scholar] [CrossRef]
- Valdimarsson, H.; Baker, B.S.; Jonsdottir, I.; Powles, A.; Fry, L. Psoriasis: A T-cell-mediated autoimmune disease induced by streptococcal superantigens? Immunol. Today 1995, 16, 145–149. [Google Scholar] [CrossRef]
- Johnston, A.; Xing, X.; Guzman, A.M.; Riblett, M.; Loyd, C.M.; Ward, N.L.; Wohn, C.; Prens, E.P.; Wang, F.; Maier, L.E.; et al. IL-1F5, F6, F8, and F9: A novel IL-1 family signaling system that is active in psoriasis and promotes keratinocyte antimicrobial peptide expression. J. Immunol. 2011, 186, 2613–2622. [Google Scholar] [CrossRef]
- Sakkas, L.I.; Bogdanos, D.P. Are psoriasis and psoriatic arthritis the same disease? The IL-23/IL-17 axis data. Autoimmun. Rev. 2017, 16, 10–15. [Google Scholar] [CrossRef]
- Sugiyama, H.; Gyulai, R.; Toichi, E.; Garaczi, E.; Shimada, S.; Stevens, S.R.; Cooper, K.D. Dysfunctional Blood and Target Tissue CD4+CD25 high Regulatory T Cells in Psoriasis: Mechanism Underlying Unrestrained Pathogenic Effector T Cell Proliferation. J. Immunol. 2005, 174, 164–173. [Google Scholar] [CrossRef]
- Han, Y.; Mora, J.; Huard, A.; da Silva, P.; Wiechmann, S.; Putyrski, M.; Scholz, T. IL-38 Ameliorates Skin Inflammation and Limits IL-17 Production from gammadelta T Cells. Cell. Rep. 2019, 27, 835–846. [Google Scholar] [CrossRef]
- Mercurio, L.; Morelli, M.; Scarponi, C.; Eisenmesser, E.Z.; Doti, N.; Pagnanelli, G.; Gubinelli, E.; Mazzanti, C.; Cavani, A.; Ruvo, M.; et al. IL-38 has an anti-inflammatory action in psoriasis and its expression correlates with disease severity and therapeutic response to anti-IL-17A treatment. Cell Death Dis. 2018, 9, 1104. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, S.H.; Park, J.; Lee, M.; Kim, D.S.; Lee, M.-G. Up-regulation of receptor antagonist interleukin-1 family members in psoriasis and their regulation by pro-inflammatory cytokines. J. Dermatol. Sci. 2016, 82, 204–206. [Google Scholar] [CrossRef]
- Keermann, M.; Kõks, S.; Reimann, E.; Abram, K.; Erm, T.; Silm, H.; Kingo, K. Expression of IL-36 family cytokines and IL-37 but not IL-38 is altered in psoriatic skin. J. Dermatol. Sci. 2015, 80, 150–152. [Google Scholar] [CrossRef]
- Blumberg, H.; Dinh, H.; Trueblood, E.S.; Pretorius, J.; Kugler, D.; Weng, N.; Kanaly, S.T.; Towne, J.E.; Willis, C.R.; Kuechle, M.K.; et al. Opposing activities of two novel members of the IL-1 ligand family regulate skin inflammation. J. Exp. Med. 2007, 204, 2603–2614. [Google Scholar] [CrossRef]
- Blumberg, H.; Dinh, H.; Dean, C.; Trueblood, E.S.; Bailey, K.; Shows, D.; Bhagavathula, N.; Aslam, M.N.; Varani, J.; Towne, J.E.; et al. IL-1RL2 and Its Ligands Contribute to the Cytokine Network in Psoriasis. J. Immunol. 2010, 185, 4354–4362. [Google Scholar] [CrossRef]
- Marrakchi, S.; Guigue, P.; Renshaw, B.R.; Puel, A.; Pei, X.-Y.; Fraitag, S.; Zribi, J.; Bal, E.; Cluzeau, C.; Chrabieh, M.; et al. Interleukin-36–Receptor Antagonist Deficiency and Generalized Pustular Psoriasis. New Engl. J. Med. 2011, 365, 620–628. [Google Scholar] [CrossRef]
- Shepherd, J.; Little, M.C.; Nicklin, M.J. Psoriasis-Like Cutaneous Inflammation in Mice Lacking Interleukin-1 Receptor Antagonist. J. Investig. Dermatol. 2004, 122, 665–669. [Google Scholar] [CrossRef]
