Maternal Leucine-Rich Diet Minimises Muscle Mass Loss in Tumour-bearing Adult Rat Offspring by Improving the Balance of Muscle Protein Synthesis and Degradation
Abstract
1. Introduction
2. Materials and Methods
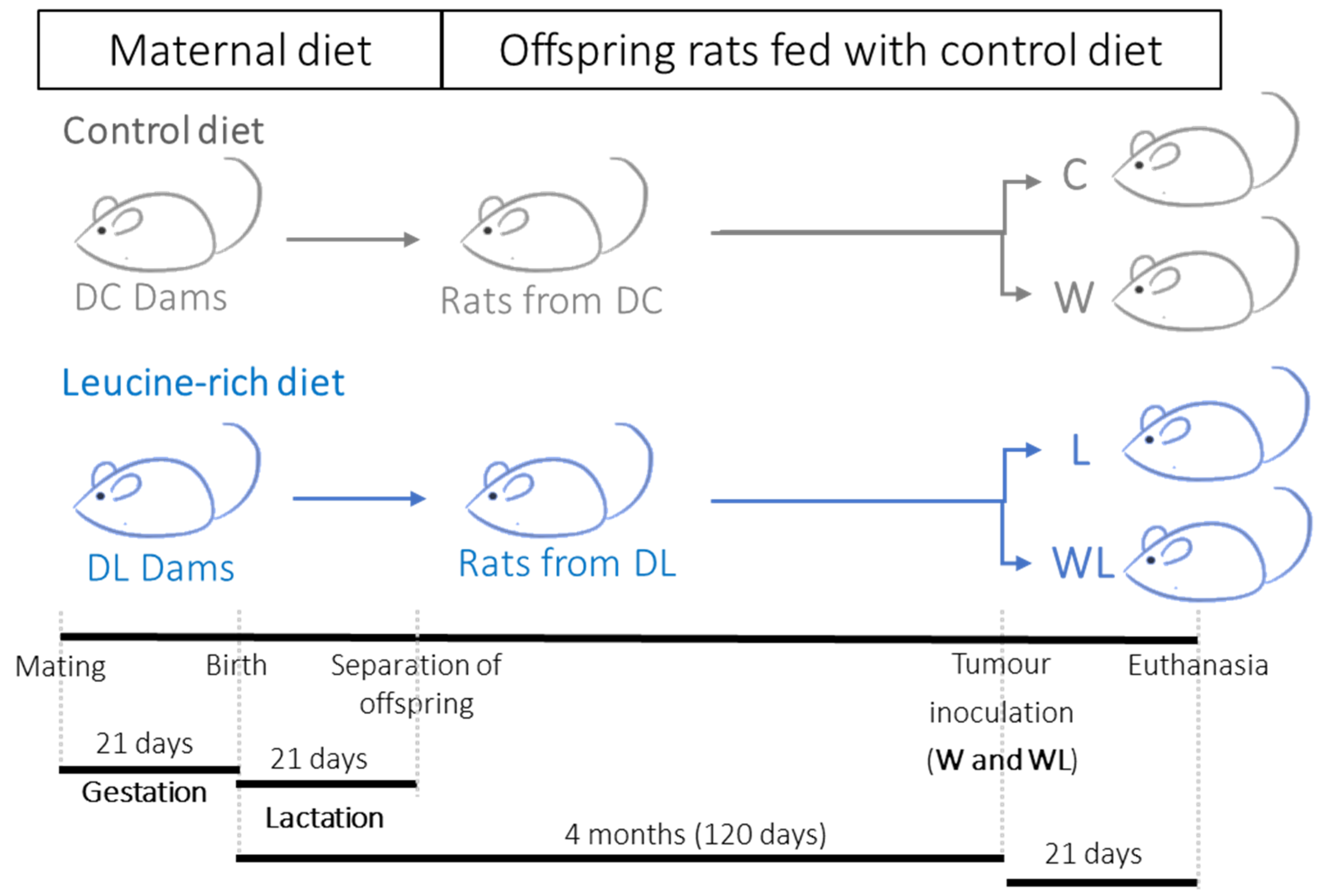
2.1. Animals
2.2. Diet
2.3. Experimental Procedure
2.4. Cachexia Indicators: Serum and Morphometric Parameters
2.5. Muscle Protein Degradation and Synthesis Analysis
2.6. Enzymatic Assays
2.7. Western Blot Assay
2.8. Statistical Analyses
3. Results
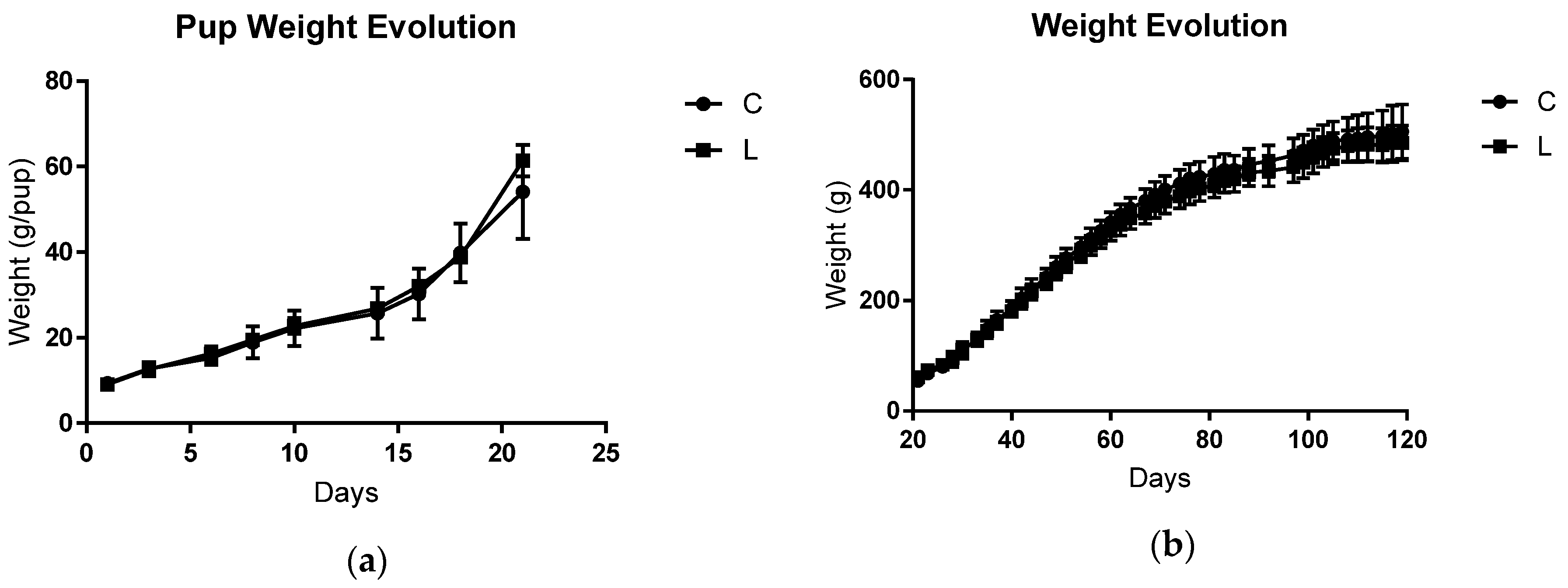
3.1. Maternal and Offspring Weight Gain, Food Intake, and Cachexia Indicators after Tumoural Evolution
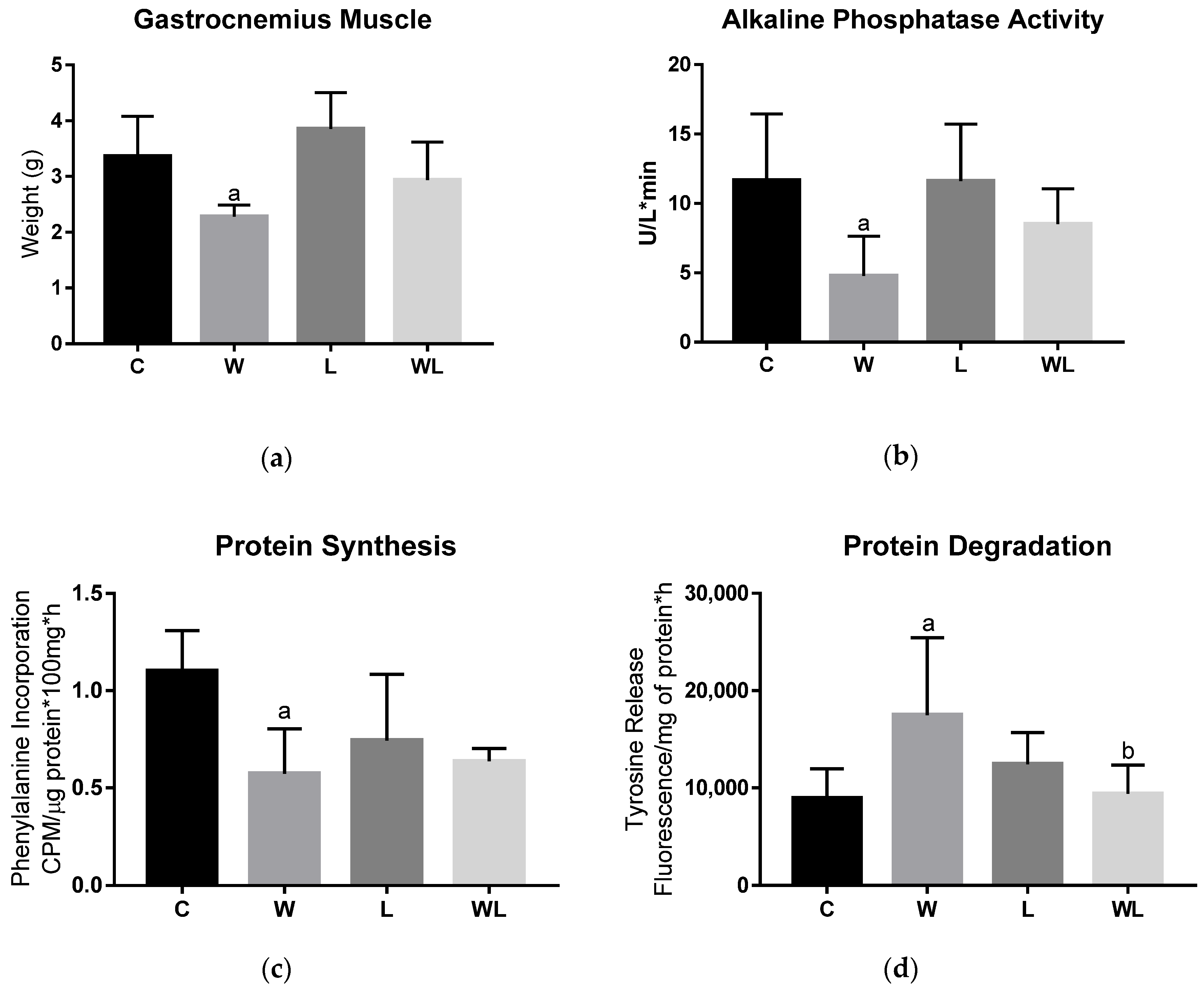
3.2. Maternal Leucine-Rich Diet Attenuates the Effects of Tumour Evolution Over the Gastrocnemius Muscle Protein Synthesis and Degradation
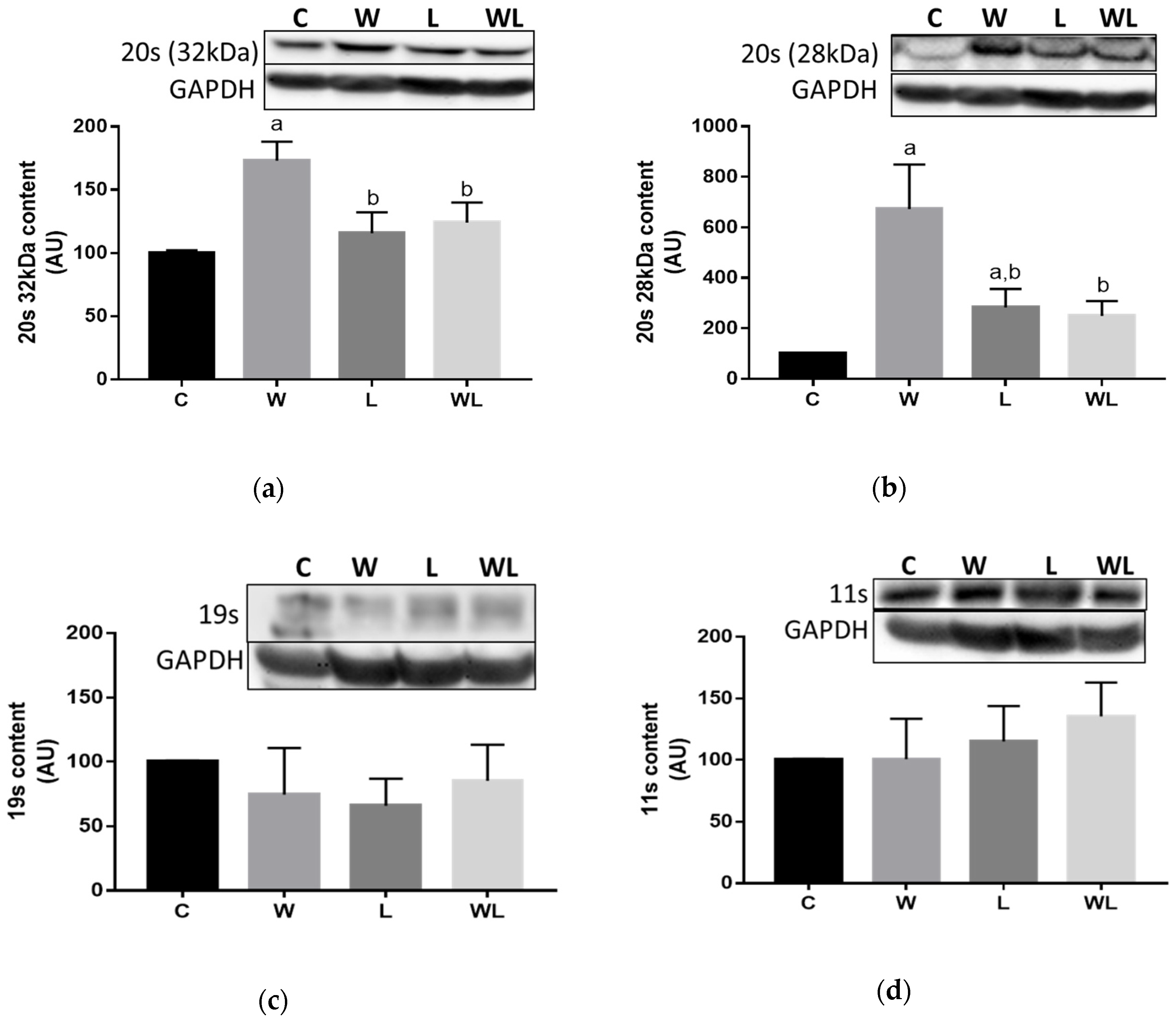
3.3. Proteasome 20S Subunit Expression and Calpain and Cathepsin Activities Were Modulated in the Rats with Maternal Leucine Supplementation
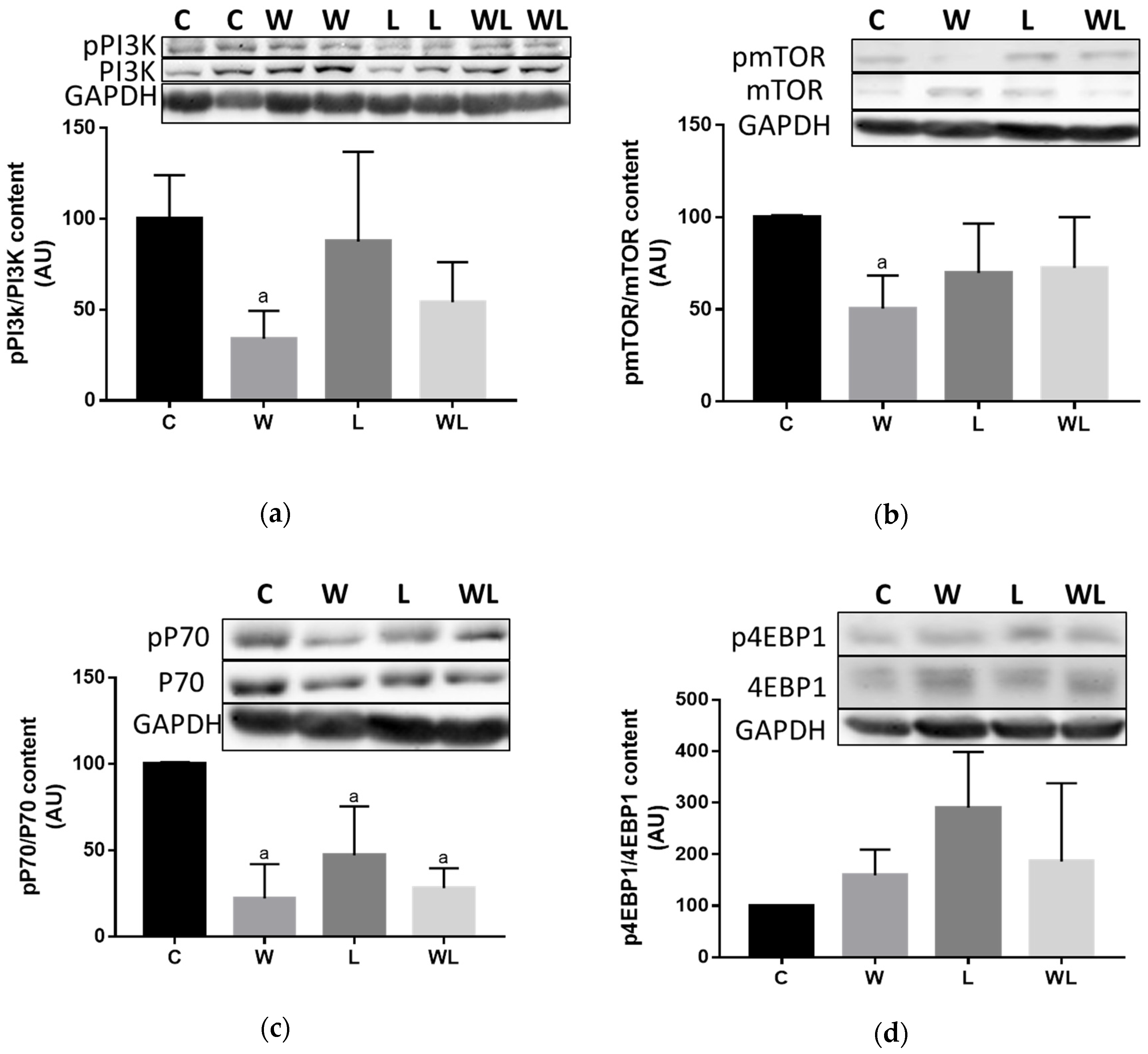
3.4. Maternal Leucine-Rich Diet Changed the mTor Muscle Protein Synthesis-Related Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Argilés, J.M.; Stemmler, B.; López-Soriano, F.J.; Busquets, S. Inter-tissue communication in cancer cachexia. Nat. Rev. Endocrinol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Remely, M.; Stefanska, B.; Lovrecic, L.; Magnet, U.; Haslberger, A.G. Nutriepigenomics. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S. Impact of maternal diet on the epigenome during in utero life and the developmental programming of diseases in childhood and adulthood. Nutrients 2015, 7, 9492–9507. [Google Scholar] [CrossRef] [PubMed]
