Molecular Pathways Modulated by Curcumin Analogue, Diarylpentanoids in Cancer
Abstract
1. Introduction
2. Bioavailability of Curcumin
3. Natural Analogues of Curcumin
4. Diarylpentanoids as Potential Therapeutic Curcumin Analogues Treatment of Cancer Cells
5. Molecular Mechanisms of Diarylpentanoids: Effect on Molecular Pathways
5.1. Effects on NF-κB Signalling Pathway
5.2. Effects on STAT3 Signalling Pathway
5.3. Effects on Akt Phosphorylation and PTEN Expression
5.4. Effects on MAPK/ERK Signalling Pathway
5.5. Effects on VEGF Signaling Pathway
5.6. Induction of Cell Cycle Arrest and Apoptosis Pathways
6. Concluding Remarks and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Phuah, N.H.; In, L.L.; Azmi, M.N.; Ibrahim, H.; Awang, K.; Nagoor, N.H. Alterations of microrna expression patterns in human cervical carcinoma cells (ca ski) toward 1’s-1’-acetoxychavicol acetate and cisplatin. Reprod. Sci. 2013, 20, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Rathaur, P.; Waseem, R.; Ramteke, P.W.; John, S.A. Turmeric: The golden spice of life. Int. J. Pharm. Sci. Res. 2012, 3, 1987–1994. [Google Scholar]
- Prasad, S.; Aggarwal, B.B. Turmeric, the golden spice: From traditional medicine to modern medicine. In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; Benzie, I., Wachtel-Galor, S., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011. [Google Scholar]
- Tonnesen, H.H.; Karlsen, J. Studies on curcumin and curcuminoids. Vi. Kinetics of curcumin degradation in aqueous solution. Z. Lebensm. Unters. Forsch. 1985, 180, 402–404. [Google Scholar]
- Gupta, S.C.; Prasad, S.; Kim, J.H.; Patchva, S.; Webb, L.J.; Priyadarsini, I.K.; Aggarwal, B.B. Multitargeting by curcumin as revealed by molecular interaction studies. Nat. Prod. Rep. 2011, 28, 1937–1955. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Harikumar, K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59. [Google Scholar] [CrossRef] [PubMed]
- Perrone, D.; Ardito, F.; Giannatempo, G.; Dioguardi, M.; Troiano, G.; Lo Russo, L.; De Lillo, A.; Laino, L.; Lo Muzio, L. Biological and therapeutic activities, and anticancer properties of curcumin. Exp. Ther. Med. 2015, 10, 1615–1623. [Google Scholar] [CrossRef] [PubMed]
- Willenbacher, E.; Khan, S.Z.; Mujica, S.C.A.; Trapani, D.; Hussain, S.; Wolf, D.; Willenbacher, W.; Spizzo, G.; Seeber, A. Curcumin: New insights into an ancient ingredient against cancer. Int. J. Mol. Sci. 2019, 20, 1808. [Google Scholar] [CrossRef]
- Siwak, D.R.; Shishodia, S.; Aggarwal, B.B.; Kurzrock, R. Curcumin-induced antiprolierative and proapoptotic effects in melanoma cells are associated with suppession of ikappab kinase and nuclear factor kappab activity and are independent of the b-raf/mitogen-activated/extracellular signal-regulated protein kinase pathway and the akt pathway. Cancer 2005, 104, 879–890. [Google Scholar]
- Plummer, S.M.; Holloway, K.A.; Manson, M.M.; Munks, R.J.; Kaptein, A.; Farrow, S.; Howells, L. Inhibition of cyclo-oxygenase 2 expression in colon cells by the chemopreventive agent curcumin involves inhibition of nf-kappab activation via the nik/ikk signalling complex. Oncogene 1999, 18, 6013–6020. [Google Scholar] [CrossRef]
