Preclinical Evaluation of the Antimicrobial-Immunomodulatory Dual Action of Xenohormetic Molecules against Haemophilus influenzae Respiratory Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Media, Growth Conditions, and Drugs
2.2. Determination of Plant Extract and Pure Polyphenol Antimicrobial Effects
2.3. Determination of Antimicrobial Synergic Effects
2.4. Polyphenol Susceptibility Assays
2.5. Serial Passage Experiment with Polyphenol
2.6. Infection of Tissue Cultured Cells
2.7. RNA Extraction and Real-Time Quantitative PCR
2.8. Western Blotting
2.9. NTHi Mouse Lung Infection
2.10. NTHi Adult Zebrafish Infection
2.11. Statistical Analysis
3. Results
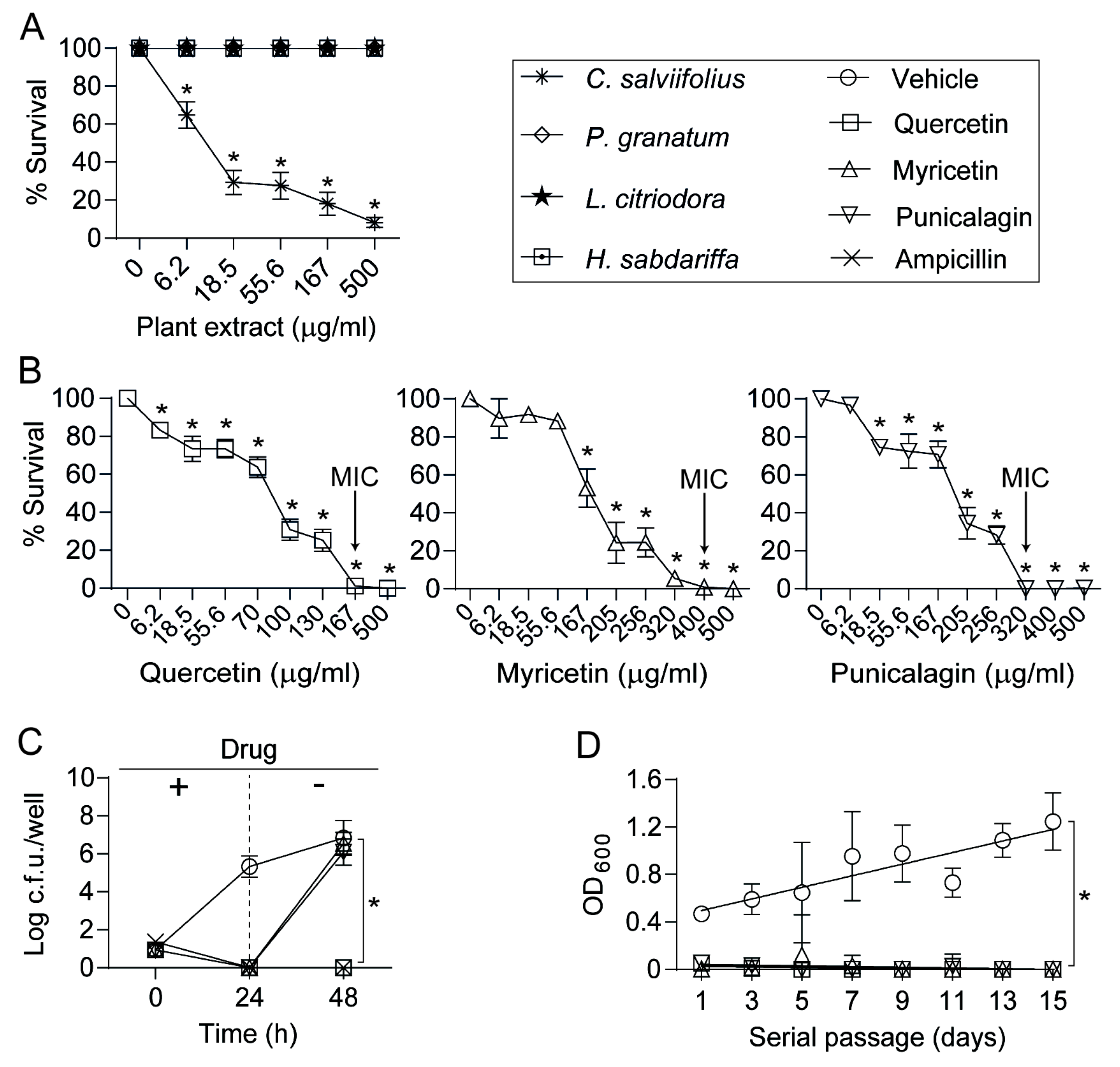
3.1. An Extract of Cistus salviifolius Rich in Polyphenols Has an Antimicrobial Effect on H. influenzae
3.2. Antimicrobial Effects of Quercetin, Myricetin, and Punicalagin against NTHi
3.3. Quercetin Modulates NTHi Infection of Cultured Airway Epithelial Cells
3.4. Quercetin Lowers Proinflammatory Gene Expression by Epithelial Cells Infected with H. influenzae
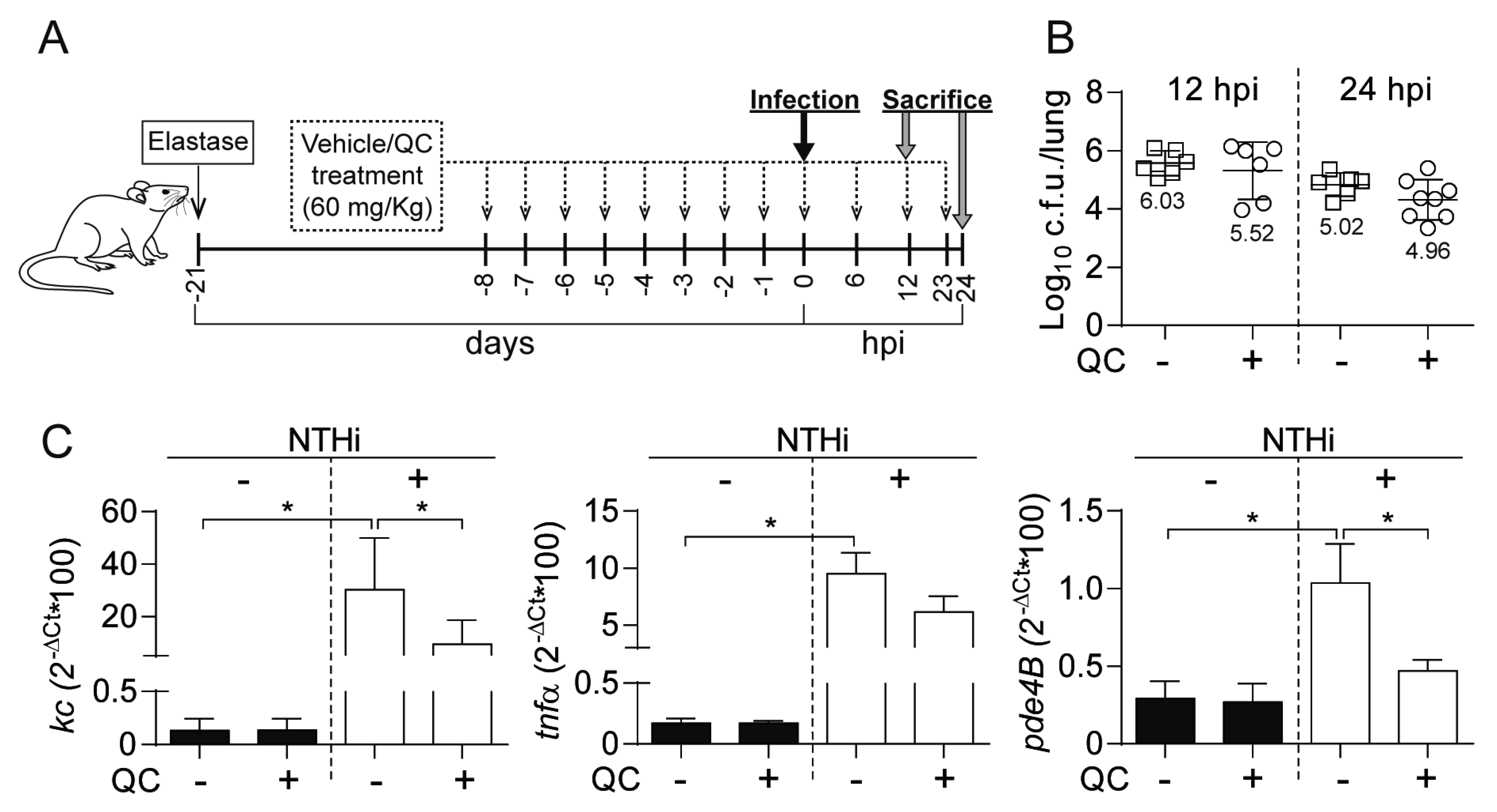
3.5. Antimicrobial and Anti-inflammatory Effects of Quercetin Administration on Mouse Lung Infection with NTHi
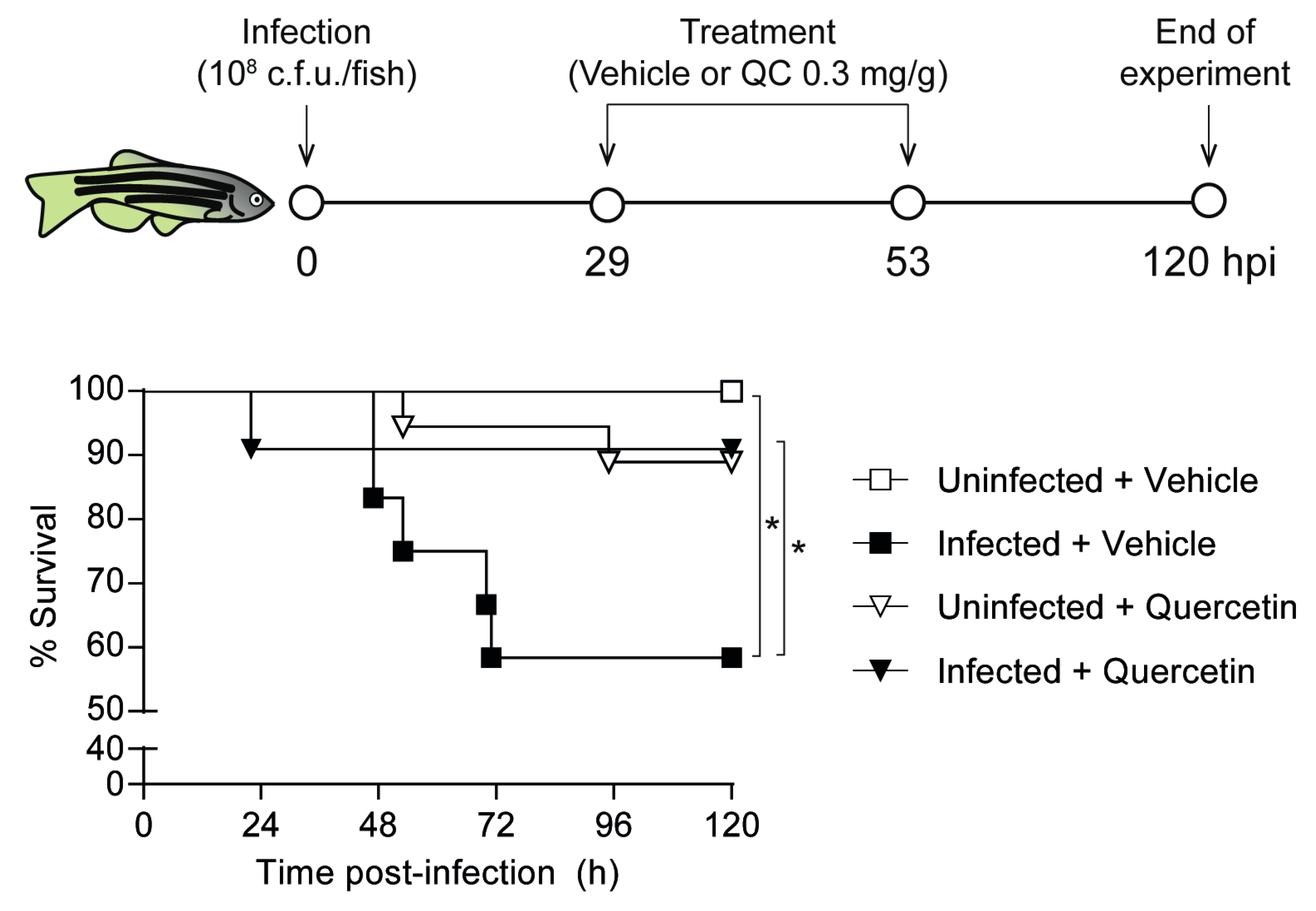
3.6. Quercetin Antimicrobial Protective Effect on Zebrafish Systemic Infection with NTHi
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mannino, D.M.; Buist, A.S. Global burden of COPD: Risk factors, prevalence, and future trends. Lancet 2007, 370, 765–773. [Google Scholar] [CrossRef]
- Hogg, J.C. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet 2004, 364, 709–721. [Google Scholar] [CrossRef]
- Hogg, J.C.; Chu, F.; Utokaparch, S.; Woods, R.; Elliott, W.M.; Buzatu, L.; Cherniack, R.M.; Rogers, R.M.; Sciurba, F.C.; Coxson, H.O.; et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N. Engl. J. Med. 2004, 350, 2645–2653. [Google Scholar] [CrossRef] [PubMed]
- Rangelov, K.; Sethi, S. Role of infections. Clin. Chest. Med. 2014, 35, 87–100. [Google Scholar] [CrossRef]
- Ahearn, C.P.; Gallo, M.C.; Murphy, T.F. Insights on persistent airway infection by non-typeable Haemophilus influenzae in chronic obstructive pulmonary disease. Pathog. Dis. 2017, 75. [Google Scholar] [CrossRef]
- Duell, B.L.; Su, Y.C.; Riesbeck, K. Host-pathogen interactions of nontypeable Haemophilus influenzae: From commensal to pathogen. FEBS Lett. 2016, 590, 3840–3853. [Google Scholar] [CrossRef]
- Aaron, S.D.; Angel, J.B.; Lunau, M.; Wright, K.; Fex, C.; Le Saux, N.; Dales, R.E. Granulocyte inflammatory markers and airway infection during acute exacerbation of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2001, 163, 349–355. [Google Scholar] [CrossRef]
