Mining for Oxysterols in Cyp7b1−/− Mouse Brain and Plasma: Relevance to Spastic Paraplegia Type 5
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Sample Preparation
2.3. Analysis
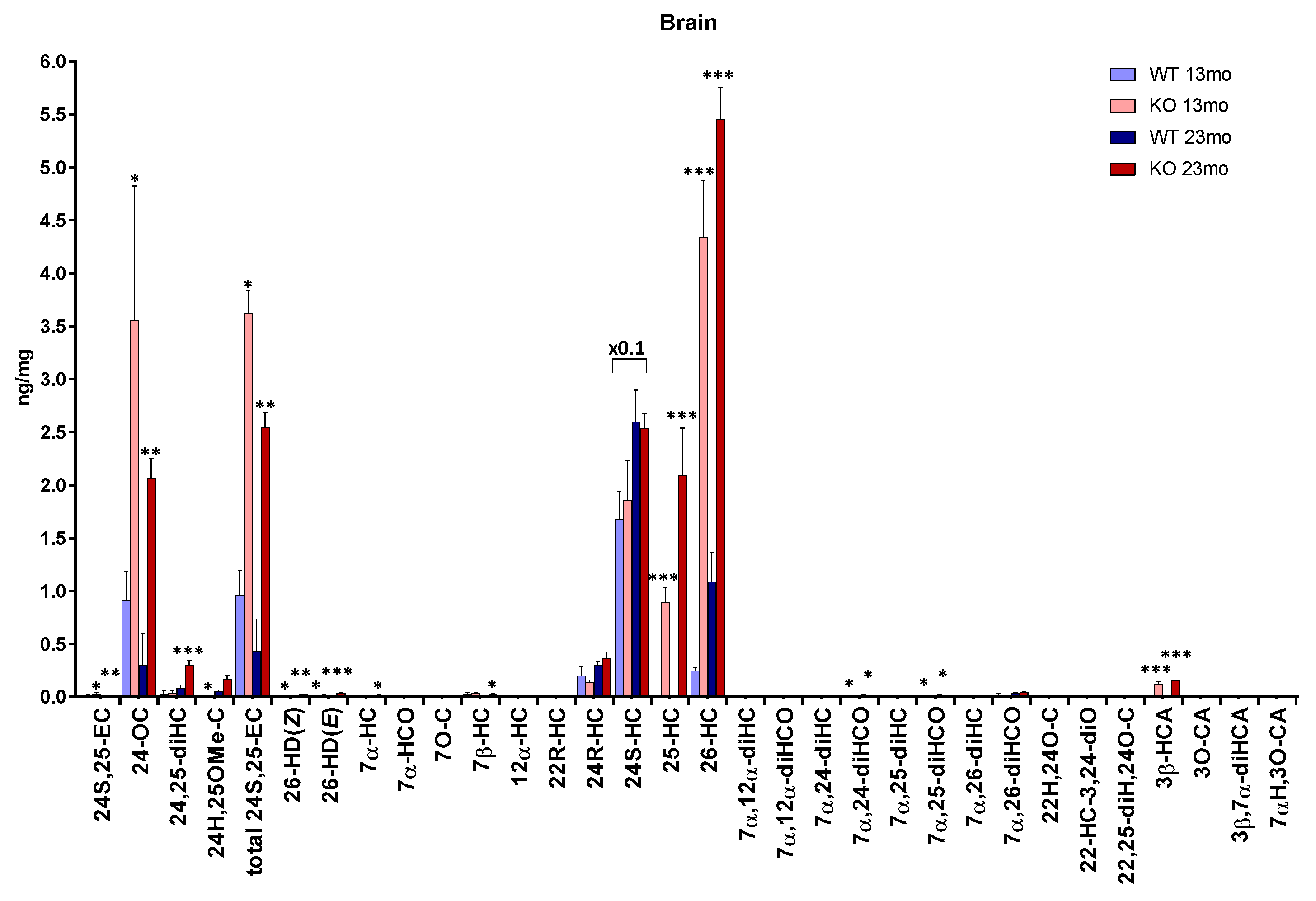
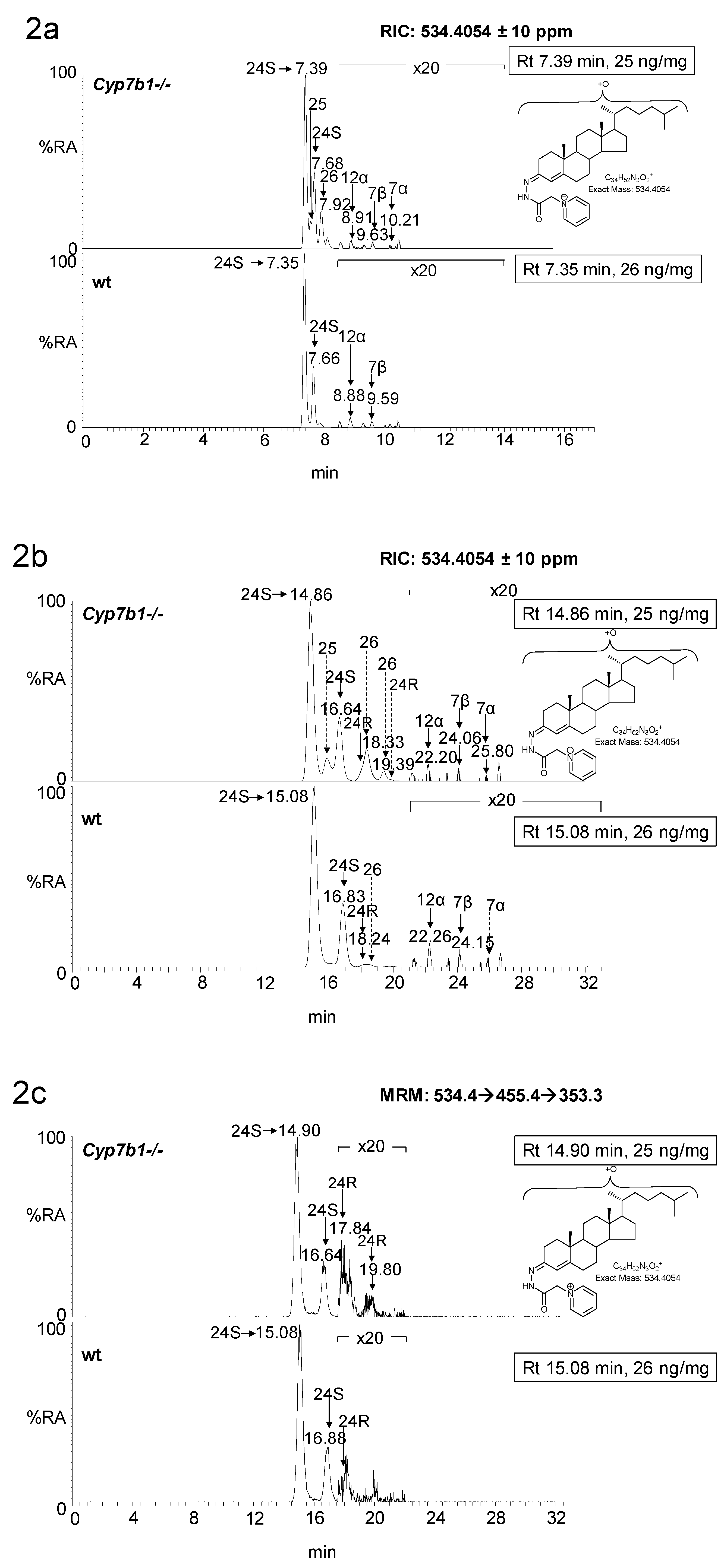
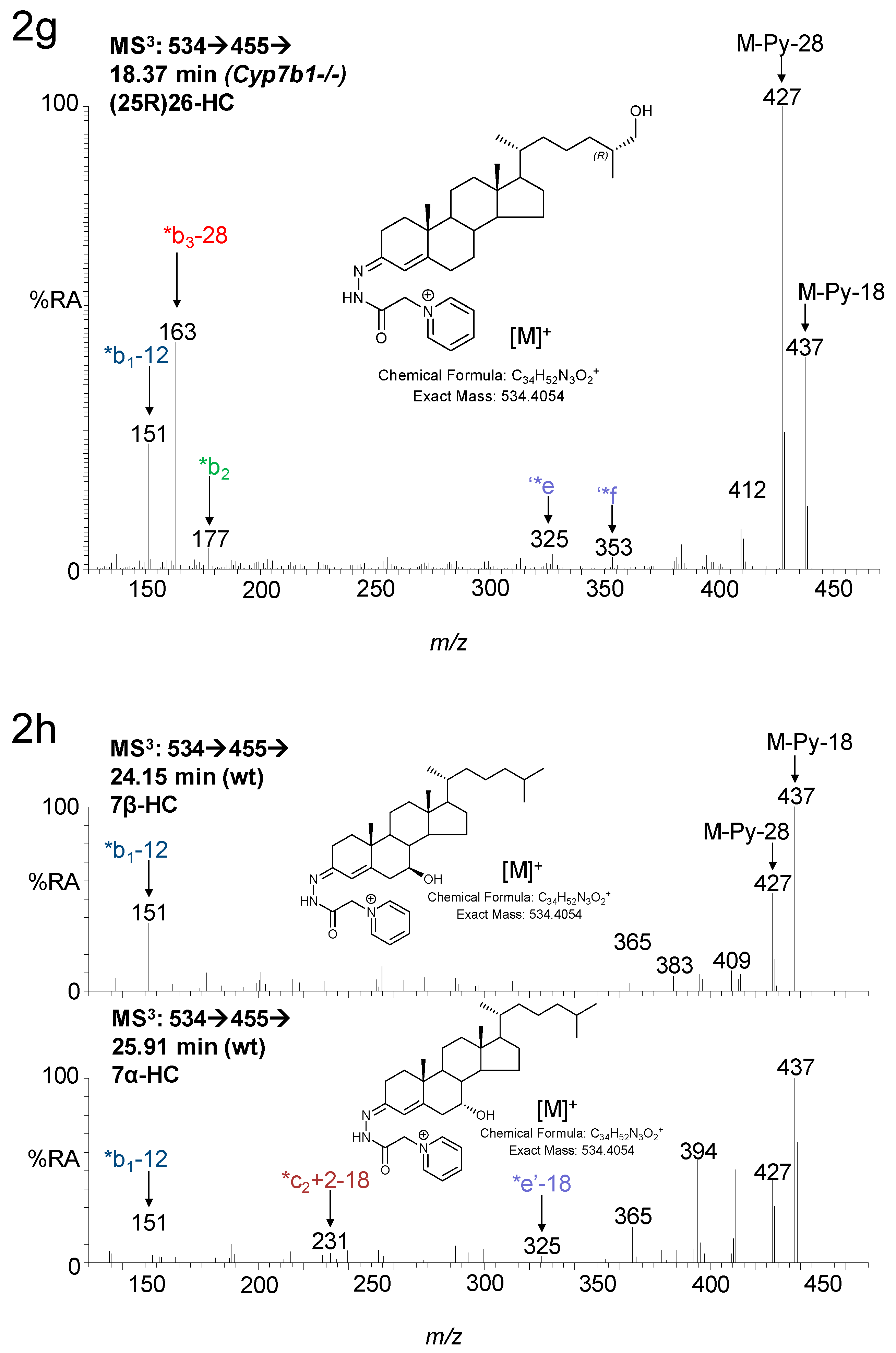
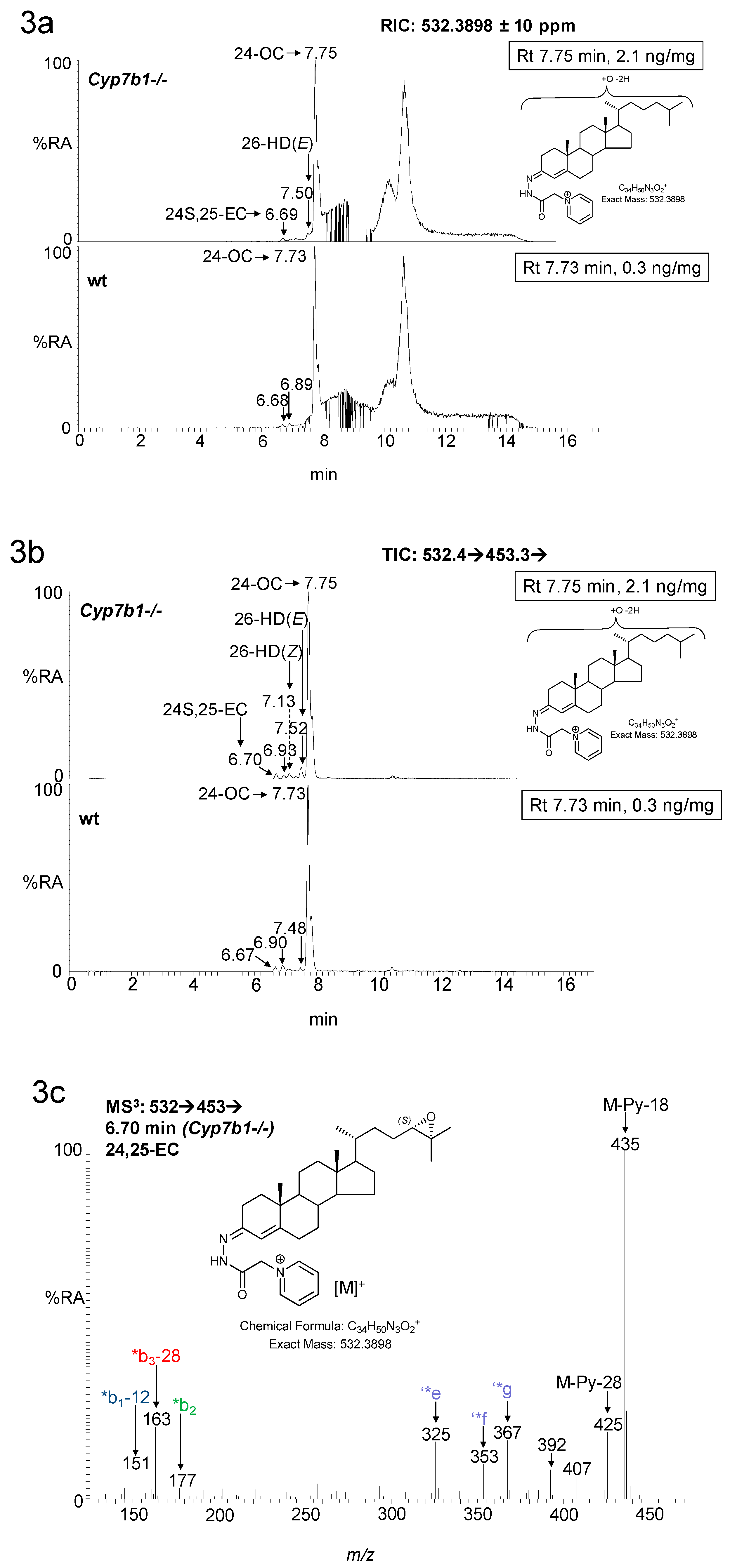
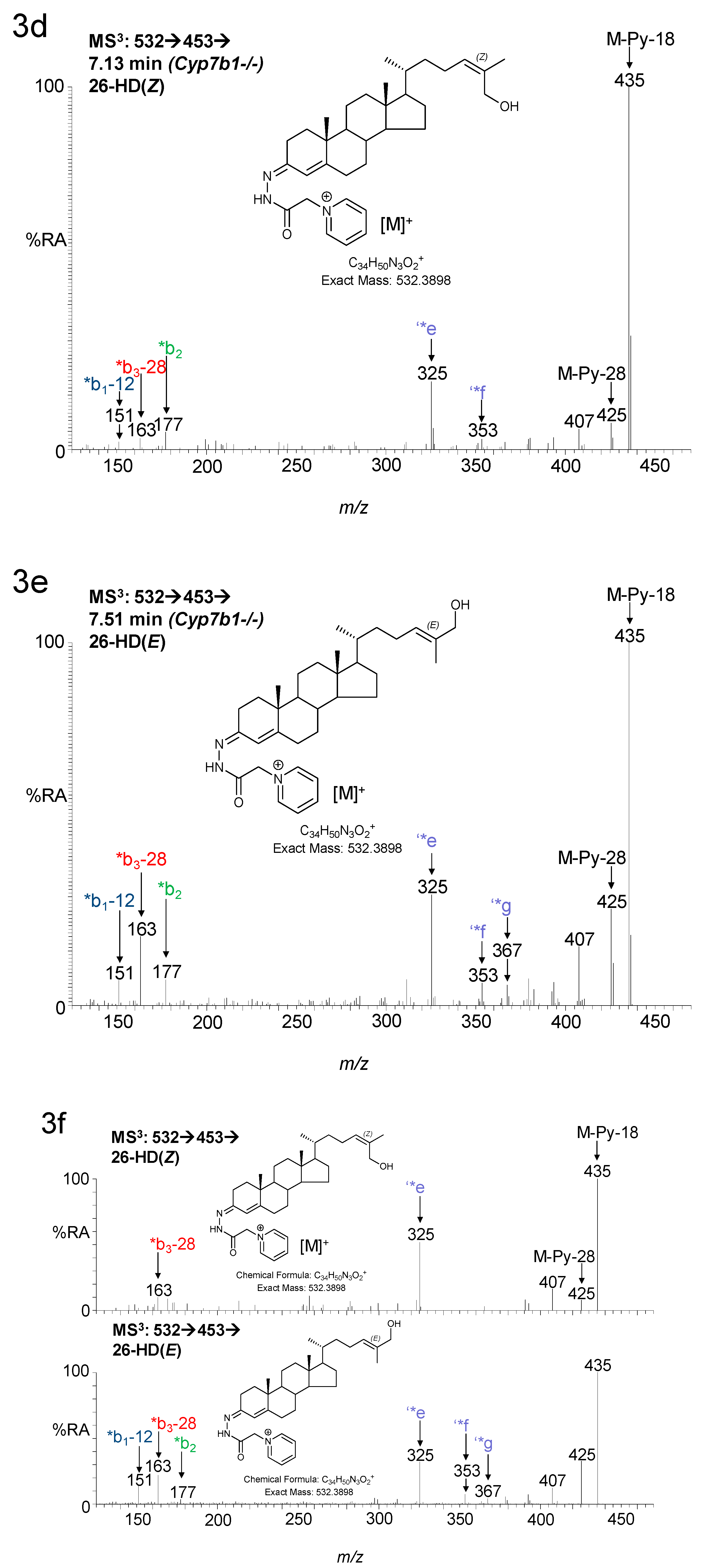
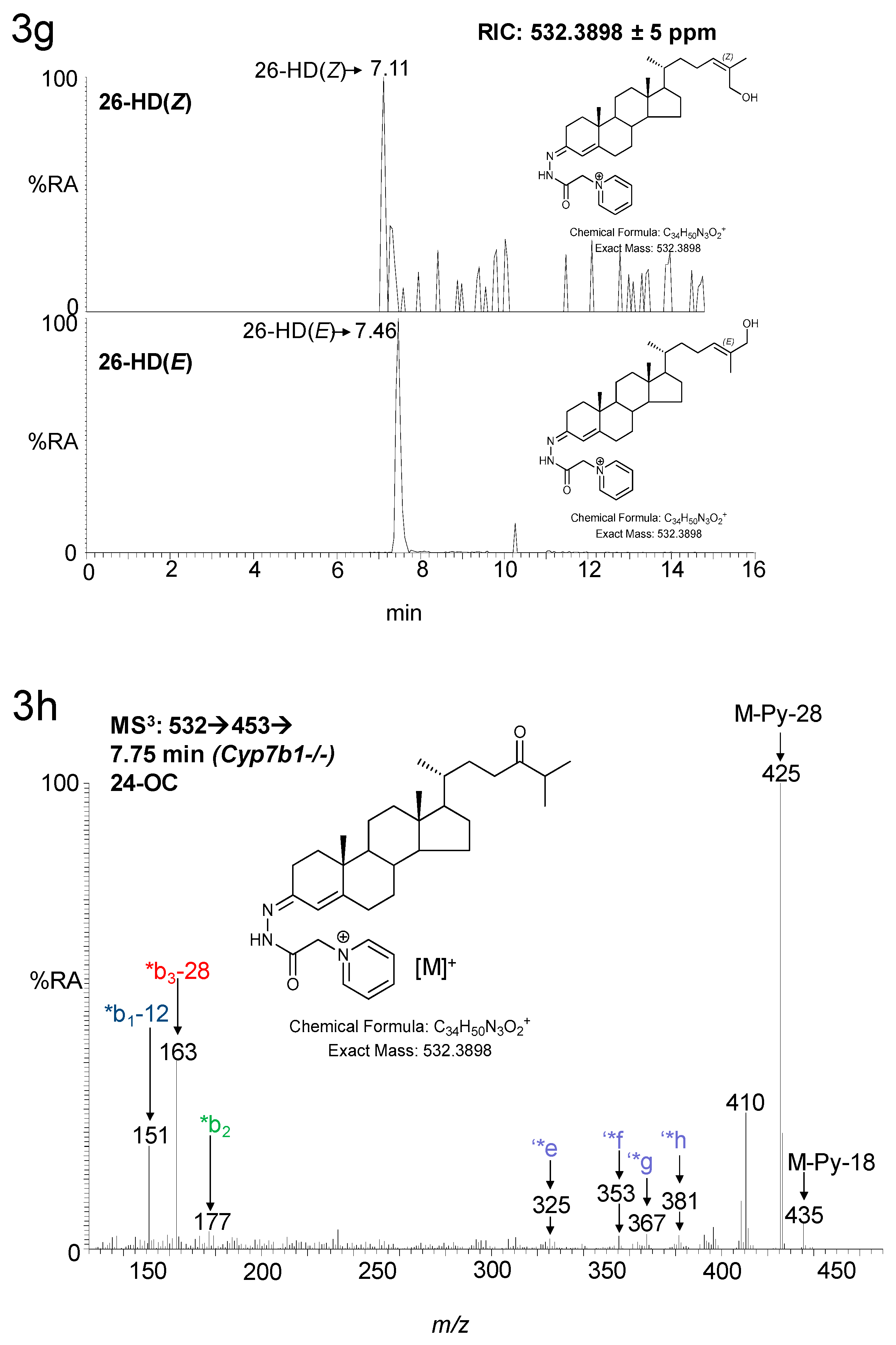
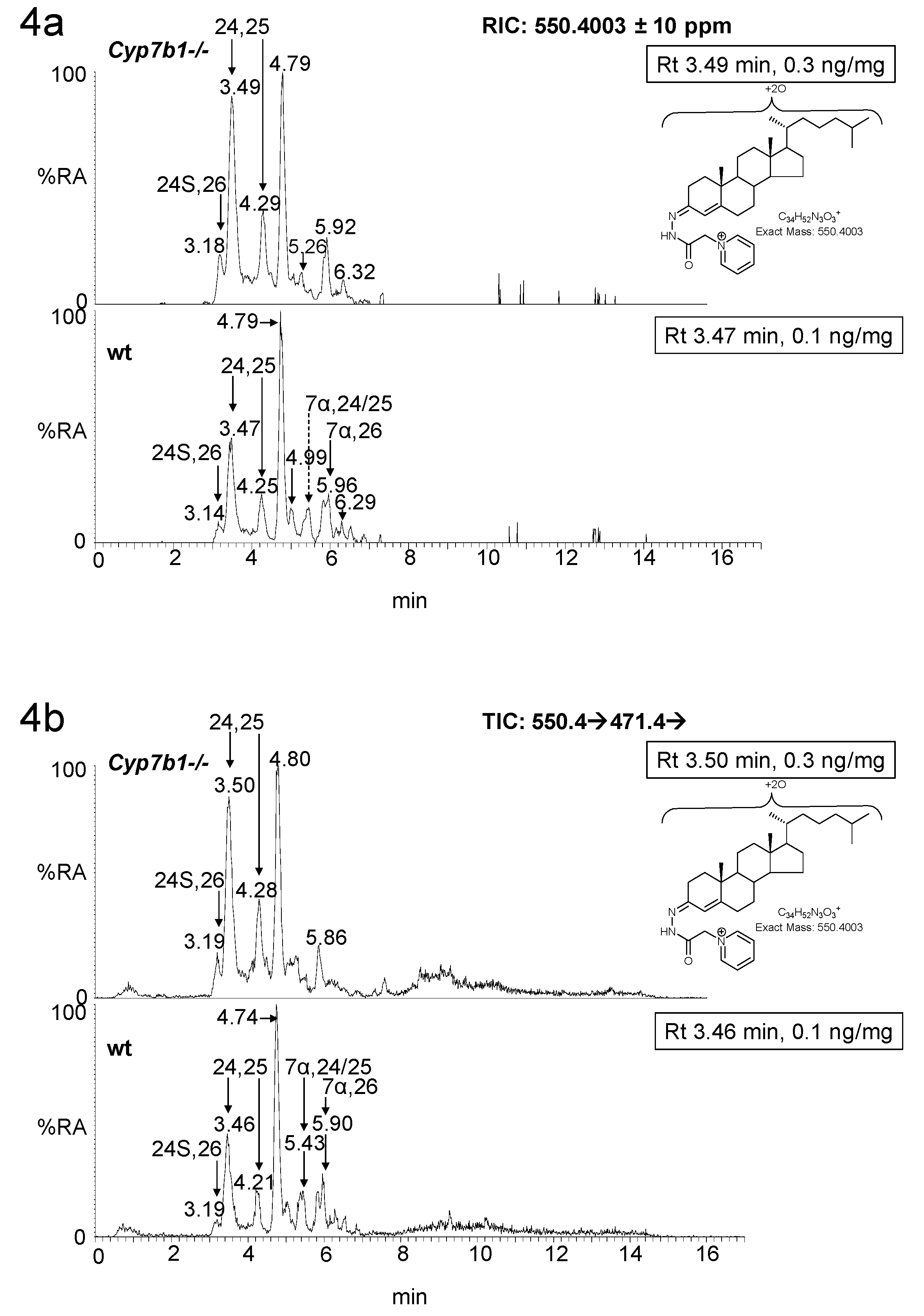
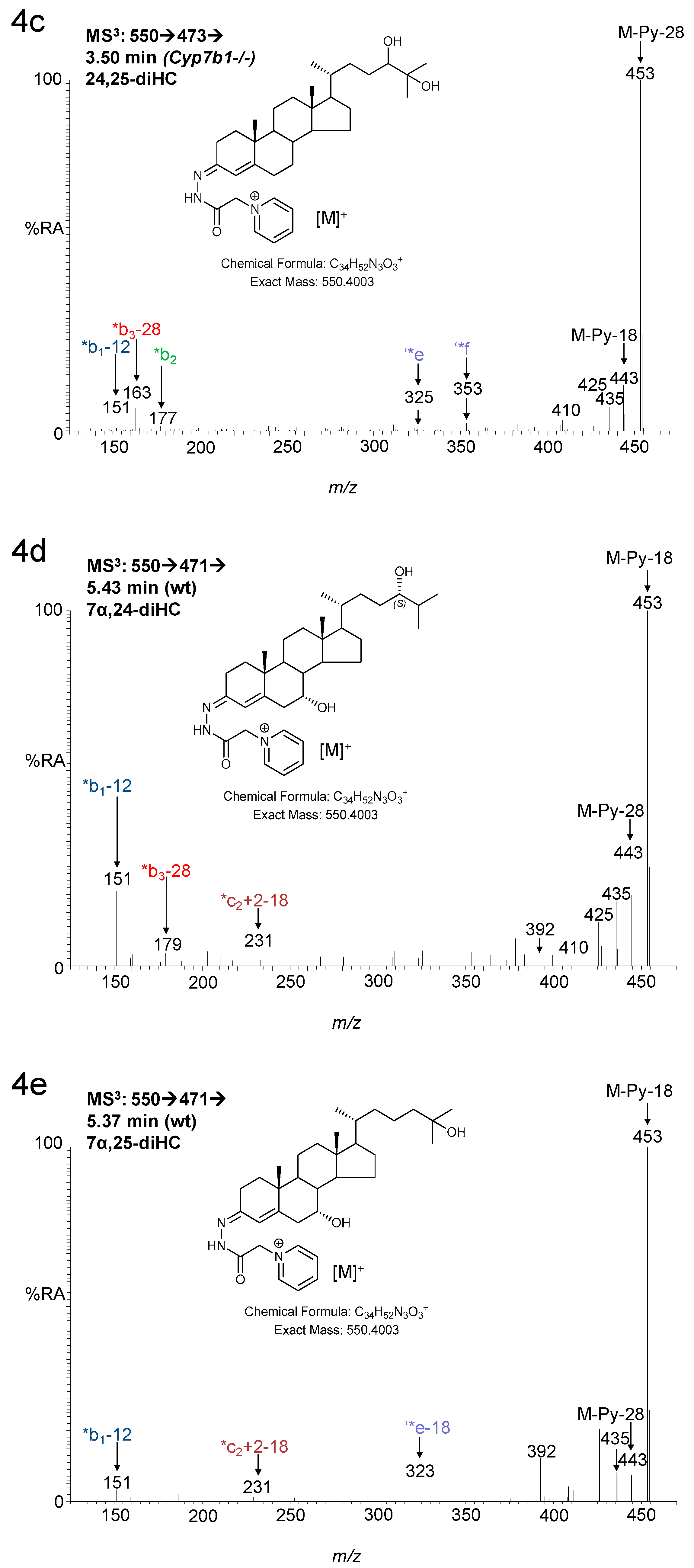
3. Results
3.1. Brain
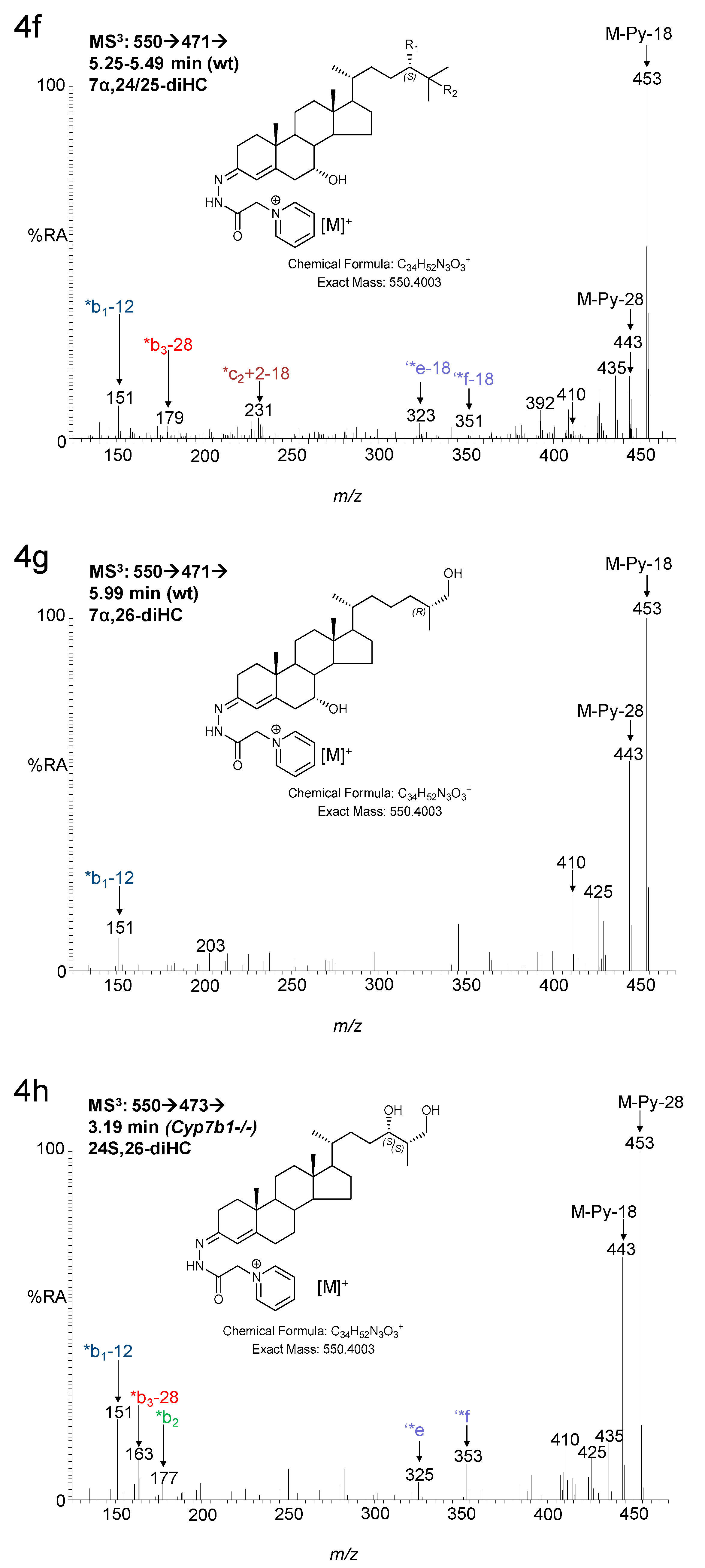
3.1.1. Oxysterols
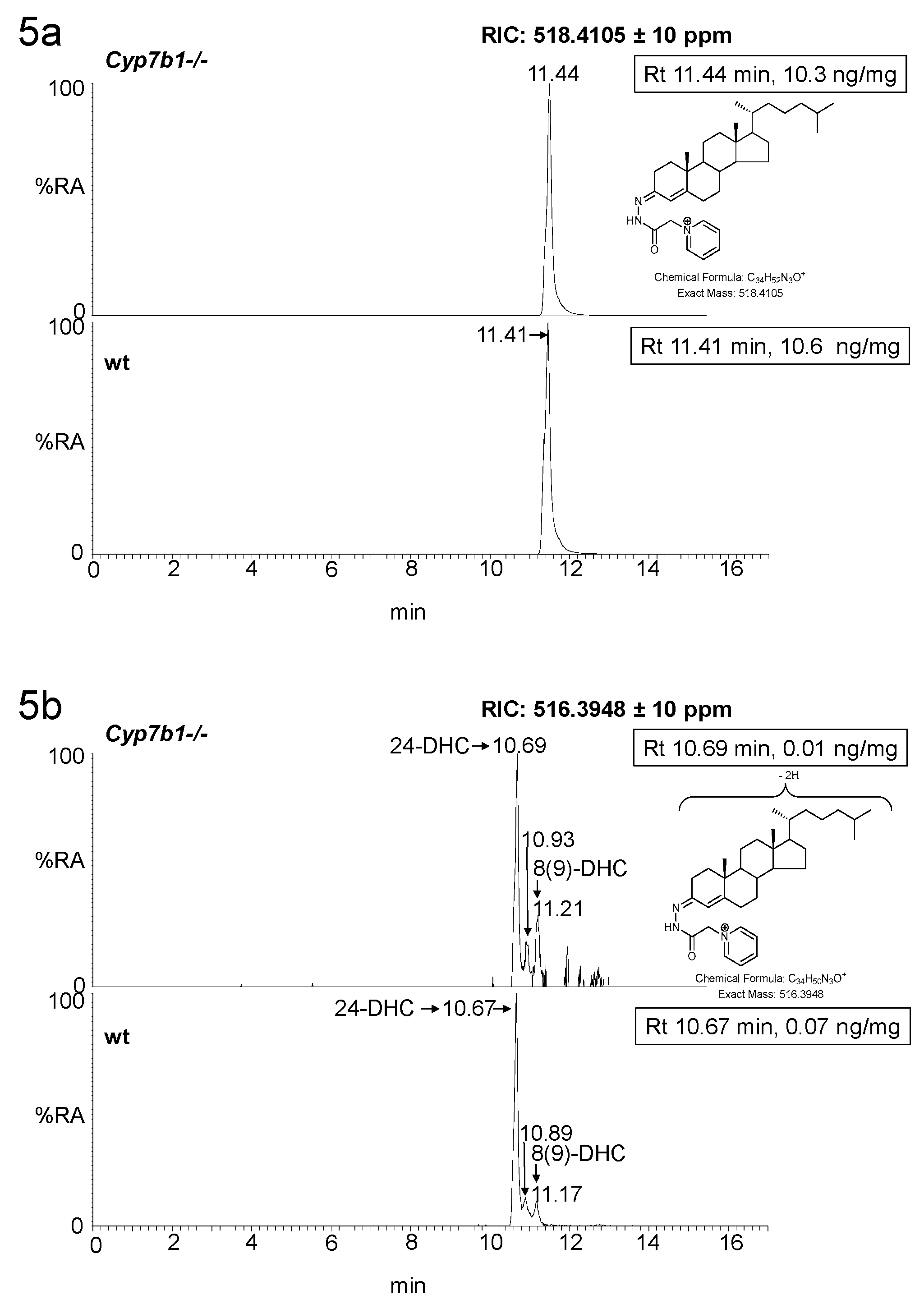
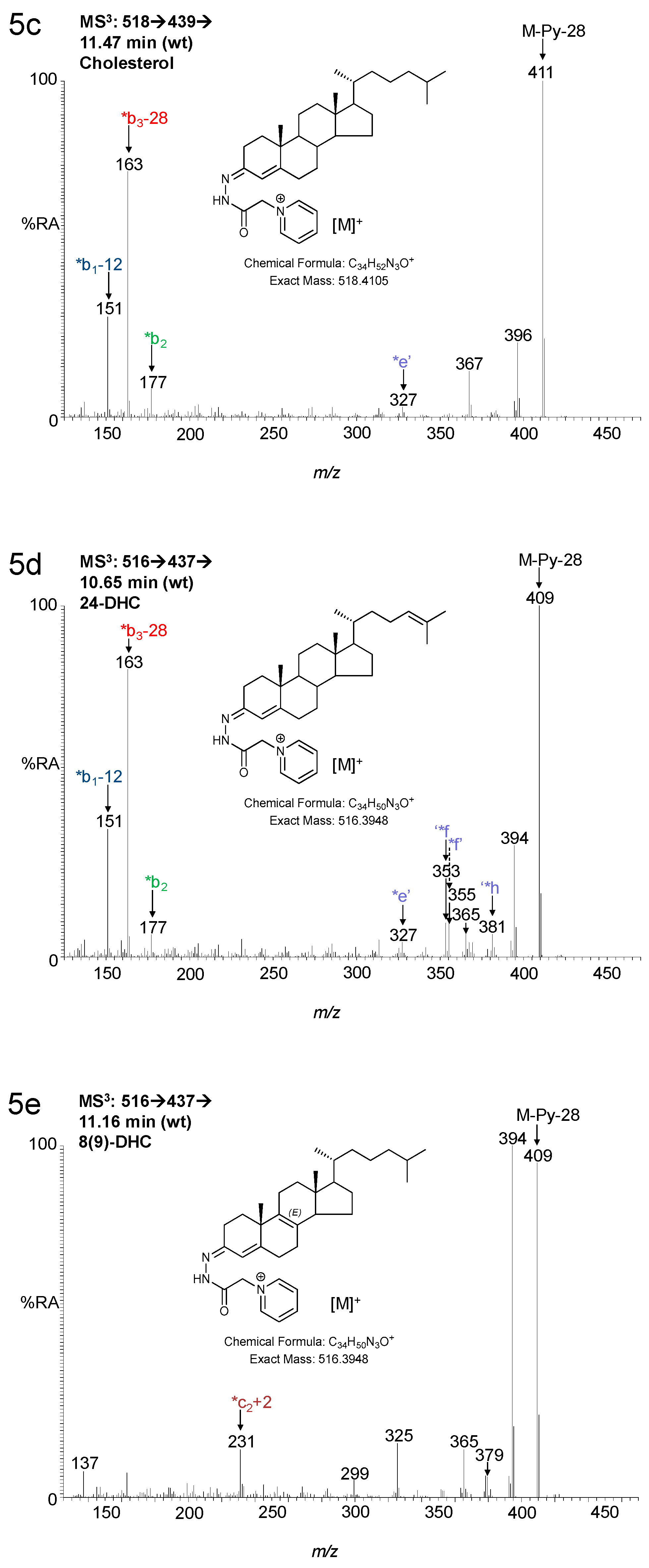
3.1.2. Sterols
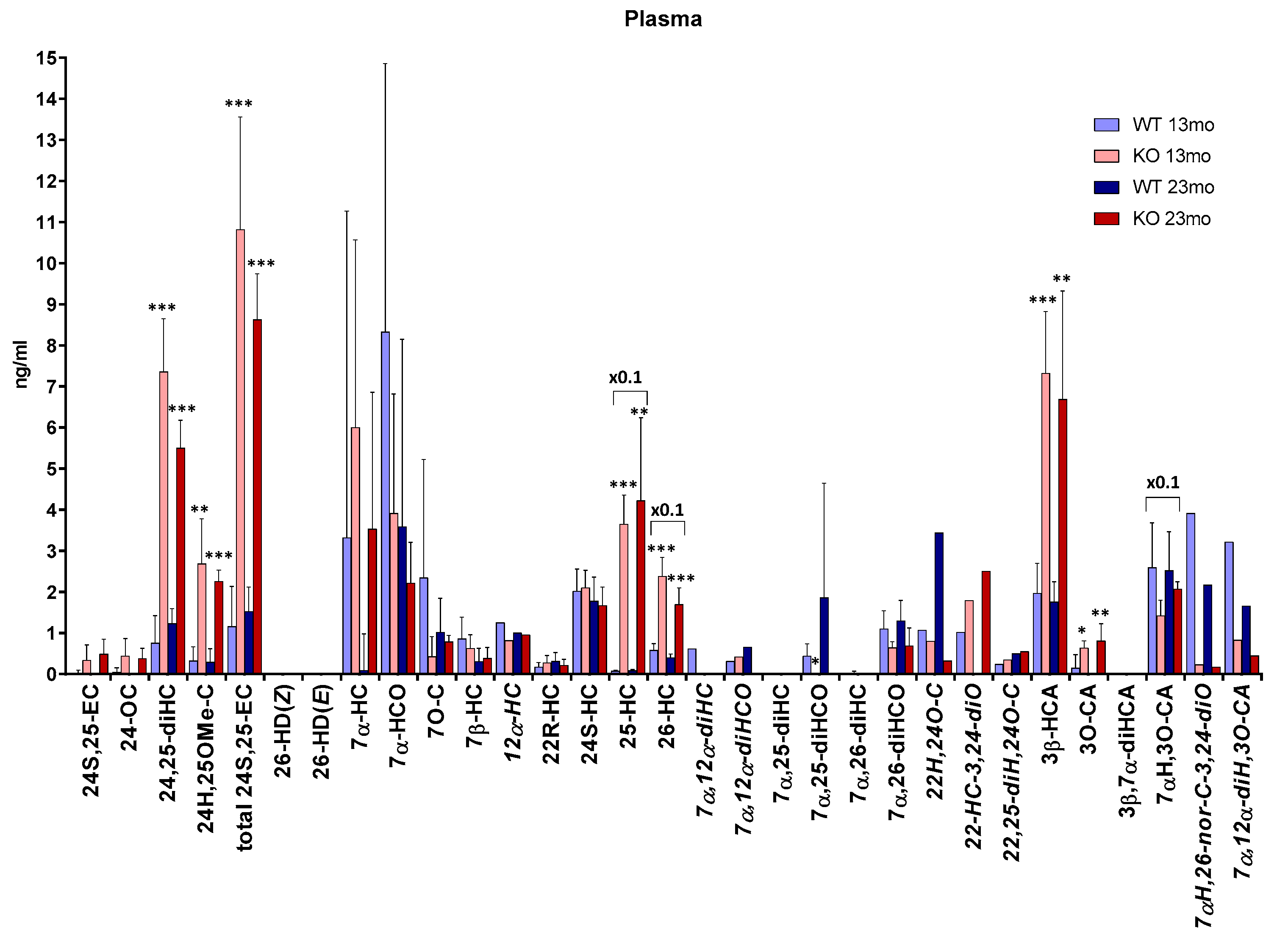
3.2. Plasma
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stapleton, G.; Steel, M.; Richardson, M.; Mason, J.O.; Rose, K.A.; Morris, R.G.; Lathe, R. A novel cytochrome P450 expressed primarily in brain. J. Biol. Chem. 1995, 270, 29739–29745. [Google Scholar]
- Rose, K.A.; Stapleton, G.; Dott, K.; Kieny, M.P.; Best, R.; Schwarz, M.; Russell, D.W.; Bjorkhem, I.; Seckl, J.; Lathe, R. Cyp7b, a novel brain cytochrome P450, catalyzes the synthesis of neurosteroids 7alpha-hydroxy dehydroepiandrosterone and 7alpha-hydroxy pregnenolone. Proc. Natl. Acad. Sci. USA 1997, 94, 4925–4930. [Google Scholar] [CrossRef]
- Rose, K.; Allan, A.; Gauldie, S.; Stapleton, G.; Dobbie, L.; Dott, K.; Martin, C.; Wang, L.; Hedlund, E.; Seckl, J.R.; et al. Neurosteroid hydroxylase CYP7B: vivid reporter activity in dentate gyrus of gene-targeted mice and abolition of a widespread pathway of steroid and oxysterol hydroxylation. J. Biol. Chem. 2001, 276, 23937–23944. [Google Scholar] [CrossRef]
