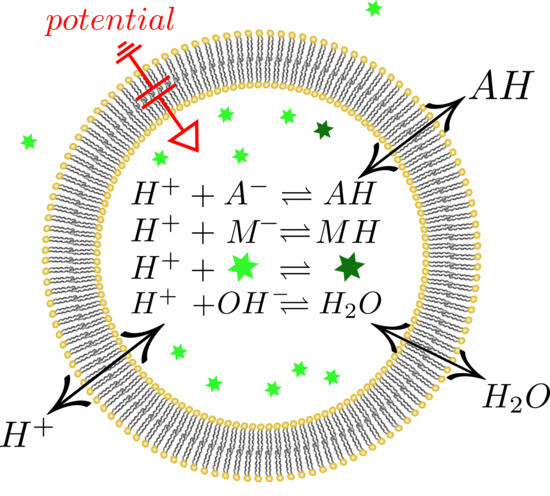
The Effect of Buffers on Weak Acid Uptake by Vesicles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Computation
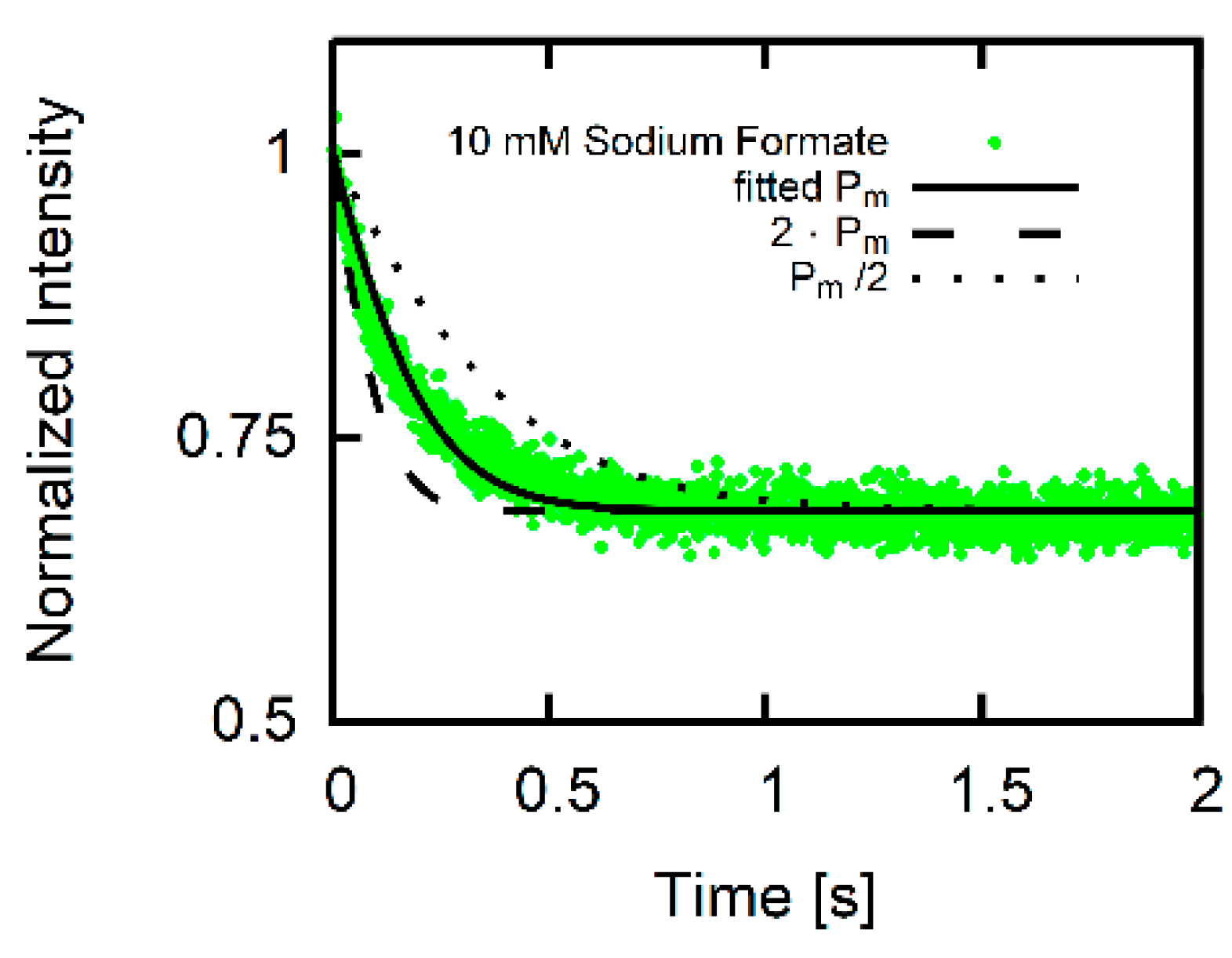
- Numerical calculation of , the time course of the concentration of fluorescent dye as function of for given initial conditions.
- is calculated from for varying .
- is fitted to using and as fitting parameters.
