Heat Shock Protein 27 Phosphorylation Regulates Tumor Cell Migration under Shear Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Establishment of Fluorescence Protein Fused Heat Shock Protein 27
2.2. Cell Culture and Transfection
2.3. Flow Systems
2.4. Transwell Migration Assay
2.5. Image Acquisition
2.6. Image Analysis
2.7. Statistical Analysis
3. Results
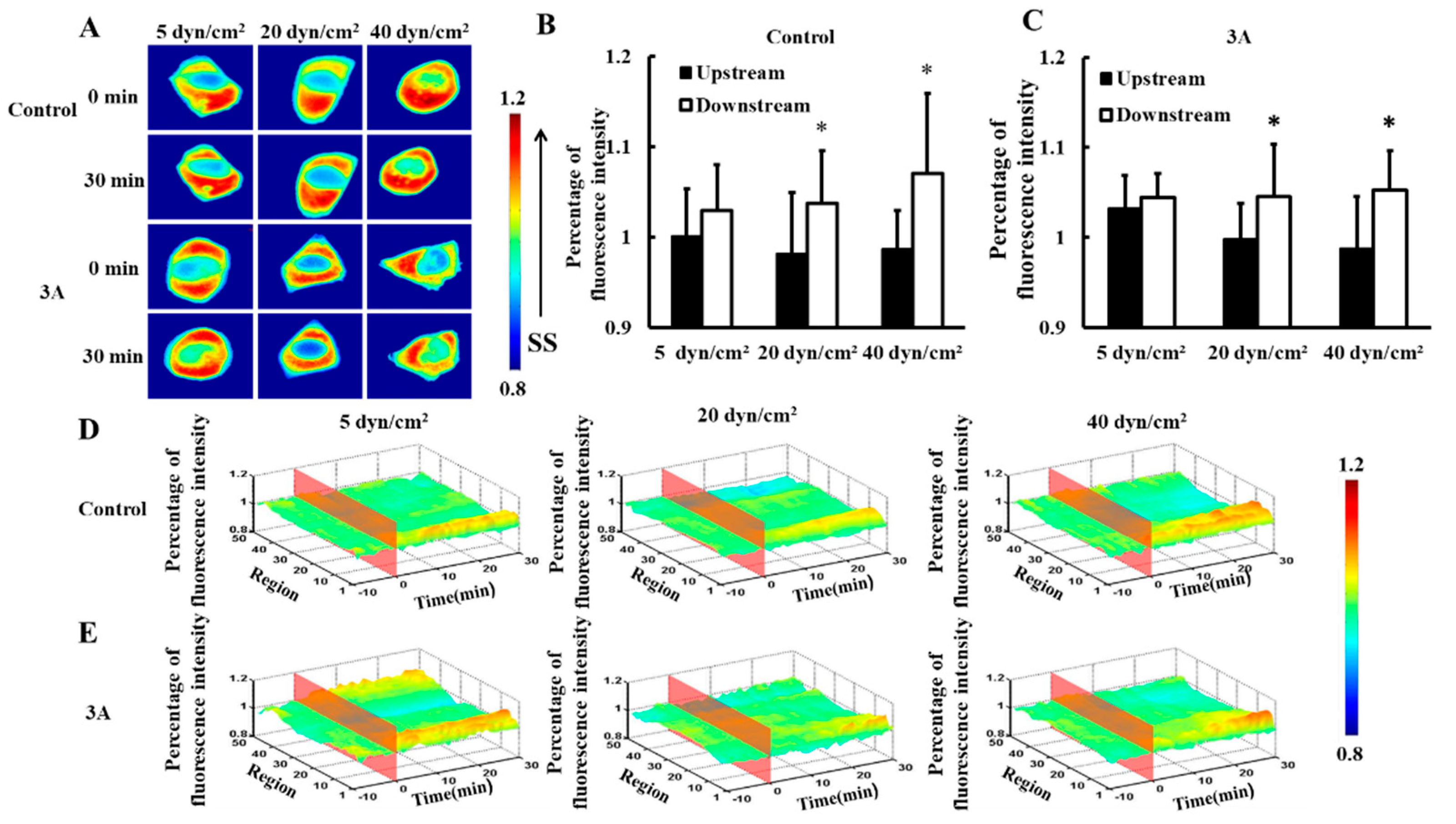
3.1. Shear Stress Induces Heat Shock Protein 27 Polarization Distribution
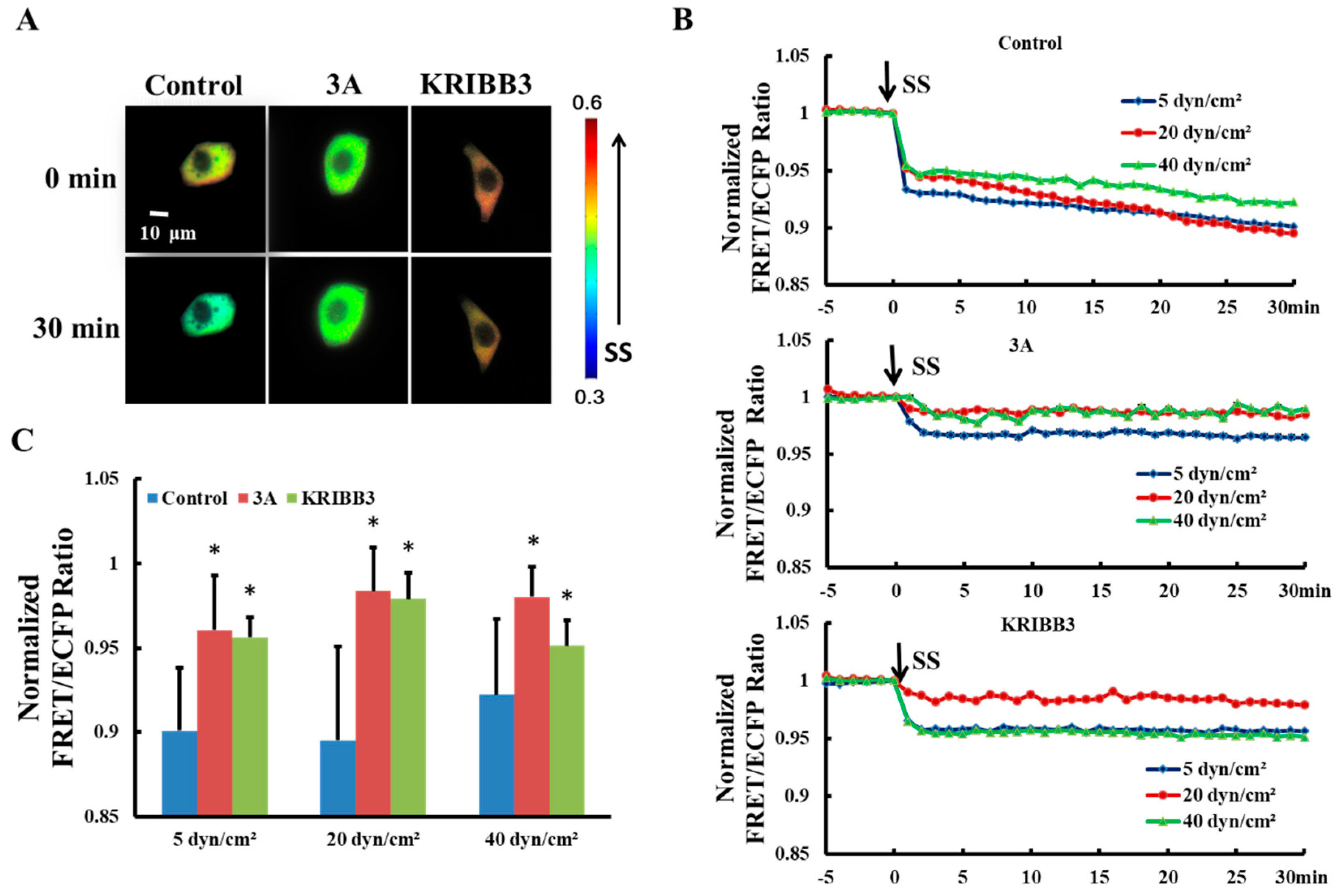
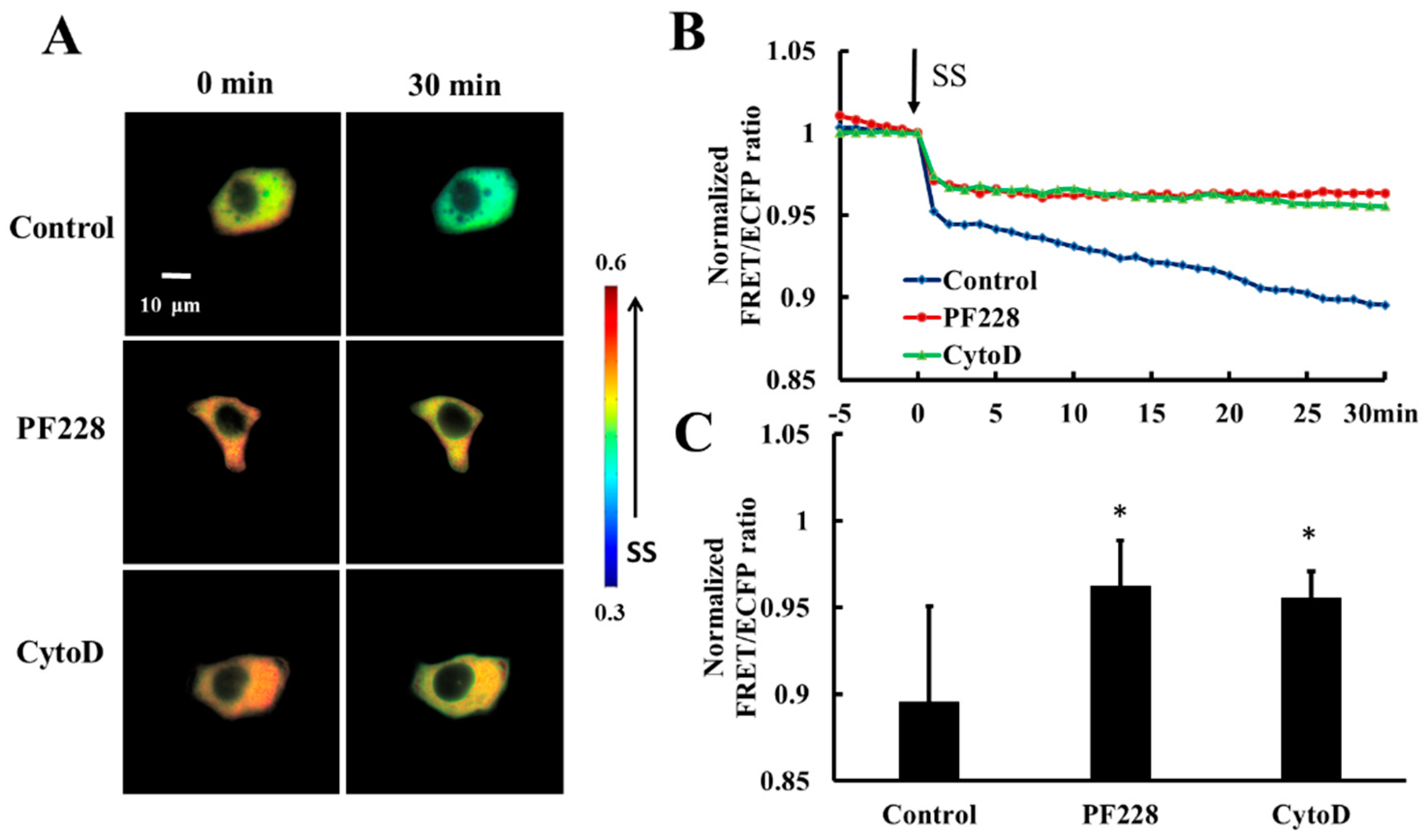
3.2. Shear-Stress-Induced Heat Shock Protein 27 Depolymerization is Regulated by its Phosphorylation
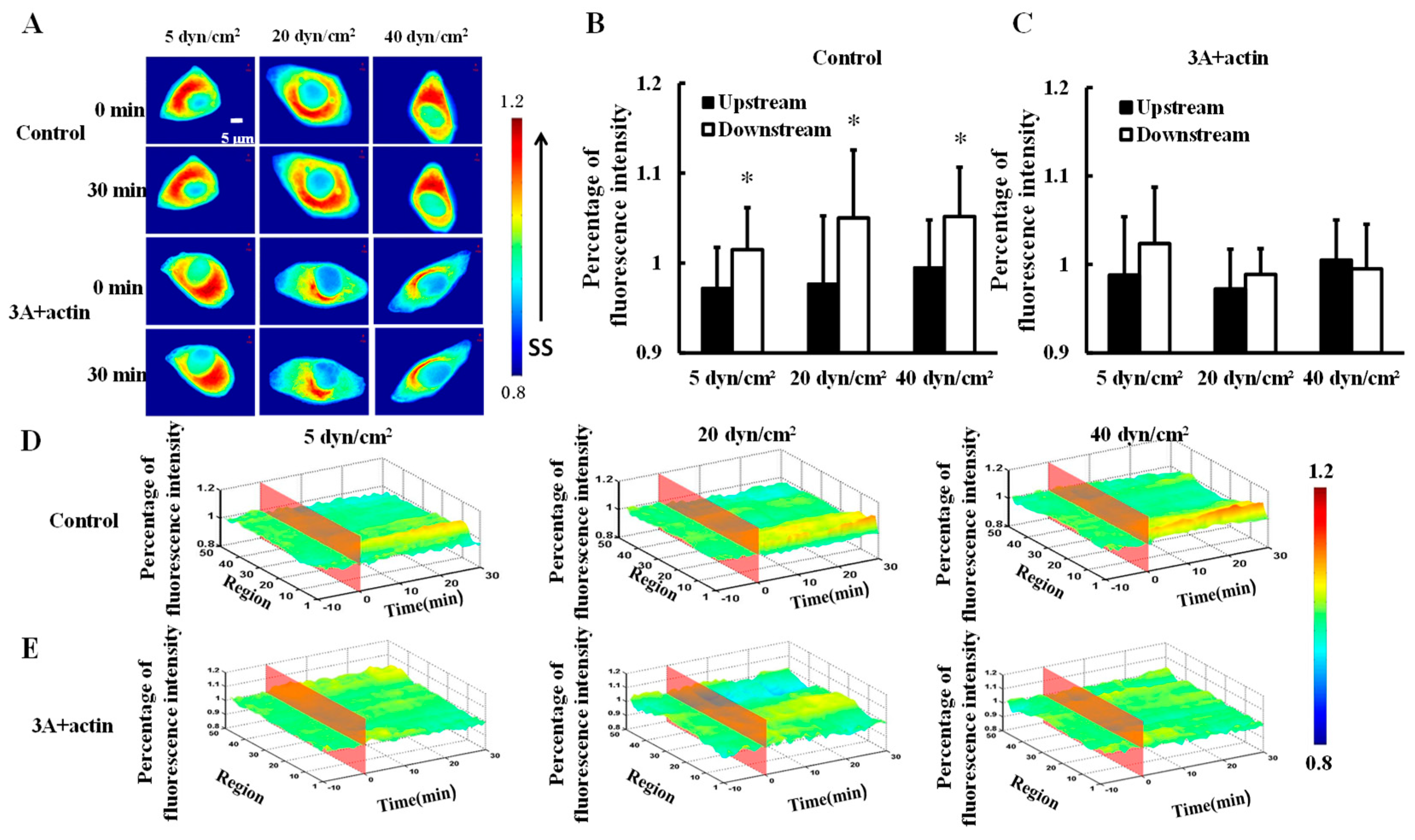
3.3. The Polarity Distribution of Actin in Response to Shear Stress is Regulated by Heat Shock Protein 27 Phosphorylation
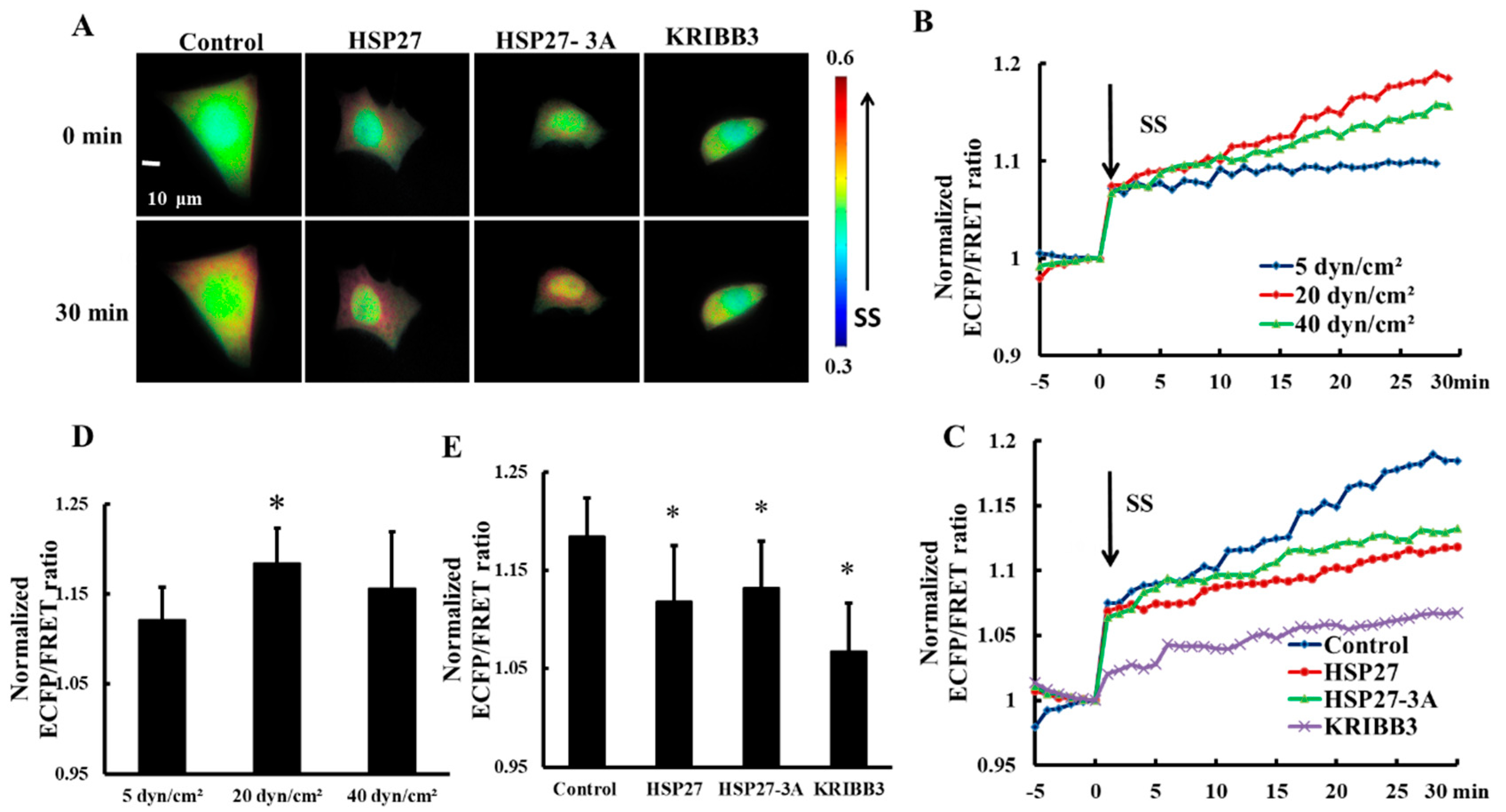
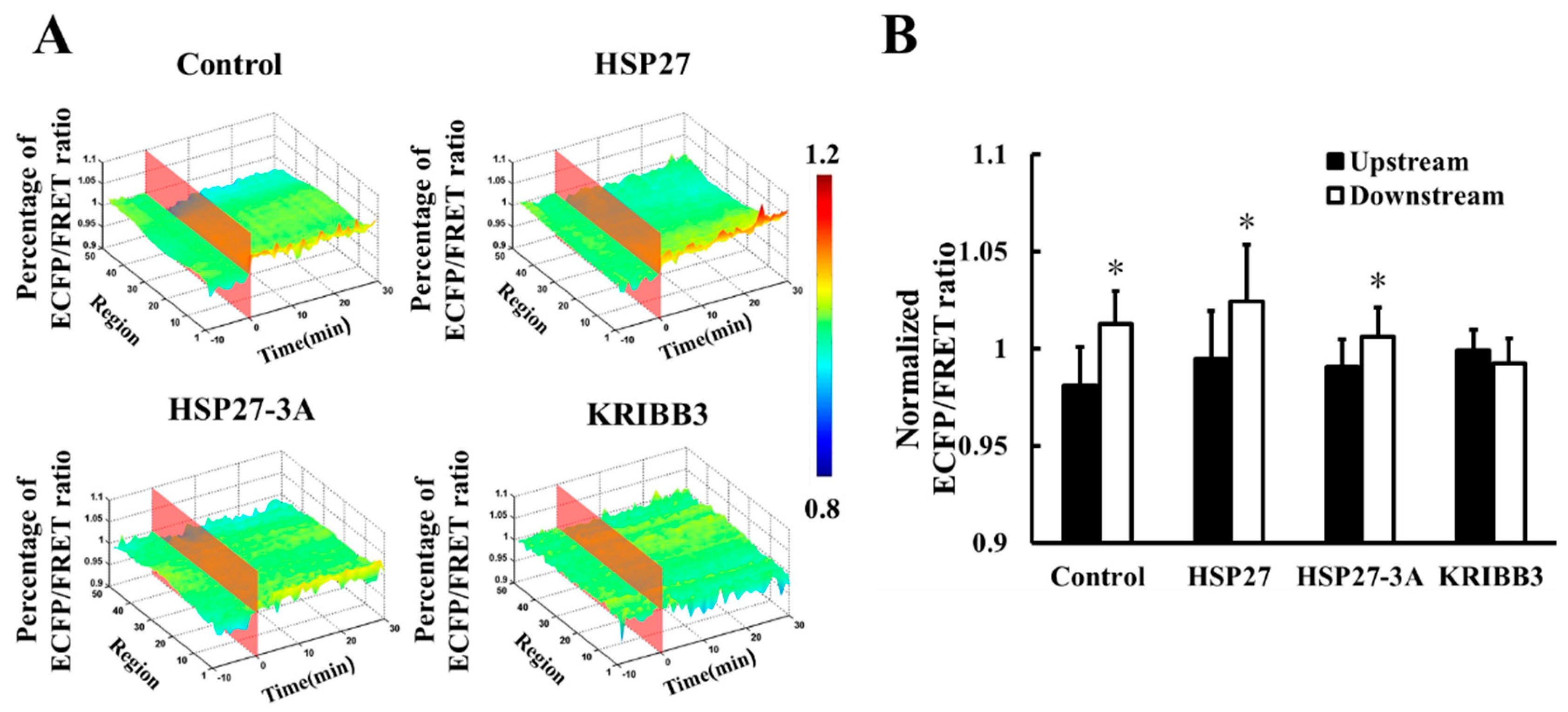
3.4. Expression and Phosphorylation of Heat Shock Protein 27 Regulates Shear-Stress-Induced Focal Adhesion Kinase Activation
3.5. Shear-Stress-Induced Heat Shock Proteain 27 Depolymerization is Regulated by Focal Adhesion Kinase Activation and Actin
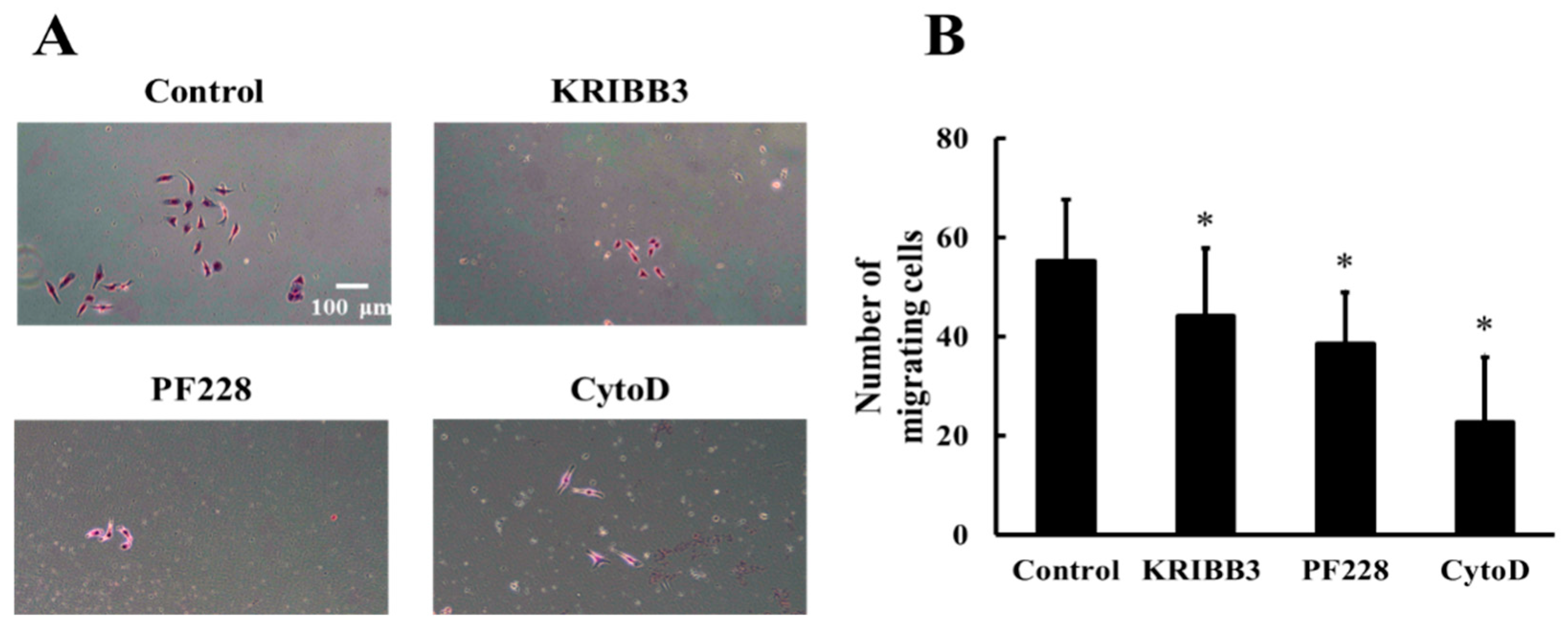
3.6. Heat Shock Protein 27 Phosphorylation is Closely Related to Cell Migration with Shear Stress Stimulation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jin, X.; Zhu, Z.; Shi, Y. Metastasis mechanism and gene/protein expression in gastric cancer with distant organs metastasis. Bull. Cancer 2014. [Google Scholar] [CrossRef]
- Miles, F.L.; Pruitt, F.L.; van Golen, K.L.; Cooper, C.R. Stepping out of the flow: Capillary extravasation in cancer metastasis. Clin. Exp. Metastasis 2008, 25, 305–324. [Google Scholar] [CrossRef]
- Mitchell, M.J.; King, M.R. Computational and experimental models of cancer cell response to fluid shear stress. Front. Oncol. 2013, 3, 44. [Google Scholar] [CrossRef]
- Ma, S.; Fu, A.; Chiew, G.G.; Luo, K.Q. Hemodynamic shear stress stimulates migration and extravasation of tumor cells by elevating cellular oxidative level. Cancer Lett. 2017, 388, 239–248. [Google Scholar] [CrossRef]
