Relationship between Circulating Serpina3g, Matrix Metalloproteinase-9, and Tissue Inhibitor of Metalloproteinase-1 and -2 with Chronic Obstructive Pulmonary Disease Severity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Measurement of Emphysema by High-Resolution Computed Tomography Scan
2.2. Laboratory Analysis
2.2.1. Sample Collection and Preparation
2.2.2. Measurement of Plasma Serpina3g Concentration
2.2.3. Measurement of MMP-9 Concentration
2.2.4. Measurement of TIMP-1 Concentration
2.2.5. Measurement of TIMP-2 Concentration
2.3. Statistical Analysis
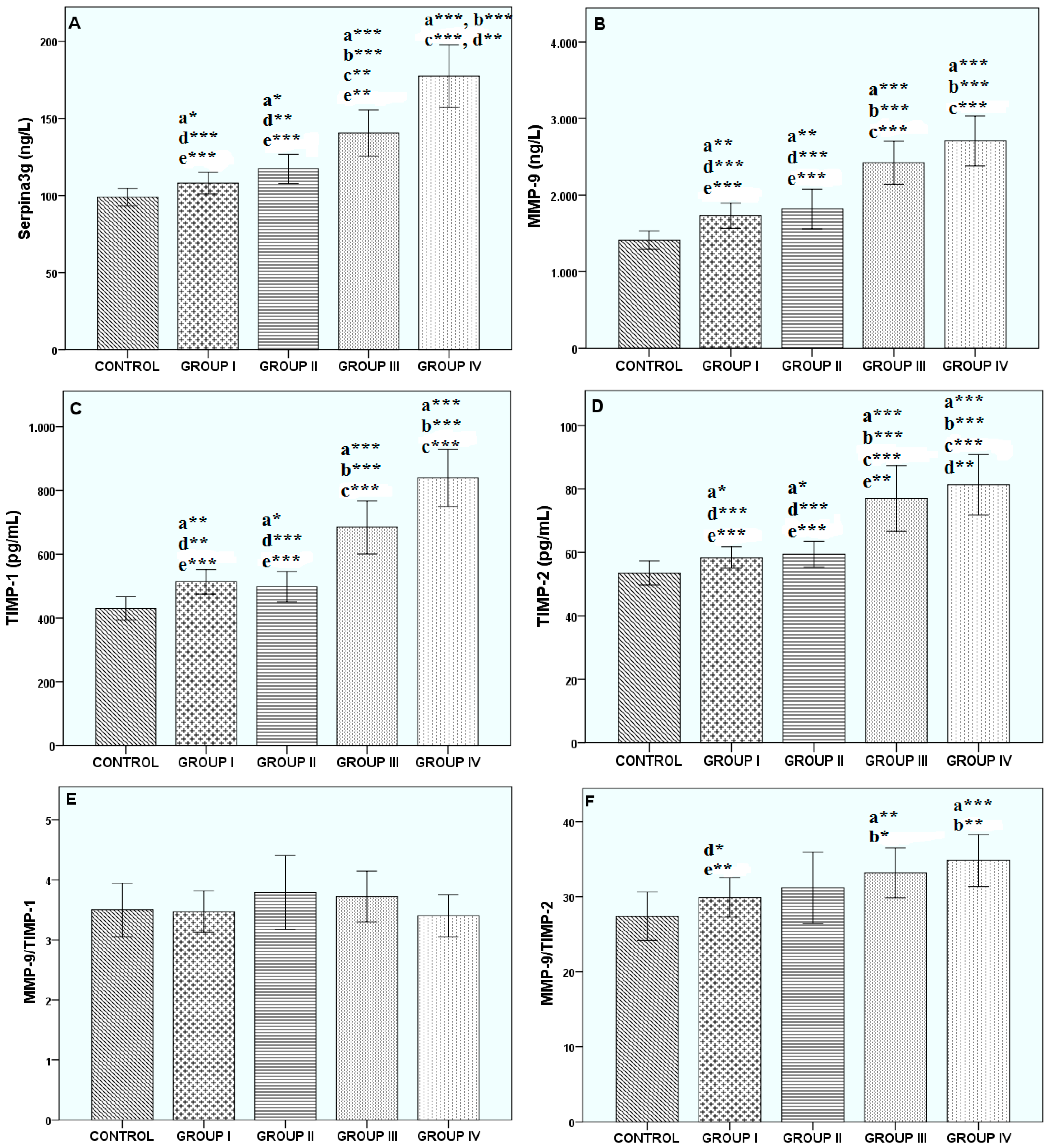
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Available online: http://www.who.int/respiratory/copd/burden/en/index.html (accessed on 24 March 2010).