- Papi, A.; Bellettato, C.M.; Braccioni, F.; Romagnoli, M.; Casolari, P.; Caramori, G.; Fabbri, L.M.; Johnston, S.L. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am. J. Respir. Crit. Care Med. 2006, 173, 1114–1121. [Google Scholar] [CrossRef]
- Sethi, S.; Muscarella, K.; Evans, N.; Klingman, K.L.; Grant, B.J.; Murphy, T.F. Airway inflammation and etiology of acute exacerbations of chronic bronchitis. Chest 2000, 118, 1557–1565. [Google Scholar] [CrossRef]
- Izquierdo, J.L.; Cosio, B.G. The dose of inhaled corticosteroids in patients with COPD: When less is better. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 3539–3547. [Google Scholar] [CrossRef]
- Liapikou, A.; Cilloniz, C.; Torres, A. Drugs that increase the risk of community-acquired pneumonia: A narrative review. Expert Opin. Drug Saf. 2018, 17, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Restrepo, M.I.; Sibila, O.; Anzueto, A. Pneumonia in patients with chronic obstructive pulmonary disease. Tuberc. Respir. Dis. (Seoul) 2018, 81, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Zhu, L. Update on molecular mechanisms of corticosteroid resistance in chronic obstructive pulmonary disease. Pulm. Pharmacol. Ther. 2016, 37, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ramos, F.L.; Criner, G.J. Use of long-term macrolide therapy in chronic obstructive pulmonary disease. Curr. Opin. Pulm. Med. 2014, 20, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Huckle, A.W.; Fairclough, L.C.; Todd, I. Prophylactic antibiotic use in COPD and the potential anti-inflammatory activities of antibiotics. Respir. Care 2018, 63, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.P.; Sellers, E.; Taylor, B.T. Azithromycin for the prevention of COPD exacerbations: The good, bad, and ugly. Am. J. Med. 2015, 128, 1362.e1–1362.e6. [Google Scholar] [CrossRef]
- Howitz, K.T.; Sinclair, D.A. Xenohormesis: Sensing the chemical cues of other species. Cell 2008, 133, 387–391. [Google Scholar] [CrossRef]
- Arts, I.C.; Hollman, P.C. Polyphenols and disease risk in epidemiologic studies. Am. J. Clin. Nutr. 2005, 81, 317S–325S. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Tabak, C.; Arts, I.C.; Smit, H.A.; Heederik, D.; Kromhout, D. Chronic obstructive pulmonary disease and intake of catechins, flavonols, and flavones: The MORGEN Study. Am. J. Respir. Crit. Care Med. 2001, 164, 61–64. [Google Scholar] [CrossRef]
- Lee, S.U.; Ryu, H.W.; Lee, S.; Shin, I.S.; Choi, J.H.; Lee, J.W.; Lee, J.; Kim, M.O.; Lee, H.J.; Ahn, K.S.; et al. Lignans isolated from flower buds of Magnolia fargesii attenuate airway inflammation induced by cigarette smoke in vitro and in vivo. Front. Pharmacol. 2018, 9, 970. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, S.; Faris, A.N.; Comstock, A.T.; Chattoraj, S.S.; Chattoraj, A.; Burgess, J.R.; Curtis, J.L.; Martinez, F.J.; Zick, S.; Hershenson, M.B.; et al. Quercetin prevents progression of disease in elastase/LPS-exposed mice by negatively regulating MMP expression. Respir. Res. 2010, 11, 131. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Betsuyaku, T.; Ito, Y.; Nagai, K.; Odajima, N.; Moriyama, C.; Nasuhara, Y.; Nishimura, M. Curcumin attenuates elastase- and cigarette smoke-induced pulmonary emphysema in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009, 296, L614–L623. [Google Scholar] [CrossRef] [PubMed]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Sun, Q.; Gong, S.; Bi, X.; Jiang, W.; Xue, W.; Fei, P. Antimicrobial activity and action approach of the olive oil polyphenol extract against Listeria monocytogenes. Front. Microbiol. 2019, 10, 1586. [Google Scholar] [CrossRef]
- Mandal, S.M.; Dias, R.O.; Franco, O.L. Phenolic compounds in antimicrobial therapy. J. Med. Food 2017, 20, 1031–1038. [Google Scholar] [CrossRef]
- Slobodnikova, L.; Fialova, S.; Rendekova, K.; Kovac, J.; Mucaji, P. Antibiofilm activity of plant polyphenols. Molecules 2016, 21, 1717. [Google Scholar] [CrossRef]
- Tomas-Menor, L.; Barrajon-Catalan, E.; Segura-Carretero, A.; Marti, N.; Saura, D.; Menendez, J.A.; Joven, J.; Micol, V. The promiscuous and synergic molecular interaction of polyphenols in bactericidal activity: An opportunity to improve the performance of antibiotics? Phytother. Res. PTR 2015, 29, 466–473. [Google Scholar] [CrossRef]
- Tomas-Menor, L.; Morales-Soto, A.; Barrajon-Catalan, E.; Roldan-Segura, C.; Segura-Carretero, A.; Micol, V. Correlation between the antibacterial activity and the composition of extracts derived from various Spanish Cistus species. Food Chem. Toxicol. 2013, 55, 313–322. [Google Scholar] [CrossRef]
- Euba, B.; Lopez-Lopez, N.; Rodriguez-Arce, I.; Fernandez-Calvet, A.; Barberan, M.; Caturla, N.; Marti, S.; Diez-Martinez, R.; Garmendia, J. Resveratrol therapeutics combines both antimicrobial and immunomodulatory properties against respiratory infection by nontypeable Haemophilus influenzae. Sci. Rep. 2017, 7, 12860. [Google Scholar] [CrossRef]
- Knobloch, J.; Sibbing, B.; Jungck, D.; Lin, Y.; Urban, K.; Stoelben, E.; Strauch, J.; Koch, A. Resveratrol impairs the release of steroid-resistant inflammatory cytokines from human airway smooth muscle cells in chronic obstructive pulmonary disease. J. Pharmacol. Exp. Ther. 2010, 335, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.G.; Wark, P.A.; Garg, M.L. Antioxidant and anti-inflammatory effects of resveratrol in airway disease. Antioxid. Redox Signal. 2010, 13, 1535–1548. [Google Scholar] [CrossRef] [PubMed]
- Euba, B.; Moleres, J.; Viadas, C.; Barberan, M.; Caballero, L.; Grillo, M.J.; Bengoechea, J.A.; de-Torres, J.P.; Linares, J.; Leiva, J.; et al. Relationship between azithromycin susceptibility and administration efficacy for nontypeable Haemophilus influenzae respiratory infection. Antimicrob. Agents Chemother. 2015, 59, 2700–2712. [Google Scholar] [CrossRef] [PubMed]
