Anti-Tumor Activity of Cembranoid-Type Diterpenes Isolated from Nicotiana tabacum L.
Abstract
:1. Introduction
2. Material and Methods
2.1. Plant Identification
2.2. Extraction and Isolation of α-CBD from Nicotiana tabacum L.
2.3. Cell Culture and Treatment with α-CBD
2.4. Detection of Viability and Clone Formation Ability of Hepatocellular Carcinoma Cells
2.5. Cellular Morphology Observation
2.6. Flow Cytometry Analysis
2.7. Analysis of Differentially Expressed Genes
2.8. Statistical Analyses
3. Results
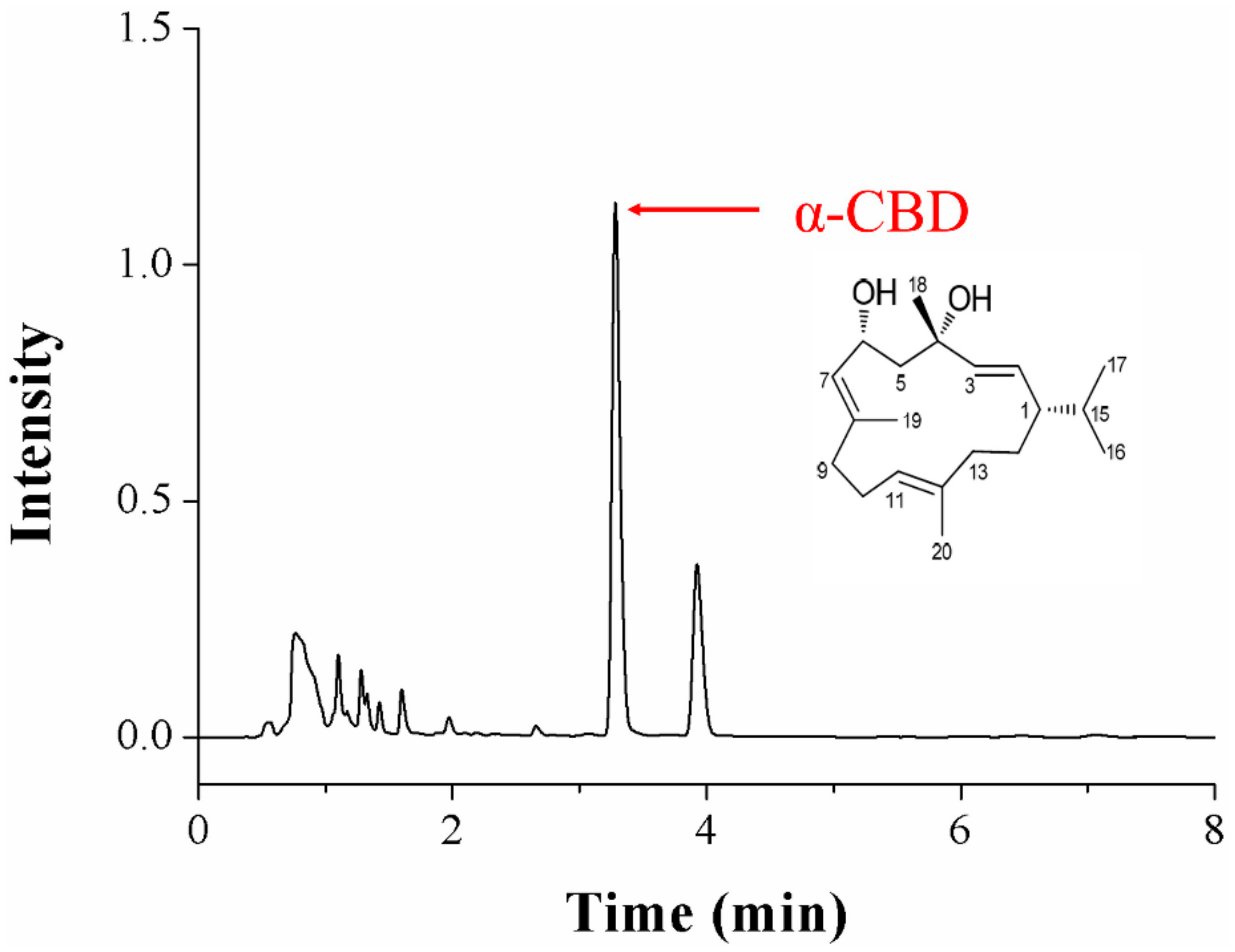
3.1. Identification of α-CBD
