Pretreatment of Wheat Straw with Phosphoric Acid and Hydrogen Peroxide to Simultaneously Facilitate Cellulose Digestibility and Modify Lignin as Adsorbents
Abstract
1. Introduction
2. Materials and Methods
2.1. Feedstock
2.2. PHP-Pretreating Wheat Straw for Fractionation
2.3. Enzymatic Hydrolysis
2.4. Analytical Methods
2.5. Lignin Characterization
2.6. Adsorption of Cationic Substances by PHPLs
3. Results and Discussion
3.1. Wheat Straw Fractionation via PHP Pretreatment
3.2. Lignin Modification During PHP Pretreatment
3.3. Adsorption of Cationic Substances by PHPLs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Farzad, S.; Mandegari, M.A.; Guo, M.; Haigh, K.F.; Shah, N.; Görgens, J.F. Multi-product biorefineries from lignocelluloses: a pathway to revitalisation of the sugar industry? Biotechnol. Biofuels 2017, 10, 87–111. [Google Scholar] [CrossRef]
- Tian, D.; Hu, J.; Chandra, R.P.; Saddler, J.N.; Lu, C. Valorizing Recalcitrant Cellulolytic Enzyme Lignin via Lignin Nanoparticles Fabrication in an Integrated Biorefinery. ACS Sustain. Chem. Eng. 2017, 5, 2702–2710. [Google Scholar] [CrossRef]
- Tian, D.; Hu, J.; Bao, J.; Chandra, R.P.; Saddler, J.N.; Lu, C. Lignin valorization: Lignin nanoparticles as high-value bio-additive for multifunctional nanocomposites. Biotechnol. Biofuels 2017, 10, 192. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Vasco, C.; Ma, R.; Quintero, M.; Guo, M.; Geleynse, S.; Ramasamy, K.K.; Wolcott, M.; Zhang, X. Unique low-molecular-weight lignin with high purity extracted from wood by deep eutectic solvents (DES): A source of lignin for valorization. Green Chem. 2016, 18, 5133–5141. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin valorization: Improving lignin processing in the biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef]
- Wang, H.; Pu, Y.; Ragauskas, A.; Yang, B. From lignin to valuable products–strategies, challenges, and prospects. Bioresour. Technol. 2019, 271, 449–461. [Google Scholar] [CrossRef]
- Schutyser, W.; Renders, T.; Van Den Bosch, S.; Koelewijn, S.F.; Beckham, G.T.; Sels, B.F. Chemicals from lignin: An interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef]