- Chotard-Ghodsnia, R.; Haddad, O.; Leyrat, A.; Drochon, A.; Verdier, C.; Duperray, A. Morphological analysis of tumor cell/endothelial cell interactions under shear flow. J. Biomech. 2007, 40, 335–344. [Google Scholar] [CrossRef]
- Fan, R.; Emery, T.; Zhang, Y.; Xia, Y.; Sun, J.; Wan, J. Circulatory shear flow alters the viability and proliferation of circulating colon cancer cells. Sci. Rep. 2016, 6, 27073. [Google Scholar] [CrossRef]
- Yu, H.; Shen, Y.; Jin, J.; Zhang, Y.; Feng, T.; Liu, X. Fluid shear stress regulates HepG2 cell migration though time-dependent integrin signaling cascade. Cell Adhes. Migr. 2018, 12, 56–68. [Google Scholar] [CrossRef]
- Sun, J.; Luo, Q.; Liu, L.; Song, G. Low-level shear stress promotes migration of liver cancer stem cells via the FAK-ERK1/2 signalling pathway. Cancer Lett. 2018, 427, 1–8. [Google Scholar] [CrossRef]
- Lee, H.J.; Diaz, M.F.; Price, K.M.; Ozuna, J.A.; Zhang, S.; Sevick-Muraca, E.M.; Hagan, J.P.; Wenzel, P.L. Fluid shear stress activates YAP1 to promote cancer cell motility. Nat. Commun. 2017, 8, 14122. [Google Scholar] [CrossRef]
- Kampinga, H.H.; Hageman, J.; Vos, M.J.; Kubota, H.; Tanguay, R.M.; Bruford, E.A.; Cheetham, M.E.; Chen, B.; Hightower, L.E. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones 2009, 14, 105–111. [Google Scholar] [CrossRef]
- Wang, X.; Chen, M.; Zhou, J.; Zhang, X. Hsp27, 70 and 90, anti-apoptotic proteins, in clinical cancer therapy (review). Int. J. Oncol. 2014, 45, 18–30. [Google Scholar] [CrossRef]
- Lianos, G.D.; Alexiou, G.A.; Mangano, A.; Mangano, A.; Rausei, S.; Boni, L.; Dionigi, G.; Roukos, D.H. The role of heat shock proteins in cancer. Cancer Lett. 2015, 360, 114–118. [Google Scholar] [CrossRef]
- Kostenko, S.; Moens, U. Heat shock protein 27 phosphorylation: Kinases, phosphatases, functions and pathology. Cell. Mol. Life Sci. 2009, 66, 3289–3307. [Google Scholar] [CrossRef]
