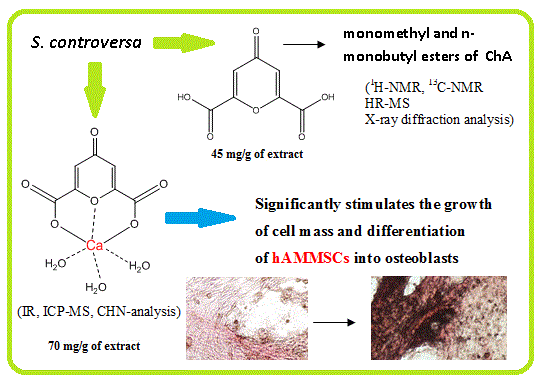
Chelidonic Acid and Its Derivatives from Saussurea Controversa: Isolation, Structural Elucidation and Influence on the Osteogenic Differentiation of Multipotent Mesenchymal Stromal Cells In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Plant Material
2.3. Extraction and Isolation
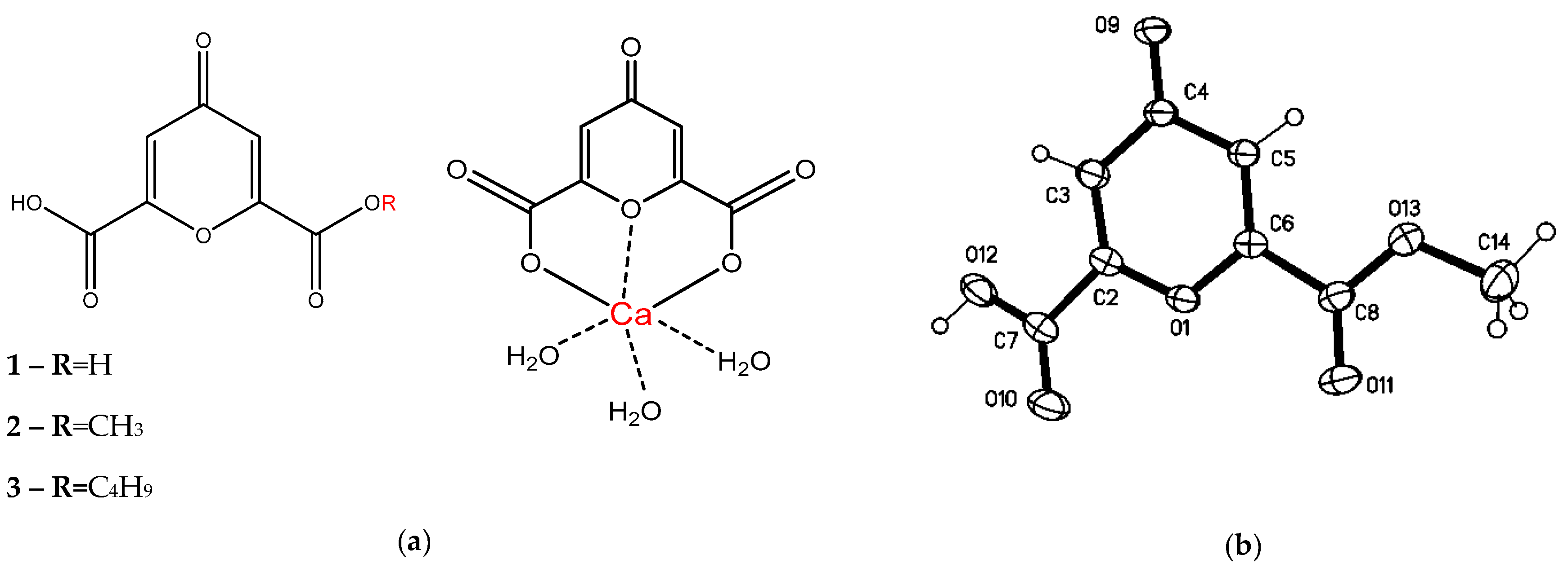
2.4. 4-oxo-4H-pyran-2.6-dicarboxylic acid (1)
2.5. 4-oxo-4H-pyran-2.6-dicarboxylic acid methyl ester (2)
2.6. 4-oxo-4H-pyran-2.6-dicarboxylic acid n-monobutyl ester (3)
2.7. [Ca(ChA)(H2O)3] (4)
2.8. Cell Culturing and In Vitro Staining
2.9. Statistical Analysis
3. Results
3.1. Isolation of Components and Determination of Their Structure
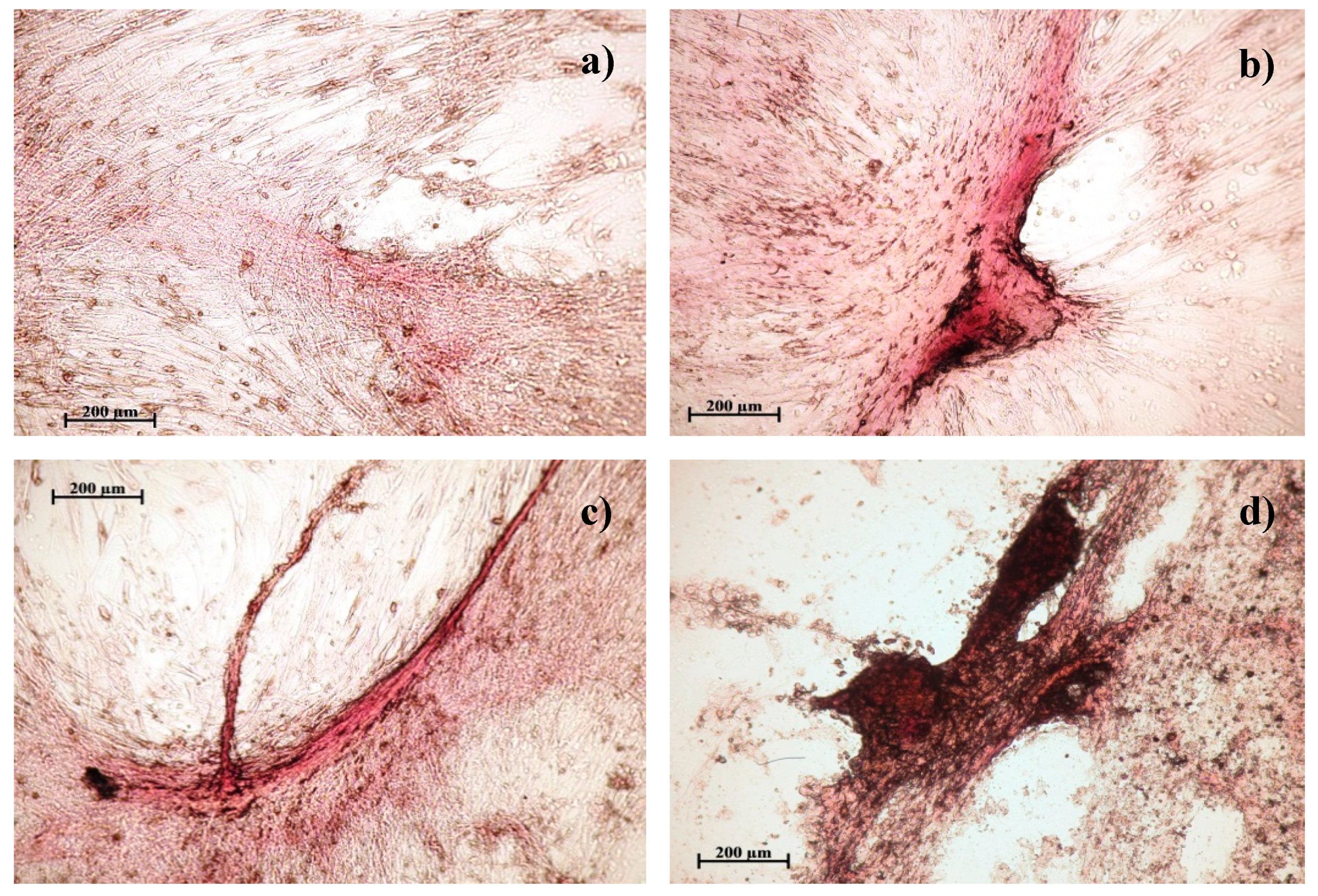
3.2. Study of Osteogenic Differentiation of hAMMSCs In Vitro
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Pandey, M.M.; Rastogi, S.; Rawat, A.K.S. Saussurea costus: Botanical, chemical and pharmacological review of an ayurvedic medicinal plant. J. Ethnopharmacol. 2007, 110, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Yi, T.; Zhao, Z.-Z.; Yu, Z.-L.; Chen, H.-B. Comparison of the anti-inflammatory and anti-nociceptive effects of three medicinal plants known as “Snow Lotus” herb in traditional Uighur and Tibetan medicines. J. Ethnopharmacol. 2010, 128, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Perevozchikova, T.V.; Avdeeva, E.Y.; Fayt, E.A.; Skorokhodova, M.G.; Krasnov, E.A. The effect of extracts of Saussurea controversa and Fillipendula ulmaria on immunological reactivity of rats with experimental osteomyelitis. Experim Clin. Pharmacol. 2016, 79, 16–20. [Google Scholar] [CrossRef]
- Avdeeva, E.; Shults, E.; Skorokhodova, M.; Reshetov, Y.; Porokhova, E.; Sukhodolo, I.; Krasnov, E.; Belousov, M. Flavonol Glycosides from Saussurea controversa and Their Efficiency in Experimental Osteomyelitis. Planta Medica Int. Open 2018, 5, e24–e29. [Google Scholar] [CrossRef][Green Version]
- Yasodha, V.; Govindarajan, S.; Low, J.N.; Glidewell, C. Cationic, neutral and anionic metal(II) complexes derived from 4-oxo-4 H -pyran-2,6-dicarboxylic acid (chelidonic acid). Acta Crystallogr. 2007, 63, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Belian, M.F.; Silva, W.E.; de Sá, G.F.; Alves, S.; de Farias, R.F. Synthesis and Characterization of Cr(III), Mn(II), Fe(III), Co(II), Ni(II), Cu(II), and Zn(II) Complexes With 2,6-Pyridinedicarboxilic Acid, Chelidamic Acid, and Chelidonic Acid. Synth. React. Inorg. Met. Org. Nano-Metal Chem. 2014, 44, 1461–1463. [Google Scholar] [CrossRef]