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease. Available online: https://www.who.int/respiratory/copd/GOLD_WR_06.pdf (accessed on 13 February 2019).
- Fujimoto, K.; Ikeda, S.; Arai, T.; Tanaka, N.; Kumasaka, T.; Ishii, T.; Kida, K.; Muramatsu, M.; Sawabe, M. Polymorphism of SERPINE2 gene is associated with pulmonary emphysema in consecutive autopsy cases. BMC Med. Genet. 2010, 11, 159. [Google Scholar] [CrossRef] [PubMed]
- Irving, J.A.; Pike, R.N.; Lesk, A.M.; Whisstock, J.C. Phylogeny of the serpin superfamily: Implications of patterns of amino acid conservation for structure and function. Genome Res. 2000, 10, 1845–1864. [Google Scholar] [CrossRef] [PubMed]
- Silverman, G.A.; Bird, P.I.; Carrell, R.W.; Church, F.C.; Coughlin, P.B.; Gettins, P.G.; Irving, J.A.; Lomas, D.A.; Luke, C.J.; Moyer, R.W.; et al. The serpins are an expanding superfamily of structurally similar but functionally diverse proteins. Evolution, mechanism of inhibition, novel functions, and a revised nomenclature. J. Biol. Chem. 2001, 276, 33293–33296. [Google Scholar] [CrossRef] [PubMed]
- Law, R.H.; Zhang, Q.; McGowan, S.; Buckle, A.M.; Silverman, G.A.; Wong, W.; Rosado, C.J.; Langendorf, C.G.; Pike, R.N.; Bird, P.I.; et al. An overview of the serpin superfamily. Genome Biol. 2006, 7, 216. [Google Scholar] [CrossRef] [PubMed]
- Poller, W.; Faber, J.P.; Weidinger, S.; Tief, K.; Scholz, S.; Fischer, M.; Olek, K.; Kirchgesser, M.; Heidtmann, H. A leucine-to-proline substitution causes a defective alpha 1-antichymotrypsin allele associated with familial obstructive lung disease. Genomics 1993, 17, 740–743. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Matsuse, T.; Teramoto, S.; Matsui, H.; Hosoi, T.; Fukuchi, Y.; Ouchi, Y. Association between alpha-1-antichymotrypsin polymorphism and susceptibility to chronic obstructive pulmonary disease. Eur. J. Clin. Investig. 2000, 30, 543–548. [Google Scholar] [CrossRef]
- Benetazzo, M.G.; Gilè, L.S.; Bombieri, C.; Malerba, G.; Massobrio, M.; Pignatti, P.F.; Luisetti, M. Alpha 1-antitrypsin TAQ I polymorphism and alpha 1-antichymotrypsin mutations in patients with obstructive pulmonary disease. Respir. Med. 1999, 93, 648–654. [Google Scholar] [CrossRef]
- Hersh, C.P.; Demeo, D.L.; Lange, C.; Litonjua, A.A.; Reilly, J.J.; Kwiatkowski, D.; Laird, N.; Sylvia, J.S.; Sparrow, D.; Speizer, F.E.; et al. Attempted replication of reported chronic obstructive pulmonary disease candidate gene associations. Am. J. Respir. Cell Mol. Biol. 2005, 33, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Demedts, I.K.; Brusselle, G.G.; Bracke, K.R.; Vermaelen, K.Y.; Pauwels, R.A. Matrix metalloproteinases in asthma and COPD. Curr. Opin. Pharmacol. 2005, 5, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Culpitt, S.V.; Rogers, D.F.; Traves, S.L.; Barnes, P.J.; Donnelly, L.E. Sputum matrix metalloproteases: Comparison between chronic obstructive pulmonary disease and asthma. Respir. Med. 2005, 99, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Sharafkhaneh, A.; Hanania, N.A.; Kim, V. Pathogenesis of emphysema: From the bench to the bedside. Proc. Am. Thorac. Soc. 2008, 5, 475–477. [Google Scholar] [CrossRef] [PubMed]