- Shashidharamurthy, R.; Koteiche, H.A.; Dong, J.; McHaourab, H.S. Mechanism of chaperone function in small heat shock proteins: Dissociation of the HSP27 oligomer is required for recognition and binding of destabilized T4 lysozyme. J. Biol. Chem. 2005, 280, 5281–5289. [Google Scholar] [CrossRef]
- Chen, W.; Ren, X.; Wu, J.; Gao, X.; Cen, X.; Wang, S.; Sheng, S.; Chen, Q.; Tang, Y.J.; Liang, X.H.; et al. HSP27 associates with epithelial-mesenchymal transition, stemness and radioresistance of salivary adenoid cystic carcinoma. J. Cell. Mol. Med. 2018, 22, 2283–2298. [Google Scholar] [CrossRef]
- Okuno, M.; Yasuda, I.; Adachi, S.; Nakashima, M.; Kawaguchi, J.; Doi, S.; Iwashita, T.; Hirose, Y.; Kozawa, O.; Yoshimi, N.; et al. The significance of phosphorylated heat shock protein 27 on the prognosis of pancreatic cancer. Oncotarget 2016, 7, 14291–14299. [Google Scholar] [CrossRef]
- Nagaraja, G.M.; Kaur, P.; Asea, A. Role of human and mouse HSPB1 in metastasis. Curr. Mol. Med. 2012, 12, 1142–1150. [Google Scholar] [CrossRef]
- Pavan, S.; Musiani, D.; Torchiaro, E.; Migliardi, G.; Gai, M.; Di Cunto, F.; Erriquez, J.; Olivero, M.; Di Renzo, M.F. HSP27 is required for invasion and metastasis triggered by hepatocyte growth factor. Int. J. Cancer 2014, 134, 1289–1299. [Google Scholar] [CrossRef]
- Stohr, N.; Huttelmaier, S. Igf2bp1: A post-transcriptional “driver” of tumor cell migration. Cell Adhes. Migr. 2012, 6, 312–318. [Google Scholar] [CrossRef]
- Tanaka, T.; Iino, M.; Goto, K. Sec6 enhances cell migration and suppresses apoptosis by elevating the phosphorylation of p38 MAPK, MK2, and HSP27. Cell. Signal. 2018, 49, 1–16. [Google Scholar] [CrossRef]
- Li, S.; Piotrowicz, R.S.; Levin, E.G.; Shyy, Y.J.; Chien, S. Fluid shear stress induces the phosphorylation of small heat shock proteins in vascular endothelial cells. Am. J. Physiol. 1996, 271, C994–C1000. [Google Scholar] [CrossRef]
- Hochberg, G.K.; Ecroyd, H.; Liu, C.; Cox, D.; Cascio, D.; Sawaya, M.R.; Collier, M.P.; Stroud, J.; Carver, J.A.; Baldwin, A.J.; et al. The structured core domain of αB-crystallin can prevent amyloid fibrillation and associated toxicity. Proc. Natl. Acad. Sci. USA 2014, 111, E1562–E1570. [Google Scholar] [CrossRef]