- Galkin, M.V.; Samec, J.S.M. Lignin Valorization through Catalytic Lignocellulose Fractionation: A Fundamental Platform for the Future Biorefinery. ChemSusChem 2016, 9, 1544–1558. [Google Scholar] [CrossRef]
- Zhang, P.; Dong, S.J.; Ma, H.H.; Zhang, B.X.; Wang, Y.F.; Hu, X.M. Fractionation of corn stover into cellulose, hemicellulose and lignin using a series of ionic liquids. Ind. Crops Prod. 2015, 76, 688–696. [Google Scholar] [CrossRef]
- Shen, X.; Wen, J.L.; Mei, Q.; Chen, X.; Sun, D.; Yuan, T.Q.; Sun, R.C. Facile fractionation of lignocelluloses by biomass-derived deep eutectic solvent (DES) pretreatment for cellulose enzymatic hydrolysis and lignin valorization. Green Chem. 2018. [Google Scholar] [CrossRef]
- Supanchaiyamat, N.; Jetsrisuparb, K.; Knijnenburg, J.T.N.; Tsang, D.C.W.; Hunt, A.J. Lignin materials for adsorption: Current trend, perspectives and opportunities. Bioresour. Technol. 2019, 272, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ding, Y.; Zhang, L.; Wang, X.; Zhao, Y.; Zhang, X.; Yu, G. A Sustainable Redox-Flow Battery with an Aluminum-Based, Deep-Eutectic-Solvent Anolyte. Angew. Chem. Int. Ed. 2017, 56, 7454–7459. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Zhang, X.; Xin, J.; Liu, G.; Wu, G.; Kong, Z.; Zhang, J. Clickable Synthesis of 1,2,4-Triazole Modified Lignin-Based Adsorbent for the Selective Removal of Cd(II). ACS Sustain. Chem. Eng. 2017, 5, 4086–4093. [Google Scholar] [CrossRef]
- Li, Z.; Xiao, D.; Ge, Y.; Koehler, S. Surface-Functionalized Porous Lignin for Fast and Efficient Lead Removal from Aqueous Solution. ACS Appl. Mater. Interfaces 2015, 7, 15000–15009. [Google Scholar] [CrossRef]
- Ge, Y.; Li, Z. Application of Lignin and Its Derivatives in Adsorption of Heavy Metal Ions in Water: A Review. ACS Sustain. Chem. Eng. 2018, 6, 7181–7192. [Google Scholar] [CrossRef]
- Upton, B.M.; Kasko, A.M. Strategies for the conversion of lignin to high-value polymeric materials: Review and perspective. Chem. Rev. 2016, 116, 2275–2306. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, R.; Pang, Y.; Qiu, X.; Yang, D. Microwave-assisted synthesis of high carboxyl content of lignin for enhancing adsorption of lead. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 553, 187–194. [Google Scholar] [CrossRef]