2.1.1. Temperature Correction
2.1.2. Additional Assumptions in the Model
- The time it takes a molecule to pass a membrane of thickness can be neglected. The time between the onset of the Fickian flux (Equation (2)) upon the application of is estimated as [25]:denotes the diffusion coefficient of a substance within the membrane. It can be approximated to be equal to ~1/10 of the aqueous diffusion coefficient [26]. amounts to ~ for L = and = . Thus, ≪ indicating that the application of gives rise to an instantaneous J.
- Solute bulk concentrations remain unaltered throughout the experiment because only 1/1000 of the volume of the suspension is encapsulated by vesicles. This can be estimated from (i) the mass concentration of lipid in the measurement cuvette (about ), (ii) the molar mass of 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC, ), and (iii) an area per lipid of approximately [27].
- Diffusion through stagnant water layers (unstirred layers) in the immediate membrane vicinity can be neglected because their width does not exceed the vesicle diameter [28]. A molecule with = crosses this distance within a few µs. is orders of magnitude larger.
- Carboxyfluorescein (CF) residues that display acidic pK’s are neglected since they do not contribute to buffer capacity at experimental pH. Only ~6.45 is considered. It is well described by the Henderson–Hasselbalch equation (Figure S1).
- For the same reason, the highly acidic pK of DOPC (2.25; [18]) is neglected.
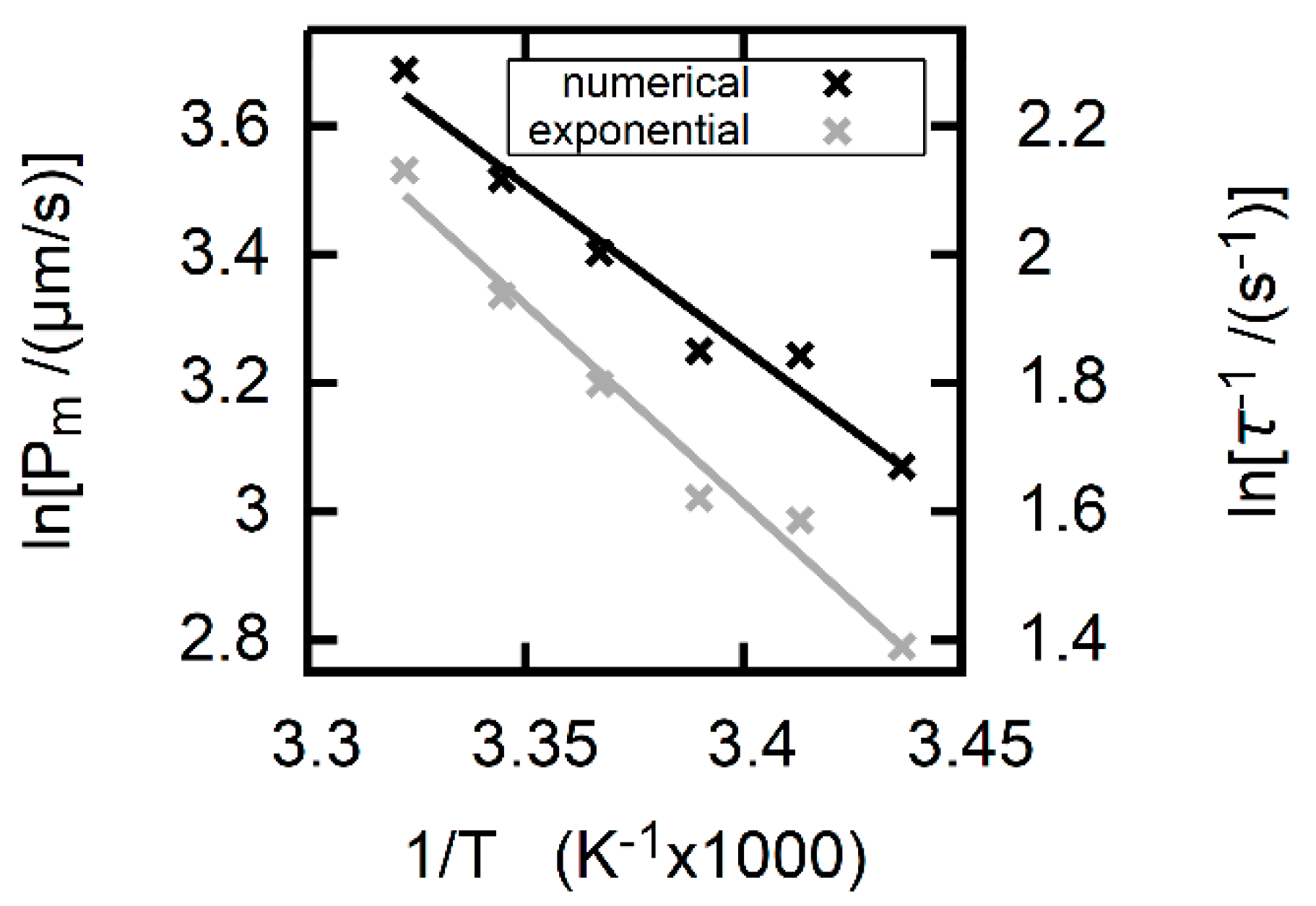
2.1.3. Activation Energy of Membrane Permeation
2.2. Buffers
2.3. Large Unilamellar Vesicles
2.4. Stopped Flow Experiments
2.5. Dynamic Light Scattering
2.6. Estimation of Proton Permeability
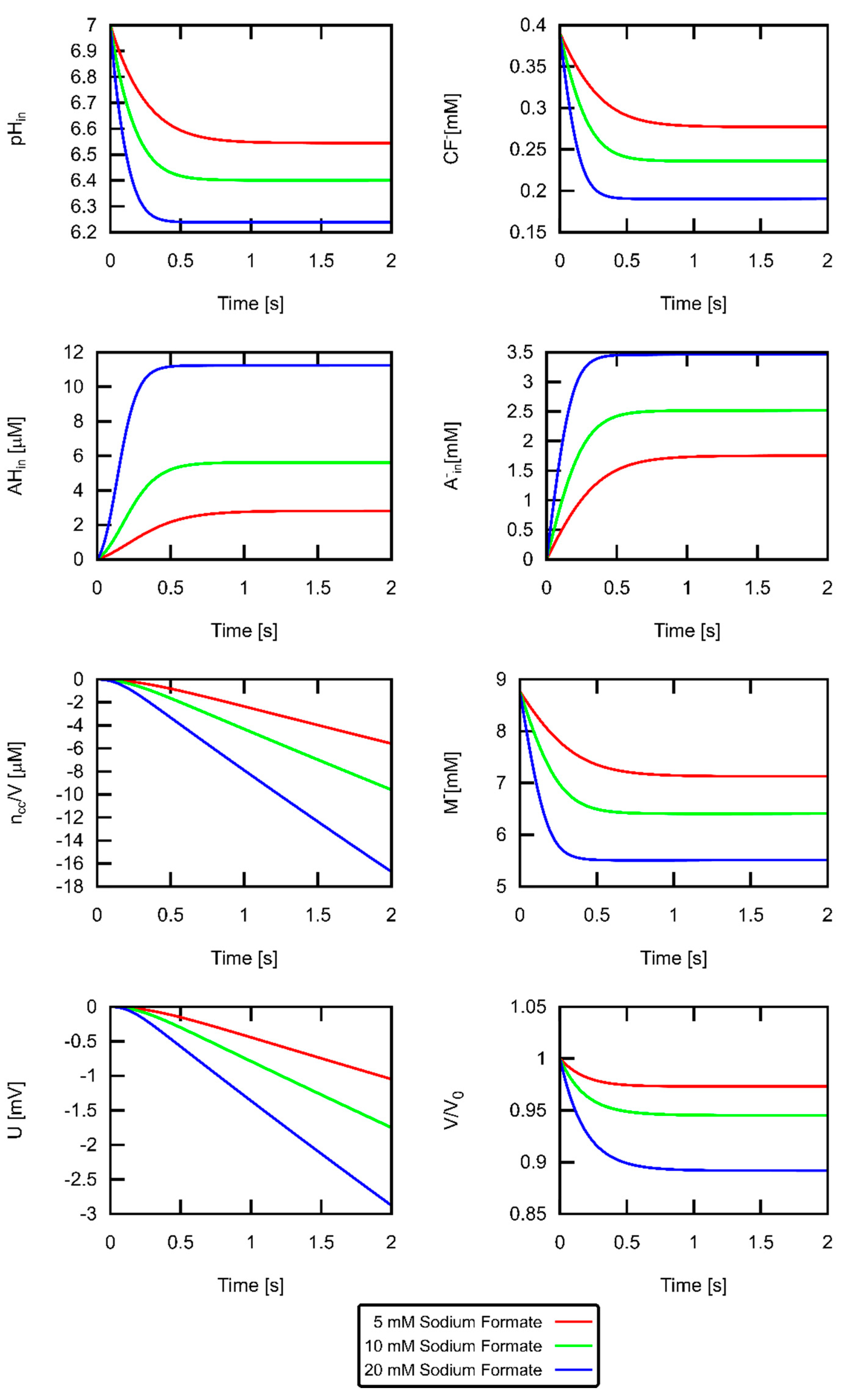
3. Results
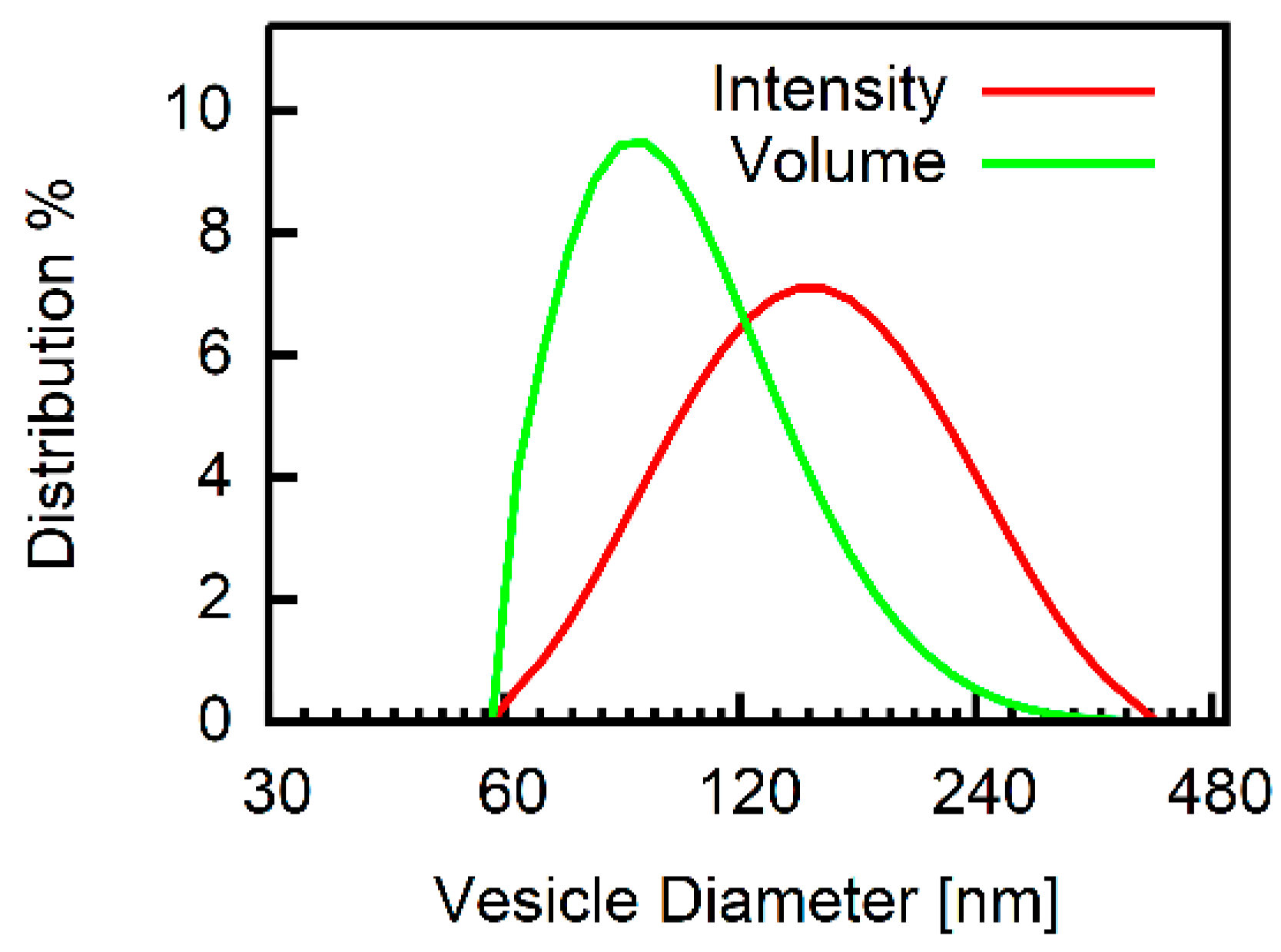
3.1. Vesicle Size
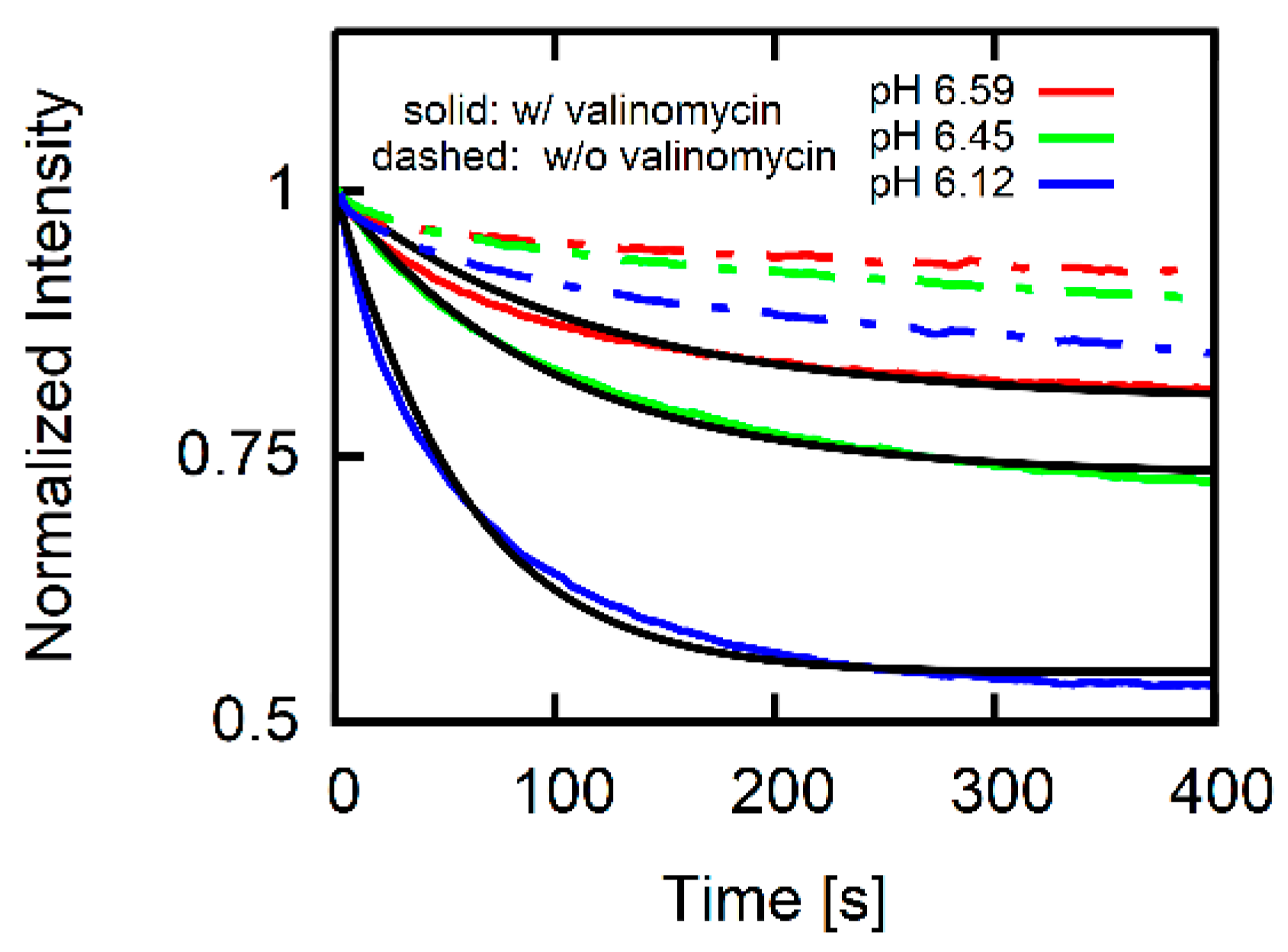
3.2. Estimate for Proton Permeability
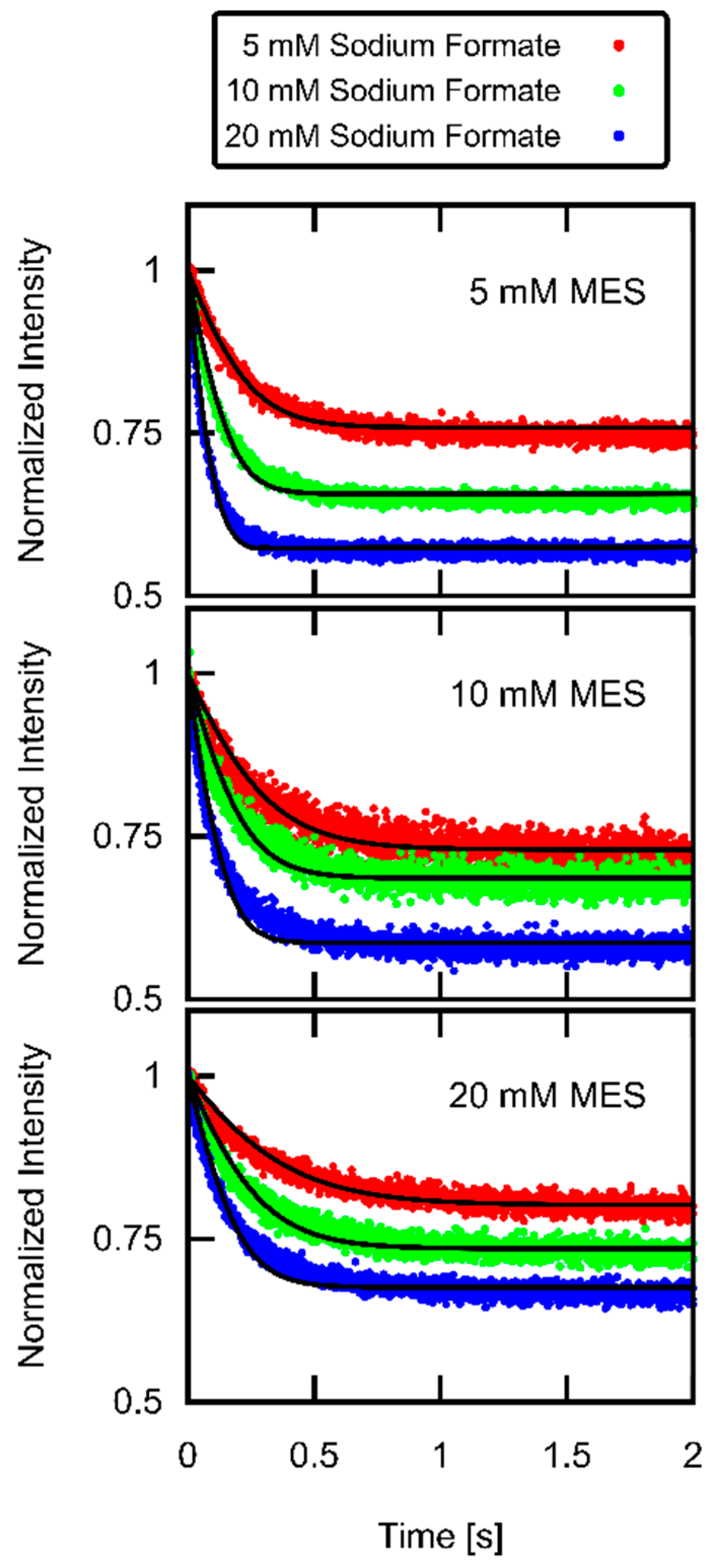
