Nanoparticle Activation Methods in Cancer Treatment
Abstract
1. Introduction
1.1. Intrinsic Activation Due to Altered Cancer Cell Metabolism
1.1.1. pH-Activated Nanoparticles for Cancer Treatments
– Organ Specific Release
– Tissue Specific Release
– Cell Specific Release
1.1.2. Enzymatic Activation of Nanoparticles
– Cathepsins
– Matrix Metalloproteinases
– Glycosyl Hydrolases
– Protein Tyrosine Kinases
– Nicotinamide Adenine Dinucleotide Phosphate (NADPH) Dehydrogenases
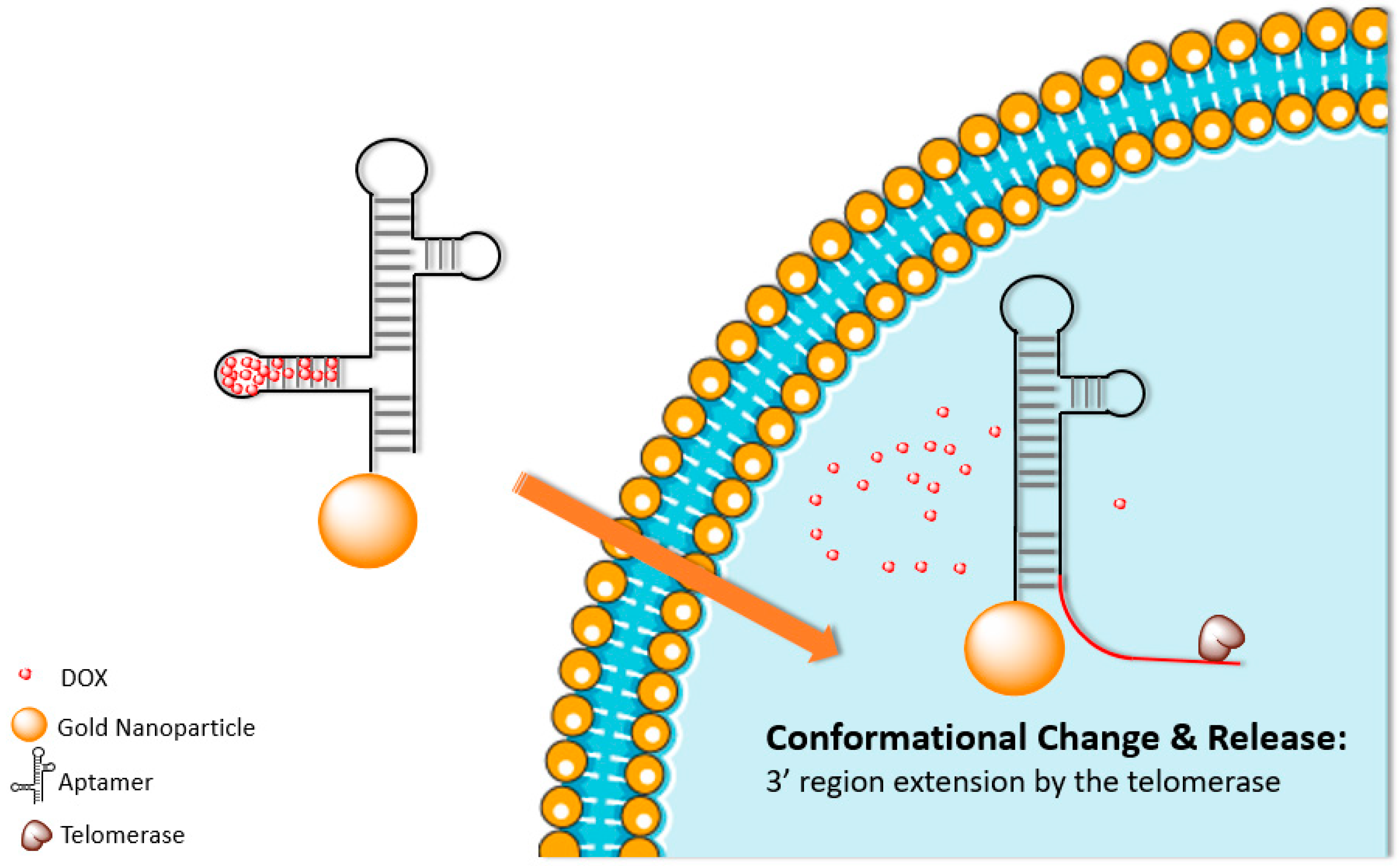
– Telomerases
– Dual Enzyme Activation
– Enzyme Loaded Nanoparticles
1.1.3. Concentration Dependent Activation
– Membrane Proteins
– Soluble Molecules
2. Extrinsic Activation
2.1. Ultrasound Activation
2.1.1. Sonoporation
2.1.2. Gas Filled Microbubbles
2.1.3. Combinatorial Ultrasound
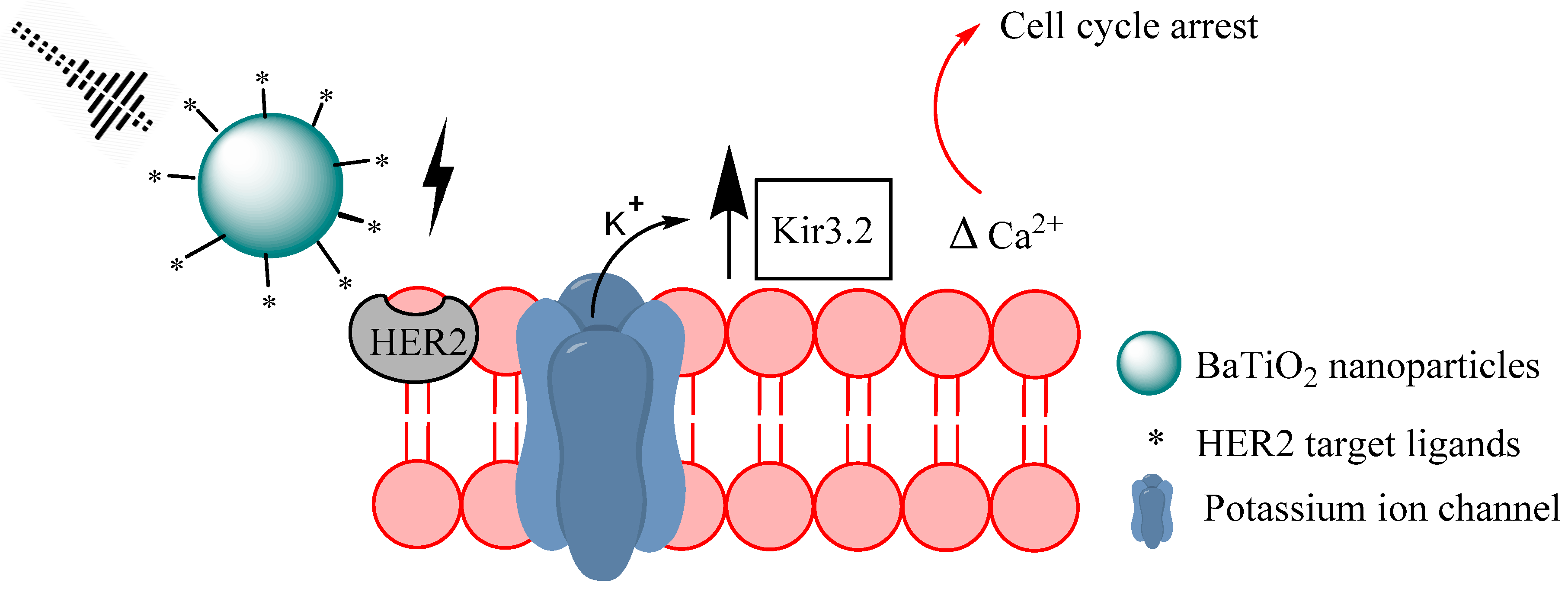
2.1.4. Piezoelectric Nanomaterials
2.2. Magnetic Field Activation
2.2.1. Magnetically Induced Hyperthermia
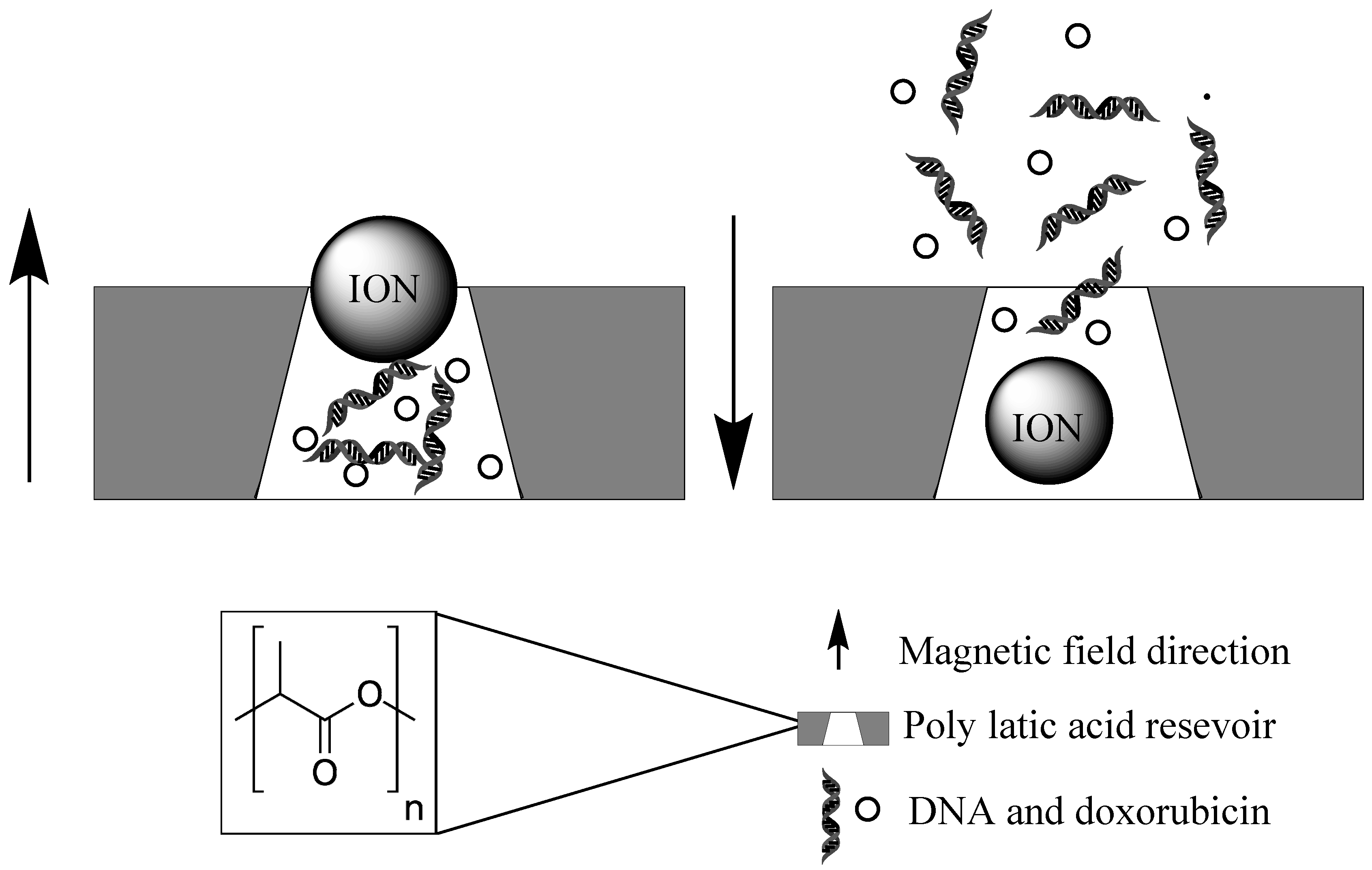
2.2.2. Magnetically Induced Localized Drug Release
2.2.3. Smart Stimulus Systems
2.3. Light Activation and Photodynamic Therapy
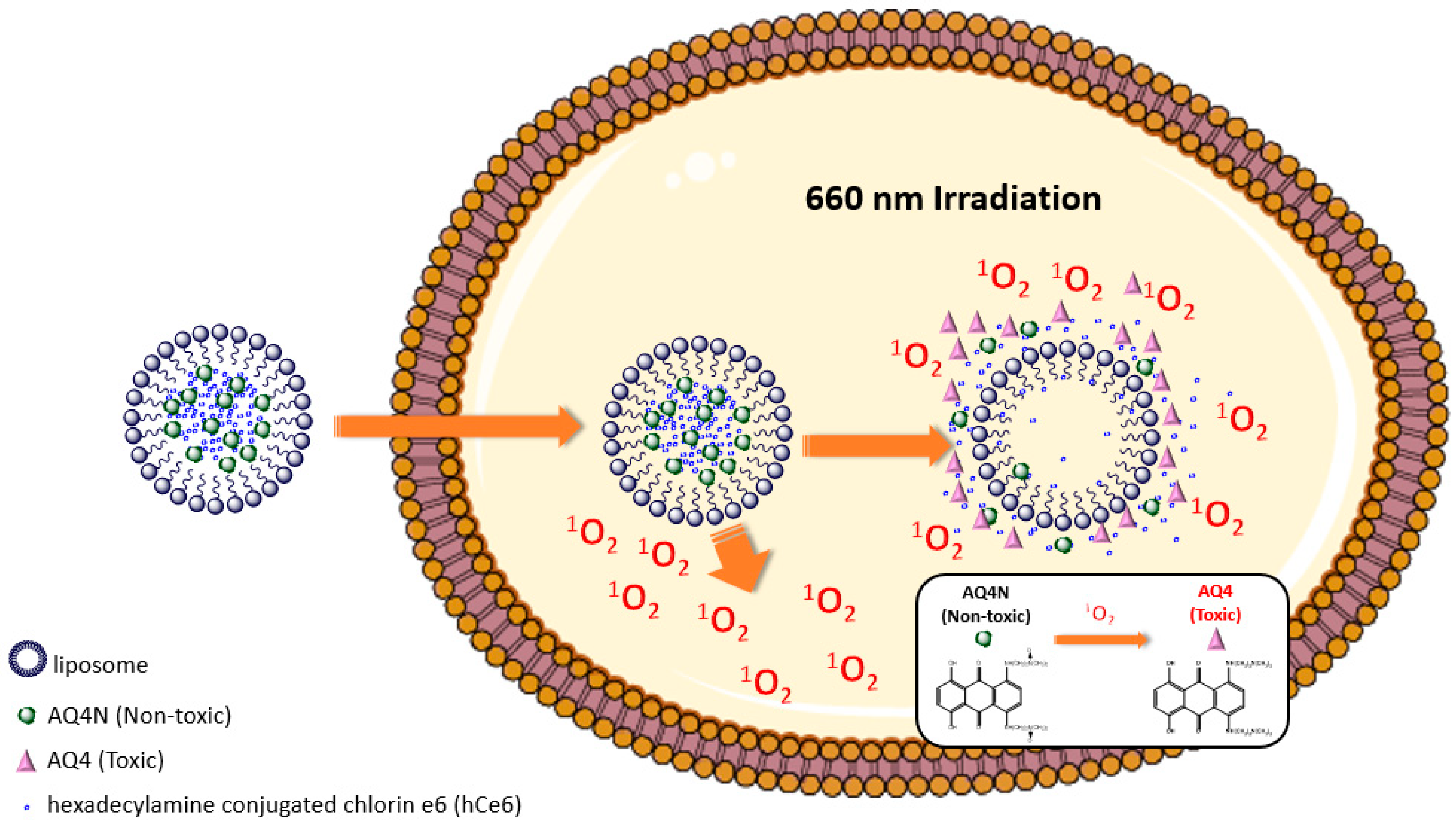
2.3.1. Near Infrared Light Activation
2.3.2. Visible Light Activation
2.3.3. X-ray Activation
3. Evaluation of Both Intrinsic and Extrinsic Activation Systems
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Boron, W.F. Regulation of intracellular pH. Adv. Physiol. Educ. 2004, 28, 160–179. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Ozawa, S.; Miyamoto, C.; Maehata, Y.; Suzuki, A.; Maeda, T.; Baba, Y. Acidic extracellular microenvironment and cancer. Cancer Cell Int. 2013, 13, 89. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-C.; Yu, D.-G.; Yang, M.-C. pH-sensitive polyelectrolyte complex gel microspheres composed of chitosan/sodium tripolyphosphate/dextran sulfate: Swelling kinetics and drug delivery properties. Colloids Surf. B Biointerfaces 2005, 44, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Q.; Zheng, L.S.; Guo, X.D.; Qian, Y.; Zhang, L.J. pH-Sensitive Micelles Self-Assembled from Amphiphilic Copolymer Brush for Delivery of Poorly Water-Soluble Drugs. Biomacromolecules 2011, 12, 116–122. [Google Scholar] [CrossRef]
- Gao, W.; Chan, J.; Farokhzad, O.C. pH-Responsive Nanoparticles for Drug Delivery. Mol. Pharm. 2010, 7, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Bradley, G.; Juranka, P.F.; Ling, V. Mechanism of multidrug resistance. Biochim. Biophys. Acta - Rev. Cancer 1988, 948, 87–128. [Google Scholar] [CrossRef]
- Wang, Z.; Deng, X.; Ding, J.; Zhou, W.; Zheng, X.; Tang, G. Mechanisms of drug release in pH-sensitive micelles for tumour targeted drug delivery system: A review. Int. J. Pharm. 2018, 535, 253–260. [Google Scholar] [CrossRef] [PubMed]
- El-Sherbiny, I.M.; Abdel-Mogib, M.; Dawidar, A.-A.M.; Elsayed, A.; Smyth, H.D.C. Biodegradable pH-responsive alginate-poly (lactic-co-glycolic acid) nano/micro hydrogel matrices for oral delivery of silymarin. Carbohydr. Polym. 2011, 83, 1345–1354. [Google Scholar] [CrossRef]
- Tian, B.; Liu, S.; Wu, S.; Lu, W.; Wang, D.; Jin, L.; Hu, B.; Li, K.; Wang, Z.; Quan, Z. pH-responsive poly (acrylic acid)-gated mesoporous silica and its application in oral colon targeted drug delivery for doxorubicin. Colloids Surf. B Biointerfaces 2017, 154, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-H.; Cheng, A.-L. Clinical studies with Curcumin. In The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease; Springer: Boston, MA, USA, 2007; pp. 471–480. [Google Scholar]
- Cui, J.; Yu, B.; Zhao, Y.; Zhu, W.; Li, H.; Lou, H.; Zhai, G. Enhancement of oral absorption of curcumin by self-microemulsifying drug delivery systems. Int. J. Pharm. 2009, 371, 148–155. [Google Scholar] [CrossRef]
- Raghunand, N.; He, X.; van Sluis, R.; Mahoney, B.; Baggett, B.; Taylor, C.W.; Paine-Murrieta, G.; Roe, D.; Bhujwalla, Z.M.; Gillies, R.J. Enhancement of chemotherapy by manipulation of tumour pH. Br. J. Cancer 1999, 80, 1005–1011. [Google Scholar] [CrossRef]
- Peppicelli, S.; Andreucci, E.; Ruzzolini, J.; Laurenzana, A.; Margheri, F.; Fibbi, G.; Del Rosso, M.; Bianchini, F.; Calorini, L. The acidic microenvironment as a possible niche of dormant tumor cells. Cell. Mol. Life Sci. 2017, 74, 2761–2771. [Google Scholar] [CrossRef]