- Mathias, P.C.F.; Elmhiri, G.; De Oliveira, J.C.; Delayre-Orthez, C.; Barella, L.F.; Tófolo, L.P.; Fabricio, G.S.; Chango, A.; Abdennebi-Najar, L. Maternal diet, bioactive molecules, and exercising as reprogramming tools of metabolic programming. Eur. J. Nutr. 2014, 53, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Mabasa, L.; Cho, K.; Walters, M.W.; Bae, S.; Park, C.S. Maternal dietary canola oil suppresses growth of mammary carcinogenesis in female rat offspring. Nutr. Cancer 2013, 65, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Porporato, P.E. Understanding cachexia as a cancer metabolism syndrome. Oncogenesis 2016, 5, e200. [Google Scholar] [CrossRef]
- Tisdale, M.J. Mechanisms of cancer cachexia. Physiol. Rev. 2009, 89, 381–410. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef]
- Sudhan, D.R.; Siemann, D.W. Cathepsin L targeting in cancer treatment. Pharmacol. Ther. 2015, 155, 105–116. [Google Scholar] [CrossRef]
- Smith, I.J.; Aversa, Z.; Hasselgren, P.-O.; Pacelli, F.; Rosa, F.; Doglietto, G.B.; Bossola, M. CALPAIN activity is increased in skeletal muscle from gastric cancer patients with no or minimal weight loss. Muscle Nerve 2011, 43, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Cruz, B.; Oliveira, A.; Gomes-Marcondes, M.C.C. L-leucine dietary supplementation modulates muscle protein degradation and increases pro-inflammatory cytokines in tumour-bearing rats. Cytokine 2017, 96, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Ventrucci, G.; Mello, M.A.R.; Gomes-Marcondes, M.C.C. Proteasome activity is altered in skeletal muscle tissue of tumour-bearing rats fed a leucine-rich diet. Endocr. Relat. Cancer 2004, 11, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Ventrucci, G.; Mello, M.A.R.; Gomes-Marcondes, M.C.C. Leucine-rich diet alters the eukaryotic translation initiation factors expression in skeletal muscle of tumour-bearing rats. BMC Cancer 2007, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, E.M.; Salomão, E.M.; Gomes-Marcondes, M.C.C. Leucine modulates the effect of Walker factor, a proteolysis-inducing factor-like protein from Walker tumours, on gene expression and cellular activity in C2C12 myotubes. Cytokine 2013, 64, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Cruz, B.L.G.; da Silva, P.C.; Tomasin, R.; Oliveira, A.G.; Viana, L.R.; Salomao, E.M.; Gomes-Marcondes, M.C.C. Dietary leucine supplementation minimises tumour-induced damage in placental tissues of pregnant, tumour-bearing rats. BMC Cancer 2016, 16, 58. [Google Scholar] [CrossRef]
- Regnault, T.R.H.; De Vrijer, B.; Battaglia, F.C. Transport and Metabolism of Amino Acids in Placenta. Endocrine 2002, 19, 23–41. [Google Scholar] [CrossRef]
- Melnik, B.C. Milk—A Nutrient System of Mammalian Evolution Promoting mTORC1-Dependent Translation. Int. J. Mol. Sci. 2015, 17048–17087. [Google Scholar] [CrossRef]
- Viana, L.R.; Gomes-Marcondes, M.C.C. Leucine-Rich Diet Improves the Serum Amino Acid Profile and Body Composition of Fetuses from Tumor-Bearing Pregnant Mice1. Biol. Reprod. 2013, 88, 1–8. [Google Scholar] [CrossRef]