- Calikoğlu, M.; Unlü, A.; Tamer, L.; Ozgür, E. MMP-9 and TIMP-1 levels in the sputum of patients with chronic obstructive pulmonary disease and asthma. Tuberk Toraks. 2006, 54, 114–121. [Google Scholar]
- Ohbayashi, H. Matrix metalloproteinases in lung diseases. Curr. Protein Pept. Sci. 2002, 3, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Kelly, E.A.; Jarjour, N.N. Role of matrix metalloproteinases in asthma. Curr. Opin. Pulm. Med. 2003, 9, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Delclaux, C.; Delacourt, C.; D’Ortho, M.P.; Boyer, V.; Lafuma, C.; Harf, A. Role of gelatinase B and elastase in human polymorphonuclear neutrophil migration across basement membrane. Am. J. Respir. Cell Mol. Biol. 1996, 14, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Triebel, S.; Bläser, J.; Gote, T.; Pelz, G.; Schüren, E.; Schmitt, M.; Tschesche, H. Evidence for the tissue inhibitor of metalloproteinases-1 (TIMP-1) in human polymorphonuclear leukocytes. Eur. J. Biochem. 1995, 231, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Labierte, R.; Rpuabhia, M.; Bosse, M.; Chakir, J. Decreased capacity of asthmatic bronchial fibroblasts to degrade collogen. Matrix Biol. 2001, 19, 743–753. [Google Scholar] [CrossRef]
- Navratilova, Z.; Kolek, V.; Petrek, M. Matrix Metalloproteinases and Their Inhibitors in Chronic Obstructive Pulmonary Disease. Arch. Immunol. Ther. Exp. 2016, 64, 177–193. [Google Scholar] [CrossRef] [PubMed]
- Somborac-Bačura, A.; Popović-Grle, S.; Zovko, V.; Žanić-Grubišić, T. Cigarette Smoke Induces Activation of Polymorphonuclear Leukocytes. Lung 2018, 196, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.C.; De, S.; Mishra, P.K. Role of Proteases in Chronic Obstructive Pulmonary Disease. Front. Pharmacol. 2017, 8, 512. [Google Scholar] [CrossRef] [PubMed]
- Gilowska, I.; Kasper, Ł.; Bogacz, K.; Szczegielniak, J.; Szymasek, T.; Kasper, M.; Czerwinski, M.; Sładek, K.; Majorczyk, E. Impact of Matrix Metalloproteinase 9 on COPD Development in Polish Patients: Genetic Polymorphism, Protein Level, and Their Relationship with Lung Function. Biomed. Res. Int. 2018, 2018, 6417415. [Google Scholar] [CrossRef] [PubMed]
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of COPD. 2017. Available online: http://goldcopd.org (accessed on 20 December 2017).
- Rabe, K.F.; Hurd, S.; Anzueto, A.; Barnes, P.J.; Buist, S.A.; Calverley, P.; Fukuchi, Y.; Jenkins, C.; Rodriguez-Roisin van Weel, C.; Zielinski, J.; Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am. J. Respir. Crit. Care Med. 2007, 176, 532–555. [Google Scholar] [CrossRef] [PubMed]
- Diagnosis of Diseases of Chronic Airflow Limitation: Asthma, COPD and Asthma-COPD Overlap Syndrome(ACOS). 2015. Available online: http://www.mscbs.gob.es/organizacion/sns/planCalidadSNS/pdf/GOLD_ACOS_2015.pdf (accessed on 13 February 2019).
