Evaluation of Physicians’ Knowledge and Attitudes Towards Biosimilars in Russia and Issues Associated with Their Prescribing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Questionnaire and Recruitment
2.2. Analyses
3. Results
3.1. Participating Physicians
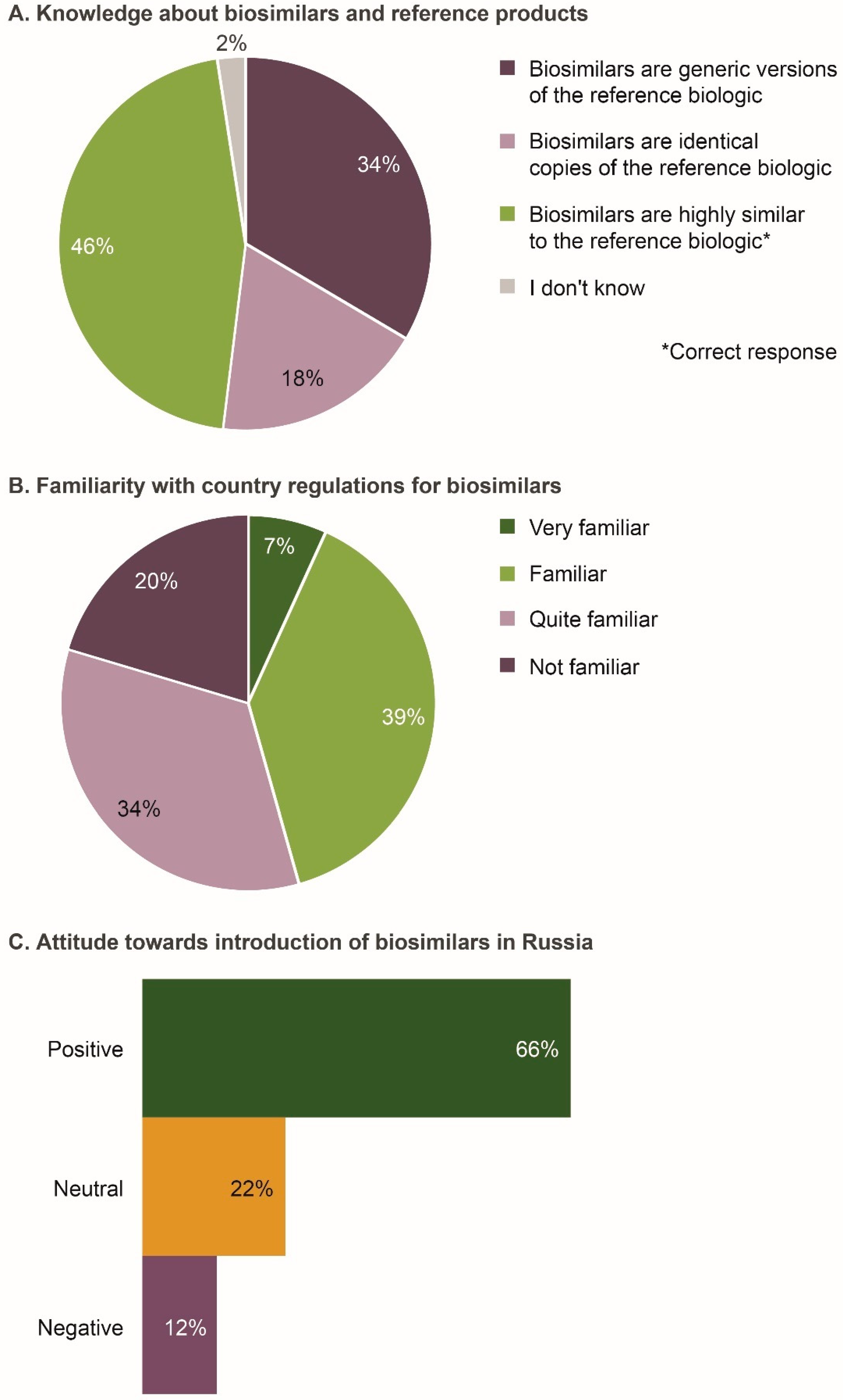
3.2. Knowledge of Biosimilars and Familiarity with Their Regulation in Russia
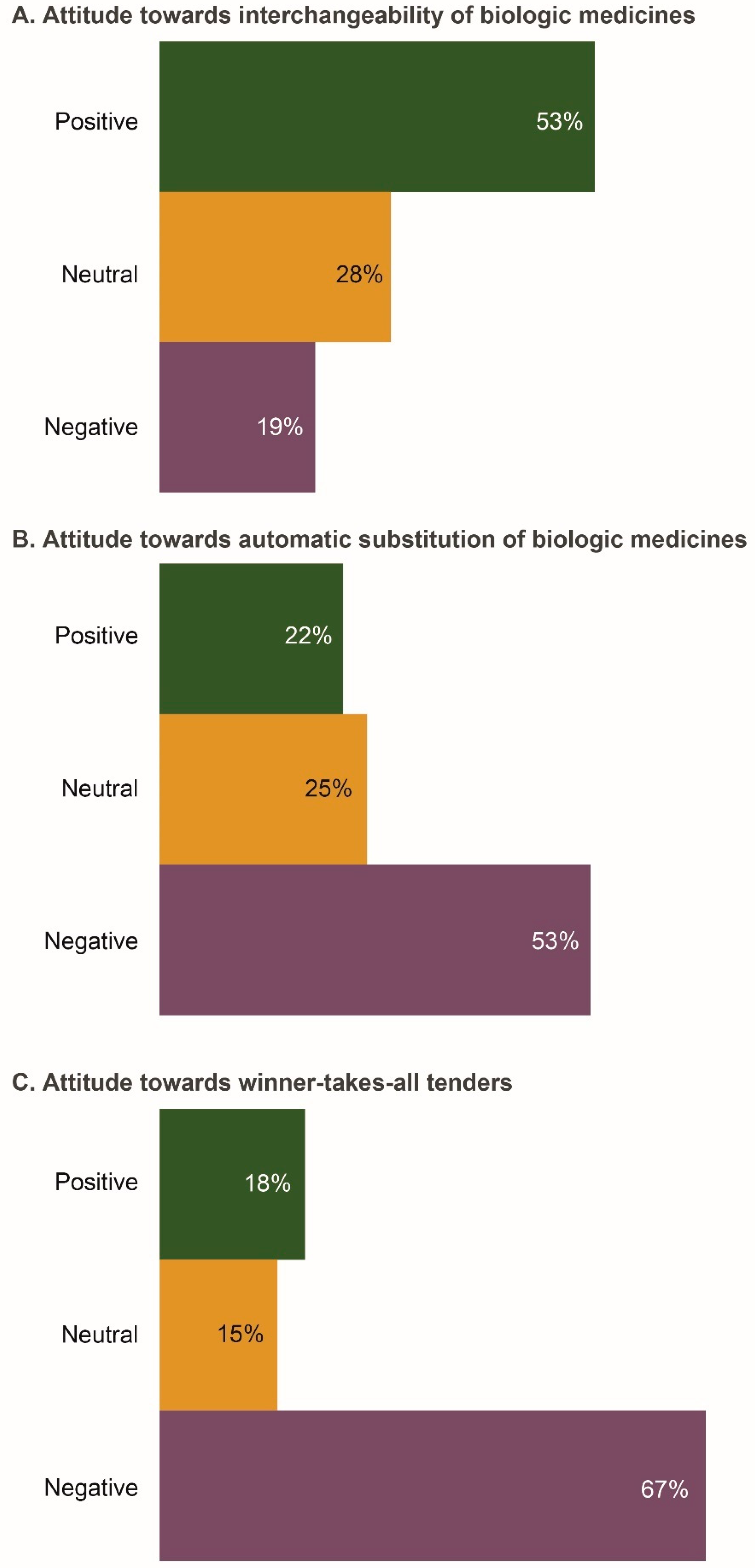
3.3. Attitudes Towards Key Policy Issues Associated with Prescribing Biosimilars
3.4. Guiding Factors for the Use of Biosimilar Products
3.5. Issues Related to Biosimilars in Professional Environments
3.6. Need for Biosimilars Education and Preferred Educational Format
3.7. Main Characteristics of Physician Groups Based on Their Knowledge of Biosimilars
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
References
- Braun, J.; Kudrin, A. Switching to biosimilar infliximab (CT-P13): Evidence of clinical safety, effectiveness and impact on public health. Biologicals 2016, 44, 257–266. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. Biosimilar Product Information. Available online: https://www.fda.gov/drugs/developmentapprovalprocess/howdrugsaredevelopedandapproved/approvalapplications/therapeuticbiologicapplications/biosimilars/ucm580432.htm (accessed on 6 August 2018).