- Wang, S.L.; Li, Y.; Wen, Y.; Chen, Y.F.; Na, L.X.; Li, S.T.; Sun, C.H. Curcumin, a potential inhibitor of up-regulation of tnf-alpha and il-6 induced by palmitate in 3t3-l1 adipocytes through nf-kappab and jnk pathway. Biomed. Environ. Sci. 2009, 22, 32–39. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Sundaram, C.; Malani, N.; Ichikawa, H. Curcumin: The indian solid gold. Adv. Exp. Med. Biol. 2007, 595, 1–75. [Google Scholar] [PubMed]
- Bordoloi, D.; Roy, N.K.; Monisha, J.; Ganesan, P.; Kunnumakkara, A.B. Multi-targeted agents in cancer cell chemosensitization: What we learnt from curcumin thus far. Recent Pat. Anti-Cancer Drug Discov. 2015, 11, 67–97. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Kumar, A.; Aggarwal, M.S.; Shishodia, S. Curcumin derived from turmeric (curcuma longa): A spice for all seasons. In Phytochemicals in Cancer Chemoprevention; CRC Press: New York, NY, USA, 2005; pp. 349–387. [Google Scholar]
- Mimeault, M.; Batra, S.K. Potential applications of curcumin and its novel synthetic analogs and nanotechnology-based formulations in cancer prevention and therapy. Chin. Med. 2011, 6, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Teiten, M.H.; Eifes, S.; Dicato, M.; Diederich, M. Curcumin-the paradigm of a multi-target natural compound with applications in cancer prevention and treatment. Toxins 2010, 2, 128–162. [Google Scholar] [CrossRef] [PubMed]
- Bimonte, S.; Barbieri, A.; Palma, G.; Luciano, A.; Rea, D.; Arra, C. Curcumin inhibits tumor growth and angiogenesis in an orthotopic mouse model of human pancreatic cancer. BioMed. Res. Int. 2013, 2013, 810423. [Google Scholar] [CrossRef] [PubMed]
- Bimonte, S.; Barbieri, A.; Leongito, M.; Piccirillo, M.; Giudice, A.; Pivonello, C.; de Angelis, C.; Granata, V.; Palaia, R.; Izzo, F. Curcumin anticancer studies in pancreatic cancer. Nutrients 2016, 8, 433. [Google Scholar] [CrossRef] [PubMed]
- Chainani-Wu, N. Safety and anti-inflammatory activity of curcumin: A component of tumeric (curcuma longa). J. Altern. Complement. Med. 2003, 9, 161–168. [Google Scholar] [CrossRef]
- Pan, M.H.; Huang, T.M.; Lin, J.K. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab. Dispos. 1999, 27, 486–494. [Google Scholar]
- Asai, A.; Miyazawa, T. Occurrence of orally administered curcuminoid as glucuronide and glucuronide/sulfate conjugates in rat plasma. Life Sci. 2000, 67, 2785–2793. [Google Scholar] [CrossRef]
- Garcea, G.; Jones, D.J.; Singh, R.; Dennison, A.R.; Farmer, P.B.; Sharma, R.A.; Steward, W.P.; Gescher, A.J.; Berry, D.P. Detection of curcumin and its metabolites in hepatic tissue and portal blood of patients following oral administration. Br. J. Cancer 2004, 90, 1011–1015. [Google Scholar] [CrossRef]
- Ravindranath, V.; Chandrasekhara, N. Absorption and tissue distribution of curcumin in rats. Toxicology 1980, 16, 259–265. [Google Scholar] [CrossRef]
- Ireson, C.R.; Jones, D.J.L.; Orr, S.; Coughtrie, M.W.H.; Boocock, D.J.; Williams, M.L.; Farmer, P.B.; Steward, W.P.; Gescher, A.J. Metabolism of the cancer chemopreventive agent curcumin in human and rat intestine. Cancer Epidemiol. Biomark. Prev. 2002, 11, 105–111. [Google Scholar]