- Jadresko, D.; Kaksa, M.; Popovic, Z. Electrochemical Characteristics of 4-oxo-4H-pyrandicarboxylic Acid (Chelidonic Acid) and some of its Metal Complexes. Electroanalysis 2017, 29, 538–547. [Google Scholar] [CrossRef]
- Aytemi, M.; Uzbay, T.; Erol, D. New 4(1H)-Pyridinone Derivatives as Analgesic Agents. Drug Res. 1999, 49, 250–254. [Google Scholar]
- Öztürk, G.; Erol, D.D.; Aytemir, M.D.; Uzbay, T. New analgesic and antiinflammatory agents 4(1H)-pyridinone derivatives. Eur. J. Med. Chem. 2002, 37, 829–834. [Google Scholar] [CrossRef]
- Liu, H.; Zheng, Y.-F.; Li, C.-Y.; Zheng, Y.-Y.; Wang, D.-Q.; Wu, Z.; Huang, L.; Wang, Y.-G.; Li, P.-B.; Peng, W.; et al. Discovery of Anti-inflammatory Ingredients in Chinese Herbal Formula Kouyanqing Granule based on Relevance Analysis between Chemical Characters and Biological Effects. Sci. Rep. 2015, 5, 18080. [Google Scholar] [CrossRef]
- Kim, D.-S.; Kim, S.-J.; Kim, M.-C.; Jeon, Y.-D.; Um, J.-Y.; Hong, S.-H. The Therapeutic Effect of Chelidonic Acid on Ulcerative Colitis. Boil. Pharm. 2012, 35, 666–671. [Google Scholar] [CrossRef]
- Singh, D.K.; Gulati, K.; Ray, A. Effects of chelidonic acid, a secondary plant metabolite, on mast cell degranulation and adaptive immunity in rats. Int. Immunopharmacol. 2016, 40, 229–234. [Google Scholar] [CrossRef]
- Porter, T.G.; Martin, D.L. Chelidonic acid and other conformationally restricted substrate analogues as inhibitors of rat brain glutamate decarboxylase. Biochem. Pharmacol. 1985, 34, 4145–4150. [Google Scholar] [CrossRef]
- Carraher, C.E.; Ayoub, M.; Roner, M.R.; Moric, A.; Trang, N.T. Synthesis, structural characterization, and ability to inhibit the growth of pancreatic cancer by organotin polymers containing chelidonic acid. J. Chin. Adv. Mater. Soc. 2013, 1, 65–73. [Google Scholar] [CrossRef]