- Lelj-Garolla, B.; Mauk, A.G. Self-association of a small heat shock protein. J. Mol. Biol. 2005, 345, 631–642. [Google Scholar] [CrossRef]
- Lambert, H.; Charette, S.J.; Bernier, A.F.; Guimond, A.; Landry, J. HSP27 multimerization mediated by phosphorylation-sensitive intermolecular interactions at the amino terminus. J. Biol. Chem. 1999, 274, 9378–9385. [Google Scholar] [CrossRef]
- Sato, S.B.; Sugiura, M.; Kurihara, T. Dimer-monomer equilibrium of human HSP27 is influenced by the in-cell macromolecular crowding environment and is controlled by fatty acids and heat. Biochim. Biophys. Acta Proteins Proteomics 2018, 1866, 692–701. [Google Scholar] [CrossRef]
- Wang, Y.X.; Shyy, J.Y.J.; Chien, S. Fluorescence proteins, live-cell imaging, and mechanobiology: Seeing is believing. Annu. Rev. Biomed. Eng. 2008, 10, 1–38. [Google Scholar] [CrossRef]
- Seong, J.; Ouyang, M.; Kim, T.; Sun, J.; Wen, P.C.; Lu, S.; Zhuo, Y.; Llewellyn, N.M.; Schlaepfer, D.D.; Guan, J.L.; et al. Detection of focal adhesion kinase activation at membrane microdomains by fluorescence resonance energy transfer. Nat. Commun. 2011, 2, 406. [Google Scholar] [CrossRef]
- Wallrabe, H.; Sun, Y.S.; Fang, X.L.; Periasamy, A.; Bloom, G.S. Three-color confocal forster (or fluorescence) resonance energy transfer microscopy: Quantitative analysis of protein interactions in the nucleation of actin filaments in live cells. Cytom. Part A 2015, 87a, 580–588. [Google Scholar] [CrossRef]
- Liu, B.; Lu, S.; Hu, Y.L.; Liao, X.; Ouyang, M.; Wang, Y. RhoA and membrane fluidity mediates the spatially polarized Src/FAK activation in response to shear stress. Sci. Rep. 2014, 4, 7008. [Google Scholar] [CrossRef]
- Liu, B.; Lu, S.; Zheng, S.; Jiang, Z.; Wang, Y. Two distinct phases of calcium signalling under flow. Cardiovasc. Res. 2011, 91, 124–133. [Google Scholar] [CrossRef]