3.3. Formic Acid Membrane Permeability of DOPC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schanker, L.S.; Shore, P.A.; Brodie, B.B.; Hogben, C.A. Absorption of drugs from the stomach. I. The rat. J. Pharmacol. Exp. Ther. 1957, 120, 528–539. [Google Scholar] [PubMed]
- Schanker, L.S.; Tocco, D.J.; Brodie, B.B.; Hogben, C.A. Absorption of drugs from the rat small intestine. J. Pharmacol. Exp. Ther. 1958, 123, 81–88. [Google Scholar] [PubMed]
- Saparov, S.M.; Antonenko, Y.N.; Pohl, P. A new model of weak acid permeation through membranes revisited: Does overton still rule? Biophys. J. 2006, 90, L86–L88. [Google Scholar] [CrossRef] [PubMed]
- Madden, T.D.; Harrigan, P.R.; Tai, L.C.; Bally, M.B.; Mayer, L.D.; Redelmeier, T.E.; Loughrey, H.C.; Tilcock, C.P.; Reinish, L.W.; Cullis, P.R. The accumulation of drugs within large unilamellar vesicles exhibiting a proton gradient: A survey. Chem. Phys. Lipids 1990, 53, 37–46. [Google Scholar] [CrossRef]
- Missner, A.; Kugler, P.; Antonenko, Y.N.; Pohl, P. Passive transport across bilayer lipid membranes: Overton continues to rule. Proc. Natl. Acad. Sci. USA 2008, 105, E123. [Google Scholar] [CrossRef] [PubMed]
- Lande, M.B.; Priver, N.A.; Zeidel, M.L. Determinants of apical membrane permeabilities of barrier epithelia. Am. J. Physiol. Cell Physiol. 1994, 267, C367–C374. [Google Scholar] [CrossRef]
- Yang, B.; Fukuda, N.; van Hoek, A.; Matthay, M.A.; Ma, T.; Verkman, A.S. Carbon dioxide permeability of aquaporin-1 measured in erythrocytes and lung of aquaporin-1 null mice and in reconstituted proteoliposomes. J. Biol. Chem. 2000, 275, 2686–2692. [Google Scholar] [CrossRef]
- Sezer, D.; Oruc, T. Protonation kinetics compromise liposomal fluorescence assay of membrane permeation. J. Phys. Chem. B 2017, 121, 5218–5227. [Google Scholar] [CrossRef]
- Antonenko, Y.N.; Denisov, G.A.; Pohl, P. Weak acid transport across bilayer lipid membrane in the presence of buffers. Theoretical and experimental pH profiles in the unstirred layers. Biophys. J. 1993, 64, 1701–1710. [Google Scholar] [CrossRef]