- Glaccum, M.B.; Stocking, K.L.; Charrier, K.; Smith, J.L.; Willis, C.R.; Maliszewski, C.; Livingston, D.J.; Peschon, J.J.; Morrissey, P.J. Phenotypic and functional characterization of mice that lack the type I receptor for IL-1. J. Immunol. 1997, 159, 364–371. [Google Scholar]
- Labow, M.; Shuster, D.; Zetterstrom, M.; Nunes, P.; Terry, R.; Cullinan, E.B.; Bartfai, T.; Solorzano, C.; Moldawer, L.L.; Chizzonite, R.; et al. Absence of IL-1 signaling and reduced inflammatory response in IL-1 type I receptor-deficient mice. J. Immunol. 1997, 159, 452–461. [Google Scholar]
- Foster, A.M.; Baliwag, J.; Chen, C.S.; Guzman, A.M.; Stoll, S.W.; Gudjonsson, J.E.; Ward, N.L.; Johnston, A. IL-36 promotes myeloid cell infiltration, activation and inflammatory activity in skin. J. Immunol. 2014, 192, 6053–6061. [Google Scholar] [CrossRef]
- Li, N.; Yamasaki, K.; Saito, R.; Fukushi-Takahashi, S.; Shimada-Omori, R.; Asano, M.; Aiba, S. Alarmin Function of Cathelicidin Antimicrobial Peptide LL37 through IL-36γ Induction in Human Epidermal Keratinocytes. J. Immunol. 2014, 193, 5140–5148. [Google Scholar] [CrossRef]
- Rabeony, H.; Petit-Paris, I.; Garnier, J.; Barrault, C.; Pedretti, N.; Guilloteau, K.; Jegou, J.-F.; Guillet, G.; Huguier, V.; Lecron, J.-C.; et al. Inhibition of Keratinocyte Differentiation by the Synergistic Effect of IL-17A, IL-22, IL-1α, TNFα and Oncostatin M. PLoS ONE 2014, 9, e101937. [Google Scholar]
- Yu, C.; Gershwin, M.E.; Chang, C. Diagnostic criteria for systemic lupus erythematosus: A critical review. J. Autoimmun. 2014, 48, 10–13. [Google Scholar] [CrossRef]
- Alunno, A.; Bartoloni, E.; Bistoni, O.; Nocentini, G.; Ronchetti, S.; Caterbi, S.; Valentini, V.; Riccardi, C.; Gerli, R. Balance between Regulatory T and Th17 Cells in Systemic Lupus Erythematosus: The Old and the New. Clin. Dev. Immunol. 2012, 2012, 1–5. [Google Scholar] [CrossRef]
- Chu, M.; Wong, C.K.; Cai, Z.; Dong, J.; Jiao, D.; Kam, N.W.; Lam, C.W.K.; Tam, L.S. Elevated Expression and Pro-Inflammatory Activity of IL-36 in Patients with Systemic Lupus Erythematosus. Molecules 2015, 20, 19588–19604. [Google Scholar] [CrossRef]
- Gresnigt, M.S.; Van De Veerdonk, F.L. Biology of IL-36 cytokines and their role in disease. Semin. Immunol. 2013, 25, 458–465. [Google Scholar] [CrossRef]
- Chu, M.; Tam, L.S.; Zhu, J.; Jiao, D.; Liu, D.H.; Cai, Z.; Dong, J.; Lam, C.W.K.; Wong, C.K. In vivo anti-inflammatory activities of novel cytokine IL-38 in Murphy Roths Large (MRL)/lpr mice. Immunobiology 2017, 222, 483–493. [Google Scholar] [CrossRef]
- Guan, Q.; Zhang, J. Recent Advances: The Imbalance of Cytokines in the Pathogenesis of Inflammatory Bowel Disease. Mediat. Inflamm. 2017, 2017, 1–8. [Google Scholar]
- Russell, S.E.; Horan, R.M.; Stefanska, A.M.; Carey, A.; Leon, G.; Aguilera, M.; Statovci, D.; Moran, T.; Fallon, P.G.; Shanahan, F.; et al. IL-36α expression is elevated in ulcerative colitis and promotes colonic inflammation. Mucosal Immunol. 2016, 9, 1193–1204. [Google Scholar] [CrossRef]
- Fonseca-Camarillo, G.; Furuzawa-Carballeda, J.; Iturriaga-Goyon, E.; Yamamoto-Furusho, J.K. Differential Expression of IL-36 Family Members and IL-38 by Immune and Nonimmune Cells in Patients with Active Inflammatory Bowel Disease. BioMed Res. Int. 2018, 2018, 1–12. [Google Scholar] [CrossRef]