- Mell, J.C.; Sinha, S.; Balashov, S.; Viadas, C.; Grassa, C.J.; Ehrlich, G.D.; Nislow, C.; Redfield, R.J.; Garmendia, J. Complete genome sequence of Haemophilus influenzae strain 375 from the middle ear of a pediatric patient with otitis media. Genome Announc. 2014, 2, e01245-14. [Google Scholar] [CrossRef] [PubMed]
- Tyc, O.; Tomas-Menor, L.; Garbeva, P.; Barrajon-Catalan, E.; Micol, V. Validation of the AlamarBlue(R) assay as a fast screening method to determine the antimicrobial activity of botanical extracts. PLoS ONE 2016, 11, e0169090. [Google Scholar] [CrossRef] [PubMed]
- Herranz-López, M.; Losada-Echeberría, M.; Barrajón-Catalán, E. The multitarget activity of natural extracts on cancer: Synergy and xenohormesis. Medicines 2019, 6, 6. [Google Scholar] [CrossRef]
- Schwalbe, R.; Steele-Moore, L.; Goodwin, A.C. Antimicrobial Susceptibility Testing Protocols, 1st ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 277–283. [Google Scholar]
- Kubicek-Sutherland, J.Z.; Lofton, H.; Vestergaard, M.; Hjort, K.; Ingmer, H.; Andersson, D.I. Antimicrobial peptide exposure selects for Staphylococcus aureus resistance to human defence peptides. J. Antimicrob. Chemother. 2017, 72, 115–127. [Google Scholar] [CrossRef]
- Morey, P.; Cano, V.; Marti-Lliteras, P.; Lopez-Gomez, A.; Regueiro, V.; Saus, C.; Bengoechea, J.A.; Garmendia, J. Evidence for a non-replicative intracellular stage of nontypeable Haemophilus influenzae in epithelial cells. Microbiology 2011, 157, 234–250. [Google Scholar] [CrossRef]
- Marti-Lliteras, P.; Regueiro, V.; Morey, P.; Hood, D.W.; Saus, C.; Sauleda, J.; Agusti, A.G.; Bengoechea, J.A.; Garmendia, J. Nontypeable Haemophilus influenzae clearance by alveolar macrophages is impaired by exposure to cigarette smoke. Infect. Immun. 2009, 77, 4232–4242. [Google Scholar] [CrossRef]
- López-Gómez, A.; Cano, V.; Moranta, D.; Morey, P.; del Portillo, F.; Bengoechea, J.A.; Garmendia, J. Host cell kinases, α5 and β1 integrins, and Rac1 signalling on the microtubule cytoskeleton are important for non-typeable Haemophilus influenzae invasion of respiratory epithelial cells. Microbiology 2012, 158, 2384–2398. [Google Scholar] [CrossRef]
- Fernandez-Calvet, A.; Rodriguez-Arce, I.; Almagro, G.; Moleres, J.; Euba, B.; Caballero, L.; Marti, S.; Ramos-Vivas, J.; Bartholomew, T.L.; Morales, X.; et al. Modulation of Haemophilus influenzae interaction with hydrophobic molecules by the VacJ/MlaA lipoprotein impacts strongly on its interplay with the airways. Sci. Rep. 2018, 8, 6872. [Google Scholar] [CrossRef] [PubMed]
- Barrajon-Catalan, E.; Fernandez-Arroyo, S.; Roldan, C.; Guillen, E.; Saura, D.; Segura-Carretero, A.; Micol, V. A systematic study of the polyphenolic composition of aqueous extracts deriving from several Cistus genus species: Evolutionary relationship. Phytochem. Anal. 2011, 22, 303–312. [Google Scholar] [CrossRef] [PubMed]