- Aoshima, Y.; Hasegawa, Y.; Hasegawa, S.; Nagasaka, A.; Kimura, T.; Hashimoto, S.; Torii, Y.; Tsukagoshi, N. Isolation of GnafC, a Polysaccharide Constituent ofGnaphalium affine, and Synergistic Effects of GnafC and Ascorbate on the Phenotypic Expression of Osteoblastic MC3T3-E1 Cells. Biosci. Biotechnol. Biochem. 2003, 67, 2068–2074. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Kuroyanagi, G.; Ohguchi, R.; Kozawa, O.; Tokuda, H.; Kainuma, S.; Fujita, K.; Matsushima-Nishiwaki, R.; Otsuka, T. Amplification by (-)-epigallocatechin gallate and chlorogenic acid of TNF-α-stimulated interleukin-6 synthesis in osteoblasts. Int. J. Mol. Med. 2015, 36, 1707–1712. [Google Scholar] [CrossRef]
- Shou, D.; Zhang, Y.; Shen, L.; Zheng, R.; Huang, X.; Mao, Z.; Yu, Z.; Wang, N.; Zhu, Y. Flavonoids of Herba Epimedii Enhances Bone Repair in a Rabbit Model of Chronic Osteomyelitis During Post-infection Treatment and Stimulates Osteoblast Proliferation in Vitro. Phytother. Res. 2017, 31, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SADABS, Program for area Detector Adsorption Correction; Institute for Inorganic Chemistry, University of Goettingen: Goettingen, Germany, 1996. [Google Scholar]
- Sheldrick, M. SHELX-97—Programs for Crystal Structure Analysis, Release 97-2; University of Goetingen: Goettingen, Germany, 1997. [Google Scholar]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage Cells from Human Adipose Tissue: Implications for Cell-Based Therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Bourin, P.; Bunnell, B.A.; Casteilla, L.; Dominici, M.; Katz, A.J.; March, K.L.; Redl, H.; Rubin, J.P.; Yoshimura, K.; Gimble, J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013, 15, 641–648. [Google Scholar] [CrossRef]
- Riggs, B.; Melton, L. Osteoporosis: Etiology, Diagnosis, and Management, 2nd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1995; p. 524. [Google Scholar]
- Khlusov, I.A.; Dekhtyar, Y.; Sharkeev, Y.P.; Pichugin, V.F.; Khlusova, M.Y.; Polyaka, N.; Tjulkins, F.; Vendinya, V.; Legostaeva, E.V.; Litvinova, L.S.; et al. Nanoscale Electrical Potential and Roughness of a Calcium Phosphate Surface Promotes the Osteogenic Phenotype of Stromal Cells. Materials 2018, 11, 978. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.J.; Kim, H.J.; Won, H.Y.; Min, Y.K.; Hwang, E.S. Acceleration of osteoblast differentiation by a novel osteogenic compound, DMP-PYT, through activation of both the BMP and Wnt pathways. Sci. Rep. 2017, 7, 8455. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Shih, Y.-R.V.; Nakasaki, M.; Kabra, H.; Varghese, S.; Shih, Y.-R.V. Small molecule–driven direct conversion of human pluripotent stem cells into functional osteoblasts. Sci. Adv. 2016, 2, e1600691. [Google Scholar] [CrossRef] [PubMed]
