A Simple and Efficient Molecularly Imprinted Electrochemical Sensor for the Selective Determination of Tryptophan
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals and Solutions
2.2. Apparatus
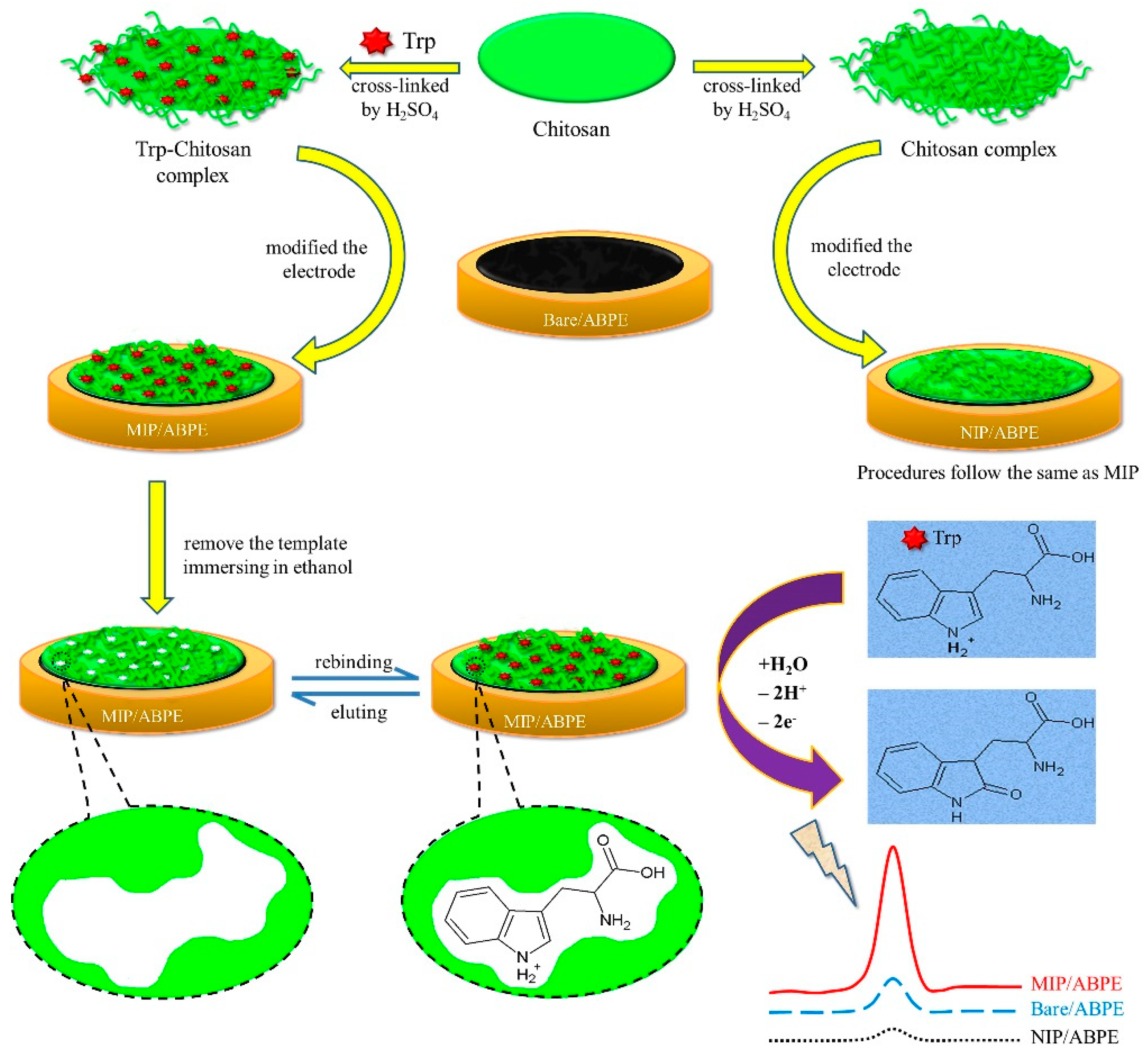
2.3. Preparation of MIP/ABPE
2.4. Electrochemical Measurements
3. Results and Discussion
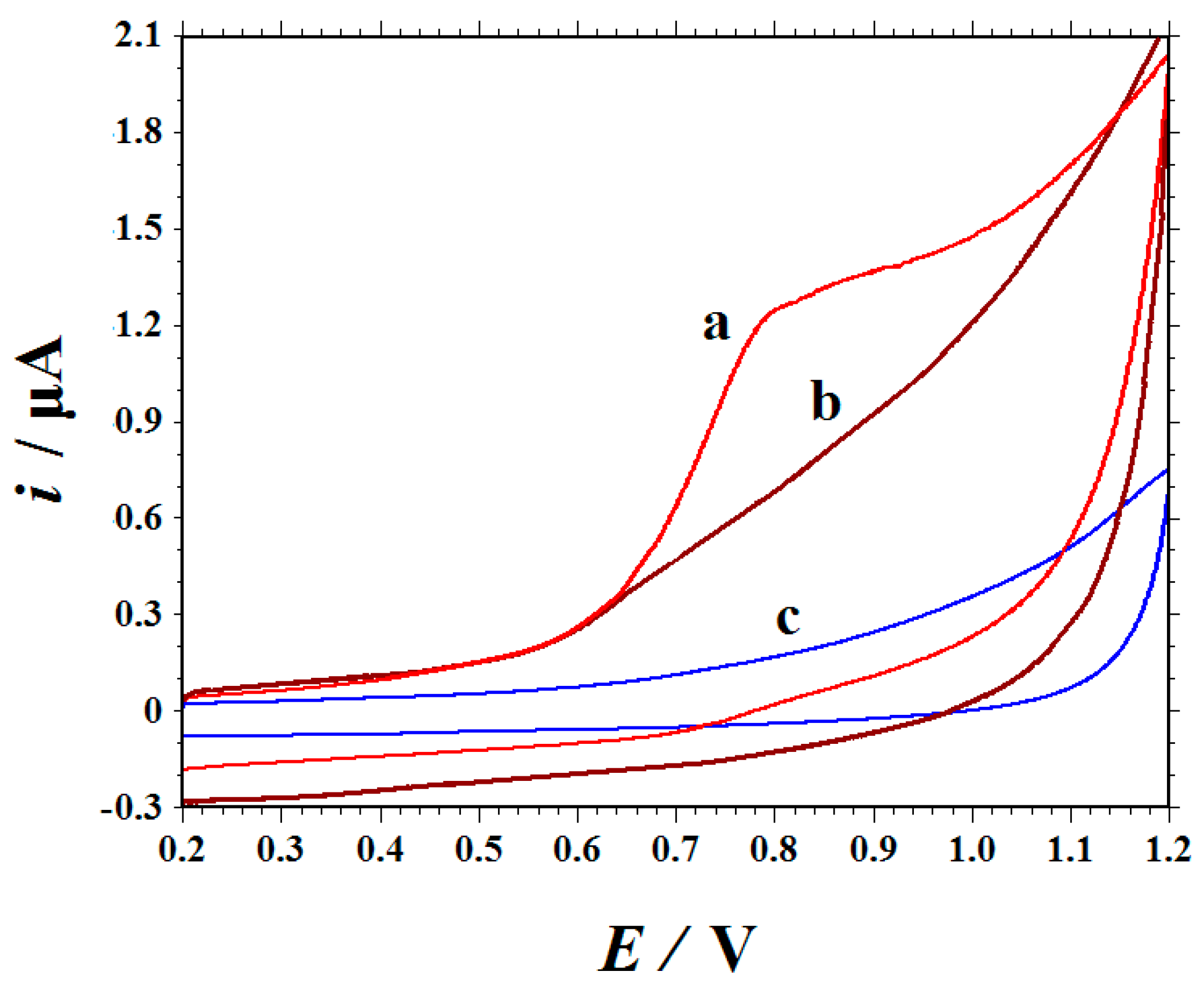
3.1. Template Removal
3.2. FT–IR Spectra
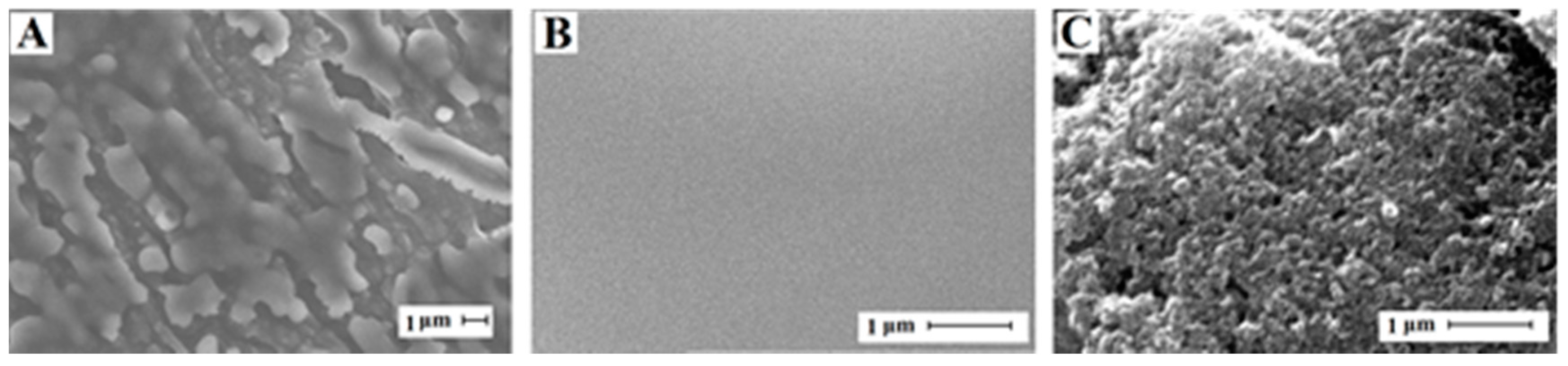
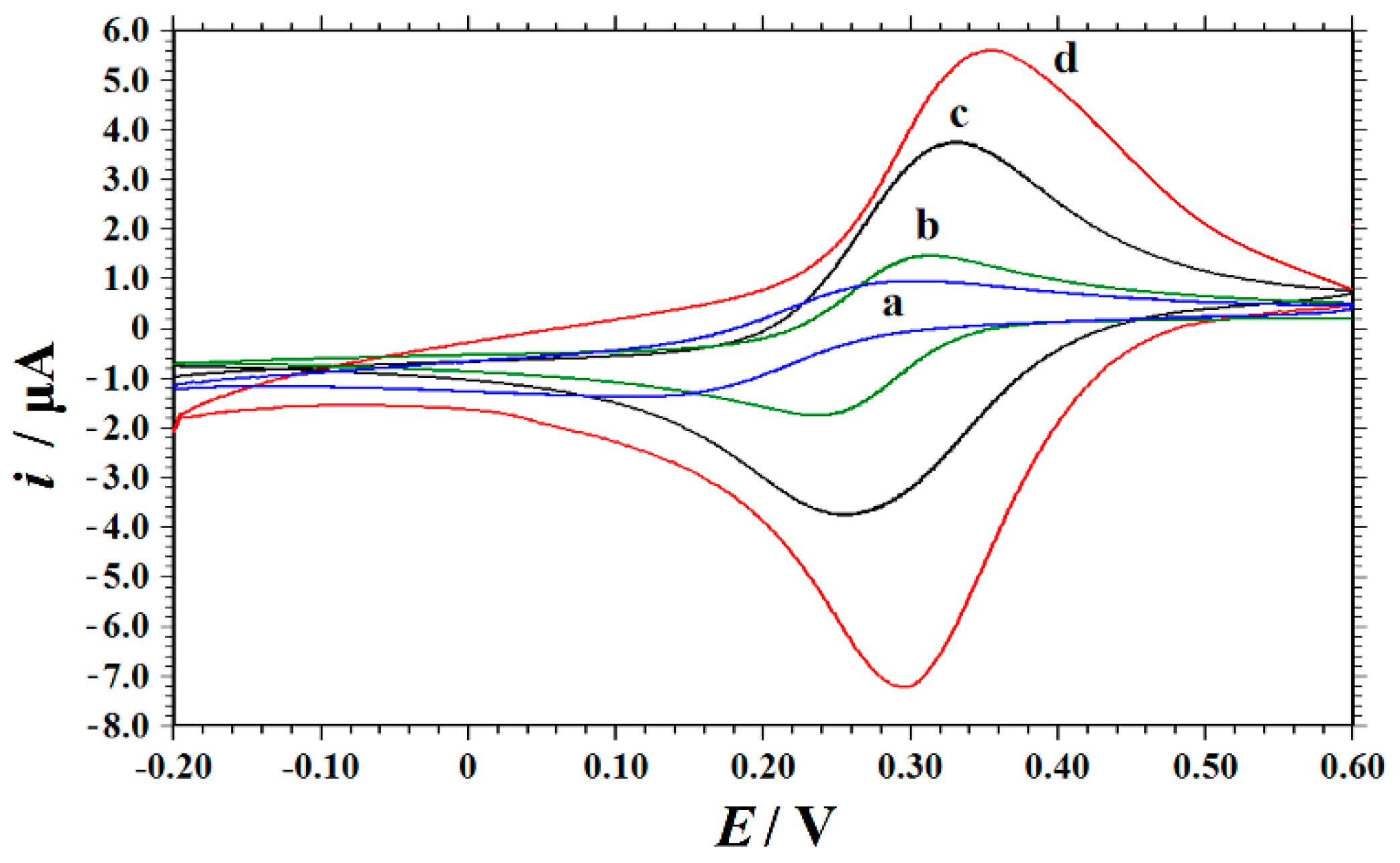
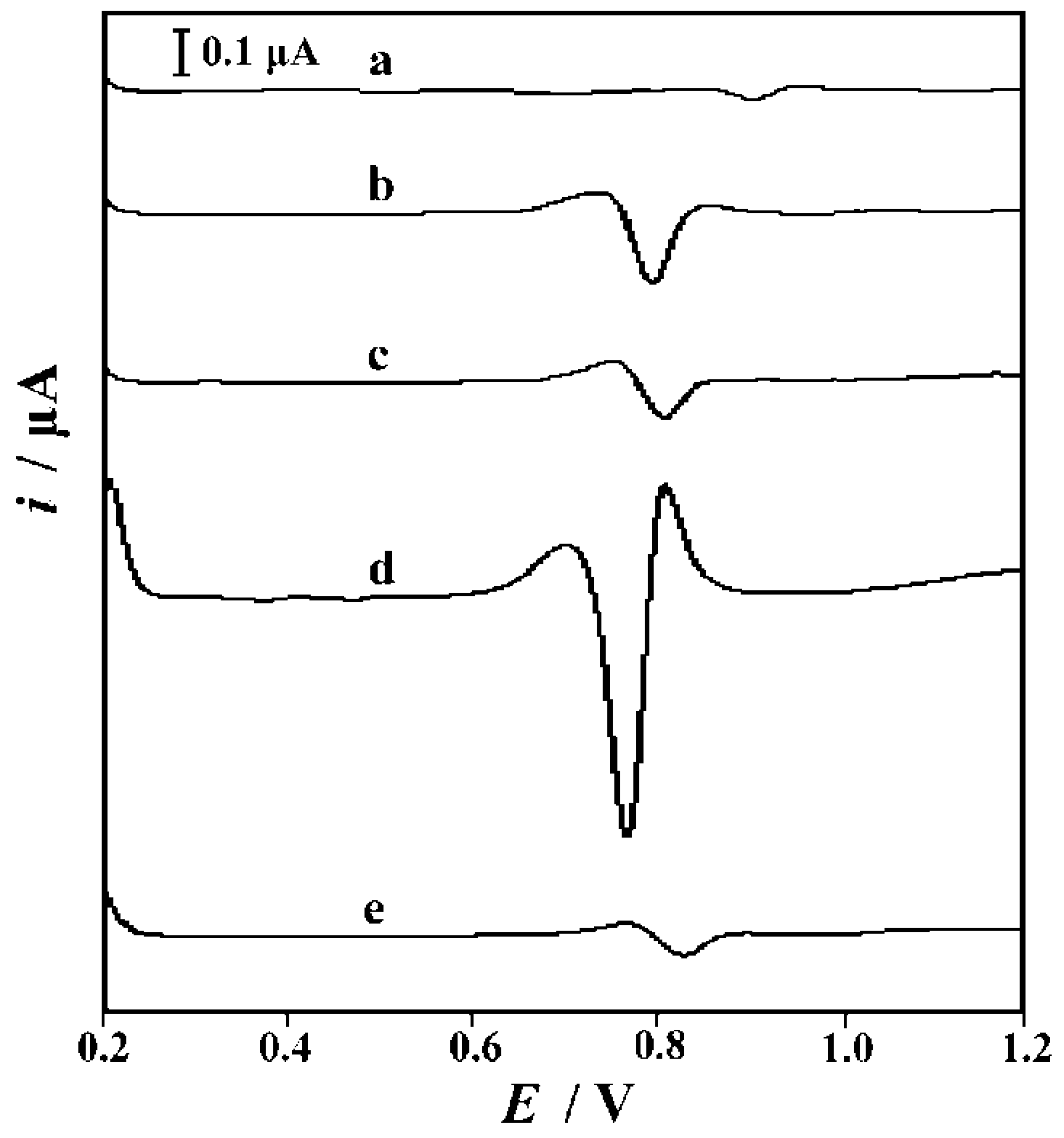
3.3. Electrode Characterizations by SEM and CV
3.4. The Imprinting Effect and the Electrode Process Mechanism
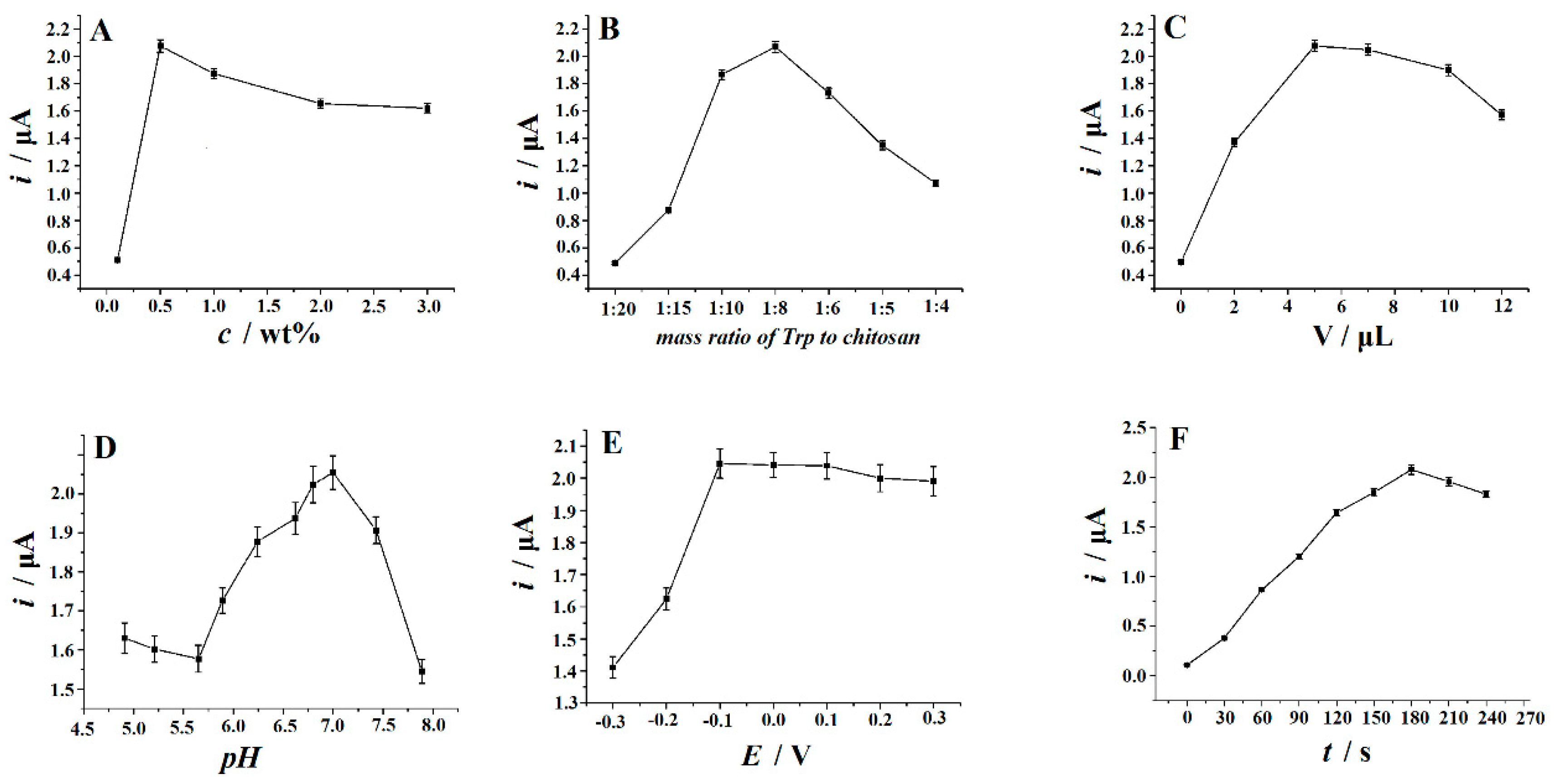
3.5. Optimization of Analytical Conditions
3.5.1. The Effect of the Concentrations of Chitosan
3.5.2. The Effect of the Mass Ratio of Trp to Chitosan
3.5.3. The Effect of Trp-Chitosan Dropping Amount
3.5.4. The Effect of the Solution pH
3.5.5. Accumulation Potential and Accumulation Time
3.6. Analytical Performance of the MIP/ABPE
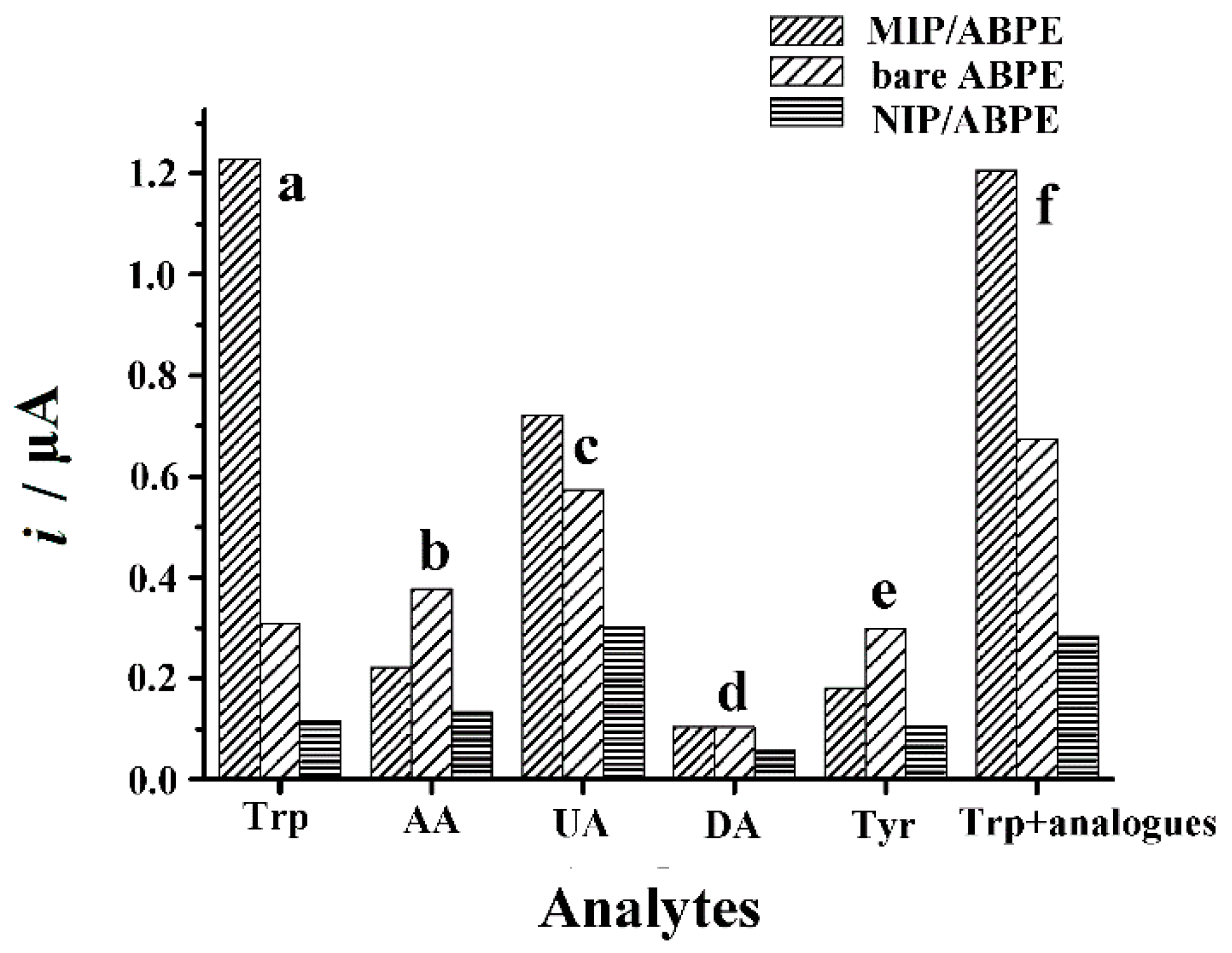
3.6.1. Interference Study
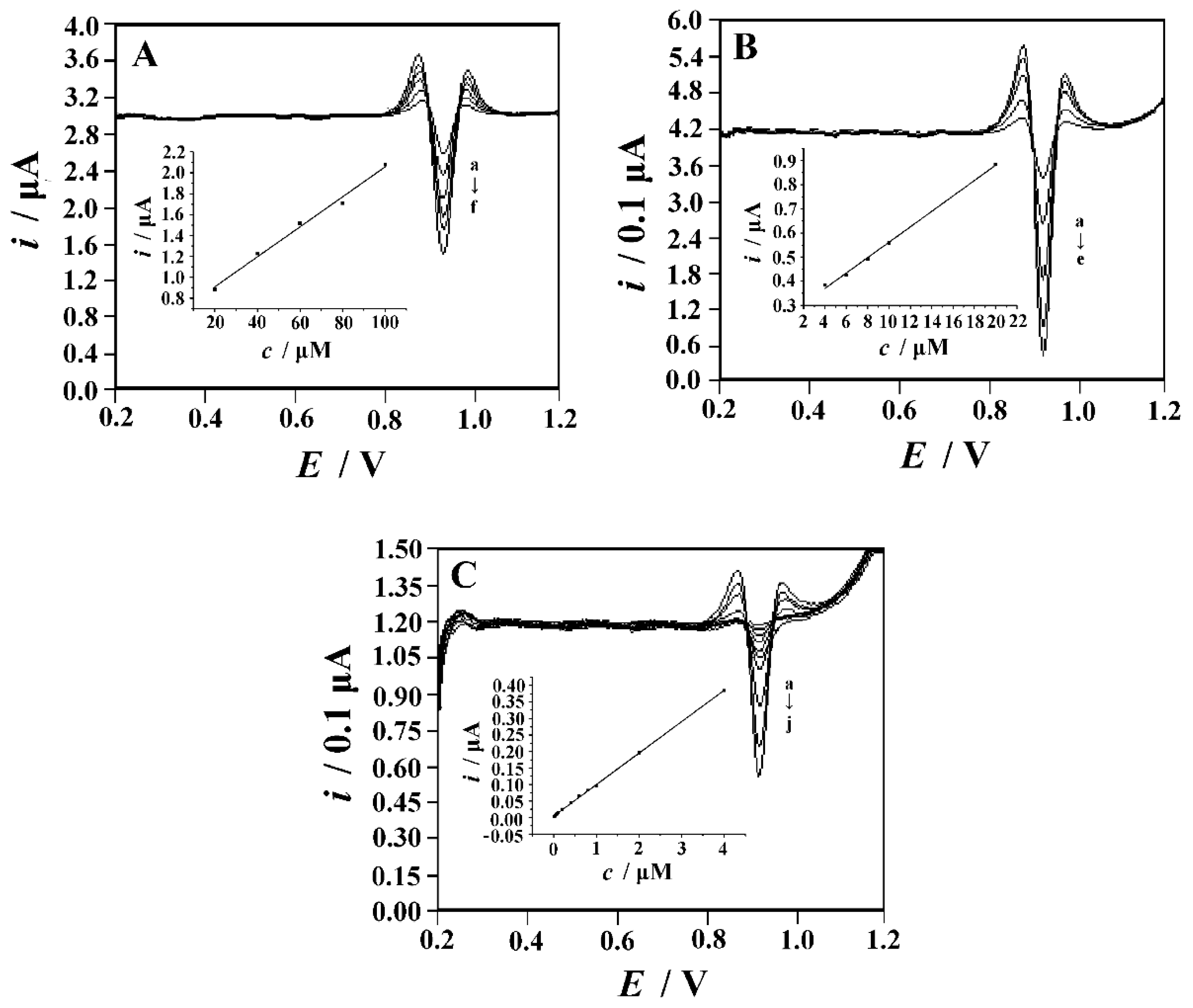
3.6.2. Linear Range and Detection Limit
3.6.3. Reproducibility, Reusability and Long-Term Stability
3.6.4. Practical Application
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fiorucci, A.R.; Cavalheiro, É.T.G. The use of carbon paste electrode in the direct voltammetric determination of tryptophan in pharmaceutical formulations. J. Pharm. Biomed. Anal. 2002, 28, 909–915. [Google Scholar] [CrossRef]
- Kochen, W.; Steinhart, H. L-Tryptophan: Current Prospects in Medicine and Drug Safety; Walter de-Gruyter: Berlin, Germany, 1994. [Google Scholar]
- Pi, L.G.; Tang, A.G.; Mo, X.M.; Luo, X.B.; Ao, X. More rapid and sensitive method for simultaneous determination of tryptophan and kynurenic acid by HPLC. Clin. Biochem. 2009, 42, 420–425. [Google Scholar] [PubMed]
- Michalczuk, L.; Bialek, K.; Cohen, J.D. Rapid determination of free tryptophan in plant samples by gas chromatography-selected ion monitoring mass spectrometry. J. Chromatogr. A 1992, 596, 294–298. [Google Scholar] [CrossRef]