- De Chiara, M.; Hood, D.; Muzzi, A.; Pickard, D.J.; Perkins, T.; Pizza, M.; Dougan, G.; Rappuoli, R.; Moxon, E.R.; Soriani, M.; et al. Genome sequencing of disease and carriage isolates of nontypeable Haemophilus influenzae identifies discrete population structure. Proc. Natl. Acad. Sci. USA 2014, 111, 5439–5444. [Google Scholar] [CrossRef] [PubMed]
- Moleres, J.; Fernandez-Calvet, A.; Ehrlich, R.L.; Marti, S.; Perez-Regidor, L.; Euba, B.; Rodriguez-Arce, I.; Balashov, S.; Cuevas, E.; Linares, J.; et al. Antagonistic pleiotropy in the bifunctional surface protein FadL (OmpP1) during adaptation of Haemophilus influenzae to chronic lung infection associated with chronic obstructive pulmonary disease. MBio 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Pettigrew, M.M.; Ahearn, C.P.; Gent, J.F.; Kong, Y.; Gallo, M.C.; Munro, J.B.; D’Mello, A.; Sethi, S.; Tettelin, H.; Murphy, T.F. Haemophilus influenzae genome evolution during persistence in the human airways in chronic obstructive pulmonary disease. Proc. Natl. Acad. Sci. USA 2018, 115, E3256–E3265. [Google Scholar] [CrossRef] [PubMed]
- Morey, P.; Viadas, C.; Euba, B.; Hood, D.W.; Barberan, M.; Gil, C.; Grillo, M.J.; Bengoechea, J.A.; Garmendia, J. Relative contributions of lipooligosaccharide inner and outer core modifications to nontypeable Haemophilus influenzae pathogenesis. Infect. Immun. 2013, 81, 4100–4111. [Google Scholar] [CrossRef]
- Rodriguez-Arce, I.; Al-Jubair, T.; Euba, B.; Fernandez-Calvet, A.; Gil-Campillo, C.; Marti, S.; Tornroth-Horsefield, S.; Riesbeck, K.; Garmendia, J. Moonlighting of Haemophilus influenzae heme acquisition systems contributes to the host airway-pathogen interplay in a coordinated manner. Virulence 2019, 10, 315–333. [Google Scholar] [CrossRef]
- Rodriguez-Arce, I.; Marti, S.; Euba, B.; Fernandez-Calvet, A.; Moleres, J.; Lopez-Lopez, N.; Barberan, M.; Ramos-Vivas, J.; Tubau, F.; Losa, C.; et al. Inactivation of the thymidylate synthase thyA in non-typeable Haemophilus influenzae modulates antibiotic resistance and has a strong impact on its interplay with the host airways. Front. Cell. Infect. Microbiol. 2017, 7, 266. [Google Scholar] [CrossRef]
- Reyes-Farias, M.; Carrasco-Pozo, C. The anti-cancer effect of quercetin: Molecular implications in cancer metabolism. Int. J. Mol. Sci. 2019, 20, 3177. [Google Scholar] [CrossRef]
- Townsend, E.A.; Emala, C.W., Sr. Quercetin acutely relaxes airway smooth muscle and potentiates b-agonist-induced relaxation via dual phosphodiesterase inhibition of PLCbeta and PDE4. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 305, L396–L403. [Google Scholar] [CrossRef]