- Antonenko, Y.N.; Pohl, P.; Denisov, G.A. Permeation of ammonia across bilayer lipid membranes studied by ammonium ion selective microelectrodes. Biophys. J. 1997, 72, 2187–2195. [Google Scholar] [CrossRef]
- Hannesschlaeger, C.; Pohl, P. Membrane permeabilities of ascorbic acid and ascorbate. Biomolecules 2018, 8, 73. [Google Scholar] [CrossRef] [PubMed]
- Rothert, M.; Ronfeldt, D.; Beitz, E. Electrostatic attraction of weak monoacid anions increases probability for protonation and passage through aquaporins. J. Biol. Chem. 2017, 292, 9358–9364. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.W.; Deamer, D.W. Net proton-hydroxyl permeability of large unilamellar liposomes measured by an acid-base titration technique. Proc. Natl. Acad. Sci. USA 1980, 77, 2038–2042. [Google Scholar] [CrossRef] [PubMed]
- Deamer, D.W.; Nichols, J.W. Proton-hydroxide permeability of liposomes. Proc. Natl. Acad. Sci. USA 1983, 80, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Goldman, D.E. Potential, impedance, and rectification in membranes. J. Gen. Physiol. 1943, 27, 37–60. [Google Scholar] [CrossRef] [PubMed]
- Hodgkin, A.L.; Katz, B. The effect of sodium ions on the electrical activity of giant axon of the squid. J. Physiol. 1949, 108, 37–77. [Google Scholar] [CrossRef] [PubMed]
- Hille, B. Ionic Channels of Excitable Membranes; Sinauer Associates: Franklin County, MA, USA, 1984; p. 230. [Google Scholar]
- Gutman, M.; Nachliel, E. The dynamic aspects of proton-transfer processes. Biochim. Biophys. Acta 1990, 1015, 391–414. [Google Scholar] [CrossRef]
- Chen, R.F.; Knutson, J.R. Mechanism of fluorescence concentration quenching of carboxyfluorescein in liposomes—Energy-transfer to nonfluorescent dimers. Anal. Biochem. 1988, 172, 61–77. [Google Scholar] [CrossRef]
- Wolfram Research, I. Mathematica, version 11.2; Wolfram Research, Inc.: Champaign, IL, USA, 2017. [Google Scholar]
- Hindmarsh, A.C.; Taylor, A.G. User documentation for IDA, a differential-algebraic equation solver for sequential and parallel computers. In Lawrence Livermore National Laboratory Report; UCRL-MA-136910; Center for Applied Scientific Computing, University of California: Okland, CA, USA, 1999. [Google Scholar]