- Qiu, J.W.; Ma, L.J.; Shen, F.; Yang, G.; Zhang, Y.Z.; Deng, S.H.; Zhang, J.; Zeng, Y.M.; Hu, Y.D. Pretreating wheat straw by phosphoric acid plus hydrogen peroxide for enzymatic saccharification and ethanol production at high solid loading. Bioresour. Technol. 2017, 238, 174–181. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, J.; Shen, F.; Mei, Z.; Yang, G.; Zhang, Y.; Hu, Y.; Zhang, J.; Deng, S. Pretreating wheat straw by the concentrated phosphoric acid plus hydrogen peroxide (PHP): Investigations on pretreatment conditions and structure changes. Bioresour. Technol. 2016, 199, 245–257. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Z.H.; Shen, F.; Hu, J.G.; Sun, F.B.; Lin, L.L.; Yang, G.; Zhang, Y.Z.; Deng, S.H. Pretreating lignocellulosic biomass by the concentrated phosphoric acid plus hydrogen peroxide (PHP) for enzymatic hydrolysis: Evaluating the pretreatment flexibility on feedstocks and particle sizes. Bioresour. Technol. 2014, 166, 420–428. [Google Scholar] [CrossRef]
- Wang, Q.; Tian, D.; Hu, J.; Shen, F.; Yang, G.; Zhang, Y.; Deng, S.; Zhang, J.; Zeng, Y.; Hu, Y. Fates of hemicellulose, lignin and cellulose in concentrated phosphoric acid with hydrogen peroxide (PHP) pretreatment. RSC Adv. 2018, 8, 12714–12723. [Google Scholar] [CrossRef]
- Wan, X.; Tian, D.; Shen, F.; Hu, J.; Yang, G.; Zhang, Y.; Deng, S.; Zhang, J.; Zeng, Y. Fractionating Wheat Straw via Phosphoric Acid with Hydrogen Peroxide Pretreatment and Structural Elucidation of the Derived Lignin. Energy Fuels 2018, 32, 5218–5225. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, W.; Hou, Q.; Chen, J.; Xu, N.; Ji, F. Effects of different pre-extractions combining with chemi-thermomechanical treatments on the enzymatic hydrolysis of wheat straw. Bioresour. Technol. 2015, 175, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Del Río, J.C.; Prinsen, P.; Rencoret, J.; Nieto, L.; Jiménez-Barbero, J.; Ralph, J.; Martínez, Á.T.; Gutiérrez, A. Structural characterization of the lignin in the cortex and pith of elephant grass (Pennisetum purpureum) stems. J. Agric. Food Chem. 2012, 60, 3619–3634. [Google Scholar] [CrossRef]