- European Medicines Agency. European Public Assessment Reports. Available online: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/landing/epar_search.jsp&mid=WC0b01ac058001d124&searchTab=searchByAuthType&keyword=Enterkeywords&searchType=name&alreadyLoaded=true&status=Authorised&status=Withdrawn&status=Suspended&status=Refused&jsenabled=false&searchGenericType=biosimilars&orderBy=authDate&pageNo=1 (accessed on 6 August 2018).
- European Medicines Agency. Guideline on Similar Biological Medicinal Products. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/10/WC500176768. pdf (accessed on 22 February 2018).
- World Health Organization. Guidelines on Evaluation of Similar Biotherapeutic Products (SBPs) [Report]. Available online: http://www.who.int/biologicals/areas/biological_therapeutics/BIOTHERAPEUTICS_FOR_WEB_22APRIL2010.pdf (accessed on 22 February 2018).
- US Food and Drug Administration. Scientific Considerations in Demonstrating Biosimilarity to a Reference Product. Guidance for industry. Available online: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM291128.pdf (accessed on 22 February 2018).
- Weise, M.; Bielsky, M.C.; De Smet, K.; Ehmann, F.; Ekman, N.; Narayanan, G.; Heim, H.K.; Heinonen, E.; Ho, K.; Thorpe, R.; et al. Biosimilars– why terminology matters. Nat. Biotechnol. 2011, 29, 690–693. [Google Scholar] [CrossRef] [PubMed]
- Health Canada. Guidance Document. Information and Submission Requirements for Biosimilar Biologic Drugs. Available online: http://www.hc-sc.gc.ca/dhp-mps/alt_formats/pdf/brgtherap/applic-demande/guides/seb-pbu/seb-pbu-2016-eng.pdf (accessed on 22 February 2018).
- Ministry of Health of Russia. (State Register of Medicines). Available online: http://grls.rosminzdrav.ru/grls.aspx (accessed on 22 February 2018).
- Castañeda-Hernández, G.; Szekanecz, Z.; Mysler, E.; Azevedo, V.F.; Guzman, R.; Gutierrez, M.; Rodriguez, W.; Karateev, D. Biopharmaceuticals for rheumatic diseases in Latin America, Europe, Russia, and India: Innovators, biosimilars, and intended copies. Joint Bone Spine 2014, 81, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.L.; Chen, B.; Hermanson, T.; Wyatt, M.D.; Schulz, R.M.; Georgantopoulos, P.; Kessler, S.; Raisch, D.W.; Qureshi, Z.P.; Lu, Z.K.; et al. Regulatory and clinical considerations for biosimilar oncology drugs. Lancet Oncol. 2014, 15, e594–e605. [Google Scholar] [CrossRef]
- Kumar, R.; Sigala, S.; Malgarini, R.B.; Pimpinella, G.; Pani, L.; Pecorelli, S.; Memo, M. Biosimilars: Regulatory status and implications across the world. J. Pharmacovigilance 2015, S3, 002. [Google Scholar] [CrossRef]
- Lopes, G. Biosimilars in Emerging Markets: India and Russia. Available online: https://connection.asco.org/blogs/biosimilars-emerging-markets-india-and-russia (accessed on 22 February 2018).