- Harusato, A.; Nishio, H.; Gewirtz, A.; Parkos, C.; Towne, J.; Nusrat, A.; Denning, T.; Medina-Contreras, O.; Chassaing, B. P-146 IL-36 Receptor Is Required for Resolution of Intestinal Damage. Inflamm. Bowel Dis. 2016, 22, S54–S55. [Google Scholar] [CrossRef][Green Version]
- Medina-Contreras, O.; Harusato, A.; Nishio, H.; Flannigan, K.L.; Ngo, V.; Leoni, G.; Chassaing, B. Cutting Edge: IL-36 Receptor Promotes Resolution of Intestinal Damage. J. Immunol. 2016, 196, 34–38. [Google Scholar] [CrossRef]
- Nguyen, C.Q.; Hu, M.H.; Li, Y.; Stewart, C.; Peck, A.B. Salivary gland tissue expression of interleukin-23 and interleukin-17 in Sjögren’s syndrome: Findings in humans and mice. Arthritis Rheum. 2008, 587, 34–43. [Google Scholar] [CrossRef]
- Hessam, S.; Sand, M.; Gambichler, T.; Skrygan, M.; Rüddel, I.; Bechara, F. Interleukin-36 in hidradenitis suppurativa: Evidence for a distinctive proinflammatory role and a key factor in the development of an inflammatory loop. Br. J. Dermatol. 2018, 178, 761–767. [Google Scholar] [CrossRef]
- Schlapbach, C.; Hänni, T.; Yawalkar, N.; Hunger, R.E. Expression of the IL-23/Th17 pathway in lesions of hidradenitis suppurativa. J. Am. Acad. Dermatol. 2011, 65, 790–798. [Google Scholar] [CrossRef]
- Robinson, P.C.; Brown, M.A. Genetics of ankylosing spondylitis. Mol. Immunol. 2014, 57, 2–11. [Google Scholar] [CrossRef]
- Hohenstein-Blaul NV, T.U.; Bell, K.; Pfeiffer, N.; Grus, F.H. Autoimmune aspects in glaucoma. Eur. J. Pharmacol. 2016, 787, 105–118. [Google Scholar] [CrossRef]
- Zhang, J.-L.; Song, X.-Y.; Chen, Y.-Y.; Nguyen, T.H.A.; Zhang, J.-Y.; Bao, S.-S.; Zhang, Y.-Y. Novel inflammatory cytokines (IL-36, 37, 38) in the aqueous humor from patients with chronic primary angle closure glaucoma. Int. Immunopharmacol. 2019, 71, 164–168. [Google Scholar] [CrossRef]
- Onishi, R.M.; Gaffen, S.L. Interleukin-17 and its target genes: Mechanisms of interleukin-17 function in disease. Immunology 2010, 129, 311–321. [Google Scholar] [CrossRef]
- Fossiez, F. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J. Exp. Med. 1996, 183, 2593–2603. [Google Scholar] [CrossRef]
- Leung, S.; Liu, X.; Fang, L.; Chen, X.; Guo, T.; Zhang, J. The cytokine milieu in the interplay of pathogenic Th1/Th17 cells and regulatory T cells in autoimmune disease. Cell. Mol. Immunol. 2010, 7, 182–189. [Google Scholar] [CrossRef]
- Laurence, A.; O’Shea, J.J. T H-17 differentiation: Of mice and men. Nat. Immunol. 2007, 89, 903. [Google Scholar] [CrossRef]
- Schett, G.; Elewaut, D.; McInnes, I.B.; Dayer, J.-M.; Neurath, M.F. How Cytokine Networks Fuel Inflammation: Toward a cytokine-based disease taxonomy. Nat. Med. 2013, 19, 822–824. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, W.; Wu, T.; Chu, H. Reduced interleukin-38 in non-small cell lung cancer is associated with tumour progression. Open Biol. 2018, 8, 180132. [Google Scholar] [CrossRef]
| Cytokine | Family Name | Alternative Names | Receptor | Coreceptor | Property | Processing Required for Optimal Bioactivity | |
|---|---|---|---|---|---|---|---|
| IL-1 subfamily | IL-1α | IL-1F1 | IL-1A, IL1 | IL-1RI | IL-1RAcp | Pro-inflammatory | No |
| IL-1β | IL-1F2 | IL-1B | IL-1RI | IL-1RAcp | Pro-inflammatory | Yes | |
| IL-1Ra | IL-1F3 | IL-1RN, ICIL-1RA, IRAP, MGC10430 | IL-1RI | NA | IL-1R antagonist | No | |