- Chachisvilis, M.; Zhang, Y.L.; Frangos, J.A. G protein-coupled receptors sense fluid shear stress in endothelial cells. Proc. Natl. Acad. Sci. USA 2006, 103, 15463–15468. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Xiang, C.; Qin, K.; Ur Rehman Aziz, A.; Liao, X.; Liu, B. Visualizing the spatiotemporal map of Rac activation in bovine aortic endothelial cells under laminar and disturbed flows. PLoS ONE 2017, 12, e0189088. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Liao, X.; Xie, F.; Deng, S.; Liu, X.; Ristaniemi, T.; Liu, B. FRET biosensor allows spatio-temporal observation of shear stress-induced polar RhoGDIα activation. Commun. Biol. 2018, 1, 224. [Google Scholar] [CrossRef] [PubMed]
- Jovcevski, B.; Kelly, M.A.; Rote, A.P.; Berg, T.; Gastall, H.Y.; Benesch, J.L.; Aquilina, J.A.; Ecroyd, H. Phosphomimics destabilize HSP27 oligomeric assemblies and enhance chaperone activity. Chem. Biol. 2015, 22, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, J.N.; Hickey, E.; Weber, L.A.; Landry, J. Modulation of actin microfilament dynamics and fluid phase pinocytosis by phosphorylation of heat shock protein 27. J. Biol. Chem. 1993, 268, 24210–24214. [Google Scholar]
- Arrigo, A.P. Structure-functions of HSPB1 (HSP27). Methods Mol. Biol. 2011, 787, 105–119. [Google Scholar]
- Qi, S.; Xin, Y.; Qi, Z.; Xu, Y.; Diao, Y.; Lan, L.; Luo, L.; Yin, Z. HSP27 phosphorylation modulates TRAIL-induced activation of Src-Akt/ERK signaling through interaction with β-arrestin2. Cell. Signal. 2014, 26, 594–602. [Google Scholar] [CrossRef]
- Shin, K.D.; Lee, M.Y.; Shin, D.S.; Lee, S.; Son, K.H.; Koh, S.; Paik, Y.K.; Kwon, B.M.; Han, D.C. Blocking tumor cell migration and invasion with biphenyl isoxazole derivative KRIBB3, a synthetic molecule that inhibits HSP27 phosphorylation. J. Biol. Chem. 2005, 280, 41439–41448. [Google Scholar] [CrossRef]
- Gardel, M.L.; Schneider, I.C.; Aratyn-Schaus, Y.; Waterman, C.M. Mechanical integration of actin and adhesion dynamics in cell migration. Annu. Rev. Cell Dev. Biol. 2010, 26, 315–333. [Google Scholar] [CrossRef]