- Theman, T.; Collins, M. The Role of the Calcium-Sensing Receptor in Bone Biology and Pathophysiology. Pharm. Biotechnol. 2009, 10, 289–301. [Google Scholar] [CrossRef]
- Shih, Y.-R.V.; Hwang, Y.; Phadke, A.; Kang, H.; Hwang, N.S.; Caro, E.J.; Nguyen, S.; Siu, M.; Theodorakis, E.A.; Gianneschi, N.C.; et al. Calcium phosphate-bearing matrices induce osteogenic differentiation of stem cells through adenosine signaling. Proc. Natl. Acad. Sci. USA 2014, 111, 990–995. [Google Scholar] [CrossRef] [PubMed]
- Takedachi, M.; Oohara, H.; Smith, B.J.; Iyama, M.; Kobashi, M.; Maeda, K.; Long, C.L.; Humphrey, M.B.; Stoecker, B.J.; Toyosawa, S.; et al. CD73-generated adenosine promotes osteoblast differentiation. J. Cell. Physiol. 2012, 227, 2622–2631. [Google Scholar] [CrossRef]
| Group, n = 3 | Dose mg/L | Number of viable cells, ×104 cells/mL, n1 = 6 | U Criterion | Equation of dose-dependent linear regression | |
|---|---|---|---|---|---|
| 1 | *Control | 0 | 9.5 ± 0.61 | - | |
| 2 | Cells + ChA | 10 | 21.7 ± 3.96 | U1<0.05 | Y = 24.0833–0.405X; P = 0.2180 |
| 30 | 8.6 ± 1.73 | ||||
| 50 | 5.5 ± 1.08 | U1<0.05 U2<0.05 | |||
| 3 | Cells + BuChA | 10 | 8.7 ± 0.23 | Y = 10.4000–0.100X; P = 0.3469 | |
| 30 | 8.8 ± 0.24 | ||||
| 50 | 4.7 ± 0.13 | U1<0.05 U2<0.05 | |||
| 4 | Cells + CaChA | 10 | 11.3 ± 1.01 | U1<0.05 | Y = 10.1083 + 0.0875X; P = 0.1934 |
| 30 | 12.1 ± 1.07 | U1<0.05 | |||
| 50 | 14.8 ± 1.26 | U1<0.05 U2<0.05 | |||
| Group, n = 3 | Dose, mg/L | Total area of the sites of cell culture mineralization, mm2, n = 3 | U criterion, statistical significance | An average area of the mineralization sites, mm2 | U criterion, statistical significance | Equation of dose-dependent linear regression | |
|---|---|---|---|---|---|---|---|
| 1 | *Control | 0 | 1.166 ± 0.433 | - | 0.027(0.013–0.095) n1 = 14 | - | - |
| 2 | Cells + ChA | 10 | 0.589 ± 0.262 | - | 0.033(0.009–0.053) n1 = 13 | - | Y = 0.098X–0.379; P = 0.0064 |
| 30 | 2.582 ± 0.470 | U1 <0.05 | 0.043(0.018–0.121) n1 = 31 | - | |||
| 50 | 4.507 ± 0.097 | U1<0.05 U2<0.05 | 0.060(0.024–0.096) n1 = 56 | - | |||