- Miyaguti, N.A.d.S.; de Oliveira, S.C.P.; Gomes-Marcondes, M.C.C. Maternal nutritional supplementation with fish oil and/or leucine improves hepatic function and antioxidant defenses, and minimizes cachexia indexes in Walker-256 tumor-bearing rats offspring. Nutr. Res. 2018, 51, 29–39. [Google Scholar] [CrossRef]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Vale, C.; Stewart, L.; Tierney, J. Trends in UK cancer trials: Results from the UK Coordinating Committee for Cancer Research National Register of Cancer Trials. Br. J. Cancer 2005, 92, 811–814. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Baker, D.E.J. Reproduction and breeding. In The Laboratory Rat; Baker, H.J., Lindsey, J.R., Weisbroth, S.H., Eds.; Academic Press: New York, NY, USA, 1979; pp. 153–168. [Google Scholar]
- Gomes-Marcondes, M.C.C.; Cury, L.; Curi, R. Consequences of Walker 256 tumour growth for the placental/foetal development in rats. Cancer Res. Ther. Control 1998, 5, 277–283. [Google Scholar]
- Viana, L.R.; Canevarolo, R.; Luiz, A.C.P.; Soares, R.F.; Lubaczeuski, C.; de Mattos Zeri, A.C.; Gomes-Marcondes, M.C.C. Leucine-rich diet alters the (1)H-NMR based metabolomic profile without changing the Walker-256 tumour mass in rats. BMC Cancer 2016, 16, 764. [Google Scholar] [CrossRef] [PubMed]