- Yang, K.Y.; Lin, L.C.; Tseng, T.Y.; Wang, S.C.; Tsai, T.H. Oral bioavailability of curcumin in rat and the herbal analysis from curcuma longa by lc-ms/ms. J. Chromatogr. B 2007, 853, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharmacol. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Tamvakopoulos, C.; Dimas, K.; Sofianos, Z.D.; Hatziantoniou, S.; Han, Z.; Liu, Z.L.; Wyche, J.H.; Pantazis, P. Metabolism and anticancer activity of the curcumin analogue, dimethoxycurcumin. Clin. Cancer Res. 2007, 13, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Hoehle, S.I.; Pfeiffer, E.; Solyom, A.M.; Metzler, M. Metabolism of curcuminoids in tissue slices and subcellular fractions from rat liver. J. Agric. Food Chem. 2006, 54, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Anto, R.J.; George, J.; Babu, K.V.; Rajasekharan, K.N.; Kuttan, R. Antimutagenic and anticarcinogenic activity of natural and synthetic curcuminoids. Mutat. Res. 1996, 370, 127–131. [Google Scholar] [CrossRef]
- Simon, A.; Allais, D.P.; Duroux, J.L.; Basly, J.P.; Durand-Fontanier, S.; Delage, C. Inhibitory effect of curcuminoids on mcf-7 cell proliferation and structure-activity relationships. Cancer Lett. 1998, 129, 111–116. [Google Scholar] [CrossRef]
- Yodkeeree, S.; Chaiwangyen, W.; Garbisa, S.; Limtrakul, P. Curcumin, demethoxycurcumin and bisdemethoxycurcumin differentially inhibit cancer cell invasion through the down-regulation of mmps and upa. J. Nutr. Biochem. 2009, 20, 87–95. [Google Scholar] [CrossRef]
- Boonrao, M.; Yodkeeree, S.; Ampasavate, C.; Anuchapreeda, S.; Limtrakul, P. The inhibitory effect of turmeric curcuminoids on matrix metalloproteinase-3 secretion in human invasive breast carcinoma cells. Arch. Pharmacal Res. 2010, 33, 989–998. [Google Scholar] [CrossRef]
- Vyas, A.; Dandawate, P.; Padhye, S.; Ahmad, A.; Sarkar, F. Perspectives on new synthetic curcumin analogs and their potential anticancer properties. Curr. Pharm. Des. 2013, 19, 2047–2069. [Google Scholar] [PubMed]
- Lin, L.; Deangelis, S.; Foust, E.; Fuchs, J.; Li, C.; Li, P.K.; Schwartz, E.B.; Lesinski, G.B.; Benson, D.; Lu, J.; et al. A novel small molecule inhibits stat3 phosphorylation and DNA binding activity and exhibits potent growth suppressive activity in human cancer cells. Mol. Cancer 2010, 9, 217. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Hutzen, B.; Zuo, M.; Ball, S.; Deangelis, S.; Foust, E.; Pandit, B.; Ihnat, M.A.; Shenoy, S.S.; Kulp, S.; et al. Novel stat3 phosphorylation inhibitors exhibit potent growth-suppressive activity in pancreatic and breast cancer cells. Cancer Res. 2010, 70, 2445–2454. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.C.; Ball, S.; Lin, L.; Liu, A.; Fuchs, J.R.; Li, P.K.; Li, C.; Lin, J. Two small molecule compounds, lll12 and flll32, exhibit potent inhibitory activity on stat3 in human rhabodomyosarcoma cells. Int. J. Oncol. 2011, 38, 279–285. [Google Scholar] [PubMed]