- Huynh, T.K.X.; Leipzig-Pagani, E. Quantitative thin layer chromatography on cellulose II. Selected applications: Lower alcohols, tryptophan enantiomers, gold and platinum. J. Chromatogr. A 1996, 746, 261–268. [Google Scholar] [CrossRef]
- Verma, K.K.; Jain, A.; Gasparič, J. Spectrophotometric determination of tryptophan by reaction with nitrous acid. Talanta 1988, 35, 35–39. [Google Scholar] [CrossRef]
- Othman, A.M.; Li, S.; Leblanc, R.M. Enhancing selectivity in spectroflurimetric determination of tryptophan by using graphene oxide nanosheets. Anal. Chim. Acta 2013, 787, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Luo, C.; Sun, M.; Lu, F.; Fan, L.; Li, X. Determination of l-tryptophan based on graphene oxide-magnetite-molecularly imprinted polymers and chemiluminescence. Talanta 2012, 98, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Makriyannis, A.A.; Kheir, A.E.; Ayad, M.M.; Hassan, W.S.; Metwally, M.M.F. Derivative spectroscopy in conjunction with thin layer chromatography applied for determination of mixtures of amino acids in baby food. Spectrosc. Lett. 1997, 30, 649–660. [Google Scholar] [CrossRef]
- López-Cueto, G.; Rodríguez-Medina, J.F.; Ubide, C. Individual kinetic determinations using partial least squares calibration. Analyst 1997, 122, 519–523. [Google Scholar] [CrossRef]
- He, Q.; Liu, J.; Liu, X.; Li, G.; Chen, D.; Deng, P.; Liang, J. Fabrication of Amine-Modified Magnetite-Electrochemically Reduced Graphene Oxide Nanocomposite Modified Glassy Carbon Electrode for Sensitive Dopamine Determination. Nanomaterials 2018, 8, 194. [Google Scholar] [CrossRef]
- He, Q.; Wu, Y.; Tian, Y.; Li, G.; Chen, D. Facile Electrochemical Sensor for Nanomolar Rutin Detection Based on Magnetite Nanoparticles and Reduced Graphene Oxide Decorated Electrode. Nanomaterials 2019, 9, 115. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Liu, J.; Liu, X.; Li, G.; Chen, D.; Deng, P.; Liang, J. A promising sensing platform toward dopamine using MnO2 nanowires/electro-reduced graphene oxide composites. Electrochim. Acta 2019, 296, 683–692. [Google Scholar] [CrossRef]