- Martin, C.; Bean, R.; Rose, K.; Habib, F.; Seckl, J. cyp7b1 catalyses the 7alpha-hydroxylation of dehydroepiandrosterone and 25-hydroxycholesterol in rat prostate. Biochem. J. 2001, 355, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.; Schwarz, M.; O’Connell, N.C.; Lund, E.G.; Davis, D.L.; Lathe, R.; Thompson, H.R.; Weslie Tyson, R.; Sokol, R.J.; Russell, D.W. Identification of a new inborn error in bile acid synthesis: Mutation of the oxysterol 7alpha-hydroxylase gene causes severe neonatal liver disease. J. Clin. Investig. 1998, 102, 1690–1703. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.; Mills, P.B.; Footitt, E.; Gissen, P.; McClean, P.; Stahlschmidt, J.; Coupry, I.; Lavie, J.; Mochel, F.; Goizet, C.; et al. Liver disease in infancy caused by oxysterol 7 α-hydroxylase deficiency: Successful treatment with chenodeoxycholic acid. J. Inherit. Metab. Dis. 2014, 37, 851–861. [Google Scholar] [CrossRef]
- Yau, J.L.; Rasmuson, S.; Andrew, R.; Graham, M.; Noble, J.; Olsson, T.; Fuchs, E.; Lathe, R.; Seckl, J.R. Dehydroepiandrosterone 7-hydroxylase CYP7B: Predominant expression in primate hippocampus and reduced expression in Alzheimer’s disease. Neuroscience 2003, 121, 307–314. [Google Scholar] [CrossRef]
- Li-Hawkins, J.; Lund, E.G.; Turley, S.D.; Russell, D.W. Disruption of the oxysterol 7alpha-hydroxylase gene in mice. J. Biol. Chem. 2000, 275, 16536–16542. [Google Scholar] [CrossRef]