- Abuzeid, W.M.; Davis, S.; Tang, A.L.; Saunders, L.; Brenner, J.C.; Lin, J.; Fuchs, J.R.; Light, E.; Bradford, C.R.; Prince, M.E.; et al. Sensitization of head and neck cancer to cisplatin through the use of a novel curcumin analog. Arch. Otolaryngol. Head Neck Surg. 2011, 137, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.P.; Garcia, G.E.; Ghosh, R.; Rajnarayanan, R.V.; Alworth, W.L.; Slaga, T.J. 4-hydroxy-3-methoxybenzoic acid methyl ester: A curcumin derivative targets akt/nf kappa b cell survival signaling pathway: Potential for prostate cancer management. Neoplasia 2003, 5, 255–266. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Wang, Z.; Ahmad, A.; Bao, B.; Padhye, S.; Sarkar, F.H. Inactivation of ar/tmprss2-erg/wnt signaling networks attenuates the aggressive behavior of prostate cancer cells. Cancer Prev. Res. 2011, 4, 1495–1506. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, A.M.; MacKenzie, D.A.; Ji, M.; Deck, L.M.; Vander Jagt, D.L.; Thompson, T.A.; Bisoffi, M. The curcumin analog ca27 down-regulates androgen receptor through an oxidative stress mediated mechanism in human prostate cancer cells. Prostate 2012, 72, 612–625. [Google Scholar] [CrossRef] [PubMed]
- Qudjani, E.; Iman, M.; Davood, A.; Ramandi, M.F.; Shafiee, A. Design and synthesis of curcumin-like diarylpentanoid analogues as potential anticancer agents. Recent Pat. Anti-Cancer Drug Discov. 2016, 11, 342–351. [Google Scholar] [CrossRef]
- Cen, L.; Hutzen, B.; Ball, S.; DeAngelis, S.; Chen, C.-L.; Fuchs, J.R.; Li, C.; Li, P.-K.; Lin, J. New structural analogues of curcumin exhibit potent growth suppressive activity in human colorectal carcinoma cells. BMC Cancer 2009, 9, 99. [Google Scholar] [CrossRef]
- Kudo, C.; Yamakoshi, H.; Sato, H.; Ohori, H.; Ishioka, C.; Iwabuchi, Y.; Shibata, H. Synthesis of 86 species of 1,5-diaryl-3-oxo-1,4-pentadienes analogs of curcumin can yield a good lead in vivo. BMC Pharm. 2011, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Moore, T.W.; Lin, X.; Morii, N.; Mancini, A.; Howard, R.B.; Culver, D.; Arrendale, R.F.; Reddy, P.; Evers, T.J.; et al. Synthetic curcumin analog ef31 inhibits the growth of head and neck squamous cell carcinoma xenografts. Integr. Biol. 2012, 4, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Hutzen, B.; Friedman, L.; Sobo, M.; Lin, L.; Cen, L.; De Angelis, S.; Yamakoshi, H.; Shibata, H.; Iwabuchi, Y.; Lin, J. Curcumin anlogue go-y030 inhibits stat3 activity and cell growth in breast and pancreatic carcinomas. Int. J. Oncol. 2009, 35, 867–872. [Google Scholar] [PubMed]
- Lin, L.; Liu, Y.; Li, P.-K.; Fuchs, J.; Shibata, H.; Iwabuchi, Y.; Lin, J. Targeting colon cancer stem cells using a new curcumin analogue, go-y030. Br. J. Cancer 2011, 105, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.; Kudo, C.; Yamakoshi, H.; Uehara, Y.; Ohori, H.; Ishioka, C.; Iwabuchi, Y.; Shibata, H. Curcumin analog go-y030 is a novel inhibitor of ikkbeta that suppresses nf-kappab signaling and induces apoptosis. Cancer Sci. 2011, 102, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