- He, Q.; Liu, J.; Liu, X.; Li, G.; Deng, P.; Liang, J. Manganese dioxide Nanorods/electrochemically reduced graphene oxide nanocomposites modified electrodes for cost-effective and ultrasensitive detection of Amaranth. Colloids Surf. B 2018, 172, 565–572. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Liu, J.; Liu, X.; Li, G.; Deng, P.; Liang, J. Preparation of Cu2O-Reduced Graphene Nanocomposite Modified Electrodes towards Ultrasensitive Dopamine Detection. Sensors 2018, 18, 199. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.P.; Lin, X.Q. The electrochemical behavior and amperometric determination of tyrosine and tryptophan at a glassy carbon electrode modified with butyrylcholine. Electrochem. Commun. 2004, 6, 454–460. [Google Scholar] [CrossRef]
- Li, C.; Ya, Y.; Zhan, G. Electrochemical investigation of tryptophan at gold nanoparticles modified electrode in the presence of sodium dodecylbenzene sulfonate. J. Colloids. Surf. B 2010, 76, 340–345. [Google Scholar] [CrossRef]
- Kooshki, M.; Abdollahi, H.; Bozorgzadeh, S.; Haghighi, B. Second-order data obtained from differential pulse voltammetry:Determination of tryptophan at a gold nanoparticles decorated multiwalled carbon nanotube modified glassy carbon electrode. Electrochim. Acta 2011, 56, 8618–8624. [Google Scholar] [CrossRef]
- Özcan, A.; Sahin, Y. A novel approach for the selective determination of tryptophan in blood serum in the presence of tyrosine based on the electrochemical reduction of oxidation product of tryptophan formed in situ on graphite electrode. Biosens. Bioelectron. 2012, 31, 26–31. [Google Scholar] [CrossRef]
- Wu, F.H.; Zhao, G.C.; Wei, X.W.; Yang, Z.S. Electrocatalysis of tryptophan at multi-walled carbon nanotube modified electrode. Microchim. Acta 2004, 144, 243–247. [Google Scholar] [CrossRef]
- Liu, X.; Luo, L.; Ding, Y.; Ye, D. Poly-glutamic acid modified carbon nanotube-doped carbon paste electrode for sensitive detection of L-tryptophan. Bioelectrochemistry 2011, 82, 38–45. [Google Scholar] [CrossRef]