- Jo, J.; Lee, C.H.; Kopelman, R.; Wang, X. In vivo quantitative imaging of tumor pH by nanosonophore assisted multispectral photoacoustic imaging. Nat. Commun. 2017, 8, 471. [Google Scholar] [CrossRef]
- Ray, P.; Confeld, M.; Borowicz, P.; Wang, T.; Mallik, S.; Quadir, M. PEG-b-poly (carbonate)-derived nanocarrier platform with pH-responsive properties for pancreatic cancer combination therapy. Colloids Surf. B Biointerfaces 2019, 174, 126–135. [Google Scholar] [CrossRef]
- Qian, Y.; Wang, Y.; Jia, F.; Wang, Z.; Yue, C.; Zhang, W.; Hu, Z.; Wang, W. Tumor-microenvironment controlled nanomicelles with AIE property for boosting cancer therapy and apoptosis monitoring. Biomaterials 2019, 188, 96–106. [Google Scholar] [CrossRef]
- Glunde, K.; Guggino, S.E.; Solaiyappan, M.; Pathak, A.P.; Ichikawa, Y.; Bhujwalla, Z.M. Extracellular acidification alters lysosomal trafficking in human breast cancer cells. Neoplasia 2003, 5, 533–545. [Google Scholar] [CrossRef]
- Griset, A.P.; Walpole, J.; Liu, R.; Gaffey, A.; Colson, Y.L.; Grinstaff, M.W. Expansile Nanoparticles: Synthesis, Characterization, and in Vivo Efficacy of an Acid-Responsive Polymeric Drug Delivery System. J. Am. Chem. Soc. 2009, 131, 2469–2471. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wu, J.; Liu, Y.; Yu, M.; Zhao, L.; Zhu, X.; Bhasin, S.; Li, Q.; Ha, E.; Shi, J.; et al. Ultra-pH-Responsive and Tumor-Penetrating Nanoplatform for Targeted siRNA Delivery with Robust Anti-Cancer Efficacy. Angew. Chemie Int. Ed. 2016, 55, 7091–7094. [Google Scholar] [CrossRef] [PubMed]
- Hwang, A.A.; Lu, J.; Tamanoi, F.; Zink, J.I. Functional Nanovalves on Protein-Coated Nanoparticles for In vitro and In vivo Controlled Drug Delivery. Small 2015, 11, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Muniswamy, V.J.; Raval, N.; Gondaliya, P.; Tambe, V.; Kalia, K.; Tekade, R.K. ‘Dendrimer-Cationized-Albumin’ encrusted polymeric nanoparticle improves BBB penetration and anticancer activity of doxorubicin. Int. J. Pharm. 2019, 555, 77–99. [Google Scholar] [CrossRef]
- Dai, Z.; Song, X.-Z.; Cao, J.; He, Y.; Wen, W.; Xu, X.; Tan, Z. Dual-stimuli-responsive TiO x /DOX nanodrug system for lung cancer synergistic therapy. RSC Adv. 2018, 8, 21975–21984. [Google Scholar] [CrossRef]
- Yue, Z.-G.; Wei, W.; You, Z.-X.; Yang, Q.-Z.; Yue, H.; Su, Z.-G.; Ma, G.-H. Iron Oxide Nanotubes for Magnetically Guided Delivery and pH-Activated Release of Insoluble Anticancer Drugs. Adv. Funct. Mater. 2011, 21, 3446–3453. [Google Scholar] [CrossRef]
- Mi, P.; Kokuryo, D.; Cabral, H.; Wu, H.; Terada, Y.; Saga, T.; Aoki, I.; Nishiyama, N.; Kataoka, K. A pH-activatable nanoparticle with signal-amplification capabilities for non-invasive imaging of tumour malignancy. Nat. Nanotechnol. 2016, 11, 724–730. [Google Scholar] [CrossRef]
- Dong, Z.; Feng, L.; Zhu, W.; Sun, X.; Gao, M.; Zhao, H.; Chao, Y.; Liu, Z. CaCO3 nanoparticles as an ultra-sensitive tumor-pH-responsive nanoplatform enabling real-time drug release monitoring and cancer combination therapy. Biomaterials 2016, 110, 60–70. [Google Scholar] [CrossRef]
- Choi, J.Y.; Ramasamy, T.; Kim, S.Y.; Kim, J.; Ku, S.K.; Youn, Y.S.; Kim, J.-R.; Jeong, J.-H.; Choi, H.-G.; Yong, C.S.; et al. PEGylated lipid bilayer-supported mesoporous silica nanoparticle composite for synergistic co-delivery of axitinib and celastrol in multi-targeted cancer therapy. Acta Biomater. 2016, 39, 94–105. [Google Scholar] [CrossRef]
- Schomburg, I.; Chang, A.; Placzek, S.; Söhngen, C.; Rother, M.; Lang, M.; Munaretto, C.; Ulas, S.; Stelzer, M.; Grote, A.; et al. BRENDA in 2013: integrated reactions, kinetic data, enzyme function data, improved disease classification: new options and contents in BRENDA. Nucleic Acids Res. 2013, 41, D764–D772. [Google Scholar] [CrossRef]
- Li, X.; Kim, J.; Yoon, J.; Chen, X. Cancer-Associated, Stimuli-Driven, Turn on Theranostics for Multimodality Imaging and Therapy. Adv. Mater. 2017, 29, 1606857. [Google Scholar] [CrossRef]
- Shin, W.S.; Han, J.; Verwilst, P.; Kumar, R.; Kim, J.-H.; Kim, J.S. Cancer Targeted Enzymatic Theranostic Prodrug: Precise Diagnosis and Chemotherapy. Bioconjug. Chem. 2016, 27, 1419–1426. [Google Scholar] [CrossRef]
- Tarro, G.; Perna, A.; Esposito, C. Early diagnosis of lung cancer by detection of tumor liberated protein. J. Cell. Physiol. 2005, 203, 1–5. [Google Scholar] [CrossRef]