| 3 | Cells + BuChA | 10 | 1.904 ± 0.306 | U1 <0.05 U3 <0.05 | 0.021(0.009–0.133) n1 = 25 | - | Y = 0.008X + 1.686 P = 0.6263 |
| 30 | 1.649 ± 0.563 | U3 <0.05 | 0.026(0.010–0.077) n1 = 32 | - | |||
| 50 | 2.222 ± 0.599 | U1 <0.05 U3 <0.05 | 0.042(0.013–0.123) n1 = 27 | - | |||
| 4 | Cells + CaChA | 10 | 3.266 ± 0.207 | U1 <0.05 U3 <0.05 | 0.036(0.021–0.069) n1 = 60 | - | Y = 0.069X + 3.228 P = 0.4365 |
| 30 | 6.620 ± 0.117 | U1 <0.05 U3 <0.05 | 0.057(0.012–0.187) n1 = 58 | U2<0.05 | |||
| 50 | 6.041 ± 0.033 | U1 <0.05 U2<0.05 U3 <0.05 | 0.049(0.027–0.124) N 1= 70 | U2<0.05 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avdeeva, E.; Shults, E.; Rybalova, T.; Reshetov, Y.; Porokhova, E.; Sukhodolo, I.; Litvinova, L.; Shupletsova, V.; Khaziakhmatova, O.; Khlusov, I.; et al. Chelidonic Acid and Its Derivatives from Saussurea Controversa: Isolation, Structural Elucidation and Influence on the Osteogenic Differentiation of Multipotent Mesenchymal Stromal Cells In Vitro. Biomolecules 2019, 9, 189. https://doi.org/10.3390/biom9050189
Avdeeva E, Shults E, Rybalova T, Reshetov Y, Porokhova E, Sukhodolo I, Litvinova L, Shupletsova V, Khaziakhmatova O, Khlusov I, et al. Chelidonic Acid and Its Derivatives from Saussurea Controversa: Isolation, Structural Elucidation and Influence on the Osteogenic Differentiation of Multipotent Mesenchymal Stromal Cells In Vitro. Biomolecules. 2019; 9(5):189. https://doi.org/10.3390/biom9050189
Chicago/Turabian StyleAvdeeva, Elena, Elvira Shults, Tatyana Rybalova, Yaroslav Reshetov, Ekaterina Porokhova, Irina Sukhodolo, Larisa Litvinova, Valeria Shupletsova, Olga Khaziakhmatova, Igor Khlusov, and et al. 2019. "Chelidonic Acid and Its Derivatives from Saussurea Controversa: Isolation, Structural Elucidation and Influence on the Osteogenic Differentiation of Multipotent Mesenchymal Stromal Cells In Vitro" Biomolecules 9, no. 5: 189. https://doi.org/10.3390/biom9050189
APA StyleAvdeeva, E., Shults, E., Rybalova, T., Reshetov, Y., Porokhova, E., Sukhodolo, I., Litvinova, L., Shupletsova, V., Khaziakhmatova, O., Khlusov, I., Guryev, A., & Belousov, M. (2019). Chelidonic Acid and Its Derivatives from Saussurea Controversa: Isolation, Structural Elucidation and Influence on the Osteogenic Differentiation of Multipotent Mesenchymal Stromal Cells In Vitro. Biomolecules, 9(5), 189. https://doi.org/10.3390/biom9050189