- Thomas, T.; Mascarenhas, R.J.; D’Souza, O.J.; Martis, P.; Dalhalle, J.; Swamy, B.E.K. Multi-walled carbon nanotube modified carbon paste electrode as a sensor for the amperometric detection of L-tryptophan in biological samples. J. Colloid. Interface. Sci. 2013, 402, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Ba, X.; Luo, L.; Ding, Y.; Liu, X. Determination of l-tryptophan in the presence of ascorbic acid and dopamine using poly(sulfosalicylic acid) modified glassy carbon electrode. Sens. Actuators B Chem. 2013, 187, 27–32. [Google Scholar] [CrossRef]
- Mattioli, I.A.; Baccarin, M.; Cervini, P.; Cavalheiro, É.T.G. Electrochemical investigation of a graphite-polyurethane composite electrode modified with electrodeposited gold nanoparticles in the voltammetric determination of tryptophan. J. Electroanal. Chem. 2019, 835, 212–219. [Google Scholar] [CrossRef]
- Mukdasai, S.; Poosittisak, S.; Ngeontae, W.; Srijaranai, S. A highly sensitive electrochemical determination of L-tryptophan in the presence of ascorbic acid and uric acid using in situ addition of tetrabutylammonium bromide on the ß-cyclodextrin incorporated multi-walled carbon nanotubes modified electrode. Sens. Actuators B Chem. 2018, 272, 518–525. [Google Scholar] [CrossRef]
- Haldorai, Y.; Yeon, S.-H.; Huh, Y.S.; Han, Y.-K. Electrochemical determination of tryptophan using a glassy carbon electrode modified with flower-like structured nanocomposite consisting of reduced graphene oxide and SnO2. Sens. Actuators B Chem. 2017, 239, 1221–1230. [Google Scholar] [CrossRef]
- Zhou, S.; Deng, Z.; Wu, Z.; Xie, M.; Tian, Y.; Wu, Y.; Liu, J.; Li, G.; He, Q. Ta2O5/rGO Nanocomposite Modified Electrodes for Detection of Tryptophan through Electrochemical Route. Nanomaterials 2019, 9, 811. [Google Scholar] [CrossRef] [PubMed]
- Hammam, M.A.; Abdel-Halim, M.; Madbouly, A.; Wagdy, H.A.; Nashar, R.M.E. Computational design of molecularly imprinted polymer for solid phase extraction of moxifloxacin hydrochloride from Avalox® tablets and spiked human urine samples. Microchem. J. 2019, 148, 51–56. [Google Scholar] [CrossRef]
- Ahmad, O.S.; Bedwell, T.S.; Esen, C.; Garcia-Cruz, A.; Piletsky, S.A. Molecularly imprinted polymers in electrochemical and optical sensors. Trends Biotechnol. 2019, 37, 294–309. [Google Scholar] [CrossRef] [PubMed]