- Ivanov, R. Russian Revolution for Biosimilars. Available online: https://themedicinemaker.com/issues/0217/russian-revolution-for-biosimilars/ (accessed on 22 February 2018).
- Mueller, L.L. An Overview of Biosimilars in the Russian Federation. Available online: https://bricwallblog.com/2014/05/29/an-overview-of-biosimilars-in-the-russian-federation/ (accessed on 22 February 2018).
- Government of Russian Federation. (Federal Law of December 22, 2014 N 429-FZ “On Amending the Federal Law”). Available online: https://rg.ru/2014/12/26/lekarstva-dok.html (accessed on 2 November 2017).
- Government of Russian Federation. (Federal Law No. 44-FZ of April 5, 2013 “On the Contract System in the Sphere of Procurement of Goods, Works, and Services for Ensuring State and Municipal Needs”). Available online: https://rg.ru/2013/04/12/goszakupki-dok.html (accessed on 2 November 2017).
- Daller, J. Biosimilars: A consideration of the regulations in the United States and European Union. Regul Toxicol Pharmacol. 2016, 76, 199–208. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. Nonproprietary Naming of Biological Products: Guidance for Industry. Available online: http://www.fda.gov/downloads/drugs/guidances/ucm459987.pdf (accessed on 22 February 2018).
- Camacho, L.H.; Frost, C.P.; Abella, E.; Morrow, P.K.; Whittaker, S. Biosimilars 101: Considerations for U.S. oncologists in clinical practice. Cancer Med. 2014, 3, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, J.G.; Green, L. Biologics, pharmacovigilance, and patient safety: it’s all in the name. J. Manag. Care Spec. Pharm. 2016, 22, 927–930. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Biological Qualifier: An INN Proposal (Report). Available online: http://www.who.int/medicines/services/inn/WHO_INN_BQ_proposal_2015.pdf?ua=1 (accessed on 22 February 2018).
- Ministry of Economic Development of Russian Federation. [Order N 155 from 25 March 2014, ‘On Conditions for the Admission of Goods Originating from Foreign Countries for the Purposes of the State Procurement’]. Available online: http://zakupki.gov.ru (accessed on 22 February 2018).
- Government of Russian Federation. (N1289 from 30 November 2015, “On Establishment of Restrictions and Conditions to Access of Medicines of Foreign Origin in the Frame of the State Procurement”). Available online: http://government.ru/ (accessed on 2 November 2017).
- Murashko, M.A.; Parkhomenko, D.V.; Asetskaya, I.L.; Kosenko, V.V.; Polivanov, V.A.; Glagolev, S.V. Topical issues of drug safety monitoring in the Russian Federation. Obstetrics and Gynecology: Russian Federation 2015, 2, 72–80. [Google Scholar]
- Karateev, D.E.; Mazurov, V.; Zonova, E.V.; Nesmeyanova, O.B.; Plaksina, T.V.; Krechikova, D.G.; Reshetko, O.V.; Denisov, L.N.; Gordeev, I.G.; Pokrovskaya, T.G.; et al. Comparative efficacy and safety of infliximab biosimilar (BCD-055) and innovator infliximab in patients with ankylosing spondylitis (results of international, multiple-center, double-blind phase I and phase III clinical studies). Sovremennaya Revmatologiya 2017, 11, 14–25. [Google Scholar] [CrossRef]
- Yoo, D.H.; Hrycaj, P.; Miranda, P.; Ramiterre, E.; Piotrowski, M.; Shevchuk, S.; Kovalenko, V.; Prodanovic, N.; Abello-Banfi, M.; Gutierrez-Urena, S.; et al. A randomised, double-blind, parallel-group study to demonstrate equivalence in efficacy and safety of CT-P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: The PLANETRA study. Ann. Rheum. Dis. 2013, 72, 1613–1620. [Google Scholar] [CrossRef] [PubMed]