- Lorite, M.J.; Thompson, M.G.; Drake, J.L.; Carling, G.; Tisdale, M.J. Mechanism of muscle protein degradation induced by a cancer cachectic factor. Br. J. Cancer 1998, 78, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Waalkes, T.P.; Udenfriend, S.A. A fluorometric method for the estimation of tyrosine in plasma and tissues. J. Lab. Clin. Med. 1957, 50, 733–736. [Google Scholar] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Buroker-Kilgore, M.; Wang, K.K.W. A coomassie brilliant blue G-250-based colorimetric assay for measuring activity of calpain and other proteases. Anal. Biochem. 1993, 208, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Orino, E.; Tanaka, K.; Tamura, T.; Sone, S.; Ogura, T.; Ichihara, A. ATP-dependent reversible association of proteasomes with multiple protein components to form 26S complexes that degrade ubiquitinated proteins in human HL-60 cells. FEBS Lett. 1991, 284, 206–210. [Google Scholar] [CrossRef]
- Barrett, A.J. Fluorimetric assays for cathepsin B and cathepsin H with methylcoumarylamide substrates. Biochem. J. 1980, 187, 909–912. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Gad, S.C.; Weil, C.S. Statistics for toxicologists. In Principles and Methods of Toxicology; WALLAC Ltd.: New York, NY, USA, 1994. [Google Scholar]
- Fearon, K.C.; Voss, A.C.; Hustead, D.S. Definition of cancer cachexia: Effect of weight loss, reduced food intake, and systemic inflammation on functional status and prognosis. Am. J. Clin. Nutr. 2006, 83, 1345–1350. [Google Scholar] [CrossRef]
- Tisdale, M.J. Cachexia in cancer patients. Nat. Rev. Cancer 2002, 2, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Cruz, B.; Gomes-Marcondes, M.C.C. Leucine-rich diet supplementation modulates foetal muscle protein metabolism impaired by Walker-256 tumour. Reprod. Biol. Endocrinol. 2014, 12, 2. [Google Scholar] [CrossRef]