- Friedman, L.; Lin, L.; Ball, S.; Bekaii-Saab, T.; Fuchs, J.; Li, P.K.; Li, C.; Lin, J. Curcumin analogues exhibit enhanced growth suppressive activity in human pancreatic cancer cells. Anti-Cancer Drugs 2009, 20, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Selvendiran, K.; Ahmed, S.; Dayton, A.; Kuppusamy, M.L.; Tazi, M.; Bratasz, A.; Tong, L.; Rivera, B.K.; Kalai, T.; Hideg, K.; et al. Safe and targeted anticancer efficacy of a novel class of antioxidant-conjugated difluorodiarylidenyl piperidones: Differential cytotoxicity in healthy and cancer cells. Free Radic. Biol. Med. 2010, 48, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- Selvendiran, K.; Tong, L.; Bratasz, A.; Kuppusamy, M.L.; Ahmed, S.; Ravi, Y.; Trigg, N.J.; Rivera, B.K.; Kalai, T.; Hideg, K.; et al. Anticancer efficacy of a difluorodiarylidenyl piperidone (ho-3867) in human ovarian cancer cells and tumor xenografts. Mol. Cancer Ther. 2010, 9, 1169–1179. [Google Scholar] [CrossRef]
- Tan, X.; Sidell, N.; Mancini, A.; Huang, R.P.; Shenming, W.; Horowitz, I.R.; Liotta, D.C.; Taylor, R.N.; Wieser, F. Multiple anticancer activities of ef24, a novel curcumin analog, on human ovarian carcinoma cells. Reprod. Sci. 2010, 17, 931–940. [Google Scholar] [CrossRef]
- Subramaniam, D.; May, R.; Sureban, S.M.; Lee, K.B.; George, R.; Kuppusamy, P.; Ramanujam, R.P.; Hideg, K.; Dieckgraefe, B.K.; Houchen, C.W.; et al. Diphenyl difluoroketone: A curcumin derivative with potent in vivo anticancer activity. Cancer Res. 2008, 68, 1962–1969. [Google Scholar] [CrossRef]
- Selvendiran, K.; Tong, L.; Vishwanath, S.; Bratasz, A.; Trigg, N.J.; Kutala, V.K.; Hideg, K.; Kuppusamy, P. Ef24 induces g2/m arrest and apoptosis in cisplatin-resistant human ovarian cancer cells by increasing pten expression. J. Biol. Chem. 2007, 282, 28609–28618. [Google Scholar] [CrossRef] [PubMed]
- Kasinski, A.L.; Du, Y.; Thomas, S.L.; Zhao, J.; Sun, S.Y.; Khuri, F.R.; Wang, C.Y.; Shoji, M.; Sun, A.; Snyder, J.P.; et al. Inhibition of ikappab kinase-nuclear factor-kappab signaling pathway by 3,5-bis(2-flurobenzylidene)piperidin-4-one (ef24), a novel monoketone analog of curcumin. Mol. Pharmacol. 2008, 74, 654–661. [Google Scholar] [CrossRef]
- Thomas, S.L.; Zhao, J.; Li, Z.; Lou, B.; Du, Y.; Purcell, J.; Snyder, J.P.; Khuri, F.R.; Liotta, D.; Fu, H. Activation of the p38 pathway by a novel monoketone curcumin analog, ef24, suggests a potential combination strategy. Biochem. Pharmacol. 2010, 80, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Olivera, A.; Moore, T.W.; Hu, F.; Brown, A.P.; Sun, A.; Liotta, D.C.; Snyder, J.P.; Yoon, Y.; Shim, H.; Marcus, A.I.; et al. Inhibition of the nf-kappab signaling pathway by the curcumin analog, 3,5-bis(2-pyridinylmethylidene)-4-piperidone (ef31): Anti-inflammatory and anti-cancer properties. Int. Immunopharmacol. 2012, 12, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Karin, M.; Cao, Y.; Greten, F.R.; Li, Z.W. Nf-kappab in cancer: From innocent bystander to major culprit. Nat. Rev. Cancer 2002, 2, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Guttridge, D.C.; Albanese, C.; Reuther, J.Y.; Pestell, R.G.; Baldwin, A.S., Jr. Nf-kappab controls cell growth and differentiation through transcriptional regulation of cyclin d1. Mol. Cell. Biol. 1999, 19, 5785–5799. [Google Scholar] [CrossRef]