- Needham, M.; Stockley, R.A. Alpha 1-antitrypsin deficiency. 3: Clinical manifestations and natural history. Thorax 2004, 59, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Potempa, I.; Korzus, E.; Travis, I. The serpin superfamily of proteinase inhibitors: Structure, function, and regulation. J. Biol. Chem. 1994, 269, 15957–15960. [Google Scholar] [PubMed]
- Vernooy, J.H.; Lindeman, J.H.; Jacobs, J.A.; Hanemaaijer, R.; Wouters, E.F. Increased activity of matrix metalloproteinase-8 and matrix metalloproteinase-9 in induced sputum from patients with COPD. Chest 2004, 126, 1802–1810. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, R.; Zhong, R. Extracellular matrix promotes proliferation, migration and adhesion of airway smooth muscle cells in a rat model of chronic obstructive pulmonary disease via upregulation of the PI3K/AKT signaling pathway. Mol. Med. Rep. 2018, 18, 3143–3152. [Google Scholar] [CrossRef] [PubMed]
- Linder, R.; Rönmark, E.; Pourazar, J.; Behndig, A.F.; Blomberg, A.; Lindberg, A. Proteolytic biomarkers are related to prognosis in COPD- report from a population-based cohort. Respir. Res. 2018, 19, 64. [Google Scholar] [CrossRef] [PubMed]
- Maskey-Warzęchowska, M.; Górska, K.; Nejman-Gryz, P.; Paplińska-Goryca, M.; Grzela, T.; Krejner, A.; Grzela, K.; Krenke, R. Matrix metalloproteinase 9 in exhaled breath condensate in patients with stable chronic obstructive pulmonary disease: An observational study. Pol. Arch. Intern. Med. 2018, 128, 427–433. [Google Scholar] [PubMed]
- Zhang, Y.; Ni, H.J.; Zhou, H.S. Study on the expression of Toll-like receptor 4 and matrix metalloproteinase-9 in patients with chronic obstructive pulmonary disease and their clinical significance. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 2185–2191. [Google Scholar] [PubMed]
- Xu, L.; Bian, W.; Gu, X.H.; Shen, C. Genetic polymorphism in matrix metalloproteinase-9 and transforming growth factor-β1 and susceptibility to combined pulmonary fibrosis and emphysema in a Chinese population. Kaohsiung J. Med. Sci. 2017, 33, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Sng, J.J.; Prazakova, S.; Thomas, P.S.; Herbert, C. MMP-8, MMP-9 and Neutrophil Elastase in Peripheral Blood and Exhaled Breath Condensate in COPD. COPD 2017, 14, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, Y.; Zhao, Z.; Wang, J.; Li, J.; Wang, W.; Li, S.; Song, L. Relationships of MMP-9 and TIMP-1 proteins with chronic obstructive pulmonary disease risk: A systematic review and meta-analysis. J. Res. Med. Sci. 2016, 21, 12. [Google Scholar] [CrossRef] [PubMed]