- Chan, Y.C.; Hsiao, M. Protease-activated nanomaterials for targeted cancer theranostics. Nanomedicine 2017, 12, 2153–2159. [Google Scholar] [CrossRef]
- Han, H.; Valdepérez, D.; Jin, Q.; Yang, B.; Li, Z.; Wu, Y.; Pelaz, B.; Parak, W.J.; Ji, J. Dual Enzymatic Reaction-Assisted Gemcitabine Delivery Systems for Programmed Pancreatic Cancer Therapy. ACS Nano 2017, 11, 1281–1291. [Google Scholar] [CrossRef]
- Nairon, K.; Samykutty, A.; McNally, M.W.; Mishra, G.; Grizzle, W.E.; McNally, L.R. Abstract 4664: Enzymatically-responsive tumor-targeted mesoporous silica nanoparticle for identification of pancreatic cancer. Cancer Res. 2018, 78, 4664. [Google Scholar]
- Liu, M.; Khan, A.R.; Ji, J.; Lin, G.; Zhao, X.; Zhai, G. Crosslinked self-assembled nanoparticles for chemo-sonodynamic combination therapy favoring antitumor, antimetastasis management and immune responses. J. Control. Release 2018, 290, 150–164. [Google Scholar] [CrossRef]
- Dzamukova, M.R.; Naumenko, E.A.; Lvov, Y.M.; Fakhrullin, R.F. Enzyme-activated intracellular drug delivery with tubule clay nanoformulation. Sci. Rep. 2015, 5, 10560. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Talalay, P. NAD(P)H:quinone acceptor oxidoreductase 1 (NQO1), a multifunctional antioxidant enzyme and exceptionally versatile cytoprotector. Arch. Biochem. Biophys. 2010, 501, 116–123. [Google Scholar] [CrossRef]
- Huang, F.; You, M.; Chen, T.; Zhu, G.; Liang, H.; Tan, W. Self-assembled hybrid nanoparticles for targeted co-delivery of two drugs into cancer cells. Chem. Commun. 2014, 50, 3103. [Google Scholar] [CrossRef]
- Shi, H.; Gao, T.; Shi, L.; Chen, T.; Xiang, Y.; Li, Y.; Li, G. Molecular imaging of telomerase and the enzyme activity-triggered drug release by using a conformation-switchable nanoprobe in cancerous cells. Sci. Rep. 2018, 8, 16341. [Google Scholar] [CrossRef]
- Mannaris, C.; Teo, B.M.; Seth, A.; Bau, L.; Coussios, C.; Stride, E. Gas-Stabilizing Gold Nanocones for Acoustically Mediated Drug Delivery. Adv. Healthc. Mater. 2018, 7, 1800184. [Google Scholar] [CrossRef]
- Decock, J.; Obermajer, N.; Vozelj, S.; Hendrickx, W.; Paridaens, R.; Kos, J. Cathepsin B, cathepsin H, cathepsin X and cystatin C in sera of patients with early-stage and inflammatory breast cancer. Int. J. Biol. Mark. 2008, 23, 161–168. [Google Scholar] [CrossRef]
- Villar-Alvarez, E.; Cambón, A.; Pardo, A.; Mosquera, V.X.; Bouzas-Mosquera, A.; Topete, A.; Barbosa, S.; Taboada, P.; Mosquera, V. Gold Nanorod-Based Nanohybrids for Combinatorial Therapeutics. ACS Omega 2018, 3, 12633–12647. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, T.; Gu, R.; Zauscher, S.; Betancourt, T. Doxorubicin-loaded protease-activated near-infrared fluorescent polymeric nanoparticles for imaging and therapy of cancer. Int. J. Nanomed. 2018, 13, 6961–6986. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Fingleton, B.; Matrisian, L.M. Matrix metalloproteinase inhibitors and cancer: Trials and tribulations. Science 2002, 295, 2387–2392. [Google Scholar] [CrossRef] [PubMed]
- Somerville, R.P.; Oblander, S.A.; Apte, S.S. Matrix metalloproteinases: Old dogs with new tricks. Genome Biol. 2003, 4, 216. [Google Scholar] [CrossRef][Green Version]
- Davies, G.; Henrissat, B. Structures and mechanisms of glycosyl hydrolases. Structure 1995, 3, 853–859. [Google Scholar] [CrossRef]
- Shin, W.-S.; Kwon, J.; Lee, H.W.; Kang, M.C.; Na, H.-W.; Lee, S.-T.; Park, J.H. Oncogenic role of protein tyrosine kinase 7 in esophageal squamous cell carcinoma. Cancer Sci. 2013, 104, 1120–1126. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lu, Y.; Abbaraju, P.L.; Azimi, I.; Lei, C.; Tang, J.; Jambhrunkar, M.; Fu, J.; Zhang, M.; Liu, Y.; et al. Stepwise Degradable Nanocarriers Enabled Cascade Delivery for Synergistic Cancer Therapy. Adv. Funct. Mater. 2018, 28, 1800706. [Google Scholar] [CrossRef]
- Lian, X.; Huang, Y.; Zhu, Y.; Fang, Y.; Zhao, R.; Joseph, E.; Li, J.; Pellois, J.-P.; Zhou, H.-C. Enzyme-MOF Nanoreactor Activates Nontoxic Paracetamol for Cancer Therapy. Angew. Chemie Int. Ed. 2018, 57, 5725–5730. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Zuckerman, J.E.; Choi, C.H.J.; Seligson, D.; Tolcher, A.; Alabi, C.A.; Yen, Y.; Heidel, J.D.; Ribas, A. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature 2010, 464, 1067–1070. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, Y.; Wang, J.; Yuan, A.; Sun, M.; Wu, J.; Hu, Y. Self-assembled IR780-loaded transferrin nanoparticles as an imaging, targeting and PDT/PTT agent for cancer therapy. Sci. Rep. 2016, 6, 27421. [Google Scholar] [CrossRef]
