Lemon Peel Polyphenol Extract Reduces Interleukin-6-Induced Cell Migration, Invasiveness, and Matrix Metalloproteinase-9/2 Expression in Human Gastric Adenocarcinoma MKN-28 and AGS Cell Lines
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Lemon Peel Polyphenol Extract (LPE) and Determination of Total Polyphenols’ Content
2.3. Cell Cultures and Treatments
2.4. Cell Proliferation Assay
2.5. RNA Extraction, Reverse Transcription (RT), and Quantitative Real-Time Polymerase Chain Reaction (qPCR)
2.6. Western Blotting Analysis
2.7. Gelatin Zymography
2.8. Wound Assay
2.9. Matrigel Invasion Assay
2.10. Acetylcholinesterase Inhibition Assay
2.11. Statistical Analysis
3. Results
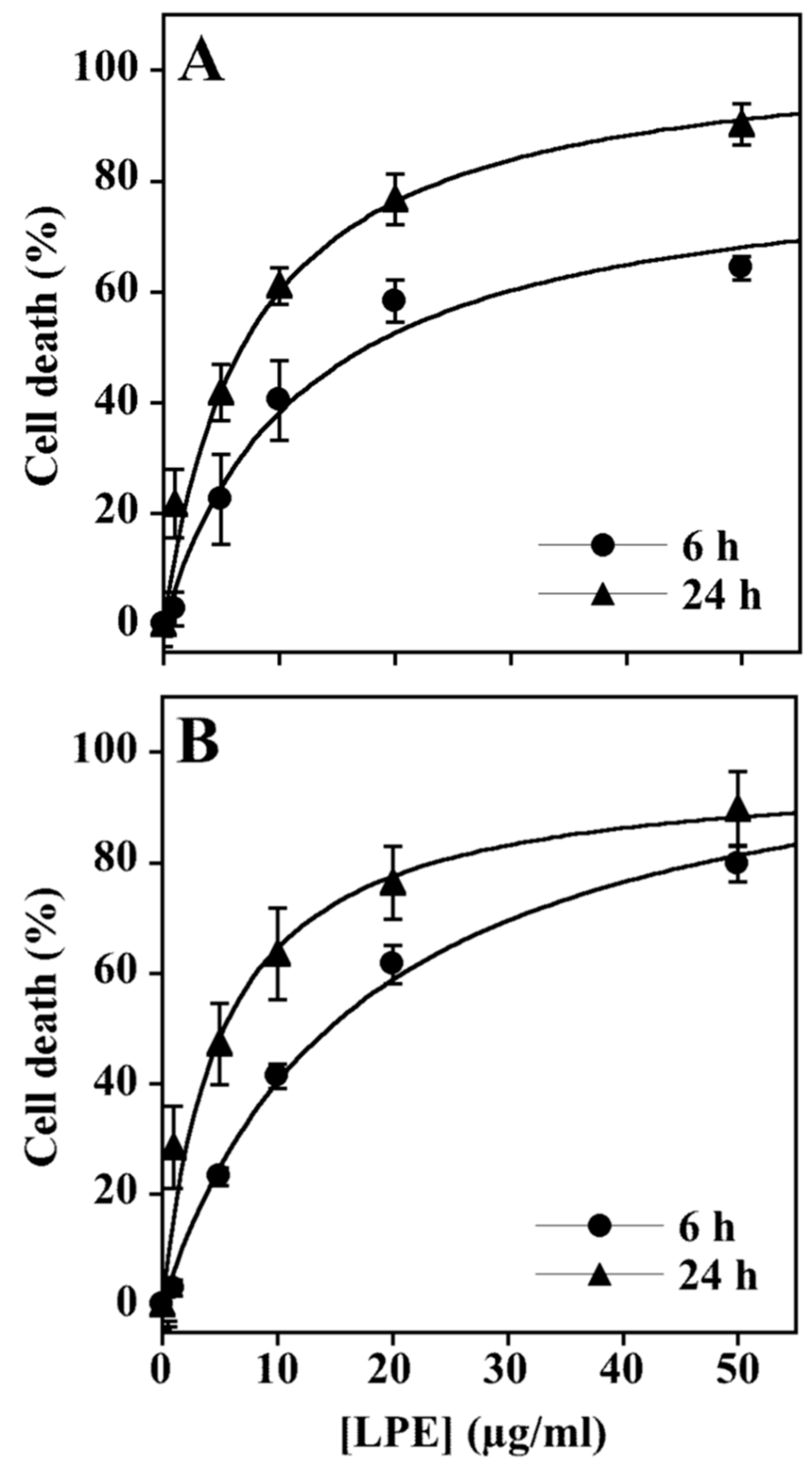
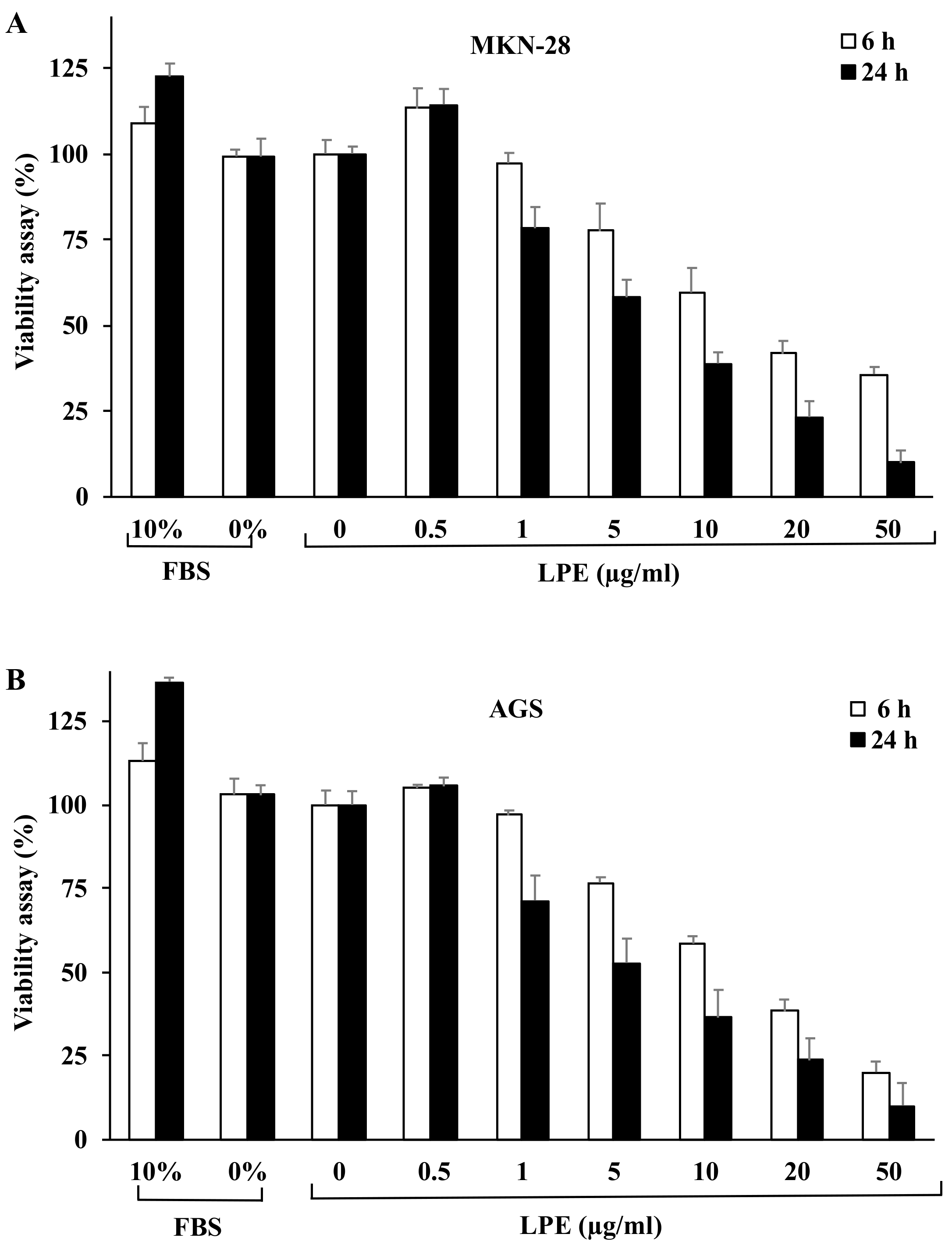
3.1. Effect of LPE Treatment on Cell Proliferation
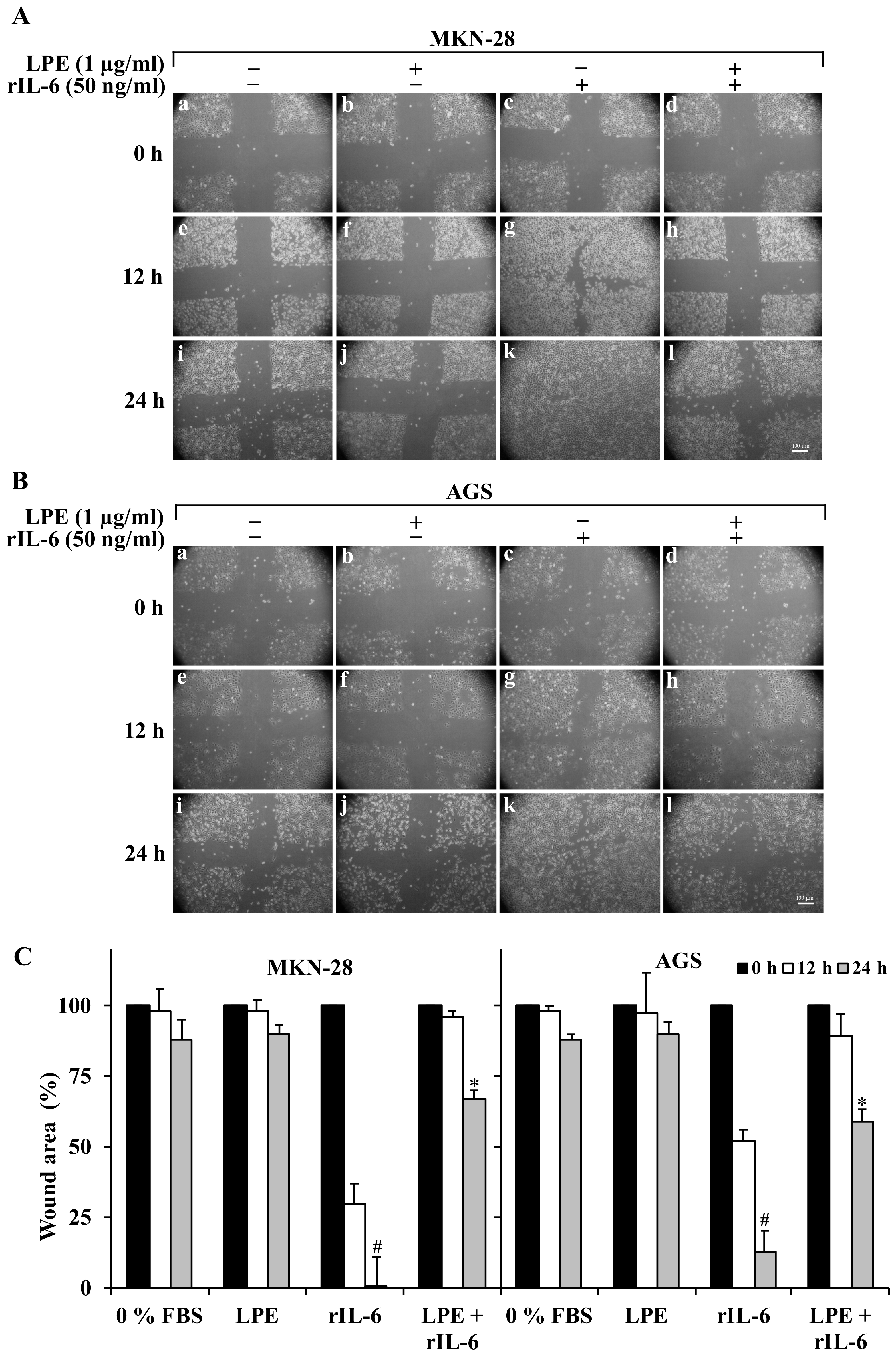
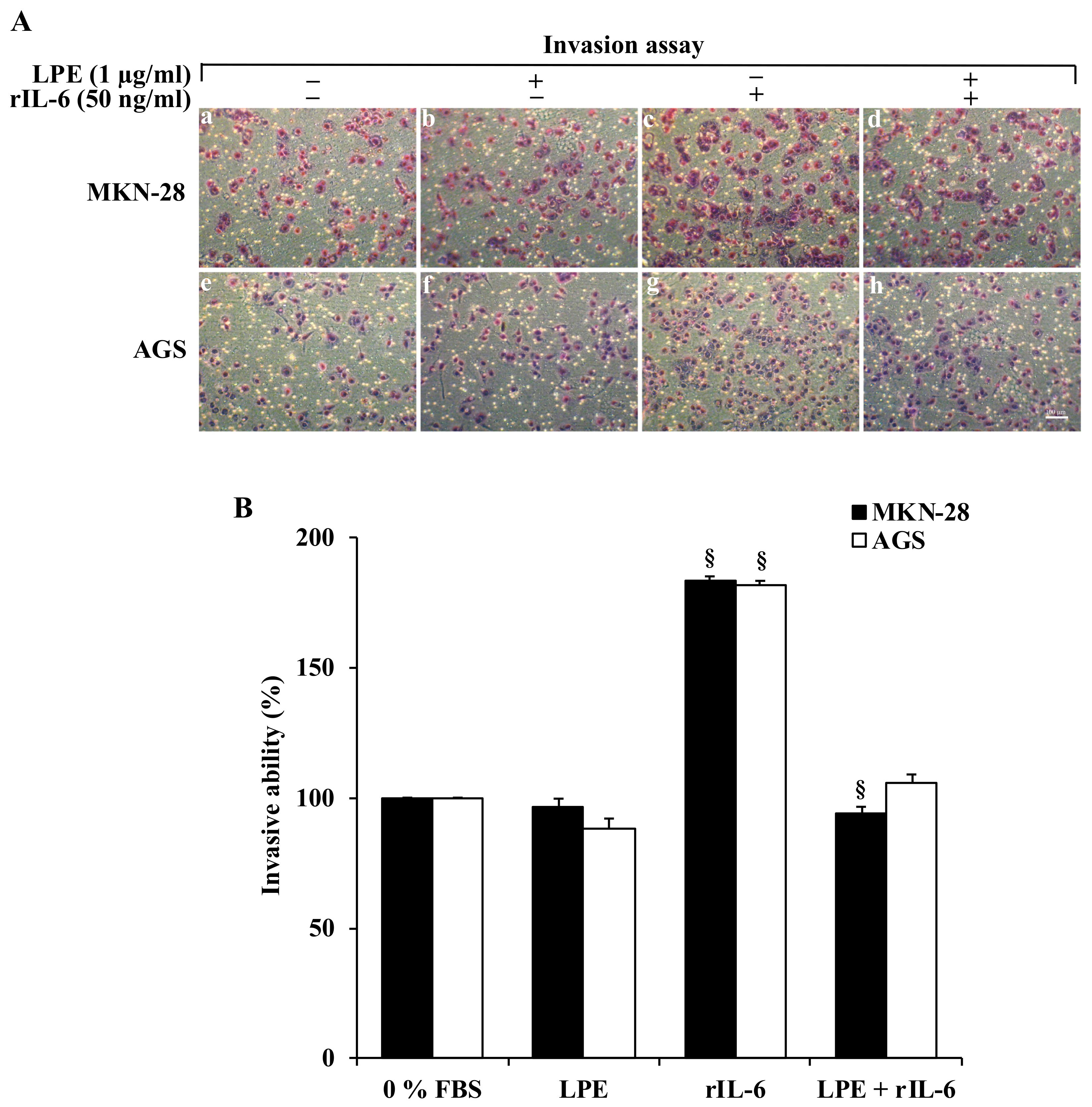
3.2. The LPE Treatment Reduces the rIL-6-Stimulated Migration and Invasiveness of MKN-28 and AGS Cells
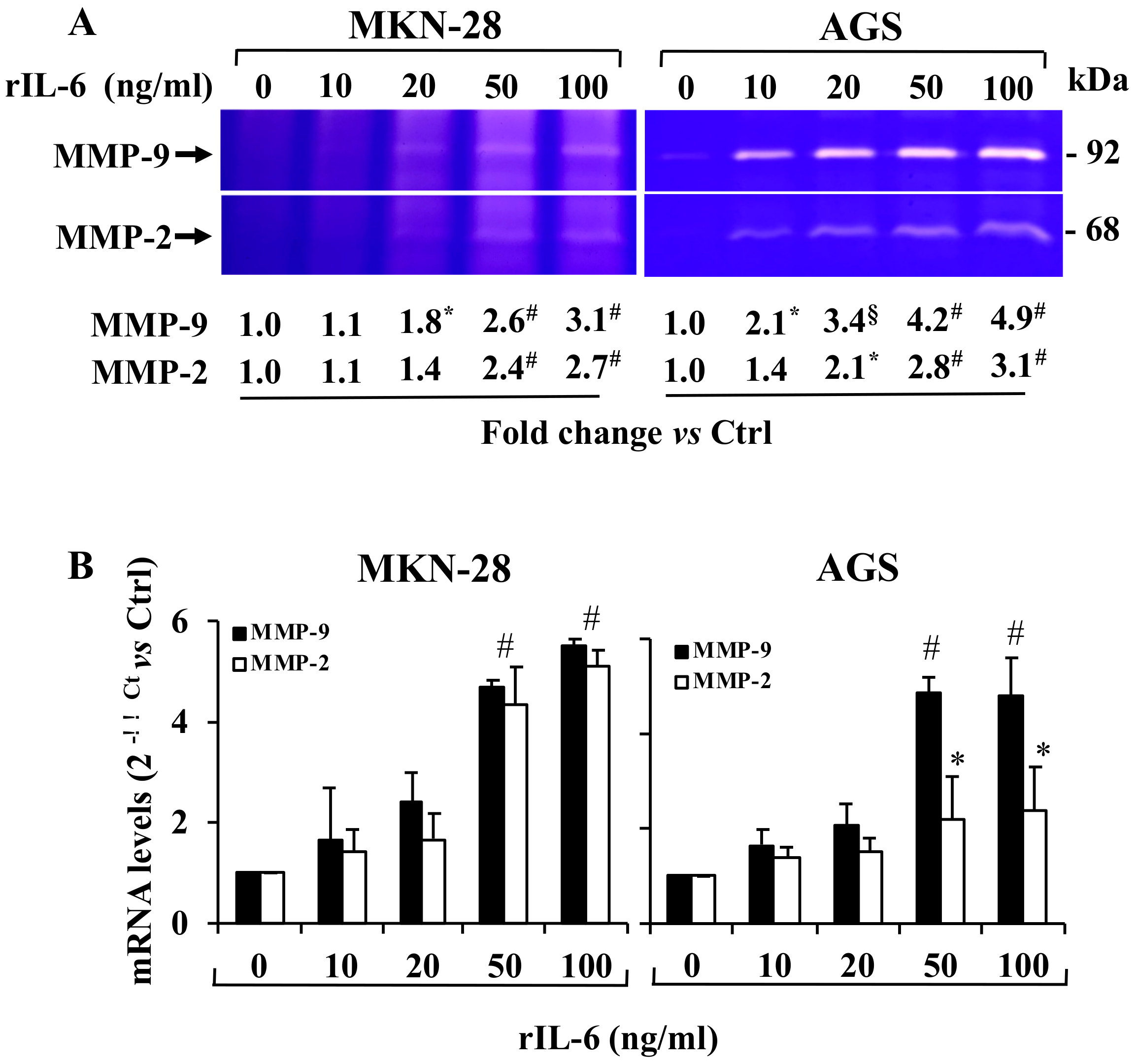