- Lee, B.Y.; Timpson, P.; Horvath, L.G.; Daly, R.J. Fak signaling in human cancer as a target for therapeutics. Pharmacol. Ther. 2015, 146, 132–149. [Google Scholar] [CrossRef]
- Lederer, P.A.; Zhou, T.; Chen, W.; Epshtein, Y.; Wang, H.; Mathew, B.; Jacobson, J.R. Attenuation of murine acute lung injury by PF-573,228, an inhibitor of focal adhesion kinase. Vasc. Pharmacol. 2018, 110, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Deevi, R.K.; Cox, O.T.; O’Connor, R. Essential function for PDLIM2 in cell polarization in three-dimensional cultures by feedback regulation of the β1-integrin-RhoA signaling axis. Neoplasia 2014, 16, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Song, K.; Jin, E.J.; Sonn, J. Staurosporine and cytochalasin D induce chondrogenesis by regulation of actin dynamics in different way. Exp. Mol. Med. 2012, 44, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.S.; Lin, J.H.; Huang, W.C.; Hsu, T.W.; Su, K.; Chiou, S.H.; Tsai, Y.T.; Hung, S.C. Chemoresistance of lung cancer stemlike cells depends on activation of HSP27. Cancer 2011, 117, 1516–1528. [Google Scholar] [CrossRef] [PubMed]
- Riehl, B.D.; Lee, J.S.; Ha, L.; Lim, J.Y. Fluid-flow-induced mesenchymal stem cell migration: Role of focal adhesion kinase and RhoA kinase sensors. J. R. Soc. Interface 2015, 12, 20141351. [Google Scholar] [CrossRef] [PubMed]
- Geum, D.; Son, G.H.; Kim, K. Phosphorylation-dependent cellular localization and thermoprotective role of heat shock protein 25 in hippocampal progenitor cells. J. Biol. Chem. 2002, 277, 19913–19921. [Google Scholar] [CrossRef] [PubMed]
- Korber, P.; Zander, T.; Herschlag, D.; Bardwell, J.C.A. A new heat shock protein that binds nucleic acids. J. Biol. Chem. 1999, 274, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Katsuno, H.; Toriyama, M.; Hosokawa, Y.; Mizuno, K.; Ikeda, K.; Sakumura, Y.; Inagaki, N. Actin migration driven by directional assembly and disassembly of membrane-anchored actin filaments. Cell Rep. 2015, 12, 648–660. [Google Scholar] [CrossRef] [PubMed]