- Tsaousidou, M.K.; Ouahchi, K.; Warner, T.T.; Yang, Y.; Simpson, M.A.; Laing, N.G.; Wilkinson, P.A.; Madrid, R.E.; Patel, H.; Hentati, F.; et al. Sequence alterations within CYP7B1 implicate defective cholesterol homeostasis in motor-neuron degeneration. Am. J. Hum. Genet. 2008, 82, 510–515. [Google Scholar] [CrossRef]
- Schule, R.; Siddique, T.; Deng, H.X.; Yang, Y.; Donkervoort, S.; Hansson, M.; Madrid, R.E.; Siddique, N.; Schols, L.; Bjorkhem, I. Marked accumulation of 27-hydroxycholesterol in SPG5 patients with hereditary spastic paresis. J. Lipid Res. 2010, 51, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Schols, L.; Rattay, T.W.; Martus, P.; Meisner, C.; Baets, J.; Fischer, I.; Jagle, C.; Fraidakis, M.J.; Martinuzzi, A.; Saute, J.A.; et al. Hereditary spastic paraplegia type 5: Natural history, biomarkers and a randomized controlled trial. Brain 2017, 140, 3112–3127. [Google Scholar] [CrossRef]
- Theofilopoulos, S.; Griffiths, W.J.; Crick, P.J.; Yang, S.; Meljon, A.; Ogundare, M.; Kitambi, S.S.; Lockhart, A.; Tuschl, K.; Clayton, P.T.; et al. Cholestenoic acids regulate motor neuron survival via liver X receptors. J. Clin. Investig. 2014, 124, 4829–4842. [Google Scholar] [CrossRef]
- Marelli, C.; Lamari, F.; Rainteau, D.; Lafourcade, A.; Banneau, G.; Humbert, L.; Monin, M.L.; Petit, E.; Debs, R.; Castelnovo, G.; et al. Plasma oxysterols: Biomarkers for diagnosis and treatment in spastic paraplegia type 5. Brain 2018, 141, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Karu, K.; Meljon, A.; Turton, J.; Yau, J.L.; Seckl, J.R.; Wang, Y.; Griffiths, W.J. 24S,25-Epoxycholesterol in mouse and rat brain. Biochem. Biophys. Res. Commun. 2014, 449, 229–234. [Google Scholar] [CrossRef][Green Version]