- Martins, M.J.; Negrão, M.R.; Hipólito-Reis, C. Alkaline phosphatase from rat liver and kidney is differentially modulated. Clin. Biochem. 2001, 34, 463–468. [Google Scholar] [CrossRef]
- Fearon, K.C.H.; Glass, D.J.; Guttridge, D.C. Cancer cachexia: Mediators, signaling, and metabolic pathways. Cell MeTable 2012, 16, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Lecker, S.H.; Solomon, V.; Mitch, W.E.; Goldberg, A.L. Muscle Protein Breakdown and the Critical Role of the Ubiquitin- Proteasome Pathway in Normal and Disease States. J. Nutr. 1999, 129, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Tardif, N.; Klaude, M.; Lundell, L.; Thorell, A.; Rooyackers, O. Autophagic-Lysosomal pathway is the main proteolytic system modified in the skeletal muscle of esophageal cancer patients1-3. Am. J. Clin. Nutr. 2013, 98, 1485–1492. [Google Scholar] [CrossRef] [PubMed]
- Op den Kamp, C.M.; Langen, R.C.; Snepvangers, F.J.; De Theije, C.C.; Schellekens, J.M.; Laugs, F.; Dingemans, A.C.; Schols, A.M. Nuclear transcription factor k B activation and protein turnover adaptations in skeletal muscle of patients with progressive stages of lung cancer cachexia. Am. J. Clin. Nutr. 2013, 98, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Costelli, P.; Reffo, P.; Penna, F.; Autelli, R.; Bonelli, G.; Baccino, F.M. Ca2+-dependent proteolysis in muscle wasting. Int. J. Biochem. Cell Biol. 2005, 37, 2134–2146. [Google Scholar] [CrossRef] [PubMed]