- Peng, X.P.; Sun, S.L.; Wen, J.L.; Yin, W.L.; Sun, R.C. Structural characterization of lignins from hydroxycinnamoyl transferase (HCT) down-regulated transgenic poplars. Fuel 2014, 134, 485–492. [Google Scholar] [CrossRef]
- Wen, J.L.; Xue, B.L.; Sun, S.L.; Sun, R.C. Quantitative structural characterization and thermal properties of birch lignins after auto-catalyzed organosolv pretreatment and enzymatic hydrolysis. J. Chem. Technol. Biotechnol. 2013, 88, 1663–1671. [Google Scholar] [CrossRef]
- Sun, S.; Huang, Y.; Sun, R.; Tu, M. The strong association of condensed phenolic moieties in isolated lignins with their inhibition of enzymatic hydrolysis. Green Chem. 2016, 18, 4276–4286. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Z.; Ge, X.; Zhang, J. Effects of lignin and surfactant on adsorption and hydrolysis of cellulases on cellulose. Biotechnol. Biofuels 2016, 9, 1–9. [Google Scholar] [CrossRef]
- Nakagame, S.; Chandra, R.P.; Kadla, J.F.; Saddler, J.N. Enhancing the enzymatic hydrolysis of lignocellulosic biomass by increasing the carboxylic acid content of the associated lignin. Biotechnol. Bioeng. 2011, 108, 538–548. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Ding, S.Y.; Mielenz, J.R.; Cui, J.B.; Elander, R.T.; Laser, M.; Himmel, M.E.; McMillan, J.R.; Lynd, L.R. Fractionating Recalcitrant Lignocellulose at Modest Reaction Conditions. Biotechnol. Bioeng. 2007, 97, 214–223. [Google Scholar] [CrossRef]
- Li, H.; McDonald, A.G. Fractionation and characterization of industrial lignins. Ind. Crops Prod. 2014, 62, 67–76. [Google Scholar] [CrossRef]
- Gabov, K.; Gosselink, R.J.A.; Smeds, A.I.; Fardim, P. Characterization of lignin extracted from birch wood by a modified hydrotropic process. J. Agric. Food Chem. 2014, 62, 10759–10767. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.Q.; Sun, S.N.; Xu, F.; Sun, R.C. Structural characterization of lignin from triploid of populus tomentosa carr. J. Agric. Food Chem. 2011, 59, 6605–6615. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.C.; Wang, M.; Sun, R.C. Toward an understanding of inhomogeneities in structure of lignin in green solvents biorefinery. Part 1: Fractionation and characterization of lignin. ACS Sustain. Chem. Eng. 2015, 3, 2443–2451. [Google Scholar] [CrossRef]