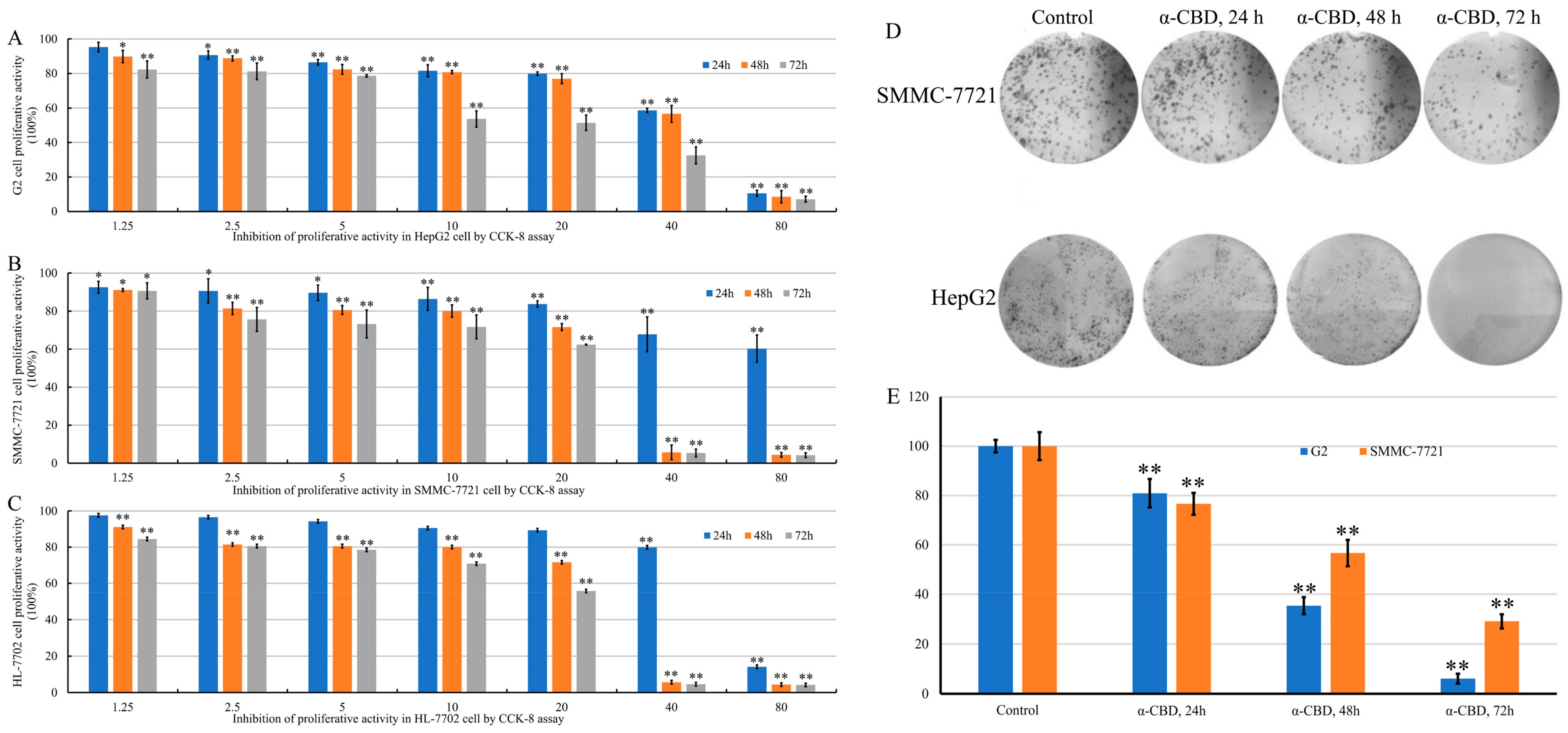
3.2. α-CBD Reduced the Viability and Prohibited the Proliferation of Hepatocellular Carcinoma Cells
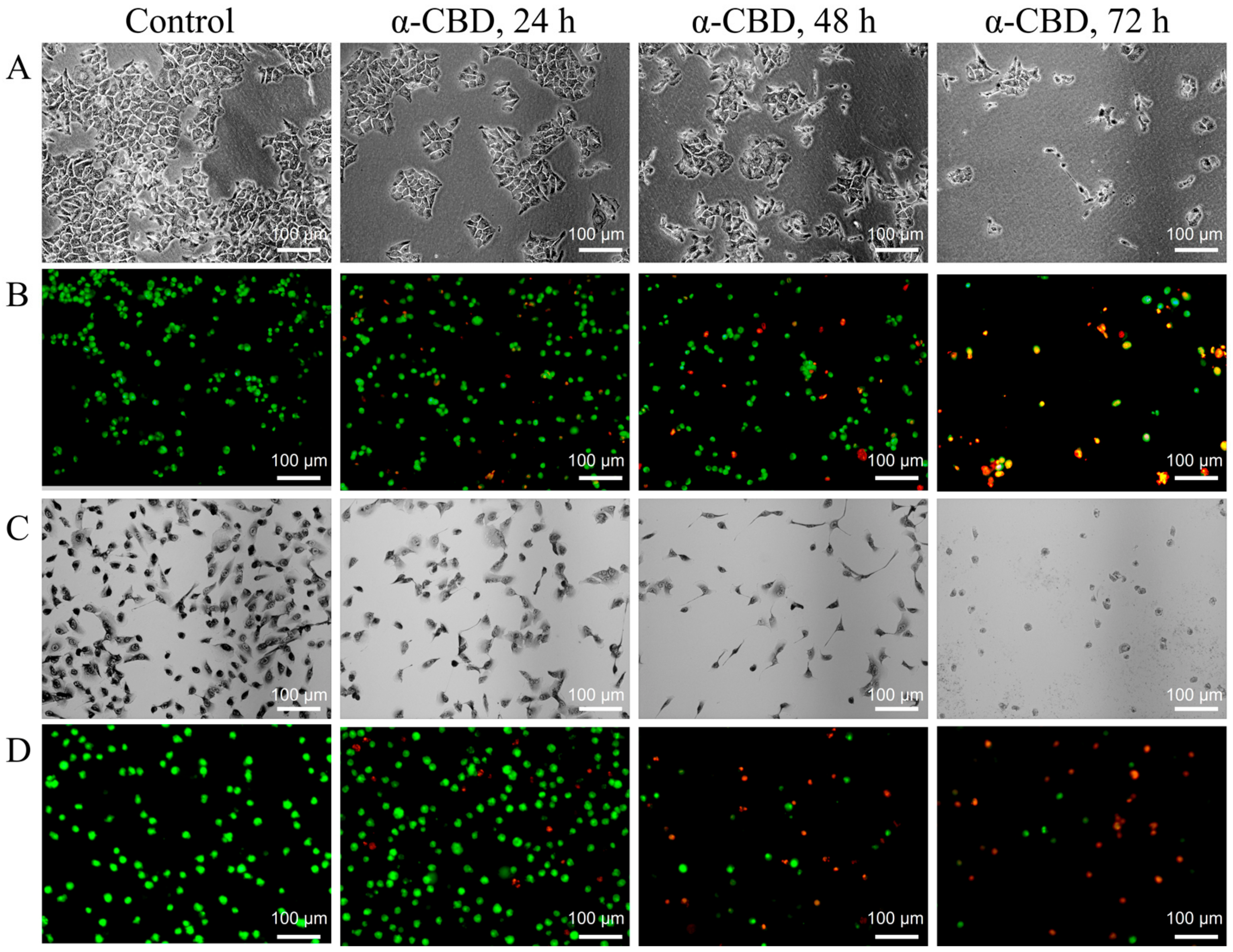
3.3. Effect of α-CBD on the Morphology of Hepatocellular Carcinoma Cells
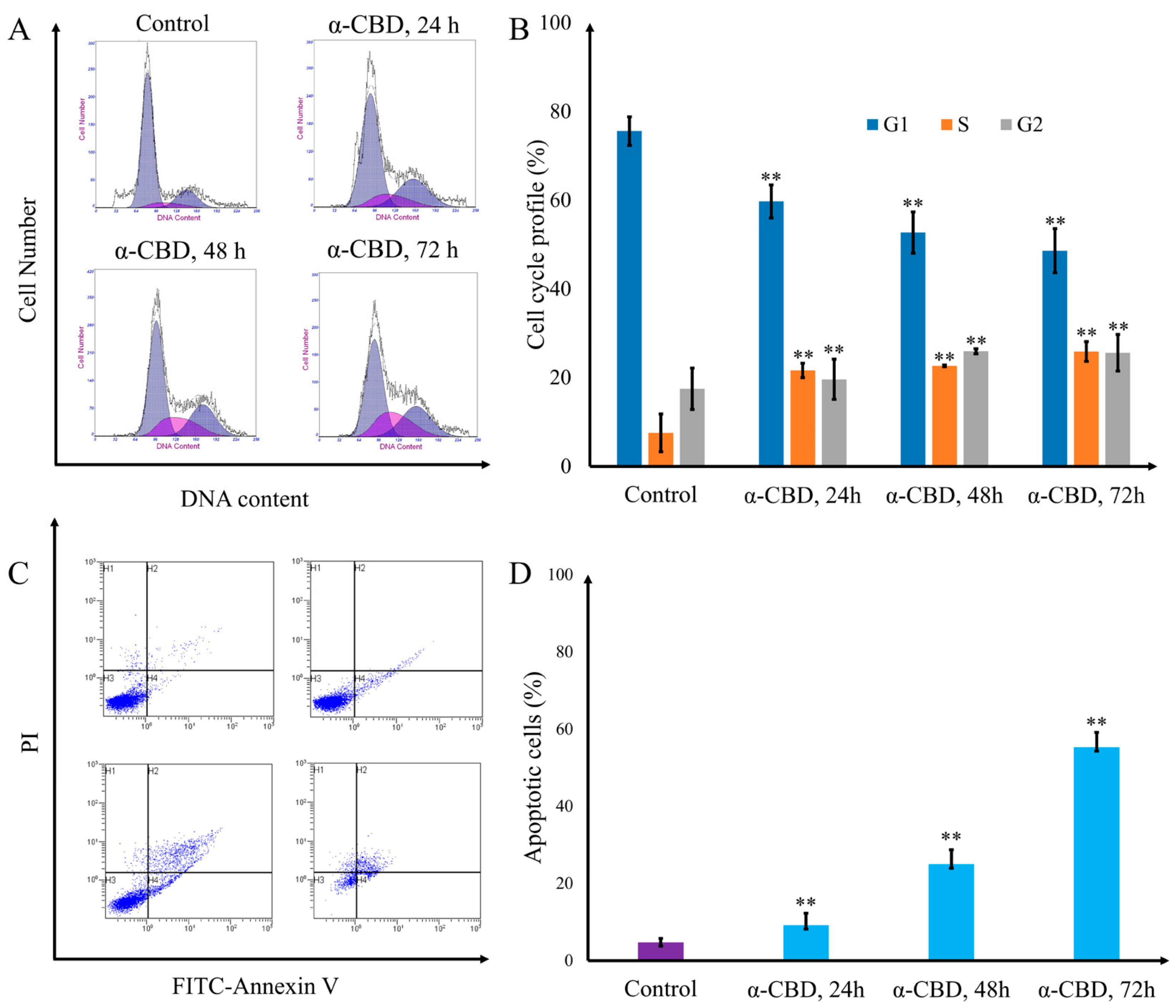