- Salomão, E.M.; Toneto, A.T.; Silva, G.O.; Gomes-, M.C.C.; Gomes-marcondes, M.C.C. Physical Exercise and a Leucine-Rich Diet Modulate the Muscle Protein Metabolism in Walker Tumor-Bearing Rats. Nutr. Cancer 2010, 37–41. [Google Scholar] [CrossRef]
- Inaba, S.; Hinohara, A.; Tachibana, M.; Tsujikawa, K.; Fukada, S. Muscle regeneration is disrupted by cancer cachexia without loss of muscle stem cell potential. PLoS ONE 2018, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]

| Parameters | DC | DL | |
|---|---|---|---|
| Gestation | Initial Food Intake (g) | 18.52 ± 3.14 | 18.02 ± 0.53 |
| Final Food Intake (g) | 19.12 ± 4.14 | 15.12 ± 2.39 | |
| Body Weight Gain (g) | 125.30±13.35 | 97.33 ± 12.44 | |
| Lactation | Initial Food Intake (g) | 20.43 ± 1.53 | 20.36 ± 3.50 |
| Final Food Intake (g) | 28.86 ± 3.37 | 34.29 ± 0.75 | |
| Body Weight Gain (g) | 42.67 ± 6.69 | 33.33 ± 3.84 |
| Morphometric and Serum Parameters | C | W | L | WL |
|---|---|---|---|---|
| Initial body weight (g) | 514.80 ± 14.13 | 531.39 ± 21.66 | 518.06 ± 10.55 | 497.35 ± 10.89 |
| Carcass weight (g) | 528.41 ± 15.20 | 458.47 ± 21.21 a | 524.00 ± 12.06 | 414.31 ± 13.45 c |
| Body weight gain (%) | 104.56 ± 3.99 | 88.10 ± 1.78 a | 101.24 ± 1.96 | 83.64 ± 3.50 c |
| Initial food intake (g) | 21.48 ± 1.24 | 22.14 ± 1.03 | 23.63 ± 3.04 | 22.96 ± 1.23 |
| Final food intake (g) | 20.52 ± 1.27 | 8.46 ± 1.08 a | 18.08 ± 0.24 | 12.60 ± 0.71 b, c |
| Tumour weight (g) | - | 71.70 ± 6.94 | - | 80.92 ± 5.54 |
| Total Protein (g/dL) | 5.82 ± 0.28 | 4.32 ± 0.12 a | 5.14 ± 0.18 | 4.25 ± 0.07 c |
| Albumin (g/dL) | 3.28 ± 0.07 | 2.81 ± 0.06 a | 3.19 ± 0.07 | 2.80 ± 0.06 c |