- Radhakrishnan, A.; Ikeda, Y.; Kwon, H.J.; Brown, M.S.; Goldstein, J.L. Sterol-regulated transport of SREBPs from endoplasmic reticulum to Golgi: Oxysterols block transport by binding to Insig. Proc. Natl. Acad. Sci. USA 2007, 104, 6511–6518. [Google Scholar] [CrossRef]
- Lehmann, J.M.; Kliewer, S.A.; Moore, L.B.; Smith-Oliver, T.A.; Oliver, B.B.; Su, J.L.; Sundseth, S.S.; Winegar, D.A.; Blanchard, D.E.; Spencer, T.A.; et al. Activation of the nuclear receptor LXR by oxysterols defines a new hormone response pathway. J. Biol. Chem. 1997, 272, 3137–3140. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Liao, S. Cholestenoic acid is a naturally occurring ligand for liver X receptor alpha. Endocrinology 2000, 141, 4180–4184. [Google Scholar] [CrossRef]
- Fu, X.; Menke, J.G.; Chen, Y.; Zhou, G.; MacNaul, K.L.; Wright, S.D.; Sparrow, C.P.; Lund, E.G. 27-hydroxycholesterol is an endogenous ligand for liver X receptor in cholesterol-loaded cells. J. Biol. Chem. 2001, 276, 38378–38387. [Google Scholar] [CrossRef]
- Martin, M.G.; Pfrieger, F.; Dotti, C.G. Cholesterol in brain disease: Sometimes determinant and frequently implicated. EMBO Rep. 2014, 15, 1036–1052. [Google Scholar] [CrossRef] [PubMed]