- Wang, C.Y.; Cusack, J.C., Jr.; Liu, R.; Baldwin, A.S., Jr. Control of inducible chemoresistance: Enhanced anti-tumor therapy through increased apoptosis by inhibition of nf-kappab. Nat. Med. 1999, 5, 412–417. [Google Scholar] [CrossRef]
- Rawlings, J.S.; Rosler, K.M.; Harrison, D.A. The jak/stat signaling pathway. J. Cell Sci. 2004, 117, 1281–1283. [Google Scholar] [CrossRef]
- Aaronson, D.S.; Horvath, C.M. A road map for those who don’t know jak-stat. Science 2002, 296, 1653–1655. [Google Scholar] [CrossRef]
- Calo, V.; Migliavacca, M.; Bazan, V.; Macaluso, M.; Buscemi, M.; Gebbia, N.; Russo, A. Stat proteins: From normal control of cellular events to tumorigenesis. J. Cell. Physiol. 2003, 197, 157–168. [Google Scholar] [CrossRef]
- Gao, T.; Furnari, F.; Newton, A.C. Phlpp: A phosphatase that directly dephosphorylates akt, promotes apoptosis, and suppresses tumor growth. Mol. Cell 2005, 18, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Ye, K. Pike/nuclear pi 3-kinase signaling in preventing programmed cell death. J. Cell Biochem. 2005, 96, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Steelman, L.S.; Abrams, S.L.; Whelan, J.; Bertrand, F.E.; Ludwig, D.E.; Basecke, J.; Libra, M.; Stivala, F.; Milella, M.; Tafuri, A.; et al. Contributions of the raf/mek/erk, pi3k/pten/akt/mtor and jak/stat pathways to leukemia. Leukemia 2008, 22, 686–707. [Google Scholar] [CrossRef] [PubMed]
- Courtney, K.D.; Corcoran, R.B.; Engelman, J.A. The pi3k pathway as drug target in human cancer. J. Clin. Oncol. 2010, 28, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Selvendiran, K.; Kuppusamy, M.L.; Bratasz, A.; Tong, L.; Rivera, B.K.; Rink, C.; Sen, C.K.; Kalai, T.; Hideg, K.; Kuppusamy, P. Inhibition of vascular smooth-muscle cell proliferation and arterial restenosis by ho-3867, a novel synthetic curcuminoid, through up-regulation of pten expression. J. Pharmacol. Exp. Ther. 2009, 329, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Wagner, E.F.; Nebreda, A.R. Signal integration by jnk and p38 mapk pathways in cancer development. Nat. Rev. Cancer 2009, 9, 537–549. [Google Scholar] [CrossRef]
- Wei, X.; Du, Z.-Y.; Zheng, X.; Cui, X.-X.; Conney, A.H.; Zhang, K. Synthesis and evaluation of curcumin-related compounds for anticancer activity. Eur. J. Med. Chem. 2012, 53, 235–245. [Google Scholar] [CrossRef]
- Saraste, A.; Pulkki, K. Morphologic and biochemical hallmarks of apoptosis. Cardiovasc. Res. 2000, 45, 528–537. [Google Scholar] [CrossRef]
- Lazebnik, Y.A.; Cole, S.; Cooke, C.A.; Nelson, W.G.; Earnshaw, W.C. Nuclear events of apoptosis in vitro in cell-free mitotic extracts: A model system for analysis of the active phase of apoptosis. J. Cell Biol. 1993, 123, 7–22. [Google Scholar] [CrossRef]
- Ohori, H.; Yamakoshi, H.; Tomizawa, M. Synthesis and biological analysis of new curcumin analogues bearing an enhanced potential for the medicinal treatment of cancer. Mol. Cancer Ther. 2006, 5, 2563–2571. [Google Scholar] [CrossRef]

 ’ symbol, while induction is represented by a green ‘+’ symbol.
’ symbol, while induction is represented by a green ‘+’ symbol.
 ’ symbol, while induction is represented by a green ‘+’ symbol.