- Euba, B.; Moleres, J.; Segura, V.; Viadas, C.; Morey, P.; Moranta, D.; Leiva, J.; de-Torres, J.P.; Bengoechea, J.A.; Garmendia, J. Genome expression profiling-based identification and administration efficacy of host-directed antimicrobial drugs against respiratory infection by nontypeable Haemophilus influenzae. Antimicrob. Agents Chemother. 2015, 59, 7581–7592. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Cao, W.; Xia, M.; Pan, S.; Xu, X. Study of structure and permeability relationship of flavonoids in Caco-2 cells. Nutrients 2017, 9, 1301. [Google Scholar] [CrossRef] [PubMed]
- Das, T.; Mukherjee, S.; Chaudhuri, K. Effect of quercetin on Vibrio cholerae induced nuclear factor-κB activation and interleukin-8 expression in intestinal epithelial cells. Microbes Infect. 2012, 14, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Shen, X.; Wei, Y.; Si, X.; Deng, X.; Wang, J. Quercetin reduces Streptococcus suis virulence by inhibiting suilysin activity and inflammation. Int. Immunopharmacol. 2019, 69, 71–78. [Google Scholar] [CrossRef]
- Wedzicha, J.A.; Calverley, P.M.; Rabe, K.F. Roflumilast: A review of its use in the treatment of COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2016, 11, 81–90. [Google Scholar] [CrossRef]
- Cosio, B.G.; Jahn, A.; Iglesias, A.; Shafiek, H.; Busquets, X.; Agusti, A. Haemophilus influenzae induces steroid-resistant inflammatory responses in COPD. BMC Pulm. Med. 2015, 15, 157. [Google Scholar] [CrossRef]
- Li, H.; Liu, D.H.; Chen, L.L.; Zhao, Q.; Yu, Y.Z.; Ding, J.J.; Miao, L.Y.; Xiao, Y.L.; Cai, H.R.; Zhang, D.P.; et al. Meta-analysis of the adverse effects of long-term azithromycin use in patients with chronic lung diseases. Antimicrob. Agents Chemother. 2014, 58, 511–517. [Google Scholar] [CrossRef]
- Boots, A.W.; Haenen, G.R.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef]
- Wang, L.; Chen, J.; Wang, B.; Wu, D.; Li, H.; Lu, H.; Wu, H.; Chai, Y. Protective effect of quercetin on lipopolysaccharide-induced acute lung injury in mice by inhibiting inflammatory cell influx. Exp. Biol. Med. 2014, 239, 1653–1662. [Google Scholar] [CrossRef]
- Wang, X.F.; Song, S.D.; Li, Y.J.; Hu, Z.Q.; Zhang, Z.W.; Yan, C.G.; Li, Z.G.; Tang, H.F. Protective effect of quercetin in LPS-induced murine acute lung injury mediated by cAMP-Epac pathway. Inflammation 2018, 41, 1093–1103. [Google Scholar] [CrossRef]
- Mitani, A.; Azam, A.; Vuppusetty, C.; Ito, K.; Mercado, N.; Barnes, P.J. Quercetin restores corticosteroid sensitivity in cells from patients with chronic obstructive pulmonary disease. Exp. Lung Res. 2017, 43, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Heinz, S.A.; Henson, D.A.; Austin, M.D.; Jin, F.; Nieman, D.C. Quercetin supplementation and upper respiratory tract infection: A randomized community clinical trial. Pharmacol. Res. 2010, 62, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Tripathi, A. Quercetin potentiates meropenem activity among pathogenic carbapenem-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. J. Appl. Microbiol. 