- Guoning, C.; Pengqi, G.; Yan, W.; Lu, W.; Qiang, F. Preparation of molecularly imprinted polymers and application in a biomimetic biotin-avidin-ELISA for the detection of bovine serum albumin. Talanta 2019, 198, 55–62. [Google Scholar] [CrossRef]
- Beluomini, M.A.; da Silva, J.L.; de Sá, A.C.; Buffon, E.; Stradiotto, N.R. Electrochemical sensors based on molecularly imprinted polymer on nanostructured carbon materials: A review. J. Electroanal. Chem. 2019, 840, 343–366. [Google Scholar] [CrossRef]
- Qader, B.; Baron, M.; Hussain, I.; Sevilla, J.M.; Gonzalez-Rodriguez, J. Electrochemical determination of disulfoton using a molecularly imprinted poly-phenol polymer. Electrochim. Acta 2019, 295, 333–339. [Google Scholar] [CrossRef]
- Roushani, M.; Jalilian, Z.; Nezhadali, A. A novel electrochemical sensor based on electrode modified with gold nanoparticles and molecularly imprinted polymer for rapid determination of trazosin. Colloids Surf. B Biointerfaces 2018, 172, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, T.N.; Das, D.; Roy, R.B.; Tudu, B.; Bandyopadhyay, R. Development of a nickel hydroxide nanopetal decorated molecular imprinted polymer based electrode for sensitive detection of epigallocatechin-3-gallate in green tea. Sens. Actuators B Chem. 2019, 283, 69–78. [Google Scholar] [CrossRef]
- Sah, A.K.; Dewangan, M.; Suresh, P.K. Potential of chitosan-based carrier for periodontal drug delivery. Colloids Surf. B Biointerfaces 2019, 178, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Jimtaisong, A.; Sarakonsri, T. Chitosan intercalated bentonite as natural adsorbent matrix for water-soluble sappanwood dye. Int. J. Biol. Macromol. 2019, 129, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhang, L.; Mao, S.; Rohani, S.; Lu, J. Zwitterionic chitosan–silica–PVA hybrid ultrafiltration membranes for protein separation. Sep. Purif. Technol. 2015, 152, 55–63. [Google Scholar] [CrossRef]
- Guo, W.; Pi, F.; Zhang, H.; Sun, J.; Sun, X. A novel molecularly imprinted electrochemical sensor modified with carbon dots, chitosan, gold nanoparticles for the determination of patulin. Biosens. Bioelectron. 