- Agmon, N. Hydrogen bonds, water rotation and proton mobility. J. Chim. Phys. Phys. Chim. Biol. 1996, 93, 1714–1736. [Google Scholar] [CrossRef]
- Sengers, J.V.; Watson, J.T.R. Improved international formulations for the viscosity and thermal-conductivity of water substance. J. Phys. Chem. Ref. Data 1986, 15, 1291–1314. [Google Scholar] [CrossRef]
- Mathai, J.C.; Sprott, G.D.; Zeidel, M.L. Molecular mechanisms of water and solute transport across archaebacterial lipid membranes. J. Biol. Chem. 2001, 276, 27266–27271. [Google Scholar] [CrossRef] [PubMed]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Clarendon Press: Oxford, UK, 1975; p. 51. [Google Scholar]
- Bittermann, K.; Goss, K.U. Predicting apparent passive permeability of Caco-2 and MDCK cell-monolayers: A mechanistic model. PLoS ONE 2017, 12, e0190319. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Tristram-Nagle, S.; Kucerka, N.; Nagle, J.F. Temperature dependence of structure, bending rigidity, and bilayer interactions of dioleoylphosphatidylcholine bilayers. Biophys. J. 2008, 94, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Missner, A.; Pohl, P. 110 years of the Meyer-Overton rule: Predicting membrane permeability of gases and other small compounds. Chemphyschem 2009, 10, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- Walter, A.; Hastings, D.; Gutknecht, J. Weak acid permeability through lipid bilayer-membranes—Role of chemical-reactions in the unstirred layer. J. Gen. Physiol. 1982, 79, 917–933. [Google Scholar] [CrossRef] [PubMed]
- Padro, J.M.; Acquaviva, A.; Tascon, M.; Gagliardi, L.G.; Castells, C.B. Effect of temperature and solvent composition on acid dissociation equilibria, i: Sequenced (s)(s)pKa determination of compounds commonly used as buffers in high performance liquid chromatography coupled to mass spectroscopy detection. Anal. Chim. Acta 2012, 725, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Gutman, M. Application of the laser-induced proton pulse for measuring the protonation rate constants of specific sites on proteins and membranes. Method Enzymol. 1986, 127, 522–538. [Google Scholar]