- Dixit, S.; Novak, T.; Miller, K.; Zhu, Y.; Kenney, M.E.; Broome, A.-M. Transferrin receptor-targeted theranostic gold nanoparticles for photosensitizer delivery in brain tumors. Nanoscale 2015, 7, 1782–1790. [Google Scholar] [CrossRef]
- Dautry-Varsat, A.; Ciechanover, A.; Lodish, H.F. pH and the recycling of transferrin during receptor-mediated endocytosis. Proc. Natl. Acad. Sci. USA 1983, 80, 2258–2262. [Google Scholar] [CrossRef]
- Sutherland, R.; Delia, D.; Schneider, C.; Newman, R.; Kemshead, J.; Greaves, M. Ubiquitous cell-surface glycoprotein on tumor cells is proliferation-associated receptor for transferrin. Proc. Natl. Acad. Sci. USA 1981, 78, 4515–4519. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, N.; Ma, M.; Luo, Y.; Chen, H. Transferrin Receptor-Mediated Sequential Intercellular Nanoparticles Relay for Tumor Deep Penetration and Sonodynamic Therapy. Adv. Ther. 2019, 1800152. [Google Scholar] [CrossRef]
- Wosikowski, K.; Schuurhuis, D.; Kops, G.J.; Saceda, M.; Bates, S.E. Altered gene expression in drug-resistant human breast cancer cells. Clin. Cancer Res. 1997, 3, 2405–2414. [Google Scholar]
- Akimoto, T.; Hunter, N.R.; Buchmiller, L.; Mason, K.; Ang, K.K.; Milas, L. Inverse relationship between epidermal growth factor receptor expression and radiocurability of murine carcinomas. Clin. Cancer Res. 1999, 5, 2884–2890. [Google Scholar] [PubMed]
- Galizia, G.; Lieto, E.; Ferraraccio, F.; De Vita, F.; Castellano, P.; Orditura, M.; Imperatore, V.; La Mura, A.; La Manna, G.; Pinto, M.; et al. Prognostic Significance of Epidermal Growth Factor Receptor Expression in Colon Cancer Patients Undergoing Curative Surgery. Ann. Surg. Oncol. 2006, 13, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, L.; Grandal, M.V.; Knudsen, S.L.J.; van Deurs, B.; Grøvdal, L.M. Internalization Mechanisms of the Epidermal Growth Factor Receptor after Activation with Different Ligands. PLoS ONE 2013, 8, e58148. [Google Scholar] [CrossRef] [PubMed]
- Merten, H.; Brandl, F.; Plückthun, A.; Zangemeister-Wittke, U. Antibody–Drug Conjugates for Tumor Targeting—Novel Conjugation Chemistries and the Promise of non-IgG Binding Proteins. Bioconjug. Chem. 2015, 26, 2176–2185. [Google Scholar] [CrossRef]
- Ducry, L.; Stump, B. Antibody−Drug Conjugates: Linking Cytotoxic Payloads to Monoclonal Antibodies. Bioconjug. Chem. 2010, 21, 5–13. [Google Scholar] [CrossRef]
- Roncato, F.; Rruga, F.; Porcù, E.; Casarin, E.; Ronca, R.; Maccarinelli, F.; Realdon, N.; Basso, G.; Alon, R.; Viola, G.; et al. Improvement and extension of anti-EGFR targeting in breast cancer therapy by integration with the Avidin-Nucleic-Acid-Nano-Assemblies. Nat. Commun. 2018, 9, 4070. [Google Scholar] [CrossRef]
- Mhawech-Fauceglia, P.; Zhang, S.; Terracciano, L.; Sauter, G.; Chadhuri, A.; Herrmann, F.R.; Penetrante, R. Prostate-specific membrane antigen (PSMA) protein expression in normal and neoplastic tissues and its sensitivity and specificity in prostate adenocarcinoma: an immunohistochemical study using mutiple tumour tissue microarray technique. Histopathology 2007, 50, 472–483. [Google Scholar] [CrossRef]
- Rauscher, I.; Maurer, T.; Fendler, W.P.; Sommer, W.H.; Schwaiger, M.; Eiber, M. 68Ga-PSMA ligand PET/CT in patients with prostate cancer: How we review and report. Cancer Imaging 2016, 16, 14. [Google Scholar] [CrossRef]
- Liu, H.; Rajasekaran, A.K.; Moy, P.; Xia, Y.; Kim, S.; Navarro, V.; Rahmati, R.; Bander, N.H. Constitutive and antibody-induced internalization of prostate-specific membrane antigen. Cancer Res. 1998, 58, 4055–4060. [Google Scholar] [PubMed]
- Mangadlao, J.D.; Wang, X.; McCleese, C.; Escamilla, M.; Ramamurthy, G.; Wang, Z.; Govande, M.; Basilion, J.P.; Burda, C. Prostate-Specific Membrane Antigen Targeted Gold Nanoparticles for Theranostics of Prostate Cancer. ACS Nano 2018, 12, 3714–3725. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M.; Giaccia, A.J. The unique physiology of solid tumors: Opportunities (and problems) for cancer therapy. Cancer Res. 1998, 58, 1408–1416. [Google Scholar]