- Pu, Y.; Cao, S.; Ragauskas, A.J. Application of quantitative 31P NMR in biomass lignin and biofuel precursors characterization. Energy Environ. Sci. 2011, 4, 3154–3166. [Google Scholar] [CrossRef]
- Wen, J.L.; Yuan, T.Q.; Sun, S.L.; Xu, F.; Sun, R.C. Understanding the chemical transformations of lignin during ionic liquid pretreatment. Green Chem. 2014, 16, 181–190. [Google Scholar] [CrossRef]
- Wen, J.L.; Sun, S.L.; Yuan, T.Q.; Sun, R.C. Structural elucidation of whole lignin from Eucalyptus based on preswelling and enzymatic hydrolysis. Green Chem. 2015, 17, 1589–1596. [Google Scholar] [CrossRef]
- Dizhbite, T.; Jashina, L.; Dobele, G.; Andersone, A.; Evtuguin, D.; Bikovens, O.; Telysheva, G. Polyoxometalate (POM)-aided modification of lignin from wheat straw biorefinery. Holzforschung 2013, 67, 539–547. [Google Scholar] [CrossRef]
- Wang, B.; Wen, J.L.; Sun, S.L.; Wang, H.M.; Wang, S.F.; Liu, Q.Y.; Charlton, A.; Sun, R.C. Chemosynthesis and structural characterization of a novel lignin-based bio-sorbent and its strong adsorption for Pb (II). Ind. Crops Prod. 2017, 108, 72–80. [Google Scholar] [CrossRef]
- Albadarin, A.B.; Collins, M.N.; Naushad, M.; Shirazian, S.; Walker, G.; Mangwandi, C. Activated lignin-chitosan extruded blends for efficient adsorption of methylene blue. Chem. Eng. J. 2017, 307, 264–272. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, G.; Jia, Q.; Zhao, C.; Zhou, W.; Li, W. Adsorption of Cu(II), Pb(II), Co(II), Ni(II), and Cd(II) from aqueous solution by poly(aryl ether ketone) containing pendant carboxyl groups (PEK-L): Equilibrium, kinetics, and thermodynamics. Chem. Eng. J. 2011, 171, 152–158. [Google Scholar] [CrossRef]
- Kriaa, A.; Hamdi, N.; Srasra, E. Adsorption studies of methylene blue dye on tunisian activated lignin. Russ. J. Phys. Chem. A 2011, 85, 279–287. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Z.; Zhang, Y.; Pan, H.; Tao, L. Adsorption of Methylene Blue on Organosolv Lignin from Rice Straw. Procedia Environ. Sci. 2016, 31, 3–11. [Google Scholar] [CrossRef]