- Park, W.; Hrycaj, P.; Jeka, S.; Kovalenko, V.; Lysenko, G.; Miranda, P.; Mikazane, H.; Gutierrez-Urena, S.; Lim, M.; Lee, Y.A.; et al. A randomised, double-blind, multicentre, parallel-group, prospective study comparing the pharmacokinetics, safety, and efficacy of CT-P13 and innovator infliximab in patients with ankylosing spondylitis: The PLANETAS study. Ann. Rheum. Dis. 2013, 72, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Danese, S.; Fiorino, G.; Michetti, P. Changes in biosimilar knowledge among European Crohn’s Colitis Organization (ECCO) members: An updated survey. J. Crohns. Colitis 2016, 10, 1362–1365. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, D.; Henderson, B.; Lam, D.; Keystone, E.C.; Thorne, C.; Jamal, S.; Pope, J.; Haraoui, B.; Lin, D.; Revers, L. Attitudes towards subsequent entry biologics/biosimilars: A survey of Canadian rheumatologists. Clin. Rheumatol. 2015, 34, 1427–1433. [Google Scholar] [CrossRef] [PubMed]
- Biosimilarscme.org. Exploring the Future of Biological Therapy and the Role of Biosimilars: White Paper. Available online: http://www.biosimilarscme.org/content/Biosimilars_whitepaper.pdf (accessed on 22 February 2018).
- Office of Research Ethics and Integrity; QUT. Human Research Ethics Guidance. Available online: http://www.orei.qut.edu.au/human/guidance/index.jsp (accessed on 22 February 2018).
- Biernacki, P.; Waldorf, D. Snowball sampling: Problems and techniques of chain referral sampling. Sociol. Methods Res. 1981, 10, 141–163. [Google Scholar] [CrossRef]
- Watt, J.H.; van den Berg, S. Chapter 6: Sampling. In Research Methods for Communication Science; Rensselaer Polytechnic Institute: Albany, NY, USA, 2002; pp. 62–81. [Google Scholar]
- Bethlehem, J. Applied Survey Methods: A Statistical Perspective; Shewhart, W.A., Wilks, S.S., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009. [Google Scholar] [CrossRef]
- Danese, S.; Fiorino, G.; Michetti, P. Viewpoint: Knowledge and viewpoints on biosimilar monoclonal antibodies among members of the European Crohn’s and Colitis Organization. J. Crohns. Colitis 2014, 8, 1548–1550. [Google Scholar] [CrossRef] [PubMed]
- Generics and Biosimilars Initiative (GaBi) Online. Non-Originator Infliximab Approved in Russia. Available online: http://www.gabionline.net/Biosimilars/News/Non-originator-infliximab-approved-in-Russia (accessed on 22 February 2018).
- Goss, P.E.; Strasser-Weippl, K.; Lee-Bychkovsky, B.L.; Fan, L.; Li, J.; Chavarri-Guerra, Y.; Liedke, P.E.; Pramesh, C.S.; Badovinac-Crnjevic, T.; Sheikine, Y.; et al. Challenges to effective cancer control in China, India, and Russia. Lancet Oncol. 2014, 15, 489–538. [Google Scholar] [CrossRef]
- Cherny, N.; Sullivan, R.; Torode, J.; Saar, M.; Eniu, A. ESMO European Consortium Study on the availability, out-of-pocket costs and accessibility of antineoplastic medicines in Europe. Ann. Oncol. 2016, 27, 1423–1443. [Google Scholar] [CrossRef] [PubMed]
- Baer Ii, W.H.; Maini, A.; Jacobs, I. Barriers to the access and use of rituximab in patients with non-Hodgkin’s lymphoma and chronic lymphocytic leukemia: A physician survey. Pharmaceuticals 2014, 7, 530–544. [Google Scholar] [CrossRef] [PubMed]
- Lammers, P.; Criscitiello, C.; Curigliano, G.; Jacobs, I. Barriers to the use of trastuzumab for HER2+ breast cancer and the potential impact of biosimilars: A physician survey in the United States and emerging markets. Pharmaceuticals 2014, 7, 943–953. [Google Scholar] [CrossRef] [PubMed]
| Statement | Agree/Strongly Agree |
|---|---|
| I am generally comfortable prescribing biologic drugs to my patients | 57% |
| If a drug has been approved by the Russian Ministry of Health, I would offer it to my patients because I am confident it is safe and efficacious | 68% |
| Biosimilars are essentially the same as generic drugs | 55% |