3.4. α-CBD Induced Cell Cycle Arrest and Apoptosis
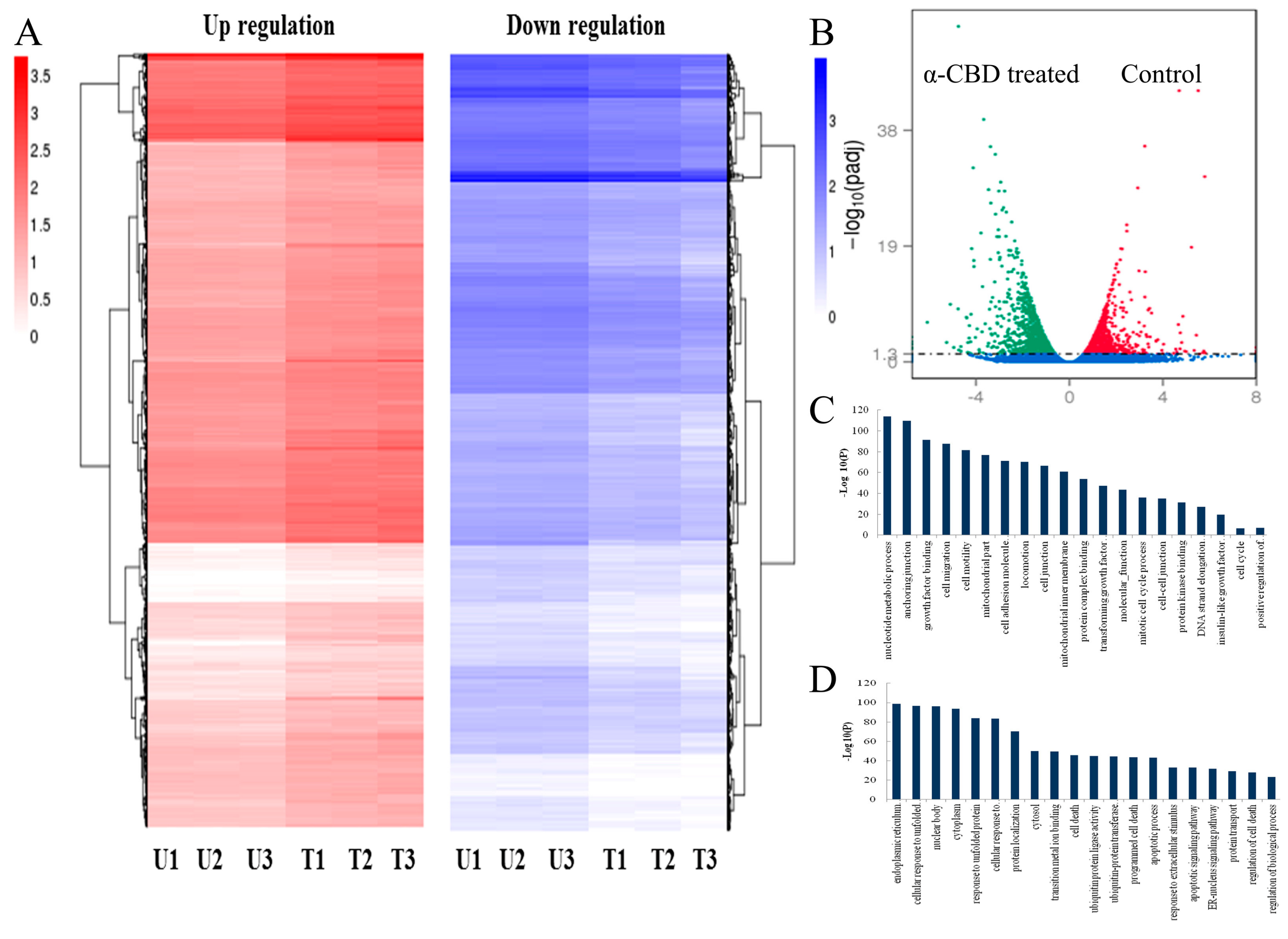
3.5. Gene Expression Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Supplementary File 1Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2017. CA Cancer J. Clin. 2015, 60, 277–300. [Google Scholar] [CrossRef] [PubMed]
- Howlader, N.; Noone, A.; Krapcho, M.; Neyman, N.; Aminou, R.; Waldron, K.W.; Altekruse, S. Surveillance, Epidemiology and End Results Program; SEER Incidence Data: Bethesda, MD, USA, 1973–2015. Available online: https://seer.cancer.gov/data/ (accessed on 7 June 2018).