- Consolin Filho, N.; Venancio, E.C.; Barriquello, M.F.; Hechenleitner, A.A.W.; Pineda, E.A.G. Methylene blue adsorption onto modified lignin from sugar cane bagasse. Eclet. Quim. 2007, 32, 63–70. [Google Scholar] [CrossRef][Green Version]
- Li, Y.; Wu, M.; Wang, B.; Wu, Y.; Ma, M.; Zhang, X. Synthesis of Magnetic Lignin-Based Hollow Microspheres: A Highly Adsorptive and Reusable Adsorbent Derived from Renewable Resources. ACS Sustain. Chem. Eng. 2016, 4, 5523–5532. [Google Scholar] [CrossRef]
- Li, S.; Barreto, V.; Li, R.; Chen, G.; Hsieh, Y.P. Nitrogen retention of biochar derived from different feedstocks at variable pyrolysis temperaturesolysis temperatures. J. Anal. Appl. Pyrolysis 2018, 133, 136–146. [Google Scholar] [CrossRef]
- Sun, J.; Lian, F.; Liu, Z.; Zhu, L.; Song, Z. Biochars derived from various crop straws: Characterization and Cd(II) removal potential. Ecotoxicol. Environ. Saf. 2014, 106, 226–231. [Google Scholar] [CrossRef]
- Shi, C.; Tao, F.; Cui, Y. Evaluation of nitriloacetic acid modified cellulose film on adsorption of methylene blue. Int. J. Biol. Macromol. 2018, 114, 400–407. [Google Scholar] [CrossRef]
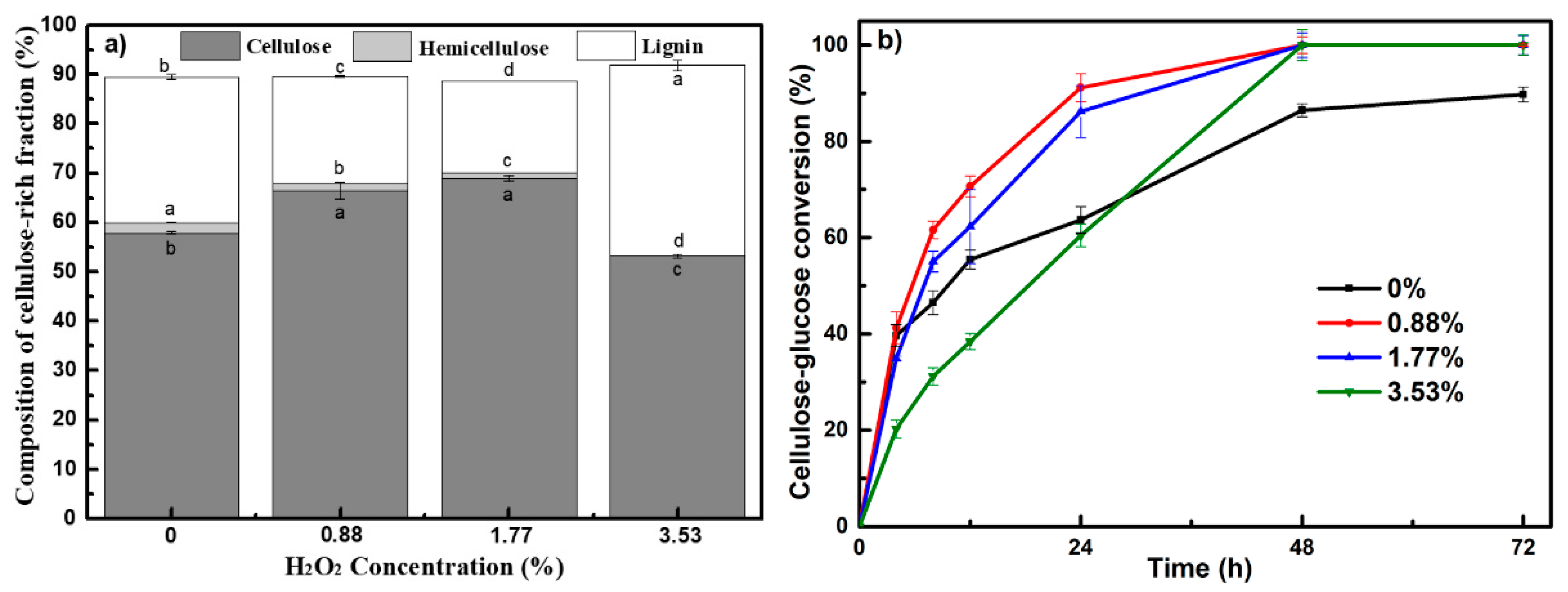
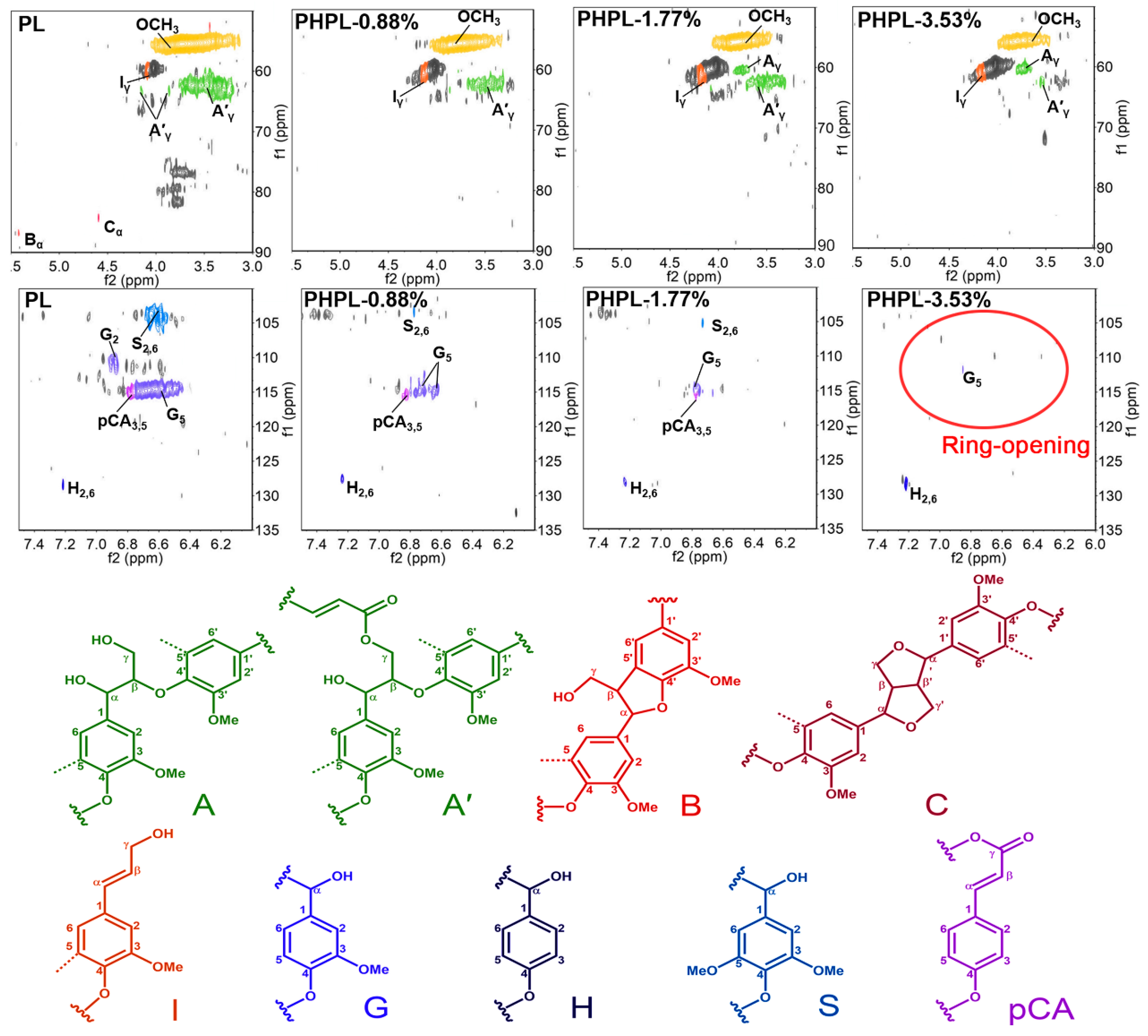

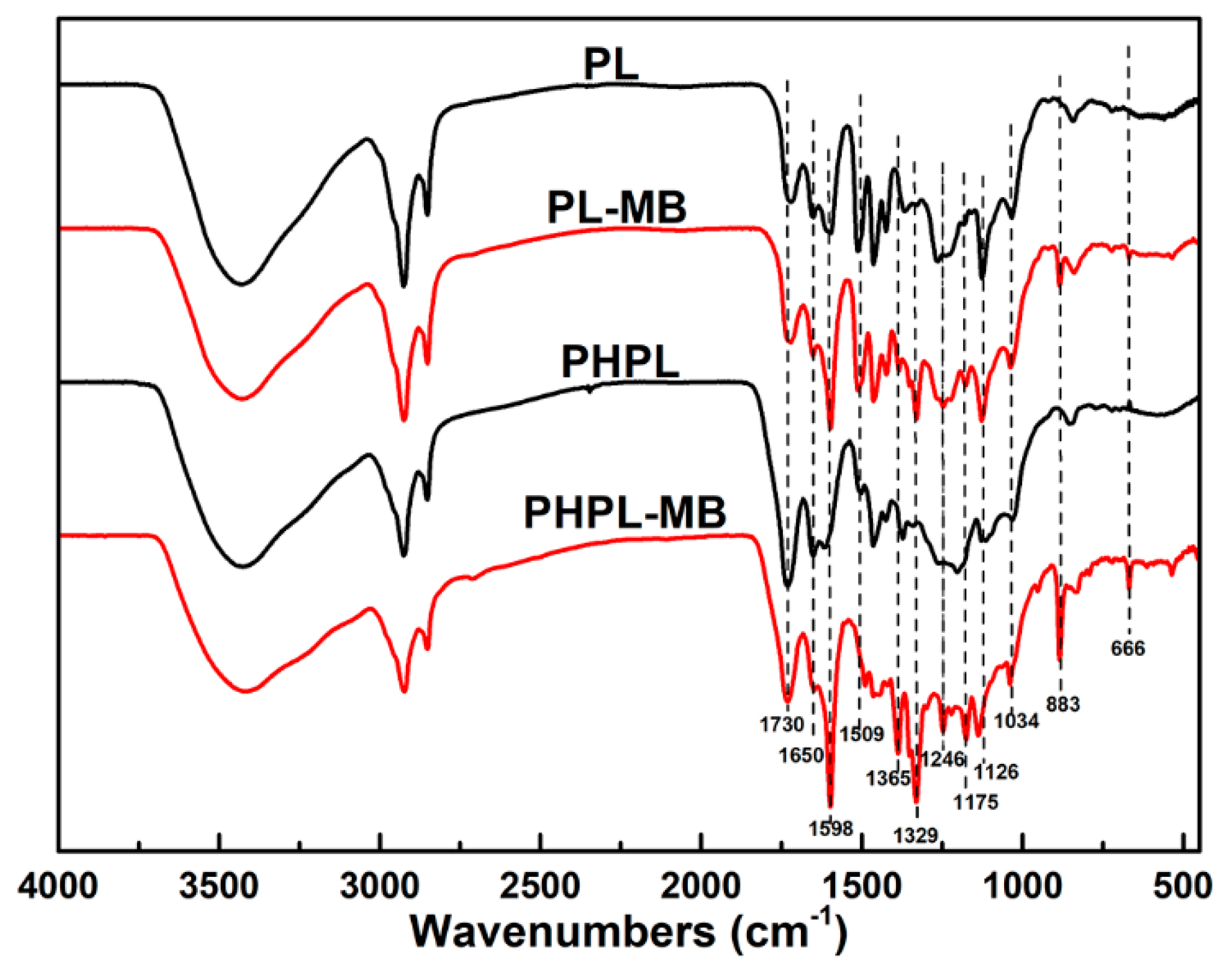

| H2O2 Input | 0% | 0.88% | 1.77% | 3.53% |
|---|---|---|---|---|
| Cellulose-rich solids (g) | 53.7 ± 0.38 a | 45.8 ± 0.29 b | 38.2 ± 0.07 c | 16.3 ± 0.59 d |
| Oligosaccharide (g) | 5.8 ± 0.03 a | 4.5 ± 0.01 a | 5.1 ± 0.01 a | 0.8 ± 0.09 b |
| PHP lignin (g) | 5.1 ± 0.48 d | 7.1 ± 0.55 c | 7.5 ± 0.01 b | 7.7 ± 0.05 a |
| Samples | Yield (%) | Carbohydrates (%) | ||
|---|---|---|---|---|
| Glucose | Xylose | Total | ||
| PL # | 23.7 ± 0.16 d | 0.49 ± 0.00 b | 0.85 ± 0.16 a | 1.33 ± 0.01 a |
| PHPL-0.88% | 32.8 ± 0.06 c | 0.30 ± 0.05 c | 0.76 ± 0.16 a | 1.06 ± 0.08 b |
| PHPL-1.77% | 34.8 ± 0.05 b | 0.20 ± 0.02 d | 0.53 ± 0.16 b | 0.74 ± 0.06 c |
| PHPL-3.53% | 35.7 ± 0.41 a | 0.72 ± 0.00 a | 0.16 ± 0.16 c | 0.87 ± 0.04 c |