- Good, N.E.; Winget, G.D.; Winter, W.; Connolly, T.N.; Izawa, S.; Singh, R.M.M. Hydrogen ion buffers for biological research. Biochemistry 1966, 5, 467–477. [Google Scholar] [CrossRef]
- Stillinger, F.H. Proton transfer reactions and kinetics in water. In Theoretical Chemistry: Advances and Perspectives; Eyring, H., Henderson, D., Eds.; Academic: New York, NY, USA, 1978; Volume 3, pp. 177–234. [Google Scholar]
- Marshall, W.L.; Franck, E.U. Ion product of water substance, 0–1000 °C, 1–10,000 bars—New international formulation and its background. J. Phys. Chem. Ref. Data 1981, 10, 295–304. [Google Scholar] [CrossRef]
- Tristram-Nagle, S.; Kim, D.J.; Akhunzada, N.; Kucerka, N.; Mathai, J.C.; Katsaras, J.; Zeidel, M.; Nagle, J.F. Structure and water permeability of fully hydrated diphytanoylPC. Chem. Phys. Lipids 2010, 163, 630–637. [Google Scholar] [CrossRef]
- Montal, M.; Mueller, P. Formation of bimolecular membranes from lipid monolayers and a study of their electrical properties. Proc. Natl. Acad. Sci. USA 1972, 69, 3561–3566. [Google Scholar] [CrossRef] [PubMed]
- Pohl, P.; Saparov, S.M.; Borgnia, M.J.; Agre, P. Highly selective water channel activity measured by voltage clamp: Analysis of planar lipid bilayers reconstituted with purified AqpZ. Proc. Natl. Acad. Sci. USA 2001, 98, 9624–9629. [Google Scholar] [CrossRef] [PubMed]
- Horner, A.; Pohl, P. Comment on “Enhanced water permeability and tunable ion selectivity in subnanometer carbon nanotube porins”. Science 2018, 359. [Google Scholar] [CrossRef] [PubMed]
- Papahadjopoulos, D.; Nir, S.; Oki, S. Permeability properties of phospholipid membranes: Effect of cholesterol and temperature. Biochim. Biophys. Acta 1972, 266, 561–583. [Google Scholar] [CrossRef]