2019, 127, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Vipin, C.; Mujeeburahiman, M.; Ashwini, P.; Arun, A.B.; Rekha, P.D. Anti-biofilm and cytoprotective activities of quercetin against Pseudomonas aeruginosa isolates. Lett. Appl. Microbiol. 2019, 68, 464–471. [Google Scholar] [CrossRef]
- Zeng, Y.; Nikitkova, A.; Abdelsalam, H.; Li, J.; Xiao, J. Activity of quercetin and kaemferol against Streptococcus mutans biofilm. Arch. Oral Biol. 2019, 98, 9–16. [Google Scholar] [CrossRef]
- Sugiyama, T.; Kawaguchi, K.; Dobashi, H.; Miyake, R.; Kaneko, M.; Kumazawa, Y. Quercetin but not luteolin suppresses the induction of lethal shock upon infection of mice with Salmonella typhimurium. FEMS Immunol. Med. Microbiol. 2008, 53, 306–313. [Google Scholar] [CrossRef]
- Brown, J.C.; Wang, J.; Kasman, L.; Jiang, X.; Haley-Zitlin, V. Activities of muscadine grape skin and quercetin against Helicobacter pylori infection in mice. J. Appl. Microbiol. 2011, 110, 139–146. [Google Scholar] [CrossRef]
- Napimoga, M.H.; Clemente-Napimoga, J.T.; Macedo, C.G.; Freitas, F.F.; Stipp, R.N.; Pinho-Ribeiro, F.A.; Casagrande, R.; Verri, W.A., Jr. Quercetin inhibits inflammatory bone resorption in a mouse periodontitis model. J. Nat. Prod. 2013, 76, 2316–2321. [Google Scholar] [CrossRef]
- Tormakangas, L.; Vuorela, P.; Saario, E.; Leinonen, M.; Saikku, P.; Vuorela, H. In vivo treatment of acute Chlamydia pneumoniae infection with the flavonoids quercetin and luteolin and an alkyl gallate, octyl gallate, in a mouse model. Biochem. Pharmacol. 2005, 70, 1222–1230. [Google Scholar] [CrossRef]
- Gaschler, G.J.; Skrtic, M.; Zavitz, C.C.; Lindahl, M.; Onnervik, P.O.; Murphy, T.F.; Sethi, S.; Stampfli, M.R. Bacteria challenge in smoke-exposed mice exacerbates inflammation and skews the inflammatory profile. Am. J. Respir. Crit. Care Med. 2009, 179, 666–675. [Google Scholar] [CrossRef]
- Konduru, A.S.; Lee, B.C.; Li, J.D. Curcumin suppresses NTHi-induced CXCL5 expression via inhibition of positive IKKβ pathway and up-regulation of negative MKP-1 pathway. Sci. Rep. 2016, 6, 31695. [Google Scholar] [CrossRef] [PubMed]
- Konduru, A.S.; Matsuyama, S.; Lee, B.C.; Komatsu, K.; Li, J.D. Curcumin inhibits NTHi-induced MUC5AC mucin overproduction in otitis media via upregulation of MAPK phosphatase MKP-1. Int. J. Inflam. 2017, 2017, 4525309. [Google Scholar] [CrossRef] [PubMed]