- Chappell, S.; Daly, L.; Morgan, K.; Guetta Baranes, T.; Roca, J.; Rabinovich, R.; Millar, A.; Donnelly, S.C.; Keatings, V.; MacNee, W.; et al. Cryptic haplotypes of SERPINA1 confer susceptibility to chronic obstructive pulmonary disease. Hum. Mutat. 2006, 27, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Hollander, C.; Westin, U.; Wallmark, A.; Piitulainen, E.; Sveger, T.; Janciauskiene, S.M. Plasma levels of alpha1-antichymotrypsin and secretory leukocyte proteinase inhibitor in healthy and chronic obstructive pulmonary disease (COPD) subjects with and without severe alpha1-antitrypsin deficiency. BMC Pulm. Med. 2007, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.Y.; Lv, J.H.; Ma, C.Y.; Yang, D.P.; Wang, T. Tissue inhibitor of metalloproteinase-1 decreased chemosensitivity of MDA-435 breast cancer cells to chemotherapeutic drugs through the PI3K/AKT/NF-κB pathway. Biomed. Pharmacother. 2011, 65, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Muller, V.; Riethdorf, S.; Rack, B.; Janni, W.; Fasching, P.A.; Solomayer, E.; Aktas, B.; Kasimir-Bauer, S.; Zeitz, J.; Pantel, K.; et al. Prospective evaluation of serum tissue inhibitor of metalloproteinase 1 and carbonic anhydrase IX in correlation to circulating tumor cells in patients with metastatic breast cancer. Breast Cancer Res. 2011, 13, R71. [Google Scholar] [CrossRef] [PubMed]
- Montano, M.; Sansores, R.H.; Becerril, C.; Cisneros, J.; Gonzalez-Avila, G.; Sommer, B.; Ochoa, L.; Herrera, I.; Ramírez-Venegas, A.; Ramos, C. FEV1 inversely correlates with metalloproteinases 1, 7, 9 and CRP in COPD by biomass smoke exposure. Respir. Res. 2014, 15, 74. [Google Scholar] [PubMed]
- Pinto-Plata, V.; Casanova, C.; Müllerova, H.; de Torres, J.P.; Corado, H.; Varo, N.; Cordoba, E.; Zeineldine, S.; Paz, H.; Baz, R.; et al. Inflammatory and repair serum biomarker pattern: Association to clinical outcomes in COPD. Respir. Res. 2012, 13, 71. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Hwang, J.W.; Sundar, I.K.; Friedman, A.E.; McBurney, M.W.; Guarente, L.; Gu, W.; Kinnula, V.L.; Rahman, I. SIRT1 redresses the imbalance of tissue inhibitor of matrix metalloproteinase-1 and matrix metalloproteinase-9 in the development of mouse emphysema and human COPD. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 305, L615–L624. [Google Scholar] [CrossRef] [PubMed]
- Hirano, K.; Sakamoto, T.; Uchida, Y.; Morishima, Y.; Masuyama, K.; Ishii, Y.; Nomura, A.; Ohtsuka, M.; Sekizawa, K. Tissue inhibitor of metalloproteinases-2 gene polymorphisms in chronic obstructive pulmonary disease. Eur. Respir. J. 2001, 18, 748–752. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M. The Role of MMPs in the Progression of Chronic Lung Inflammatory Diseases. In Lung Inflammation; Ong, K.C., Ed.; IntechOpen Limited: London, UK, 2014; Available online: https://www.intechopen.com/books/lung-inflammation/the-role-of-mmps-in-the-progression-of-chronic-lung-inflammatory-diseases (accessed on 10 May 2018).