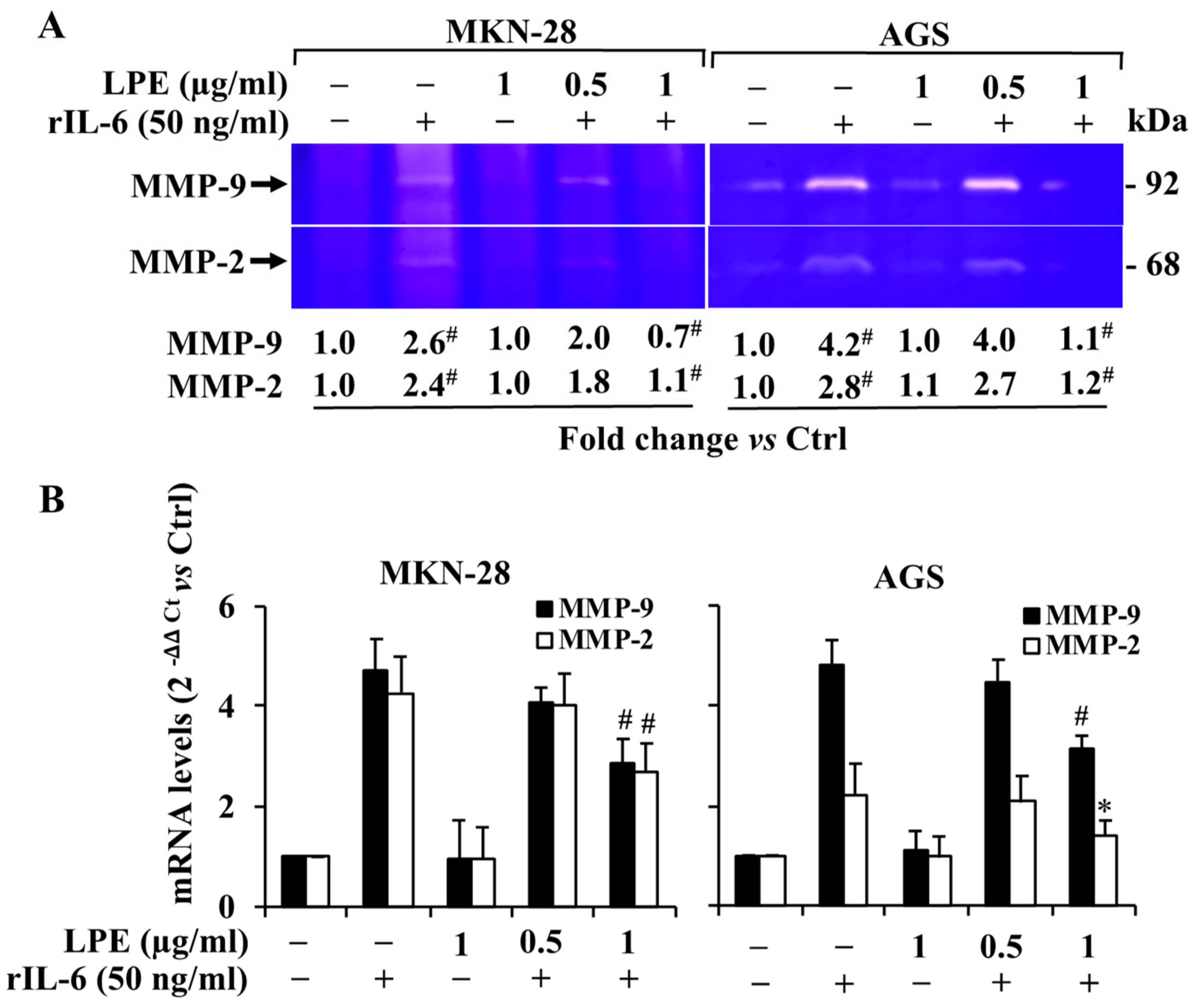
3.3. Effect of Exposure to rIL-6 on MMP-9 and MMP-2 Gelatinolytic Activity and Expression Levels in MKN-28 and AGS Cells
3.4. Effect of LPE on rIL-6 Induced Up-Regulation of MMP-9/2 Expression Levels in MKN-28 and AGS Cells
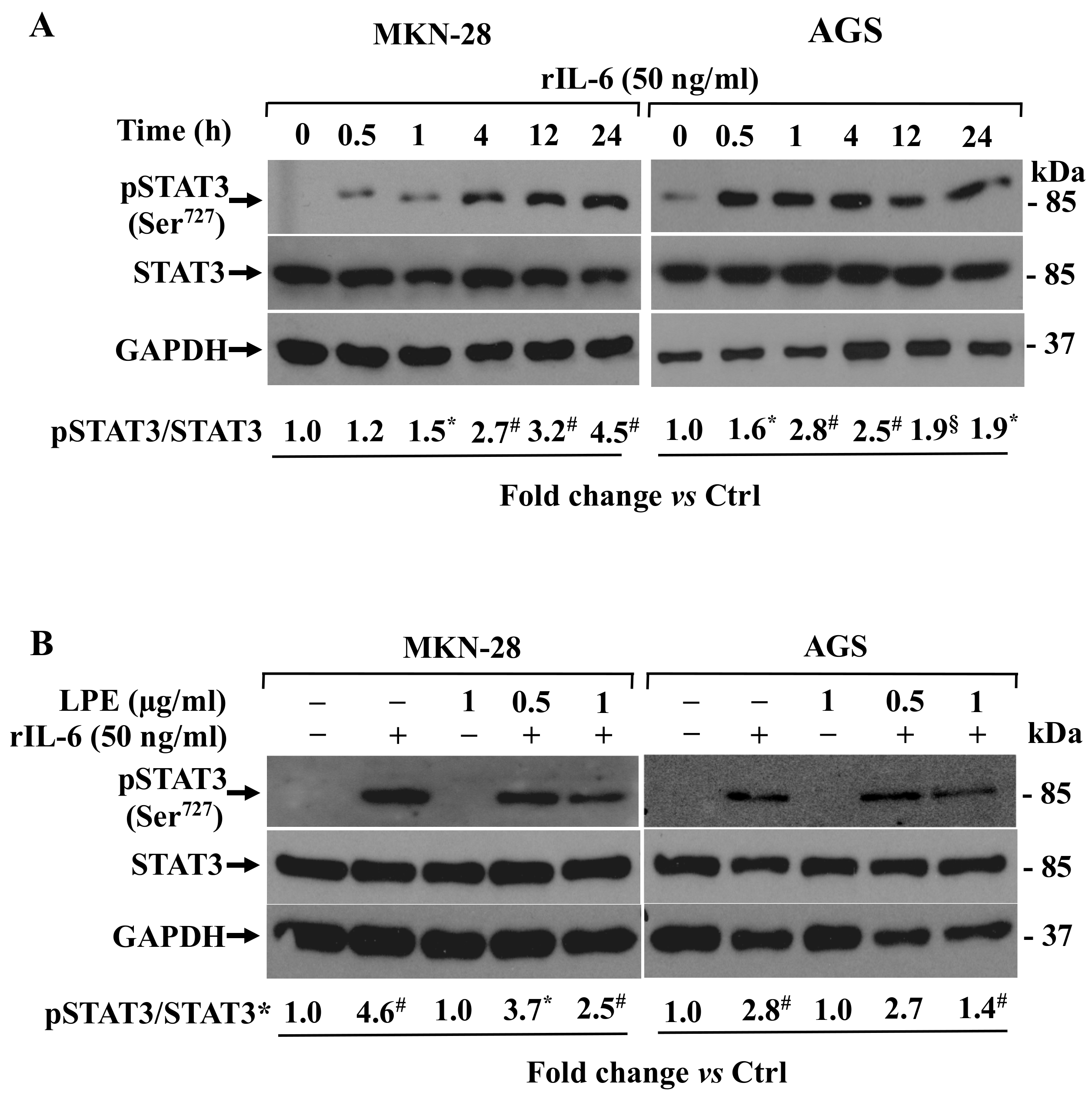
3.5. Effects of LPE on IL-6-Dependent STAT3 Phosphorylation Levels in MKN-28 and AGS Cells
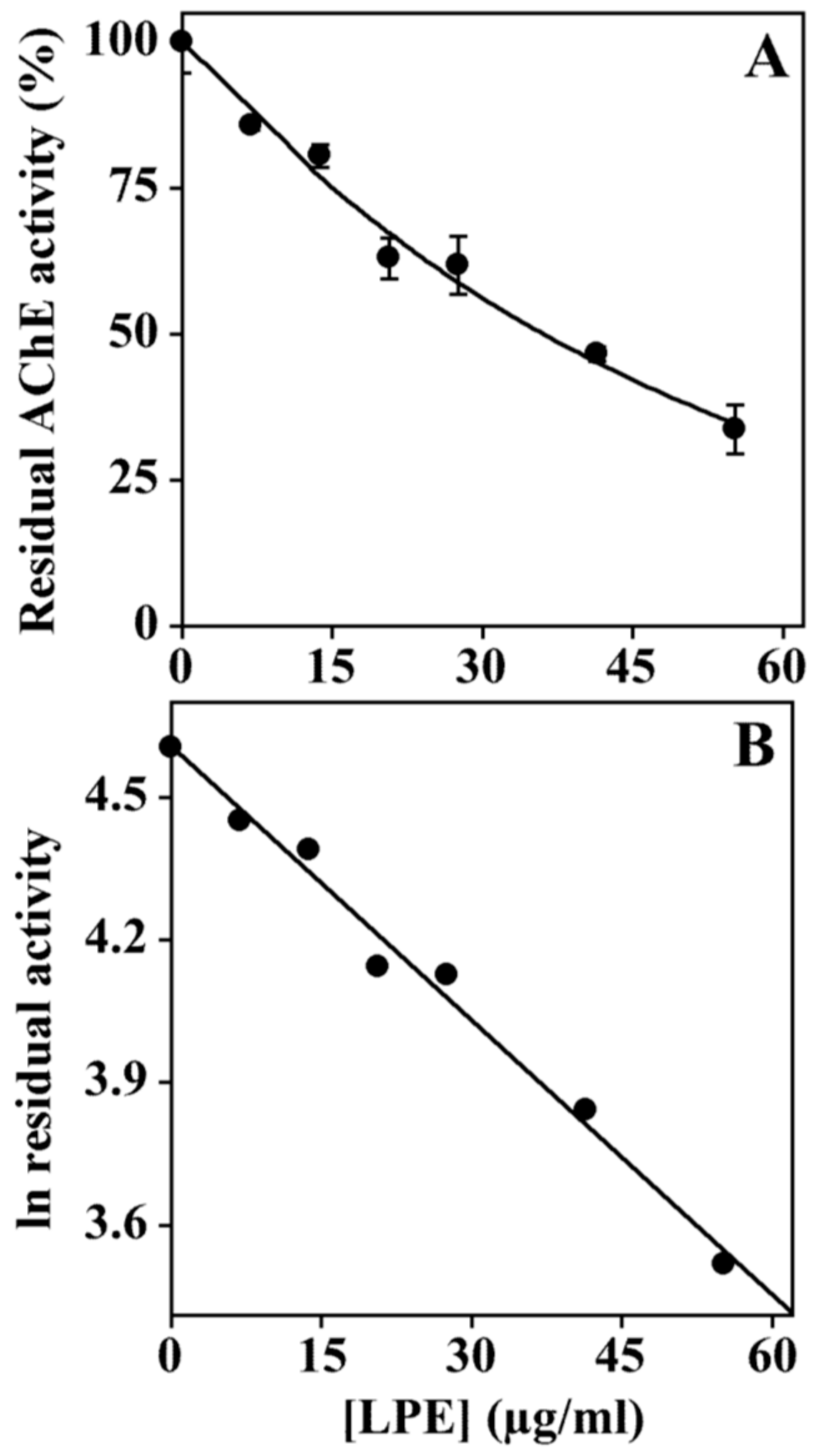
3.6. Effect of LPE on Acetylcholinesterase Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
References
- Yang, T.; Zhang, J.; Zhou, J.; Zhu, M.; Wang, L.; Yan, L. Resveratrol inhibits Interleukin-6 induced invasion of human gastric cancer cells. Biomed. Pharmacother. 2018, 99, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska-Trypuć, A.; Matejczyk, M.; Rosochacki, S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J. Enzyme Inhib. Med. Chem. 2016, 31, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Z.K.; Li, C.L.; Jin, Y.; Liu, Y.Z.; Zhang, X.; Zhang, F.; Ning, L.N.; Liang, E.S.; Ma, M.; Gao, W.; et