| Usually it is difficult to obtain information on clinical efficacy and safety for a biosimilar | 58% |
| Biosimilars clinical trial data should be included in labeling to guide physician and patient decisions | 93% |
| Publication (transparency) of clinical trial reports for biosimilars should be mandatory | 94% |
| The risk for side effects is greater with a biosimilar than for the reference product | 51% |
| Biosimilars should be subject to rigorous post-marketing surveillance, including establishing efficient patient registries | 98% |
| Biosimilars will have a significant impact on clinical practice in Russia for another 3–5 years | 83% |
| I would feel comfortable prescribing biosimilars if I am confident in their quality, efficacy, safety, and similar immunogenicity against the reference product | 91% |
| Statement | Important/Extremely Important | Selected as Being in the Three Most Important Statements |
|---|---|---|
| Studies that provide clinical immunogenicity data for the biosimilar and reference product | 97% | 42% |
| Studies that directly compare clinical efficacy and safety between reference products and biosimilars | 96% | 68% |
| Studies that show pharmacokinetic similarities between reference products and biosimilars | 96% | 30% |
| Inclusion in international and Russian clinical practice guidelines and standards of treatment | 95% | 55% |
| Studies that show chemical/physical similarities between reference products and biosimilars | 89% | 24% |
| Studies that compare activity with in vitro functional assays between reference products and biosimilars | 87% | 21% |
| Acquisition cost differences | 78% | 31% |
| Colleague and expert opinion | 78% | 8% |
| Payer decisions and requirements | 69% | 21% |
| Statement | Important/Extremely Important | Selected as Being in the Three Most Important Statements |
|---|---|---|
| Tracking safety events with biosimilars | 99% | 49% |
| Access to information on studies comparing biosimilars with reference biologics | 96% | 54% |
| Establish reasonable and scientifically justified approach to interchangeability and automatic substitution | 93% | 53% |
| Physician authority to decide on the most suitable biologic for each patient | 89% | 47% |
| Knowledge about biosimilars among interdisciplinary colleagues | 86% | 24% |
| Preparing (educating about biosimilars, which includes patients) to integrate biosimilars into clinical practice | 84% | 18% |
| Switching between reference biologics and biosimilars | 74% | 19% |
| Naming conventions for biosimilars (unique vs. same non-proprietary names) | 74% | 16% |
| Tender policy with preference for Russian manufacturers | 54% | 19% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karateev, D.; Belokoneva, N. Evaluation of Physicians’ Knowledge and Attitudes Towards Biosimilars in Russia and Issues Associated with Their Prescribing. Biomolecules 2019, 9, 57. https://doi.org/10.3390/biom9020057
Karateev D, Belokoneva N. Evaluation of Physicians’ Knowledge and Attitudes Towards Biosimilars in Russia and Issues Associated with Their Prescribing. Biomolecules. 2019; 9(2):57. https://doi.org/10.3390/biom9020057
Chicago/Turabian StyleKarateev, Dmitry, and Natalia Belokoneva. 2019. "Evaluation of Physicians’ Knowledge and Attitudes Towards Biosimilars in Russia and Issues Associated with Their Prescribing" Biomolecules 9, no. 2: 57. https://doi.org/10.3390/biom9020057
APA StyleKarateev, D., & Belokoneva, N. (2019). Evaluation of Physicians’ Knowledge and Attitudes Towards Biosimilars in Russia and Issues Associated with Their Prescribing. Biomolecules, 9(2), 57. https://doi.org/10.3390/biom9020057