- Burstein, H.J.; Krilov, L.; Aragon-Ching, J.B.; Baxter, N.N.; Chiorean, E.G.; Chow, W.A.; De Groot, J.F.; Devine, S.M.; Dubois, S.G.; El-Deiry, W.S. Clinical Cancer Advances 2017: Annual Report on Progress Against Cancer from the American Society of Clinical Oncology. J. Clin. Oncol. 2017, 35, 1341–1367. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; Deimling, A.V.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Yang, J.; Kong, A.N. Phytochemicals in Traditional Chinese Herbal Medicine: Cancer Prevention and Epigenetics Mechanisms. Curr. Pharmacol. Rep. 2017, 3, 77–91. [Google Scholar] [CrossRef]
- Nie, J.; Zhao, C.; Deng, L.I.; Chen, J.; Yu, B.; Wu, X.; Pang, P.; Chen, X. Efficacy of traditional Chinese medicine in treating cancer (Review). Biomed. Rep. 2016, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Oncology, T.L. Rethinking traditional Chinese medicines for cancer. Lancet Oncol. 2015, 16, 1439. [Google Scholar] [CrossRef]
- Nagata, S. Apoptosis and Clearance of Apoptotic Cells. Annu. Rev. Immunol. 2018, 36, 489–517. [Google Scholar] [CrossRef]
- Zhong, Z.F.; Yu, H.B.; Wang, C.M.; Qiang, W.A.; Wang, S.P.; Zhang, J.M.; Yu, H.; Cui, L.; Wu, T.; Li, D.Q. Furanodiene Induces Extrinsic and Intrinsic Apoptosis in Doxorubicin-Resistant MCF-7 Breast Cancer Cells via NF-κB-Independent Mechanism. Front. Pharm. 2017, 8, 934. [Google Scholar] [CrossRef]
- Lee, C.W.; Hsu, L.F.; Lee, M.H.; Lee, I.T.; Liu, J.F.; Chiang, Y.C.; Tsai, M.H. Extracts of Artocarpus communis Induce Mitochondria-Associated Apoptosis via Pro-oxidative Activity in Human Glioblastoma Cells. Front. Pharmacol. 2018, 9, 411. [Google Scholar] [CrossRef]
- Rudra, K.; Bart, R.; Frederique, L.; Joanna, F.; Ane Marcos, C.; Audrey, R.; Pascale, Z.; Rania, G. Syntenin controls migration, growth, proliferation, and cell cycle progression in cancer cells. Front. Pharmacol. 2015, 6, 241. [Google Scholar]
- Pawlak, A.; De, D.M.; Kutkowska, J.; Obmińskamrukowicz, B.; Rapak, A.; Lostao, L.M. Flavopiridol Strongly Sensitizes Canine Lymphoma Cells to TRAIL-induced Apoptosis. Anticancer Res. 2017, 37, 6655. [Google Scholar] [PubMed]
- Yuan, X.L.; Zhang, P.; Liu, X.M.; Du, Y.M.; Hou, X.D.; Cheng, S.; Zhang, Z.F. Cytological Assessments and Transcriptome Profiling Demonstrate that Evodiamine Inhibits Growth and Induces Apoptosis in a Renal Carcinoma Cell Line. Sci. Rep. 2017, 7, 12572. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Hao, F.; Dai, S.; Tian, L. Combination therapy Eve and Pac to induce apoptosis in cervical cancer cells by targeting PI3K/AKT/mTOR pathways. J. Recept. Signal Transduct. Res. 2018, 38, 83. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Yang, X.M.; Yin, D.H.; Tang, Q.L.; Wang, L.; Huang, C.; Li, P.; Li, S.S. Beclin1 enhances cisplatin-induced apoptosis via Bcl-2-modulated autophagy in laryngeal carcinoma cells Hep-2. Neoplasma 2018, 65, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Akiyoshi, S. Thunbergene, a Macrocyclic Diterpene. Bull. Chem. Soc. Jpn. 1962, 35, 1044–1045. [Google Scholar] [CrossRef]