al. Paeonol exerts potential activities to inhibit the growth, migration and invasion of human gastric cancer BGC823 cells via downregulating MMP-2 and MMP-9. Mol. Med. Rep. 2017, 16, 7513–7519. [Google Scholar] [CrossRef] [PubMed]
- Cui, N.; Min, H.; Khalil, R.A. Biochemical and Biological Attributes of Matrix Metalloproteinases. Prog. Mol. Biol. Transl. Sci. 2017, 147, 1–73. [Google Scholar] [CrossRef]
- Sier, C.F.; Kubben, F.J.; Ganesh, S.; Heerding, M.M.; Griffioen, G.; Hanemaaijer, R.; van Krieken, J.H.; Lamers, C.B.; Verspaget, H.W. Tissue levels of matrix metalloproteinases MMP-2 and MMP-9 are related to the overall survival of patients with gastric carcinoma. Br. J. Cancer 1996, 74, 413–417. [Google Scholar] [CrossRef]
- Chen, Z.; He, T.; Zhao, K.; Xing, C. Anti-metastatic activity of fangchinoline in human gastric cancer AGS cells. Oncol. Lett. 2017, 13, 655–660. [Google Scholar] [CrossRef]
- Pagliara, V.; Adornetto, A.; Mammì, M.; Masullo, M.; Sarnataro, D.; Pietropaolo, C.; Arcone, R. Protease Nexin-1 affects migration and invasion of C6 glioma cells through regulation of urokinase Plasminogen Activator and Matrix Metalloprotease-9/2. Biochim. Biophys. Acta 2014, 26, 2631–2644. [Google Scholar] [CrossRef]
- Shiozaki, A.; Shimizu, H.; Ichikawa, D.; Konishi, H.; Komatsu, S.; Kubota, T.; Fujiwara, H.; Okamoto, K.; Iitaka, D.; Nakashima, S.; et al. Claudin 1 mediates tumor necrosis factor alpha-induced cell migration in human gastric cancer cells. World J. Gastroenterol. 2014, 21, 17863–17876. [Google Scholar] [CrossRef]
- Vainer, N.; Dehlendorff, C.; Johansen, J.S. Systematic literature review of IL-6 as a biomarker or treatment target in patients with gastric, bile duct, pancreatic and colorectal cancer. Oncotarget 2018, 9, 29820–29841. [Google Scholar] [CrossRef]
- Ashizawa, T.; Okada, R.; Suzuki, Y.; Takagi, M.; Yamazaki, T.; Sumi, T.; Aoki, T.; Ohnuma, S.; Aoki, T. Clinical significance of interleukin-6 (IL-6) in the spread of gastric cancer: Role of IL-6 as a prognostic factor. Gastric Cancer 2005, 8, 124–131. [Google Scholar] [CrossRef]