- Vecer, J.; Herman, P.; Holoubek, A. Diffusion membrane potential in liposomes: Setting by ion gradients, absolute calibration and monitoring of fast changes by spectral shifts of diS-C3(3) fluorescence maximum. Biochim. Biophys. Acta 1997, 1325, 155–164. [Google Scholar] [CrossRef]
- Gutknecht, J. Proton conductance through phospholipid-bilayers—Water wires or weak acids. J. Bioenerg. Biomembr. 1987, 19, 427–442. [Google Scholar] [PubMed]
- Walter, A.; Gutknecht, J. Monocarboxylic acid permeation through lipid bilayer membranes. J. Membr. Biol. 1984, 77, 255–264. [Google Scholar] [CrossRef]
- Li, S.; Hu, P.C.; Malmstadt, N. Imaging molecular transport across lipid bilayers. Biophys. J. 2011, 101, 700–708. [Google Scholar] [CrossRef]
- Tsiavaliaris, G.; Itel, F.; Hedfalk, K.; Al-Samir, S.; Meier, W.; Gros, G.; Endeward, V. Low CO2 permeability of cholesterol-containing liposomes detected by stopped-flow fluorescence spectroscopy. FASEB J. 2015, 29, 1780–1793. [Google Scholar] [CrossRef]
- Missner, A.; Kugler, P.; Saparov, S.M.; Sommer, K.; Mathai, J.C.; Zeidel, M.L.; Pohl, P. Carbon dioxide transport through membranes. J. Biol. Chem. 2008, 283, 25340–25347. [Google Scholar] [CrossRef]
- Zocher, F.; Zeidel, M.L.; Missner, A.; Sun, T.T.; Zhou, G.; Liao, Y.; von Bodungen, M.; Hill, W.G.; Meyers, S.; Pohl, P.; et al. Uroplakins do not restrict CO2 transport through urothelium. J. Biol. Chem. 2012, 287, 11011–11017. [Google Scholar] [CrossRef] [PubMed]
- Gutknecht, J.; Bisson, M.A.; Tosteson, F.C. Diffusion of carbon dioxide through lipid bilayer membranes: Effects of carbonic anhydrase, bicarbonate, and unstirred layers. J. Gen. Physiol. 1977, 69, 779–794. [Google Scholar] [CrossRef] [PubMed]
- Widomska, J.; Raguz, M.; Subczynski, W.K. Oxygen permeability of the lipid bilayer membrane made of calf lens lipids. Biochim. Biophys. Acta (BBA)—Biomembr. 2007, 1768, 2635–2645. [Google Scholar] [CrossRef]
- Werber, J.R.; Elimelech, M. Permselectivity limits of biomimetic desalination membranes. Sci. Adv. 2018, 4, eaar8266. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S.; Dix, J.A.; Seifter, J.L. Water and urea transport in renal microvillus membrane vesicles. Am. J. Physiol. 1985, 248, F650–F655. [Google Scholar] [CrossRef] [PubMed]