| IL-33 | IL-1F11 | C9orf26, DKFZp586H0523, DVS27, NF-HEV | ST2 | IL-1RAcp | Pro-inflammatory, transcription regulating factor | No | |
| IL-18 subfamily | IL-18 | IL-1F4 | IGIF, IL-1 g, IL1F4 | IL-18Rα | IL-18Rβ | Pro-inflammatory | Yes |
| IL-37 | IL-1F7 | FIL1Z, FIL-1ζ, IL-1H4, IL-1RP1 | IL-18Rα | SIGIRR | Anti-inflammatory, transcription regulating factor | Yes | |
| IL-36 subfamily | IL-36Ra | IL-1F5 | FIL1δ, FIL1D, IL1HY1, IL-1L1, IL-1RP3, IL-36RN, IL-1H3, MGC29840 | IL-36R | NA | IL-36R antagonist | Yes |
| IL-36α | IL-1F6 | FIL1E, IL-1ε, MGC129552, MGC129553 | IL-36R | IL-1RAcp | Pro-inflammatory | Yes | |
| IL-36β | IL-1F8 | FIL1η, IL1-ETA, IL-1H2, MGC126880, MGC126882 | IL-36R | IL-1RAcp | Pro-inflammatory | Yes | |
| IL-36γ | IL-1F9 | IL-1RP2, IL1E, IL-36G, IL-1H1 | IL-36R | IL-1RAcp | Pro-inflammatory | Yes | |
| IL-38 | IL-1F10 | IL-1HY2, FKSG75, MGC11983, MGC119832, MGC119833 | IL-36R, IL1RAPL1, IL1R1? | Unknown | Anti-inflammatory, proinflammatory? | Yes | |
| NA, not applicable | |||||||
| Inflammatory Autoimmune Diseases | IL-38 and Related Cytokines Levels | Function |
|---|---|---|
| Rheumatoid arthritis (RA) | Increased IL-38, IL-36R, IL-36 procytokines, and IL-36Ra levels in the serum and synovial fibroblasts [29,75,77]. Elevated IL-38 levels correlate with IL-1β, TNF-α, IL-6, IL-1Ra, CCL3, CCL4, M-CSF, erythrocyte sedimentation rate and C-reactive protein [29,78]. IL-38, IL-36 procytokines and IL-36Ra correlate with each other [29]. Th17 numbers are elevated but Treg numbers decreased in RA [71]. | IL-38 overexpression ameliorates collagen-induced arthritis (CIA) and K/BxN-serum-transfer-induced arthritis (STIA) but not antigen-induced arthritis (AIA) and has no influence on gristle or bone damage [40]. IL-38 neutralization exacerbates RA syndromes [40], and induces greater disease severity in STIA [76]. IL-38 reduces proinflammatory cytokine and chemokine expression in PBMCs, SFs and THP-1 cells [40]. IL-38 decreases Mφ infiltration to reduce expression of Th17 cytokines, TNF-α, chemokine ligand 1 and nuclear factor kappa-B ligand. IL-38 might be a biomarker for RA [78]. Th17 neutralization suppresses the development of CIA and AIA [83,88]. IL-36 signaling neutralization has no effect on RA [63]. IL-1R1 neutralization attenuates progression and severity of CIA [64]. |
| Psoriasis | IL-38 levels are significantly reduced in skin lesions and in circulating of psoriasis [29,44,96] but are increased in pustular psoriasis [56]. Levels of IL-36R, IL-36 procytokines, and IL-36Ra [92,98,99], but not IL-38, are increased in psoriasis [98]. Increased Th1 and Th17 numbers and decreased Treg numbers occur in psoriasis [94]. Anti-TNF-α therapy improves psoriasis and decreases IL-36R, IL-36 procytokine, and IL-36Ra levels [92]. Anti-IL-22 therapy diminishes psoriasis symptoms and reduces IL-36 procytokine expression [51]. | IL-38 dampens Th17 responses [94]. IL-38 knockout mice show delayed disease resolution and exacerbated IL-17 inflammation, which can be reversed by adding IL-38 or γδ T cell-receptor antibodies [95]. IL-38 expression is decreased in dedifferentiated KCs [56] and correlates with reduced expression of CK10 [29]. IL-38 correlates with disease severity [97]. IL-38 knockout shows