’ symbol, while induction is represented by a green ‘+’ symbol.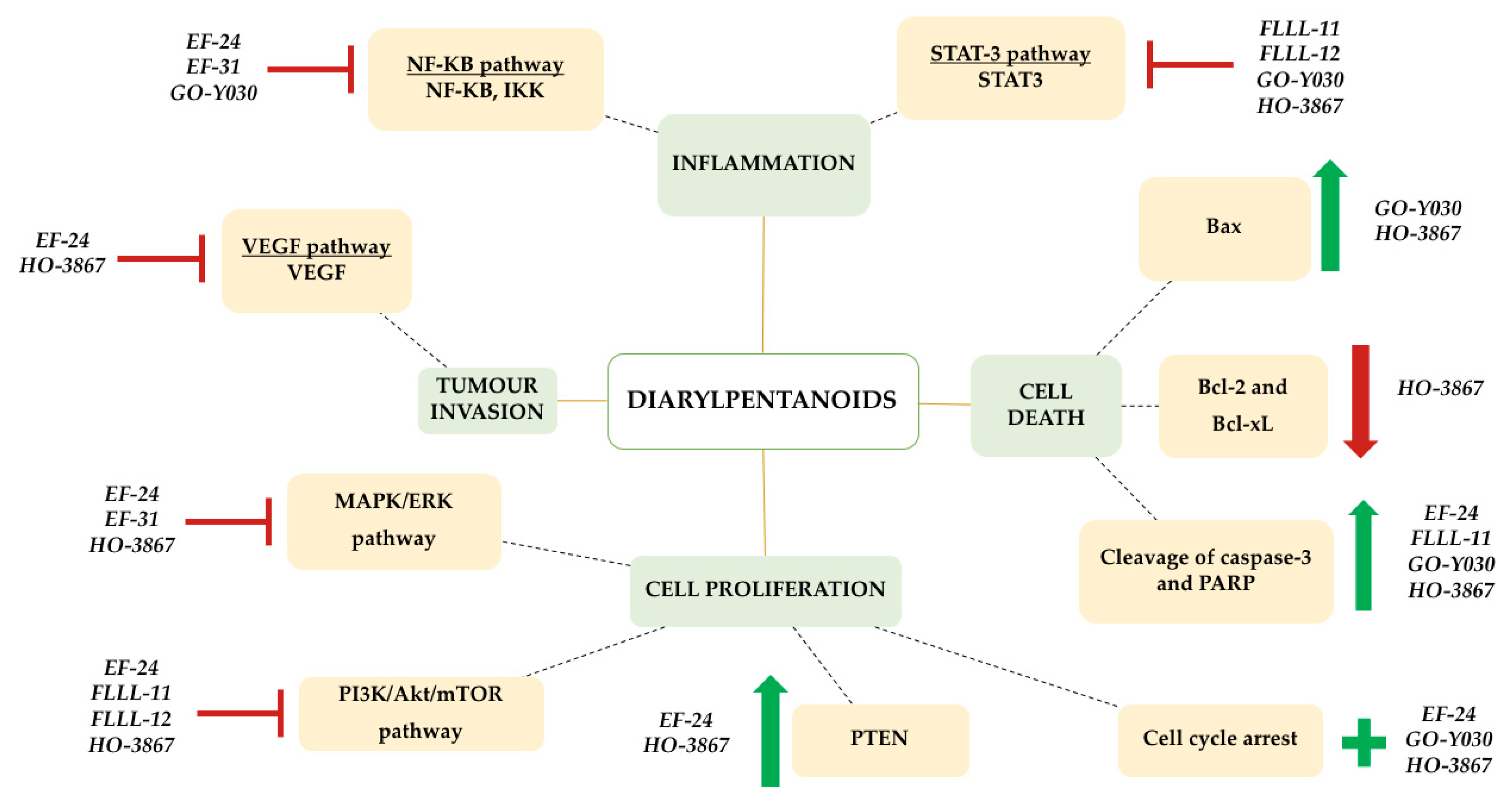
| DAP | Human Cancer Cell Line (In Vitro) | Source | In Vivo | Proposed Modulated Molecular Pathways | Reference |
|---|---|---|---|---|---|
| GO-Y030 | SW480 HT29 HCT 116 | Colorectal Colorectal Colorectal | - | NF-κB, STAT3, Cell cycle arrest and apoptotic pathways | [42] |
| HCT116 | Colorectal | - | [43] | ||
| HCT116 | Colorectal | - | [44] | ||
| MDA-MB-231 | Breast | - | [45] | ||
| ALDH+/CD133+ stem cells from cell lines SW480, HCT-116, DLD-1 and HT29 | Colorectal Cancer stem cells | - | [46] | ||
| SW620 SH-10-TC MCF7 PC3 PK-1 8505c HuCCT-1 | Colon Stomach Breast Prostate Pancreas Thyroid Bile duct | - | [47] | ||
| FLLL-11 | SW480 HT29 HCT 116 | Colorectal Colorectal Colorectal | - | STAT3, PI3K/PTEN/Akt/mTOR, cell cycle arrest and apoptotic pathways | [42] |
| PANC-1 BXPC-3 MIA-PACA-2 ASPC-1 HPAC | Pancreas Pancreas Pancreas Pancreas Pancreas | - | [48] | ||
| FLLL-12 | SW480 HT29 HCT 116 | Colorectal Colorectal Colorectal | - | STAT3, PI3K/PTEN/Akt/mTOR, cell cycle arrest and apoptotic pathways | [42] |
| PANC-1 BXPC-3 MIA-PACA-2 ASPC-1 HPAC | Pancreas Pancreas Pancreas Pancreas Pancreas | - | [48] | ||
| HO-3867 | A2780 A2780R MCF7 HCT116 PC3 HepG2 A549 SCC4 | Ovarian Ovarian Breast Colorectal Prostate Liver Lung Squamous Cell | - | STAT3, PI3K/PTEN/Akt/mTOR, MAPK/ERK pathway VEGF signalling, cell cycle arrest and apoptotic pathways | [49] |
| A2780 SKOV3 OV4 OVCAR3 | Ovarian Ovarian Ovarian Ovarian | Human ovarian xenograft (A2780) grown in back of BALB/C nude mice | [50] | ||