- Meljon, A.; Theofilopoulos, S.; Shackleton, C.H.; Watson, G.L.; Javitt, N.B.; Knolker, H.J.; Saini, R.; Arenas, E.; Wang, Y.; Griffiths, W.J. Analysis of bioactive oxysterols in newborn mouse brain by LC/MS. J. Lipid Res. 2012, 53, 2469–2483. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, W.J.; Crick, P.J.; Meljon, A.; Theofilopoulos, S.; Abdel-Khalik, J.; Yutuc, E.; Parker, J.E.; Kelly, D.E.; Kelly, S.L.; Arenas, E.; et al. Additional pathways of sterol metabolism: Evidence from analysis of Cyp27a1-/-mouse brain and plasma. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 191–211. [Google Scholar] [CrossRef]
- Autio, K.J.; Schmitz, W.; Nair, R.R.; Selkala, E.M.; Sormunen, R.T.; Miinalainen, I.J.; Crick, P.J.; Wang, Y.; Griffiths, W.J.; Reddy, J.K.; et al. Role of AMACR (alpha-methylacyl-CoA racemase) and MFE-1 (peroxisomal multifunctional enzyme-1) in bile acid synthesis in mice. Biochem. J. 2014, 461, 125–135. [Google Scholar] [CrossRef]
- Crick, P.J.; Beckers, L.; Baes, M.; Van Veldhoven, P.P.; Wang, Y.; Griffiths, W.J. The oxysterol and cholestenoic acid profile of mouse cerebrospinal fluid. Steroids 2015, 99, 172–177. [Google Scholar] [CrossRef]
- Crick, P.J.; William Bentley, T.; Abdel-Khalik, J.; Matthews, I.; Clayton, P.T.; Morris, A.A.; Bigger, B.W.; Zerbinati, C.; Tritapepe, L.; Iuliano, L.; et al. Quantitative charge-tags for sterol and oxysterol analysis. Clin. Chem. 2015, 61, 400–411. [Google Scholar] [CrossRef]
- Cali, J.J.; Russell, D.W. Characterization of human sterol 27-hydroxylase. A mitochondrial cytochrome P-450 that catalyzes multiple oxidation reaction in bile acid biosynthesis. J. Biol. Chem. 1991, 266, 7774–7778. [Google Scholar]