2017, 98, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Dai, X.; Cheng, T.; Li, S. Highly sensitive and selective ion-imprinted polymers based on one-step electrodeposition of chitosan-graphene nanocomposites for the determination of Cr(VI). Carbohyd. Polym. 2018, 195, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Lian, H.T.; Yin, J.F.; Sun, X.Y. Dopamine molecularly imprinted electrochemical sensor based on graphene–chitosan composite. Electrochim. Acta 2012, 75, 108–114. [Google Scholar] [CrossRef]
- Xia, J.; Cao, X.; Wang, Z.; Yang, M.; Xia, Y. Molecularly imprinted electrochemical biosensor based on chitosan/ionic liquid–graphene composites modified electrode for determination of bovine serum albumin. Sens. Actuators B Chem. 2016, 225, 305–311. [Google Scholar] [CrossRef]
- Deng, P.; Xu, Z.; Feng, Y. Acetylene black paste electrode modified with graphene as the voltammetric sensor for selective determination of tryptophan in the presence of high concentrations of tyrosine. Mater. Sci. Eng. C 2014, 35, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.; Xu, Z.; Zeng, R.; Ding, C. Electrochemical behavior and voltammetric determination of vanillin based on an acetylene black paste electrode modified with graphene–polyvinylpyrrolidone composite film. Food Chem. 2015, 180, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Chen, R.; Zheng, X.; Youn, H.; Chen, Z. Preparation of molecularly imprinted CS membrane for recognizing naringin in aqueous media. Polym. Bull. 2011, 66, 853–863. [Google Scholar] [CrossRef]
- Motahari, A.; Omrani, A.; Rostami, A.A.; Ehsani, M. Preparation and characterization of a novel epoxy based nanocomposite using tryptophan as an eco-friendly curing agent. Thermochim. Acta 2013, 574, 38–46. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Y.; Liu, S.; Wang, L.; Gao, F.; Gao, F.; Sun, W. Voltammetric detection of bisphenol a by a chitosan-graphene composite modified carbon ionic liquid electrode. Thin Solid Films 2012, 520, 4459–4464. [Google Scholar] [CrossRef]
- Laviron, E. Adsorption, autoinhibition and autocatalysis in polarography and in linear potential sweep voltammetry. J. Electroanal. Chem. Interf. Electrochem. 1974, 52, 355–393. [Google Scholar] [CrossRef]