- Dauben, W.G.; Thiessen, W.E.; Resnick, P.R. Cembrene, A 14-Membered Ring Diterpene Hydrocarbon. J. Am. Chem. Soc. 2002, 84, 2015–2016. [Google Scholar] [CrossRef]
- Januar, H.I.; Zamani, N.P.; Soedharma, D.; Chasanah, E. New Cytotoxic Cembranoid from Indonesian Soft Coral Sarcophyton sp. Pharmacogn. Res. 2017, 9, 65–68. [Google Scholar] [CrossRef]
- Al-Lihaibi, S.S.; Alarif, W.M.; Abdel-Lateff, A.; Ayyad, S.E.N.; Abdel-Naim, A.B.; El-Senduny, F.F.; Badria, F.A. Three new cembranoid-type diterpenes from Red Sea soft coral Sarcophyton glaucum: Isolation and antiproliferative activity against HepG2 cells. Eur. J. Med. Chem. 2015, 45, 314–322. [Google Scholar]
- Ghouri, Y.A.; Mian, I.; Rowe, J.H. Review of hepatocellular carcinoma: Epidemiology, etiology, and carcinogenesis. J. Carcinog. 2017, 16, 1. [Google Scholar]
- Maluccio, M.; Covey, A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J. Clin. 2012, 62, 394–399. [Google Scholar] [CrossRef]
- Kiraz, Y.; Adan, A.; Yandim, M.K.; Baran, Y. Major apoptotic mechanisms and genes involved in apoptosis. Tumour Biol. 2016, 37, 8471–8486. [Google Scholar] [CrossRef] [PubMed]
- Aqil, F.; Zahin, M.; Sayed, K.A.E.; Ahmad, I.; Orabi, K.Y.; Arif, J.M. Antimicrobial, antioxidant, and antimutagenic activities of selected marine natural products and tobacco cembranoids. Drug Chem. Toxicol. 2011, 34, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Li, H.; Wu, J.; Xu, X.; Sun, X.; Zhao, X.; Xu, N. Transcriptome Profiling Reveals the Antitumor Mechanism of Polysaccharide from Marine Algae Gracilariopsis lemaneiformis. PLoS ONE 2016, 11, e0158279. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, Q.; Wang, Q.; He, Y.; Ren, D.; Liu, S.; Wu, L. Structural characterization and antitumor effects of fucoidans from brown algae Kjellmaniella crassifolia farmed in northern China. Int. J. Biol. Macromol. 2018, 119, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C.; Hsieh, M.J.; Chen, C.J.; Lin, J.T.; Lo, Y.S.; Chuang, Y.C.; Chien, S.Y.; Chen, M.K. Polyphyllin G induce apoptosis and autophagy in human nasopharyngeal cancer cells by modulation of AKT and mitogen-activated protein kinase pathways in vitro and in vivo. Oncotarget 2016, 7, 70276–70289. [Google Scholar] [CrossRef] [PubMed]
- Recek, N.; Andjelić, S.; Hojnik, N.; Filipič, G.; Lazović, S.; Vesel, A.; Primc, G.; Mozetič, M.; Hawlina, M.; Petrovski, G. Microplasma Induced Cell Morphological Changes and Apoptosis of Ex Vivo Cultured Human Anterior Lens Epithelial Cells—Relevance to Capsular Opacification. PLoS ONE 2016, 11, e0165883. [Google Scholar] [CrossRef]