- Feng, L.; Cheng, L.; Dong, Z.; Tao, D.; Barnhart, T.E.; Cai, W.; Chen, M.; Liu, Z. Theranostic Liposomes with Hypoxia-Activated Prodrug to Effectively Destruct Hypoxic Tumors Post-Photodynamic Therapy. ACS Nano 2017, 11, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wan, J.; Niu, Y.; Gu, Z.; Zhang, J.; Yu, M.; Yu, C. Structure-Dependent and Glutathione-Responsive Biodegradable Dendritic Mesoporous Organosilica Nanoparticles for Safe Protein Delivery. Chem. Mater. 2016, 28, 9008–9016. [Google Scholar] [CrossRef]
- Robinson, R.K.; Batt, C.A.; Patel, P.D. Encyclopedia of Food Microbiology; Academic Press: San Diego, CA, USA, 2000; ISBN 0122270703. [Google Scholar]
- Mullin, L.B.; Phillips, L.C.; Dayton, P.A. Nanoparticle delivery enhancement with acoustically activated microbubbles. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2013, 60, 65–77. [Google Scholar] [CrossRef]
- Deng, C.X.; Sieling, F.; Pan, H.; Cui, J. Ultrasound-induced cell membrane porosity. Ultrasound Med. Biol. 2004, 30, 519–526. [Google Scholar] [CrossRef] [PubMed]
- De Temmerman, M.-L.; Dewitte, H.; Vandenbroucke, R.E.; Lucas, B.; Libert, C.; Demeester, J.; De Smedt, S.C.; Lentacker, I.; Rejman, J. mRNA-Lipoplex loaded microbubble contrast agents for ultrasound-assisted transfection of dendritic cells. Biomaterials 2011, 32, 9128–9135. [Google Scholar] [CrossRef]
- Burke, C.W.; Alexander, E.; Timbie, K.; Kilbanov, A.L.; Price, R.J.; Price, R.J. Ultrasound-activated agents comprised of 5FU-bearing nanoparticles bonded to microbubbles inhibit solid tumor growth and improve survival. Mol. Ther. 2014, 22, 321–328. [Google Scholar] [CrossRef]
- Kwan, J.J.; Myers, R.; Coviello, C.M.; Graham, S.M.; Shah, A.R.; Stride, E.; Carlisle, R.C.; Coussios, C.C. Ultrasound-Propelled Nanocups for Drug Delivery. Small 2015, 11, 5305–5314. [Google Scholar] [CrossRef]
- Burke, C.W.; Hsiang, Y.-H.J.; Alexander, E.; Kilbanov, A.L.; Price, R.J.; Price, R.J. Covalently linking poly(lactic-co-glycolic acid) nanoparticles to microbubbles before intravenous injection improves their ultrasound-targeted delivery to skeletal muscle. Small 2011, 7, 1227–1235. [Google Scholar] [CrossRef]
- Chappell, J.C.; Song, J.; Burke, C.W.; Klibanov, A.L.; Price, R.J. Targeted delivery of nanoparticles bearing fibroblast growth factor-2 by ultrasonic microbubble destruction for therapeutic arteriogenesis. Small 2008, 4, 1769–1777. [Google Scholar] [CrossRef]
- Price, R.J.; Skyba, D.M.; Kaul, S.; Skalak, T.C. Delivery of colloidal particles and red blood cells to tissue through microvessel ruptures created by targeted microbubble destruction with ultrasound. Circulation 1998, 98, 1264–1267. [Google Scholar] [CrossRef]
- Cochran, M.C.; Eisenbrey, J.; Ouma, R.O.; Soulen, M.; Wheatley, M.A. Doxorubicin and paclitaxel loaded microbubbles for ultrasound triggered drug delivery. Int. J. Pharm. 2011, 414, 161–170. [Google Scholar] [CrossRef]
- Yuan, P.; Song, D. MRI tracing non-invasive TiO2-based nanoparticles activated by ultrasound for multi-mechanism therapy of prostatic cancer. Nanotechnology 2018, 29, 125101. [Google Scholar] [CrossRef]
- Doxorubicin Hydrochloride. Available online: https://www.drugs.com/monograph/doxorubicin-hydrochloride.html (accessed on 14 December 2018).
- Lee, J.; Min, H.-S.; You, D.G.; Kim, K.; Kwon, I.C.; Rhim, T.; Lee, K.Y. Theranostic gas-generating nanoparticles for targeted ultrasound imaging and treatment of neuroblastoma. J. Control. Release 2016, 223, 197–206. [Google Scholar] [CrossRef]
- Marshalek, J.P.; Sheeran, P.S.; Ingram, P.; Dayton, P.A.; Witte, R.S.; Matsunaga, T.O. Intracellular delivery and ultrasonic activation of folate receptor-targeted phase-change contrast agents in breast cancer cells in vitro. J. Control. Release 2016, 243, 69–77. [Google Scholar] [CrossRef]
- Wang, X.; Song, J.; Liu, J.; Wang, Z.L. Direct-Current Nanogenerator Driven by Ultrasonic Waves. Science 2007, 316, 102–105. [Google Scholar] [CrossRef]
- Cucullo, L.; Dini, G.; Hallene, K.L.; Fazio, V.; Ilkanich, E.V.; Igboechi, C.; Kight, K.M.; Agarwal, M.K.; Garrity-Moses, M.; Janigro, D. Very low intensity alternating current decreases cell proliferation. Glia 2005, 51, 65–72. [Google Scholar] [CrossRef]
- Marino, A.; Battaglini, M.; De Pasquale, D.; Degl’Innocenti, A.; Ciofani, G. Ultrasound-Activated Piezoelectric Nanoparticles Inhibit Proliferation of Breast Cancer Cells. Sci. Rep. 2018, 8, 6257. [Google Scholar] [CrossRef]