- Taniguchi, K.; Karin, M. IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin. Immunol. 2014, 26, 54–74. [Google Scholar] [CrossRef]
- Hirano, T.; Ishihara, K.; Hibi, M. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene 2000, 19, 2548–2556. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; O’Keefe, R.A.; Grandis, J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018, 15, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wu, W.K.; Liu, X.; To, K.F.; Chen, G.G.; Yu, J.; Ng, E.K. Increased serum chemerin level promotes cellular invasiveness in gastric cancer: A clinical and experimental study. Peptides 2014, 51, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Zhu, G.; Huang, Y.; Zheng, W.; Hua, J.; Yang, S.; Zhuang, J.; Ye, J. IL-6 mediates the signal pathway of JAK-STAT3-VEGF-C promoting growth, invasion and lymphangiogenesis in gastric cancer. Oncol. Rep. 2016, 35, 1787–1795. [Google Scholar] [CrossRef]
- Fresco, P.; Borges, F.; Diniz, C.; Marques, M.P. New insights on the anticancer properties of dietary polyphenols. Med. Res. Rev. 2006, 26, 747–766. [Google Scholar] [CrossRef]
- Arcone, R.; Palma, M.; Pagliara, V.; Graziani, G.; Masullo, M.; Nardone, G. Green tea polyphenols affect invasiveness of human gastric MKN-28 cells by inhibition of LPS or TNF-α induced Matrix Metalloproteinase-9/2. Biochim. Open 2016, 3, 56–63. [Google Scholar] [CrossRef]
- Di Donato, P.; Taurisano, V.; Tommonaro, G.; Pasquale, V.; Jiménez, J.M.S.; de Pascual, S.; Poli, T.A.; Nicolaus, B. Biological Properties of Polyphenols Extracts from Agro Industry’s Wastes. Waste Biomass Valor. 2017, 9, 1567–1578. [Google Scholar] [CrossRef]
- Arcone, R.; Pucci, P.; Zappacosta, F.; Fontaine, V.; Malorni, A.; Marino, G.; Ciliberto, G. Single-step purification and structural characterization of human interleukin-6 produced in Escherichia coli from a T7 RNA polymerase expression vector. Eur. J. Biochem. 1991, 198, 541–547. [Google Scholar] [CrossRef]