| Samples | CEL | PL | PHPL-0.88% | PHPL-1.77% | PHPL-3.53% |
|---|---|---|---|---|---|
| Aliphatic OH | 4.81 | 1.73 | 1.76 | 1.79 | 1.54 |
| C-5 substitution | 0.35 | 0.87 | 1.17 | 1.39 | 1.36 |
| Guaiacyl phenolic OH | 0.60 | 0.61 | 0.68 | 0.70 | 0.62 |
| p-Hydroxyphenyl OH | 0.46 | 0.30 | 0.42 | 0.45 | 0.39 |
| Carboxylic acids OH | 0.15 | 0.46 | 0.66 | 0.69 | 0.76 |
| Total phenolic OH | 1.40 | 1.79 | 2.27 | 2.54 | 2.38 |
| Adsorbent | T (K) | Adsorption Capacity Qmax (mg g−1) | Refs. |
|---|---|---|---|
| PHPL | 298 | 201.1 | This study |
| Natural lignin | a | 80.6 | [42] |
| Organosolv lignin | 293 | 40.0 | [43] |
| Lignin-chitosan blended extrudates | 293 | 36.3 | [40] |
| Modified lignin | 323 | 34.2 | [44] |
| Lignin-based hollow microsphere | 313 | 31.2 | [45] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, X.; Yao, F.; Tian, D.; Shen, F.; Hu, J.; Zeng, Y.; Yang, G.; Zhang, Y.; Deng, S. Pretreatment of Wheat Straw with Phosphoric Acid and Hydrogen Peroxide to Simultaneously Facilitate Cellulose Digestibility and Modify Lignin as Adsorbents. Biomolecules 2019, 9, 844. https://doi.org/10.3390/biom9120844
Wan X, Yao F, Tian D, Shen F, Hu J, Zeng Y, Yang G, Zhang Y, Deng S. Pretreatment of Wheat Straw with Phosphoric Acid and Hydrogen Peroxide to Simultaneously Facilitate Cellulose Digestibility and Modify Lignin as Adsorbents. Biomolecules. 2019; 9(12):844. https://doi.org/10.3390/biom9120844
Chicago/Turabian StyleWan, Xue, Fengpei Yao, Dong Tian, Fei Shen, Jinguang Hu, Yongmei Zeng, Gang Yang, Yanzong Zhang, and Shihuai Deng. 2019. "Pretreatment of Wheat Straw with Phosphoric Acid and Hydrogen Peroxide to Simultaneously Facilitate Cellulose Digestibility and Modify Lignin as Adsorbents" Biomolecules 9, no. 12: 844. https://doi.org/10.3390/biom9120844
APA StyleWan, X., Yao, F., Tian, D., Shen, F., Hu, J., Zeng, Y., Yang, G., Zhang, Y., & Deng, S. (2019). Pretreatment of Wheat Straw with Phosphoric Acid and Hydrogen Peroxide to Simultaneously Facilitate Cellulose Digestibility and Modify Lignin as Adsorbents. Biomolecules, 9(12), 844. https://doi.org/10.3390/biom9120844