- Pearce, J.; Giustini, A.; Stigliano, R.; Jack Hoopes, P. Magnetic Heating of Nanoparticles: The Importance of Particle Clustering to Achieve Therapeutic Temperatures. J. Nanotechnol. Eng. Med. 2013, 4, 011005. [Google Scholar] [CrossRef]
- Shah, R.R.; Davis, T.P.; Glover, A.L.; Nikles, D.E.; Brazel, C.S. Impact of magnetic field parameters and iron oxide nanoparticle properties on heat generation for use in magnetic hyperthermia. J. Magn. Magn. Mater. 2015, 387, 96–106. [Google Scholar] [CrossRef]
- Noto, V.; Taper, H.S.; Yi-Hua, J.; Janssens, J.; Bonte, J.; De Loecker, W. Effects of sodium ascorbate (vitamin C) and 2-methyl-1,4-naphthoquinone (vitamin K3) treatment on human tumor cell growth in vitro. I. Synergism of combined vitamin C and K3 action. Cancer 1989, 63, 901–906. [Google Scholar] [CrossRef]
- Johannsen, M.; Gneveckow, U.; Eckelt, L.; Feussner, A.; WaldÖFner, N.; Scholz, R.; Deger, S.; Wust, P.; Loening, S.A.; Jordan, A. Clinical hyperthermia of prostate cancer using magnetic nanoparticles: Presentation of a new interstitial technique. Int. J. Hyperth. 2005, 21, 637–647. [Google Scholar] [CrossRef]
- Bañobre-López, M.; Teijeiro, A.; Rivas, J. Magnetic nanoparticle-based hyperthermia for cancer treatment. Rep. Pract. Oncol. Radiother. 2013, 18, 397–400. [Google Scholar] [CrossRef]
- Vedova, P.D.; Ilieva, M.; Zhurbenko, V.; Mateiu, R.; Faralli, A.; Dufva, M.; Hansen, O. Gold Nanoparticle-Based Sensors Activated by External Radio Frequency Fields. Small 2015, 11, 248–256. [Google Scholar] [CrossRef]
- Raoof, M.; Corr, S.J.; Kaluarachchi, W.D.; Massey, K.L.; Briggs, K.; Zhu, C.; Cheney, M.A.; Wilson, L.J.; Curley, S.A. Stability of antibody-conjugated gold nanoparticles in the endolysosomal nanoenvironment: implications for noninvasive radiofrequency-based cancer therapy. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 1096–1105. [Google Scholar] [CrossRef]
- Mironava, T.; Arachchilage, V.T.; Myers, K.J.; Suchalkin, S. Gold Nanoparticles and Radio Frequency Field Interactions: Effects of Nanoparticle Size, Charge, Aggregation, Radio Frequency, and Ionic Background. Langmuir 2017, 33, 13114–13124. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, K.; Imai, Y.; Koumoto, K.; Kaiden, T.; Kono, K.; Aoshima, S. Magnetoresponsive On-Demand Release of Hybrid Liposomes Formed from Fe3O4 Nanoparticles and Thermosensitive Block Copolymers. Small 2011, 7, 1683–1689. [Google Scholar] [CrossRef]
- Pradhan, P.; Giri, J.; Rieken, F.; Koch, C.; Mykhaylyk, O.; Döblinger, M.; Banerjee, R.; Bahadur, D.; Plank, C. Targeted temperature sensitive magnetic liposomes for thermo-chemotherapy. J. Control. Release 2010, 142, 108–121. [Google Scholar] [CrossRef]
- Plassat, V.; Wilhelm, C.; Marsaud, V.; Ménager, C.; Gazeau, F.; Renoir, J.-M.; Lesieur, S. Anti-Estrogen-Loaded Superparamagnetic Liposomes for Intracellular Magnetic Targeting and Treatment of Breast Cancer Tumors. Adv. Funct. Mater. 2011, 21, 83–92. [Google Scholar] [CrossRef]
- Hu, S.-H.; Chen, S.-Y.; Gao, X. Multifunctional Nanocapsules for Simultaneous Encapsulation of Hydrophilic and Hydrophobic Compounds and On-Demand Release. ACS Nano 2012, 6, 2558–2565. [Google Scholar] [CrossRef] [PubMed]
- Hua, M.-Y.; Liu, H.-L.; Yang, H.-W.; Chen, P.-Y.; Tsai, R.-Y.; Huang, C.-Y.; Tseng, I.-C.; Lyu, L.-A.; Ma, C.-C.; Tang, H.-J.; et al. The effectiveness of a magnetic nanoparticle-based delivery system for BCNU in the treatment of gliomas. Biomaterials 2011, 32, 516–527. [Google Scholar] [CrossRef]
- Yang, H.-W.; Hua, M.-Y.; Liu, H.-L.; Huang, C.-Y.; Tsai, R.-Y.; Lu, Y.-J.; Chen, J.-Y.; Tang, H.-J.; Hsien, H.-Y.; Chang, Y.-S.; et al. Self-protecting core-shell magnetic nanoparticles for targeted, traceable, long half-life delivery of BCNU to gliomas. Biomaterials 2011, 32, 6523–6532. [Google Scholar] [CrossRef]
- Nigam, S.; Bahadur, D. Dendrimer-conjugated iron oxide nanoparticles as stimuli-responsive drug carriers for thermally-activated chemotherapy of cancer. Colloids Surf. B Biointerfaces 2017, 155, 182–192. [Google Scholar] [CrossRef]
- Stanley, S.A.; Gagner, J.E.; Damanpour, S.; Yoshida, M.; Dordick, J.S.; Friedman, J.M. Radio-Wave Heating of Iron Oxide Nanoparticles Can Regulate Plasma Glucose in Mice. Science 2012, 336, 604–608. [Google Scholar] [CrossRef]