- Mulyadi, S.; Azhary, M.; Yunus, F.; Nurwidya, F. The correlation of age and body mass index with the level of both protease MMP3 and anti-protease TIMP-1 among Indonesian patients with chronic obstructive pulmonary disease: A preliminary findings. BMC Res. Notes 2018, 11, 551. [Google Scholar] [CrossRef] [PubMed]
| Control (n = 35) | Group I (Mild) (n = 45) | Group II (Moderate) (n = 35) | Group III (Severe) (n = 35) | Group IV (Very Severe) (n = 35) | |
|---|---|---|---|---|---|
| Age (years) | 46.91 ± 7.96 | 57.78 ± 11.88 a***,c* | 63.31 ±11.98 a***, b* | 67.03 ± 11.13 a***, b*** | 65.44 ± 10.45 a***, b** |
| Female/Male | 15/20 | 16/34 | 13/19 | 10/20 | 11/27 |
| FEV1 (% predicted) | 102.06 ± 8.40 | 78.84 ± 15.25 a***,d***,e*** | 75.50 ± 10.50 a***,d***,e*** | 40.33 ± 9.36 a***,b***,c***,e* | 35.00 ± 10.17 a***,b***,c***,d* |
| FEV1/FVC | 83.91 ± 4.34 | 65.31 ± 7.16 a***,d***,e*** | 67.41 ± 3.77 a***,d***,e*** | 55.20 ± 10.04 a***,b***,c***,e* | 49.54 ± 9.90 a***,b***,c***,d* |
| WBC (×103/μL) | 7.43 ± 1.19 | 8.30 ± 3.03 | 7,59 ± 2,07 e* | 8,97 ± 3.51 | 9.14 ± 2.99 a***,c* |
| Total Protein (g/dL) | 8.06 ± 0.36 | 7.38 ± 0.48 a***,e*** | 7,31 ± 0,60 a***,e*** | 7,28 ± 0,45 a***,e** | 6.91 ± 0,64 a***,b***,c***,d** |
| Albumin (g/dL) | 4.06 ± 0.28 | 3.62 ± 0.38 a***,e** | 3,66 ± 0,43 a***,e*** | 3,47 ± 0,44 a*** | 3.39 ± 0.50 a***,b**,c*** |
| ESR (mm/h) | 11.47 ± 5.90 | 18.18 ± 11.38 a*** | 18.73 ± 11.13 a*** | 22.30 ± 12.59 a*** | 21.95 ± 15.83 a*** |
| CRP (mg/L) | 0.33 ± 0.19 | 0.65 ± 0.55 a***,d* | 0.52 ± 0.50 | 0.74 ± 0.61 a*** | 0.58 ± 0.50 a** |
| Serpina3g (ng/mL) | MMP-9 (ng/mL) | TIMP-1 (pg/mL) | TIMP-2 (pg/mL) | MMP-9/TIMP-1 | MMP-9/TIMP-2 | FEV1 (% Predicted) | FEV1/FVC | ||
|---|---|---|---|---|---|---|---|---|---|
| Serpina3g (ng/mL) | r | 1 | 0.576 ** | 0.734 ** | 0.839 ** | −0.197 | −0.259 | −0.666 ** | −0.431 ** |
| p | 0.000 | 0.000 | 0.000 | 0.230 | 0.111 | 0.000 | 0.006 | ||
| MMP-9 (ng/mL) | r | 0.576 ** | 1 | 0.578 ** | 0.627 ** | 0.384 * | 0.426 ** | −0.477 ** | −0.305 |
| p | 0.000 | 0.000 | 0.000 | 0.016 | 0.007 | 0.002 | 0.059 | ||
| TIMP-1 (ng/mL) | r | 0.734 ** | 0.578 ** | 1 | 0.773 ** | −0.508 ** | −0.212 | −0.934 ** | −0.699 ** |
| p | 0.000 | 0.000 | 0.000 | 0.001 | 0.195 | 0.000 | 0.000 | ||
| TIMP-2 | r | 0.839 ** | 0.627 ** | 0.773 ** | 1 | −0.202 | −0.416 ** | −0.656 ** | −0.423 ** |
| p | 0.000 | 0.000 | 0.000 | 0.218 | 0.008 | 0.000 | 0.007 | ||
| MMP-9/TIMP-1 | r | −0.197 | 0.384 * | −0.508 ** | −0.202 | 1 | 0.670 ** | 0.553 ** | 0.507 ** |
| p | 0.230 | 0.016 | 0.001 | 0.218 | 0.000 | 0.000 | 0.001 | ||
| MMP-9/TIMP-2 | r | −0.259 | 0.426 ** | −0.212 | −0.416 ** | 0.670 ** | 1 | 0.192 | 0.117 |
| p | 0.111 | 0.007 | 0.195 | 0.008 | 0.000 | 0.241 | 0.479 | ||
| FEV1 (% predicted) | r | −0.666 ** | −0.477 ** | −0.934 ** | −0.656 ** | 0.553 ** | 0.192 | 1 | 0.741 ** |
| p | 0.000 | 0.002 | 0.000 | 0.000 | 0.000 | 0.241 | 0.000 | ||