no impact in a mouse model of psoriasis [44]. Th17 numbers and serum IL-17 levels are strongly related to the systemic disease activity of psoriasis and psoriatic arthritis [73,93]. Th17 cytokines can inhibit KC differentiation [107]. IL-1RACP knockout mice show attenuated inflammation by γδ T cells [95]. IL-36R neutralization attenuates psoriasis [100]. IL-36Ra neutralization strongly exacerbates skin inflammation [101], but IL1R1 and IL1RAcP neutralization produces no obvious skin abnormalities [104]. |
| Systemic lupus erythematosus (SLE) | IL-38 and IL-36 procytokine levels are significantly increased in the serum and correlate with disease activity [30,110]. IL-38 expression is significantly decreased in Murphy Roths Large /lpr mice [112]. IL-38 expression decreases after treatment in juvenile-onset SLE patients. [54]. | IL-38 treatment attenuates disease severity due to a reduction in PBMC and Th17 numbers and promotes Treg expansion, with no influence for Th1s and Th2s [30,112]. IL-38 neutralization increases proinflammatory cytokine IL-6, CCL2, and APRIL production in PBMCs [30]. IL-38 is associated with complications in the renal and central nervous systems [30]. |
| Inflammatory bowel disease | Increased IL-38, IL-36α/γ and IL-36Ra expression in colonic biopsies from Crohn’s disease patients correlate with IL-1β and IL-17 [29]. IL-38 and IL-36α/γ levels are increased in dextran sulfate sodium-induced colitis [29,114]. IL-36Ra expression in lamina propria mononuclear cells is decreased in ulcerative colitis [29]. | IL-36R knockout mice exhibit reduced intestinal inflammation with decreased inflammatory cell infiltration [114,116,117]. IL-36R neutralization elevates Th17 responses while reducing Th1 responses by decreasing IL-2 levels [114]. |
| Other inflammatory autoimmune diseases | IL-38 expression is increased in primary Sjogren’s syndrome (pSS) [57] and hidradenitis suppurativa (HS) patients [119]. Increased IL-36α levels in the serum and salivary glands of pSS patients correlate with disease activity [57]. IL-36 procytokine and IL-36Ra levels are significantly higher in HS [119]. IL-38 and IL-36 procytokine levels are increased in the aqueous humor of glaucoma patients [123]. | The IL-36 axis is important in pSS [57]. IL-23/Th17 pathway components are overexpressed in pSS [118] and HS [120]. Th17 numbers as well as IL-17 levels correlate greatly with ankylosing spondylitis activity [73]. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, L.; Huang, Z.; Li, H.; Liu, X.; Guo Zheng, S.; Su, W. IL-38: A New Player in Inflammatory Autoimmune Disorders. Biomolecules 2019, 9, 345. https://doi.org/10.3390/biom9080345
Xie L, Huang Z, Li H, Liu X, Guo Zheng S, Su W. IL-38: A New Player in Inflammatory Autoimmune Disorders. Biomolecules. 2019; 9(8):345. https://doi.org/10.3390/biom9080345
Chicago/Turabian StyleXie, Lihui, Zhaohao Huang, He Li, Xiuxing Liu, Song Guo Zheng, and Wenru Su. 2019. "IL-38: A New Player in Inflammatory Autoimmune Disorders" Biomolecules 9, no. 8: 345. https://doi.org/10.3390/biom9080345
APA StyleXie, L., Huang, Z., Li, H., Liu, X., Guo Zheng, S., & Su, W. (2019). IL-38: A New Player in Inflammatory Autoimmune Disorders. Biomolecules, 9(8), 345. https://doi.org/10.3390/biom9080345