- Clarke, J.P.; Mearow, K.M. Cell stress promotes the association of phosphorylated HSPB1 with F-actin. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huk, D.J.; Wang, Q.; Lincoln, J.; Zhao, Y. A microfluidic shear device that accommodates parallel high and low stress zones within the same culturing chamber. Biomicrofluidics 2014, 8, 054106. [Google Scholar] [CrossRef] [PubMed]
- During, R.L.; Gibson, B.G.; Li, W.; Bishai, E.A.; Sidhu, G.S.; Landry, J.; Southwick, F.S. Anthrax lethal toxin paralyzes actin-based motility by blocking HSP27 phosphorylation. EMBO J. 2007, 26, 2240–2250. [Google Scholar] [CrossRef] [PubMed]
- Frankenberg, N.T.; Lamb, G.D.; Overgaard, K.; Murphy, R.M.; Vissing, K. Small heat shock proteins translocate to the cytoskeleton in human skeletal muscle following eccentric exercise independently of phosphorylation. J. Appl. Physiol. 2014, 116, 1463–1472. [Google Scholar] [CrossRef] [PubMed]
- Arrigo, A.P. Analysis of HSPB1 (HSP27) oligomerization and phosphorylation patterns and its interaction with specific client polypeptides. Methods Mol. Biol. 2018, 1709, 163–178. [Google Scholar] [PubMed]
- Rogalla, T.; Ehrnsperger, M.; Preville, X.; Kotlyarov, A.; Lutsch, G.; Ducasse, C.; Paul, C.; Wieske, M.; Arrigo, A.P.; Buchner, J.; et al. Regulation of HSP27 oligomerization, chaperone function, and protective activity against oxidative stress/tumor necrosis factor alpha by phosphorylation. J. Biol. Chem. 1999, 274, 18947–18956. [Google Scholar] [CrossRef]
- Choi, C.K.; Vicente-Manzanares, M.; Zareno, J.; Whitmore, L.A.; Mogilner, A.; Horwitz, A.R. Actin and α-actinin orchestrate the assembly and maturation of nascent adhesions in a myosin II motor-independent manner. Nat. Cell Biol. 2008, 10, 1039–1050. [Google Scholar] [CrossRef] [PubMed]
- Tomar, A.; Schlaepfer, D.D. Focal adhesion kinase: Switching between GAPs and GEFs in the regulation of cell motility. Curr. Opin. Cell Biol. 2009, 21, 676–683. [Google Scholar] [CrossRef]