- Heverin, M.; Meaney, S.; Brafman, A.; Shafir, M.; Olin, M.; Shafaati, M.; von Bahr, S.; Larsson, L.; Lovgren-Sandblom, A.; Diczfalusy, U.; et al. Studies on the cholesterol-free mouse: Strong activation of LXR-regulated hepatic genes when replacing cholesterol with desmosterol. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2191–2197. [Google Scholar] [CrossRef]
- Terwel, D.; Steffensen, K.R.; Verghese, P.B.; Kummer, M.P.; Gustafsson, J.A.; Holtzman, D.M.; Heneka, M.T. Critical role of astroglial apolipoprotein E and liver X receptor-alpha expression for microglial Abeta phagocytosis. J. Neurosci. 2011, 31, 7049–7059. [Google Scholar] [CrossRef]
- Schwarz, M.; Lund, E.G.; Russell, D.W. Two 7 alpha-hydroxylase enzymes in bile acid biosynthesis. Curr. Opin. Lipidol. 1998, 9, 113–118. [Google Scholar] [CrossRef]
- Shinkyo, R.; Xu, L.; Tallman, K.A.; Cheng, Q.; Porter, N.A.; Guengerich, F.P. Conversion of 7-dehydrocholesterol to 7-ketocholesterol is catalyzed by human cytochrome P450 7A1 and occurs by direct oxidation without an epoxide intermediate. J. Biol. Chem. 2011, 286, 33021–33028. [Google Scholar] [CrossRef]
- Schweizer, R.A.; Zurcher, M.; Balazs, Z.; Dick, B.; Odermatt, A. Rapid hepatic metabolism of 7-ketocholesterol by 11beta-hydroxysteroid dehydrogenase type 1: Species-specific differences between the rat, human, and hamster enzyme. J. Biol. Chem. 2004, 279, 18415–18424. [Google Scholar] [CrossRef]
- Zerbinati, C.; Iuliano, L. Cholesterol and related sterols autoxidation. Free Radic. Biol. Med. 2017, 111, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Ferdinandusse, S.; Houten, S.M. Peroxisomes and bile acid biosynthesis. Biochim. Biophys. Acta 2006, 1763, 1427–1440. [Google Scholar] [CrossRef] [PubMed]