- González, A.G.; Herrador, M.Á. A practical guide to analytical method validation, including measurement uncertainty and accuracy profiles. Trend. Anal. Chem. 2007, 26, 227–238. [Google Scholar] [CrossRef]
- Deng, P.; Fei, J.; Feng, Y. Sensitive voltammetric determination of tryptophan using an acetylene black paste electrode modified with a Schiff’s base derivative of chitosan. Analyst 2011, 136, 5211–5217. [Google Scholar] [CrossRef]
- He, Q.; Tian, Y.; Wu, Y.; Liu, J.; Li, G.; Deng, P.; Chen, D. Electrochemical sensor for rapid and sensitive detection of tryptophan by a Cu2O nanoparticles-coated reduced graphene oxide nanocomposite. Biomolecules 2019, 9, 176. [Google Scholar] [CrossRef]
- Magesa, F.; Wu, Y.; Tian, Y.; Vianney, J.-M.; Buza, J.; He, Q.; Tan, Y. Graphene and graphene like 2D graphitic carbon nitride: Electrochemical detection of food colorants and toxic substances in environment. Trends Environ. Anal. Chem. 2019. [Google Scholar] [CrossRef]
- He, Q.; Liu, J.; Xia, Y.; Tuo, D.; Deng, P.; Tian, Y.; Chen, D. Rapid and sensitive voltammetric detection of rhodamine B in chili-containing foodstuffs using MnO2 nanorods/electro-reduced graphene oxide composite. J. Electrochem. Soc. 2019. [Google Scholar] [CrossRef]


| Electrode | Technique | Supporting Electrolyte | Linear Range/μM | Detection Limit/μM | Effect of Tyr | References |
|---|---|---|---|---|---|---|
| a BuCh/GCE | m DPV | phosphate buffer (pH 7.0) | 2–60 | 0.6 | seriously interfered | [16] |
| b Au-NPs/GCE | DPV | phosphate buffer (pH 2.5) | 0.09–50 | 0.08 | seriously interfered | [17] |
| c nanoAu-MWCNTs/ GCE | DPV | phosphate buffer (pH 7.4) | 5–100 | 3 | seriously interfered | [18] |
| d ETPGE | DPV | phosphate buffer (pH 3.0) | 0.5–50 | 0.05 | 10-fold concentration did not interfere | [19] |
| e MWCNTs/GCE | DPV | phosphate Buffer (pH 3.5) | 0.25–100 | 0.027 | 5-fold concentration did not interfere | [20] |
| f PGA/CNTPE | n CV | phosphate buffer (pH 6.0) | 0.05–100 | 0.01 | not mentioned | [21] |
| g MWCNTs/CPE | Amperometry | phosphate buffer (pH 3.0) | 0.6–9.0; 10.0–100 | 0.033 | seriously interfered | [22] |
| h PSA/GCE | DPV | phosphate buffer (pH 3.5) | 0.05–10 | 0.0068 | not mentioned | [23] |