- Lazari, D.; Alexiou, G.A.; Markopoulos, G.S.; Vartholomatos, E.; Hodaj, E.; Chousidis, I.; Leonardos, I.; Galani, V.; Kyritsis, A.P. N-(p-coumaroyl) serotonin inhibits glioblastoma cells growth through triggering S-phase arrest and apoptosis. J. Neuro-Oncol. 2017, 132, 373–381. [Google Scholar] [CrossRef]
- Wang, Y.; Compton, C.; Rankin, G.O.; Cutler, S.J.; Rojanasakul, Y.; Tu, Y.; Chen, Y.C. 3-Hydroxyterphenyllin, a natural fungal metabolite, induces apoptosis and S phase arrest in human ovarian carcinoma cells. Int. J. Oncol. 2017, 50, 1392. [Google Scholar] [CrossRef]
- Song, H.; Wei, M.; Liu, W.; Shen, S.; Li, J.; Wang, L. Cisplatin induced apoptosis of ovarian cancer A2780s cells by activation of ERK/p53/PUMA signals. Histol. Histopathol. 2018, 33, 73–79. [Google Scholar]
- Lien, E.C.; Dibble, C.C.; Toker, A. PI3K signaling in cancer: Beyond AKT. Curr. Opin. Cell Biol. 2017, 45, 62. [Google Scholar] [CrossRef]
- Li, Q.; Yang, G.; Feng, M.; Zheng, S.; Cao, Z.; Qiu, J.; You, L.; Zheng, L.; Hu, Y.; Zhang, T. NF-κB in pancreatic cancer: Its Key Role in Chemoresistance. Cancer Lett. 2018, 421, 127. [Google Scholar] [CrossRef] [PubMed]
| Term | ID | p-Value |
|---|---|---|
| Upregulated | ||
| NF-kappa B signaling pathway | hsa04064 | 0.660557 |
| HIF-1 signaling pathway | hsa04066 | 0.660557 |
| Apoptosis | hsa04210 | 0.90181 |
| Pathways in cancer | hsa05200 | 0.90181 |
| Regulation of autophagy | hsa04140 | 0.938176 |
| Downregulated | ||
| Adherens junction | hsa04520 | 0.283208 |
| Citrate cycle (TCA cycle) | hsa00020 | 0.573324 |
| p53 signaling pathway | hsa04115 | 0.759371 |
| AMPK signaling pathway | hsa04152 | 0.771113 |
| TGF-beta signaling pathway | hsa04350 | 0.946339 |
| PI3K-Akt signaling pathway | hsa04151 | 0.952199 |
| Cell cycle | hsa04110 | 0.952652 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, X.-L.; Mao, X.-X.; Du, Y.-M.; Yan, P.-Z.; Hou, X.-D.; Zhang, Z.-F. Anti-Tumor Activity of Cembranoid-Type Diterpenes Isolated from Nicotiana tabacum L. Biomolecules 2019, 9, 45. https://doi.org/10.3390/biom9020045
Yuan X-L, Mao X-X, Du Y-M, Yan P-Z, Hou X-D, Zhang Z-F. Anti-Tumor Activity of Cembranoid-Type Diterpenes Isolated from Nicotiana tabacum L. Biomolecules. 2019; 9(2):45. https://doi.org/10.3390/biom9020045
Chicago/Turabian StyleYuan, Xiao-Long, Xin-Xin Mao, Yong-Mei Du, Pei-Zhen Yan, Xiao-Dong Hou, and Zhong-Feng Zhang. 2019. "Anti-Tumor Activity of Cembranoid-Type Diterpenes Isolated from Nicotiana tabacum L." Biomolecules 9, no. 2: 45. https://doi.org/10.3390/biom9020045
APA StyleYuan, X.-L., Mao, X.-X., Du, Y.-M., Yan, P.-Z., Hou, X.-D., & Zhang, Z.-F. (2019). Anti-Tumor Activity of Cembranoid-Type Diterpenes Isolated from Nicotiana tabacum L. Biomolecules, 9(2), 45. https://doi.org/10.3390/biom9020045