- Vaz, F.M.; Ferdinandusse, S. Bile acid analysis in human disorders of bile acid biosynthesis. Mol. Asp. Med. 2017, 56, 10–24. [Google Scholar] [CrossRef]
- Stiles, A.R.; McDonald, J.G.; Bauman, D.R.; Russell, D.W. CYP7B1: One cytochrome P450, two human genetic diseases, and multiple physiological functions. J. Biol. Chem. 2009, 284, 28485–28489. [Google Scholar] [CrossRef]
- Wu, Z.; Martin, K.O.; Javitt, N.B.; Chiang, J.Y. Structure and functions of human oxysterol 7alpha-hydroxylase cDNAs and gene CYP7B1. J. Lipid Res. 1999, 40, 2195–2203. [Google Scholar] [PubMed]
- Lund, E.G.; Guileyardo, J.M.; Russell, D.W. cDNA cloning of cholesterol 24-hydroxylase, a mediator of cholesterol homeostasis in the brain. Proc. Natl. Acad. Sci. USA 1999, 96, 7238–7243. [Google Scholar] [CrossRef]
- Xie, C.; Lund, E.G.; Turley, S.D.; Russell, D.W.; Dietschy, J.M. Quantitation of two pathways for cholesterol excretion from the brain in normal mice and mice with neurodegeneration. J. Lipid Res. 2003, 44, 1780–1789. [Google Scholar] [CrossRef] [PubMed]
- Iuliano, L.; Crick, P.J.; Zerbinati, C.; Tritapepe, L.; Abdel-Khalik, J.; Poirot, M.; Wang, Y.; Griffiths, W.J. Cholesterol metabolites exported from human brain. Steroids 2015, 99, 189–193. [Google Scholar] [CrossRef]
- Li-Hawkins, J.; Lund, E.G.; Bronson, A.D.; Russell, D.W. Expression cloning of an oxysterol 7alpha-hydroxylase selective for 24-hydroxycholesterol. J. Biol. Chem. 2000, 275, 16543–16549. [Google Scholar] [CrossRef]