| EF24 | IGROV1 SK-OV-3 | Ovarian Ovarian | - | NF-κB, PI3K/PTEN/Akt/mTOR, MAPK/ERK pathway, VEGF signalling, cell cycle arrest and apoptotic pathways | [51] |
| HCT-116 HT-29 AGS | Colorectal Colorectal Stomach | HCT-116 colon cancer xenografts established in athymic nude mice | [52] | ||
| A2780R | Ovarian | - | [53] | ||
| A549, H460 Calu-1 1A9 MDA-MB-231 HeLa | Lung Lung Ovarian Breast Cervical | - | [54] | ||
| A549 | Lung | - | [55] | ||
| EF31 | A2780 MDA-MB-231 | Ovarian Breast | - | NF-κB, MAPK/ERK pathway | [56] |
| Human head and neck squamous cell carcinoma Tu212 xenograft tumors established in athymic nude mice | [44] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paulraj, F.; Abas, F.; H. Lajis, N.; Othman, I.; Naidu, R. Molecular Pathways Modulated by Curcumin Analogue, Diarylpentanoids in Cancer. Biomolecules 2019, 9, 270. https://doi.org/10.3390/biom9070270
Paulraj F, Abas F, H. Lajis N, Othman I, Naidu R. Molecular Pathways Modulated by Curcumin Analogue, Diarylpentanoids in Cancer. Biomolecules. 2019; 9(7):270. https://doi.org/10.3390/biom9070270
Chicago/Turabian StylePaulraj, Felicia, Faridah Abas, Nordin H. Lajis, Iekhsan Othman, and Rakesh Naidu. 2019. "Molecular Pathways Modulated by Curcumin Analogue, Diarylpentanoids in Cancer" Biomolecules 9, no. 7: 270. https://doi.org/10.3390/biom9070270
APA StylePaulraj, F., Abas, F., H. Lajis, N., Othman, I., & Naidu, R. (2019). Molecular Pathways Modulated by Curcumin Analogue, Diarylpentanoids in Cancer. Biomolecules, 9(7), 270. https://doi.org/10.3390/biom9070270