- Huang, H.-Y.; Hu, S.-H.; Chian, C.-S.; Chen, S.-Y.; Lai, H.-Y.; Chen, Y.-Y. Self-assembling PVA-F127 thermosensitive nanocarriers with highly sensitive magnetically-triggered drug release for epilepsy therapy in vivo. J. Mater. Chem. 2012, 22, 8566. [Google Scholar] [CrossRef]
- Satarkar, N.S.; Zach Hilt, J. Hydrogel nanocomposites as remote-controlled biomaterials. Acta Biomater. 2008, 4, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.-H.; Liu, D.-M.; Tung, W.-L.; Liao, C.-F.; Chen, S.-Y. Surfactant-Free, Self-Assembled PVA-Iron Oxide/Silica Core-Shell Nanocarriers for Highly Sensitive, Magnetically Controlled Drug Release and Ultrahigh Cancer Cell Uptake Efficiency. Adv. Funct. Mater. 2008, 18, 2946–2955. [Google Scholar] [CrossRef]
- Hu, S.-H.; Chen, S.-Y.; Liu, D.-M.; Hsiao, C.-S. Core/Single-Crystal-Shell Nanospheres for Controlled Drug Release via a Magnetically Triggered Rupturing Mechanism. Adv. Mater. 2008, 20, 2690–2695. [Google Scholar] [CrossRef]
- Bringas, E.; Köysüren, Ö.; Quach, D.V.; Mahmoudi, M.; Aznar, E.; Roehling, J.D.; Marcos, M.D.; Martínez-Máñez, R.; Stroeve, P. Triggered release in lipid bilayer-capped mesoporous silica nanoparticles containing SPION using an alternating magnetic field. Chem. Commun. 2012, 48, 5647. [Google Scholar] [CrossRef] [PubMed]
- Cai, K.; Luo, Z.; Hu, Y.; Chen, X.; Liao, Y.; Yang, L.; Deng, L. Magnetically Triggered Reversible Controlled Drug Delivery from Microfabricated Polymeric Multireservoir Devices. Adv. Mater. 2009, 21, 4045–4049. [Google Scholar] [CrossRef]
- Thomas, C.R.; Ferris, D.P.; Lee, J.-H.; Choi, E.; Cho, M.H.; Kim, E.S.; Stoddart, J.F.; Shin, J.-S.; Cheon, J.; Zink, J.I. Noninvasive Remote-Controlled Release of Drug Molecules in Vitro Using Magnetic Actuation of Mechanized Nanoparticles. J. Am. Chem. Soc. 2010, 132, 10623–10625. [Google Scholar] [CrossRef]
- Walker, E.L.; Wang, Z.; Neogi, A. Radio-frequency actuated polymer-based phononic meta-materials for control of ultrasonic waves. NPG Asia Mater. 2017, 9, e350. [Google Scholar] [CrossRef]
- Park, J.W.; Bae, K.H.; Kim, C.; Park, T.G. Clustered magnetite nanocrystals cross-linked with PEI for efficient siRNA delivery. Biomacromolecules 2011, 12, 457–465. [Google Scholar] [CrossRef]
- Allison, R.R.; Sibata, C.H. Oncologic photodynamic therapy photosensitizers: A clinical review. Photodiagn. Photodyn. Ther. 2010, 7, 61–75. [Google Scholar] [CrossRef]
- Wang, M.; Abbineni, G.; Clevenger, A.; Mao, C.; Xu, S. Upconversion nanoparticles: Synthesis, surface modification and biological applications. Nanomedicine 2011, 7, 710–729. [Google Scholar] [CrossRef] [PubMed]
- Haynes, W.M. CRC Handbook of Chemistry and Physics: A Ready-Reference Book of Chemical and Physical Data; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Meng, Q.; Meng, J.; Ran, W.; Wang, J.; Zhai, Y.; Zhang, P.; Li, Y. Light-Activated Core–Shell Nanoparticles for Spatiotemporally Specific Treatment of Metastatic Triple-Negative Breast Cancer. ACS Nano 2018, 12, 2789–2802. [Google Scholar] [CrossRef] [PubMed]
- Lucky, S.S.; Muhammad Idris, N.; Li, Z.; Huang, K.; Soo, K.C.; Zhang, Y. Titania Coated Upconversion Nanoparticles for Near-Infrared Light Triggered Photodynamic Therapy. ACS Nano 2015, 9, 191–205. [Google Scholar] [CrossRef]
- Dai, Y.; Bi, H.; Deng, X.; Li, C.; He, F.; Ma, P.; Yang, P.; Lin, J. 808 nm near-infrared light controlled dual-drug release and cancer therapy in vivo by upconversion mesoporous silica nanostructures. J. Mater. Chem. B 2017, 5, 2086–2095. [Google Scholar] [CrossRef]
- Huang, L.; Li, Z.; Zhao, Y.; Zhang, Y.; Wu, S.; Zhao, J.; Han, G. Ultralow-Power Near Infrared Lamp Light Operable Targeted Organic Nanoparticle Photodynamic Therapy. J. Am. Chem. Soc. 2016, 138, 14586–14591. [Google Scholar] [CrossRef] [PubMed]
- Vankayala, R.; Kuo, C.-L.; Nuthalapati, K.; Chiang, C.-S.; Hwang, K.C. Nucleus-Targeting Gold Nanoclusters for Simultaneous In Vivo Fluorescence Imaging, Gene Delivery, and NIR-Light Activated Photodynamic Therapy. Adv. Funct. Mater. 2015, 25, 5934–5945. [Google Scholar] [CrossRef]