| i EGPU-tAuNP | DPV | Britton-Robinson buffer (pH 7.4) | 0.6–2.0 | 0.053 | not mentioned | [24] |
| j ß-CD/MWCNTs/GCE | DPV | phosphate buffer (pH 3.0) | 1.5–30.5 | 0.07 | 4-fold concentration did not interfere | [25] |
| k rGO/SnO2/GCE | DPV | phosphate buffer (pH 7.0) | 1–100 | 0.04 | 30-fold concentration did not interfere | [26] |
| l Ta2O5-rGO-GCE | second-order derivative LSV | phosphate buffer (pH 6.0) | 1–8; 8–80; 80–800 | 0.87 | N.A | [27] |
| MIP/ABPE | second-order derivative LSV | phosphate buffer (pH 3.0) | 0.01–4; 4–20; 20–100 | 0.008 | 20-fold concentration did not interfere | This work |
| Sample ID | Label Values/g L−1 | Found/gL−1 | RSD/% | Recovery/% |
|---|---|---|---|---|
| Aa | 0.430 | 0.438 | 2.4 | 102.4 |
| Ba | 0.700 | 0.695 | 2.8 | 98.6 |
| Ca | 0.900 | 0.913 | 3.1 | 97.3 |
| Db | 1.000 | 0.988 | 2.2 | 101.8 |
| Sample ID | Found/µM | RSD/% | Added/µM | Total Found/µM | Recovery/% |
|---|---|---|---|---|---|
| A | 2.67 | 2.7 | 3.0 | 5.74 | 102.3 |
| B | 3.45 | 2.3 | 4.0 | 7.52 | 101.8 |
| C | 4.28 | 2.5 | 4.0 | 8.45 | 104.2 |
| D | 3.37 | 2.9 | 3.0 | 6.32 | 98.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Deng, P.; Wu, Y.; Ding, Z.; Li, G.; Liu, J.; He, Q. A Simple and Efficient Molecularly Imprinted Electrochemical Sensor for the Selective Determination of Tryptophan. Biomolecules 2019, 9, 294. https://doi.org/10.3390/biom9070294
Tian Y, Deng P, Wu Y, Ding Z, Li G, Liu J, He Q. A Simple and Efficient Molecularly Imprinted Electrochemical Sensor for the Selective Determination of Tryptophan. Biomolecules. 2019; 9(7):294. https://doi.org/10.3390/biom9070294
Chicago/Turabian StyleTian, Yaling, Peihong Deng, Yiyong Wu, Ziyu Ding, Guangli Li, Jun Liu, and Quanguo He. 2019. "A Simple and Efficient Molecularly Imprinted Electrochemical Sensor for the Selective Determination of Tryptophan" Biomolecules 9, no. 7: 294. https://doi.org/10.3390/biom9070294
APA StyleTian, Y., Deng, P., Wu, Y., Ding, Z., Li, G., Liu, J., & He, Q. (2019). A Simple and Efficient Molecularly Imprinted Electrochemical Sensor for the Selective Determination of Tryptophan. Biomolecules, 9(7), 294. https://doi.org/10.3390/biom9070294