- Wang, L.; Schuster, G.U.; Hultenby, K.; Zhang, Q.; Andersson, S.; Gustafsson, J.A. Liver X receptors in the central nervous system: From lipid homeostasis to neuronal degeneration. Proc. Natl. Acad. Sci. USA 2002, 99, 13878–13883. [Google Scholar] [CrossRef]
- Russell, D.W. The enzymes, regulation, and genetics of bile acid synthesis. Annu. Rev. Biochem. 2003, 72, 137–174. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meljon, A.; Crick, P.J.; Yutuc, E.; Yau, J.L.; Seckl, J.R.; Theofilopoulos, S.; Arenas, E.; Wang, Y.; Griffiths, W.J. Mining for Oxysterols in Cyp7b1−/− Mouse Brain and Plasma: Relevance to Spastic Paraplegia Type 5. Biomolecules 2019, 9, 149. https://doi.org/10.3390/biom9040149
Meljon A, Crick PJ, Yutuc E, Yau JL, Seckl JR, Theofilopoulos S, Arenas E, Wang Y, Griffiths WJ. Mining for Oxysterols in Cyp7b1−/− Mouse Brain and Plasma: Relevance to Spastic Paraplegia Type 5. Biomolecules. 2019; 9(4):149. https://doi.org/10.3390/biom9040149
Chicago/Turabian StyleMeljon, Anna, Peter J. Crick, Eylan Yutuc, Joyce L. Yau, Jonathan R. Seckl, Spyridon Theofilopoulos, Ernest Arenas, Yuqin Wang, and William J. Griffiths. 2019. "Mining for Oxysterols in Cyp7b1−/− Mouse Brain and Plasma: Relevance to Spastic Paraplegia Type 5" Biomolecules 9, no. 4: 149. https://doi.org/10.3390/biom9040149
APA StyleMeljon, A., Crick, P. J., Yutuc, E., Yau, J. L., Seckl, J. R., Theofilopoulos, S., Arenas, E., Wang, Y., & Griffiths, W. J. (2019). Mining for Oxysterols in Cyp7b1−/− Mouse Brain and Plasma: Relevance to Spastic Paraplegia Type 5. Biomolecules, 9(4), 149. https://doi.org/10.3390/biom9040149