- Qiu, M.; Wang, D.; Liang, W.; Liu, L.; Zhang, Y.; Chen, X.; Sang, D.K.; Xing, C.; Li, Z.; Dong, B.; et al. Novel concept of the smart NIR-light-controlled drug release of black phosphorus nanostructure for cancer therapy. Proc. Natl. Acad. Sci. USA 2018, 115, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Detrembleur, C.; De Pauw-Gillet, M.-C.; Mornet, S.; Jérôme, C.; Duguet, E. Gold Nanorods Coated with Mesoporous Silica Shell as Drug Delivery System for Remote Near Infrared Light-Activated Release and Potential Phototherapy. Small 2015, 11, 2323–2332. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Liu, D.; Lin, W. Self-Assembled Core–Shell Nanoparticles for Combined Chemotherapy and Photodynamic Therapy of Resistant Head and Neck Cancers. ACS Nano 2015, 9, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; Yu, J.; Chen, Y.; Hu, Q.; Xiao, X.; Sun, W.; Wang, C.; Feng, P.; Shen, Q.-D.; Gu, Z. Light-Activated Hypoxia-Responsive Nanocarriers for Enhanced Anticancer Therapy. Adv. Mater. 2016, 28, 3313–3320. [Google Scholar] [CrossRef]
- Guan, M.; Dong, H.; Ge, J.; Chen, D.; Sun, L.; Li, S.; Wang, C.; Yan, C.; Wang, P.; Shu, C. Multifunctional upconversion–nanoparticles–trismethylpyridylporphyrin–fullerene nanocomposite: A near-infrared light-triggered theranostic platform for imaging-guided photodynamic therapy. NPG Asia Mater. 2015, 7, e205. [Google Scholar] [CrossRef]
- Yue, C.; Yang, Y.; Zhang, C.; Alfranca, G.; Cheng, S.; Ma, L.; Liu, Y.; Zhi, X.; Ni, J.; Jiang, W.; et al. ROS-Responsive Mitochondria-Targeting Blended Nanoparticles: Chemo- and Photodynamic Synergistic Therapy for Lung Cancer with On-Demand Drug Release upon Irradiation with a Single Light Source. Theranostics 2016, 6, 2352–2366. [Google Scholar] [CrossRef]
- Lu, K.; He, C.; Lin, W. A Chlorin-Based Nanoscale Metal–Organic Framework for Photodynamic Therapy of Colon Cancers. J. Am. Chem. Soc. 2015, 137, 7600–7603. [Google Scholar] [CrossRef]
- Solís-López, M.; Ramírez-Zamora, R.M. Fenton Reaction—An Overview | ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/chemistry/fenton-reaction (accessed on 24 January 2019).
- Kim, J.; Cho, H.R.; Jeon, H.; Kim, D.; Song, C.; Lee, N.; Choi, S.H.; Hyeon, T. Continuous O2-Evolving MnFe2O4 Nanoparticle-Anchored Mesoporous Silica Nanoparticles for Efficient Photodynamic Therapy in Hypoxic Cancer. J. Am. Chem. Soc. 2017, 139, 10992–10995. [Google Scholar] [CrossRef]
- Espinosa, A.; Di Corato, R.; Kolosnjaj-Tabi, J.; Flaud, P.; Pellegrino, T.; Wilhelm, C. Duality of Iron Oxide Nanoparticles in Cancer Therapy: Amplification of Heating Efficiency by Magnetic Hyperthermia and Photothermal Bimodal Treatment. ACS Nano 2016, 10, 2436–2446. [Google Scholar] [CrossRef]
- Goodman, A.M.; Neumann, O.; Nørregaard, K.; Henderson, L.; Choi, M.-R.; Clare, S.E.; Halas, N.J. Near-infrared remotely triggered drug-release strategies for cancer treatment. Proc. Natl. Acad. Sci. USA 2017, 114, 12419–12424. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.; Sun, X.; Feng, L.; Zhu, W.; Liu, Z.; Chen, M. Near-infrared light-activated cancer cell targeting and drug delivery with aptamer-modified nanostructures. Nano Res. 2016, 9, 139–148. [Google Scholar] [CrossRef]
- Chen, H.; Wang, G.D.; Chuang, Y.-J.; Zhen, Z.; Chen, X.; Biddinger, P.; Hao, Z.; Liu, F.; Shen, B.; Pan, Z.; et al. Nanoscintillator-Mediated X-ray Inducible Photodynamic Therapy for In Vivo Cancer Treatment. Nano Lett. 2015, 15, 2249–2256. [Google Scholar] [CrossRef]
- Kotagiri, N.; Cooper, M.L.; Rettig, M.; Egbulefu, C.; Prior, J.; Cui, G.; Karmakar, P.; Zhou, M.; Yang, X.; Sudlow, G.; et al. Radionuclides transform chemotherapeutics into phototherapeutics for precise treatment of disseminated cancer. Nat. Commun. 2018, 9, 275. [Google Scholar] [CrossRef]
- Stolik, S.; Delgado, J.; Pérez, A.; Anasagasti, L. Measurement of the penetration depths of red and near infrared light in human “ex vivo” tissues. J. Photochem. Photobiol. B Biol. 2000, 57, 90–93. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
White, B.D.; Duan, C.; Townley, H.E. Nanoparticle Activation Methods in Cancer Treatment. Biomolecules 2019, 9, 202. https://doi.org/10.3390/biom9050202
White BD, Duan C, Townley HE. Nanoparticle Activation Methods in Cancer Treatment. Biomolecules. 2019; 9(5):202. https://doi.org/10.3390/biom9050202
Chicago/Turabian StyleWhite, Benjamin D, Chengchen Duan, and Helen E Townley. 2019. "Nanoparticle Activation Methods in Cancer Treatment" Biomolecules 9, no. 5: 202. https://doi.org/10.3390/biom9050202
APA StyleWhite, B. D., Duan, C., & Townley, H. E. (2019). Nanoparticle Activation Methods in Cancer Treatment. Biomolecules, 9(5), 202. https://doi.org/10.3390/biom9050202