- Contreras, M.; Borras-Linares, I.; Herranz-Lopez, M.; Micol, V.; Segura-Carretero, A. Further exploring the absorption and enterocyte metabolism of quercetin forms in the Caco-2 model using nano-LC-TOF-MS. Electrophoresis 2016, 37, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Herranz-Lopez, M.; Borras-Linares, I.; Olivares-Vicente, M.; Galvez, J.; Segura-Carretero, A.; Micol, V. Correlation between the cellular metabolism of quercetin and its glucuronide metabolite and oxidative stress in hypertrophied 3T3-L1 adipocytes. Phytomedicine 2017, 25, 25–28. [Google Scholar] [CrossRef]


| Combination (A + B) | MICA (µg/mL) | MICA (A + B Combination) | MICB (µg/mL) | MICB (A + B Combination) | ΣFIC | Result | |
|---|---|---|---|---|---|---|---|
| A | B | ||||||
| Quercetin | Punicalagin | 167 | 83.5 | 320 | 160 | 1 | Indiferent |
| Quercetin | Myricetin | 250 ± 118 | 167 | 800 | 125 ± 106 | 0.91 ± 0.22 | Indiferent |
| Punicalagin | Myricetin | 320 | 80 | 800 | 800 | 1.25 | Indiferent |
| Quercetin | Azm | 167 | 125.25 ± 59 | 1.5 ± 0.71 | 1.5 ± 0.71 | 2 ± 1.41 | Indiferent |
| Myricetin | Azm | 400 | 12.5 | 1.5 ± 0.71 | 2 | 1.53 ± 0.71 | Indiferent |
| Punicalagin | Azm | 320 | 50 ± 42.4 | 2 | 1.5 ± 0.71 | 0.91 ± 0.49 | Indiferent |
| Quercetin | Amp | 125 ± 59 | 10.45 | 1 | 1.5 ± 0.71 | 1.6 ± 0.75 | Indiferent |
| Myricetin | Amp | 400 | 800 | 1 | 2 | 4 | Indiferent |
| Punicalagin | Amp | 320 | 640 | 1 | 0.13 | 2.13 | Indiferent |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Calvet, A.; Euba, B.; Caballero, L.; Díez-Martínez, R.; Menéndez, M.; Ortiz de Solórzano, C.; Leiva, J.; Micol, V.; Barrajón-Catalán, E.; Garmendia, J. Preclinical Evaluation of the Antimicrobial-Immunomodulatory Dual Action of Xenohormetic Molecules against Haemophilus influenzae Respiratory Infection. Biomolecules 2019, 9, 891. https://doi.org/10.3390/biom9120891
Fernández-Calvet A, Euba B, Caballero L, Díez-Martínez R, Menéndez M, Ortiz de Solórzano C, Leiva J, Micol V, Barrajón-Catalán E, Garmendia J. Preclinical Evaluation of the Antimicrobial-Immunomodulatory Dual Action of Xenohormetic Molecules against Haemophilus influenzae Respiratory Infection. Biomolecules. 2019; 9(12):891. https://doi.org/10.3390/biom9120891
Chicago/Turabian StyleFernández-Calvet, Ariadna, Begoña Euba, Lucía Caballero, Roberto Díez-Martínez, Margarita Menéndez, Carlos Ortiz de Solórzano, José Leiva, Vicente Micol, Enrique Barrajón-Catalán, and Junkal Garmendia. 2019. "Preclinical Evaluation of the Antimicrobial-Immunomodulatory Dual Action of Xenohormetic Molecules against Haemophilus influenzae Respiratory Infection" Biomolecules 9, no. 12: 891. https://doi.org/10.3390/biom9120891
APA StyleFernández-Calvet, A., Euba, B., Caballero, L., Díez-Martínez, R., Menéndez, M., Ortiz de Solórzano, C., Leiva, J., Micol, V., Barrajón-Catalán, E., & Garmendia, J. (2019). Preclinical Evaluation of the Antimicrobial-Immunomodulatory Dual Action of Xenohormetic Molecules against Haemophilus influenzae Respiratory Infection. Biomolecules, 9(12), 891. https://doi.org/10.3390/biom9120891