- Lee, J.W.; Kwak, H.J.; Lee, J.J.; Kim, Y.N.; Lee, J.W.; Park, M.J.; Jung, S.E.; Hong, S.I.; Lee, J.H.; Lee, J.S. HSP27 regulates cell adhesion and invasion via modulation of focal adhesion kinase and MMP-2 expression. Eur. J. Cell Biol. 2008, 87, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Yoon, J.H.; Lim, Y.S.; Hwang, H.K.; Kim, S.A.; Ahn, S.G.; Yoon, J.H. TAT-HSP27 promotes adhesion and migration of murine dental papilla-derived MDPC-23 cells through β1 integrin-mediated signaling. Int. J. Mol. Med. 2010, 26, 373–378. [Google Scholar] [PubMed]
- Piotrowicz, R.S.; Hickey, E.; Levin, E.G. Heat shock protein 27 kDa expression and phosphorylation regulates endothelial cell migration. FASEB J. 1998, 12, 1481–1490. [Google Scholar] [CrossRef]
- Swaminathan, V.; Fischer, R.S.; Waterman, C.M. The FAK-Arp2/3 interaction promotes leading edge advance and haptosensing by coupling nascent adhesions to lamellipodia actin. Mol. Biol. Cell 2016, 27, 1085–1100. [Google Scholar] [CrossRef]
- Dwyer, S.F.; Gelman, I.H. Cross-phosphorylation and interaction between Src/FAK and MAPKAP5/PRAK in early focal adhesions controls cell motility. J. Cancer Biol. Res. 2014, 2, 1045. [Google Scholar] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Xie, F.; Aziz, A.u.R.; Shao, S.; Li, W.; Deng, S.; Liao, X.; Liu, B. Heat Shock Protein 27 Phosphorylation Regulates Tumor Cell Migration under Shear Stress. Biomolecules 2019, 9, 50. https://doi.org/10.3390/biom9020050
Zhang B, Xie F, Aziz AuR, Shao S, Li W, Deng S, Liao X, Liu B. Heat Shock Protein 27 Phosphorylation Regulates Tumor Cell Migration under Shear Stress. Biomolecules. 2019; 9(2):50. https://doi.org/10.3390/biom9020050
Chicago/Turabian StyleZhang, Baohong, Fei Xie, Aziz ur Rehman Aziz, Shuai Shao, Wang Li, Sha Deng, Xiaoling Liao, and Bo Liu. 2019. "Heat Shock Protein 27 Phosphorylation Regulates Tumor Cell Migration under Shear Stress" Biomolecules 9, no. 2: 50. https://doi.org/10.3390/biom9020050
APA StyleZhang, B., Xie, F., Aziz, A. u. R., Shao, S., Li, W., Deng, S., Liao, X., & Liu, B. (2019). Heat Shock Protein 27 Phosphorylation Regulates Tumor Cell Migration under Shear Stress. Biomolecules, 9(2), 50. https://doi.org/10.3390/biom9020050