| A/G ratio | 1.43 ± 0.07 | 1.87 ± 0.06 a | 1.67 ± 0.01 | 1.82 ± 0.09 |
| Triglycerides (mg/dL) | 118.89 ± 4.95 | 165.39 ± 10.20 a | 125.18 ± 5.41 | 154.50 ± 8.01 |
| Cholesterol (mg/dL) | 67.05 ± 2.65 | 58.91 ± 0.58 a | 67.69 ± 2.14 | 57.28 ± 1.43 c |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyaguti, N.A.d.S.; Oliveira, S.C.P.d.; Gomes-Marcondes, M.C.C. Maternal Leucine-Rich Diet Minimises Muscle Mass Loss in Tumour-bearing Adult Rat Offspring by Improving the Balance of Muscle Protein Synthesis and Degradation. Biomolecules 2019, 9, 229. https://doi.org/10.3390/biom9060229
Miyaguti NAdS, Oliveira SCPd, Gomes-Marcondes MCC. Maternal Leucine-Rich Diet Minimises Muscle Mass Loss in Tumour-bearing Adult Rat Offspring by Improving the Balance of Muscle Protein Synthesis and Degradation. Biomolecules. 2019; 9(6):229. https://doi.org/10.3390/biom9060229
Chicago/Turabian StyleMiyaguti, Natália Angelo da Silva, Sarah Christine Pereira de Oliveira, and Maria Cristina Cintra Gomes-Marcondes. 2019. "Maternal Leucine-Rich Diet Minimises Muscle Mass Loss in Tumour-bearing Adult Rat Offspring by Improving the Balance of Muscle Protein Synthesis and Degradation" Biomolecules 9, no. 6: 229. https://doi.org/10.3390/biom9060229
APA StyleMiyaguti, N. A. d. S., Oliveira, S. C. P. d., & Gomes-Marcondes, M. C. C. (2019). Maternal Leucine-Rich Diet Minimises Muscle Mass Loss in Tumour-bearing Adult Rat Offspring by Improving the Balance of Muscle Protein Synthesis and Degradation. Biomolecules, 9(6), 229. https://doi.org/10.3390/biom9060229