| FEV1/FVC | r | −0.431 ** | −0.305 | −0.699 ** | −0.423 ** | 0.507 ** | 0.117 | 0.741 ** | 1 |
| p | 0.006 | 0.059 | 0.000 | 0.007 | 0.001 | 0.479 | 0.000 | ||
| Serpina3g (ng/mL) | MMP-9 (ng/mL) | TIMP-1 (pg/mL) | TIMP-2 (pg/mL) | MMP-9/TIMP-1 | MMP-9/TIMP-2 | FEV1 (% Predicted) | FEV1/FVC | ||
|---|---|---|---|---|---|---|---|---|---|
| Serpina3g (ng/mL) | r | 1 | 0.597 ** | 0.719 ** | 0.674 ** | −0.062 | 0.047 | −0.625 ** | −0.567 ** |
| p | 0.000 | 0.000 | 0.000 | 0.445 | 0.566 | 0.000 | 0.000 | ||
| MMP-9 (ng/mL) | r | 0.597 ** | 1 | 0.628 ** | 0.629 ** | 0.456 ** | 0.587 ** | −0.583 ** | −0.467 ** |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| TIMP-1 (ng/mL) | r | 0.719 ** | 0.628 ** | 1 | 0.755 ** | −0.341 ** | 0.021 | −0.691 ** | −0.684 ** |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.801 | 0.000 | 0.000 | ||
| TIMP-2 | r | 0.674 ** | 0.629 ** | 0.755 ** | 1 | −0.103 | −0.217 ** | −0.539 ** | −0.526 ** |
| p | 0.000 | 0.000 | 0.000 | 0.208 | 0.007 | 0.000 | 0.000 | ||
| MMP-9/TIMP-1 | r | −0.062 | 0.456 ** | −0.341 ** | −0.103 | 1 | 0.695 ** | 0.000 | 0.163 * |
| p | 0.445 | 0.000 | 0.000 | 0.208 | 0.000 | 0.996 | 0.045 | ||
| MMP-9/TIMP-2 | r | 0.047 | 0.587 ** | 0.021 | −0.217 ** | 0.695 ** | 1 | −0.221 ** | −0.055 |
| p | 0.566 | 0.000 | 0.801 | 0.007 | 0.000 | 0.006 | 0.504 | ||
| FEV1 (% predicted) | r | −0.625 ** | −0.583 ** | −0.691 ** | −0.539 ** | 0.000 | −0.221 ** | 1 | 0.731 ** |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.996 | 0.006 | 0.000 | ||
| FEV1/FVC | r | −0.567 ** | −0.467 ** | −0.684 ** | −0.526 ** | 0.163 * | −0.055 | 0.731 ** | 1 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.045 | 0.504 | 0.000 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uysal, P.; Uzun, H. Relationship between Circulating Serpina3g, Matrix Metalloproteinase-9, and Tissue Inhibitor of Metalloproteinase-1 and -2 with Chronic Obstructive Pulmonary Disease Severity. Biomolecules 2019, 9, 62. https://doi.org/10.3390/biom9020062
Uysal P, Uzun H. Relationship between Circulating Serpina3g, Matrix Metalloproteinase-9, and Tissue Inhibitor of Metalloproteinase-1 and -2 with Chronic Obstructive Pulmonary Disease Severity. Biomolecules. 2019; 9(2):62. https://doi.org/10.3390/biom9020062
Chicago/Turabian StyleUysal, Pelin, and Hafize Uzun. 2019. "Relationship between Circulating Serpina3g, Matrix Metalloproteinase-9, and Tissue Inhibitor of Metalloproteinase-1 and -2 with Chronic Obstructive Pulmonary Disease Severity" Biomolecules 9, no. 2: 62. https://doi.org/10.3390/biom9020062
APA StyleUysal, P., & Uzun, H. (2019). Relationship between Circulating Serpina3g, Matrix Metalloproteinase-9, and Tissue Inhibitor of Metalloproteinase-1 and -2 with Chronic Obstructive Pulmonary Disease Severity. Biomolecules, 9(2), 62. https://doi.org/10.3390/biom9020062