- Eyer, K.; Paech, F.; Schuler, F.; Kuhn, P.; Kissner, R.; Belli, S.; Dittrich, P.S.; Kramer, S.D. A liposomal fluorescence assay to study permeation kinetics of drug-like weak bases across the lipid bilayer. J. Control Release 2014, 173, 102–109. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Symbol | Value | Unit | Reference |
|---|---|---|---|---|
| Acid dissociation constant formic acid | 3.75 | 1 | [29] | |
| Temperature shift of | 0.001 | [30] | ||
| Deprotonation rate formic acid | After [18] | |||
| Acid dissociation constant carboxyfluorescein | 6.45 | 1 | [19], Figure S1 | |
| Temperature shift of | −0.005 | Figure S1 | ||
| Deprotonation rate carboxyfluorescein | [31] | |||
| Acid dissociation constant MES | 6.15 | [32] | ||
| Temperature shift of | −0.011 | [32] | ||
| Deprotonation rate MES | After [18] | |||
| Water dissociation rate | [33] | |||
| Temperature shift of water dissociation constant | −0.033 | Linear approximation in relevant temperature range [34] | ||
| DOPC water permeability | 16 | [35] | ||
| Specific membrane capacity | 1 | [36] | ||
| Membrane proton permeability | This study |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hannesschlaeger, C.; Barta, T.; Pechova, H.; Pohl, P. The Effect of Buffers on Weak Acid Uptake by Vesicles. Biomolecules 2019, 9, 63. https://doi.org/10.3390/biom9020063
Hannesschlaeger C, Barta T, Pechova H, Pohl P. The Effect of Buffers on Weak Acid Uptake by Vesicles. Biomolecules. 2019; 9(2):63. https://doi.org/10.3390/biom9020063
Chicago/Turabian StyleHannesschlaeger, Christof, Thomas Barta, Hana Pechova, and Peter Pohl. 2019. "The Effect of Buffers on Weak Acid Uptake by Vesicles" Biomolecules 9, no. 2: 63. https://doi.org/10.3390/biom9020063
APA StyleHannesschlaeger, C., Barta, T., Pechova, H., & Pohl, P. (2019). The Effect of Buffers on Weak Acid Uptake by Vesicles. Biomolecules, 9(2), 63. https://doi.org/10.3390/biom9020063