- Pagliara, V.; Parafati, M.; Adornetto, A.; White, M.C.; Masullo, M.; Grimaldi, M.; Arcone, R. Dibutyryl cAMP- or Interleukin-6-induced astrocytic differentiation enhances mannose binding lectin (MBL)-associated serine protease (MASP)-1/3 expression in C6 glioma cells. Arch. Biochem. Biophys. 2018, 653, 39–49. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar]
- Boncler, M.; Różalski, M.; Krajewska, U.; Podsędek, A.; Watala, C. Comparison of PrestoBlue and MTT assays of cellular viability in the assessment of anti-proliferative effects of plant extracts on human endothelial cells. J. Pharmacol. Toxicol. Methods 2014, 69, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of proteins utilizing the principle of protein dye binding. Ann. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Tam, J.C.; Lau, K.M.; Liu, C.L.; To, M.H.; Kwok, H.F.; Lai, K.K.; Lau, C.P.; Ko, C.H.; Leung, P.C.; Fung, K.P.; et al. The in vivo and in vitro diabetic wound healing effects of a 2-herb formula and its mechanisms of action. J. Ethnopharmacol. 2011, 134, 831–838. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–90. [Google Scholar] [CrossRef]
- Lin, M.T.; Lin, B.R.; Chang, C.C.; Chu, C.Y.; Su, H.J.; Chen, S.T.; Jeng, Y.M.; Kuo, M.L. IL-6 induces AGS gastric cancer cell invasion via activation of the c-Src/RhoA/ROCK signaling pathway. Int. J. Cancer 2007, 120, 2600–2608. [Google Scholar] [CrossRef]
- Luo, J.; Yan, R.; He, X.; He, J. Constitutive activation of STAT3 and cyclin D1 overexpression contribute to proliferation, migration and invasion in gastric cancer cells. Am. J. Transl. Res. 2017, 9, 5671–5677. [Google Scholar]
- Adornetto, A.; Pagliara, V.; Di Renzo, G.; Arcone, R. Polychlorinated biphenyls impair dibutyryl cAMP-induced astrocytic differentiation in rat C6 glial cell line. FEBS Open Bio 2013, 29, 459–466. [Google Scholar] [CrossRef]
- Alcaro, S.; Arcone, R.; Costa, G.; De Vita, D.; Iannone, M.; Ortuso, F.; Procopio, A.; Pasceri, R.; Rotiroti, D.; Scipione, L. Simple choline esters as potential anti-Alzheimer agents. Curr. Pharm. Des. 2010, 16, 692–697. [Google Scholar] [CrossRef]
- Vitale, R.M.; Rispoli, V.; Desiderio, D.; Sgammato, R.; Thellung, S.; Canale, C.; Vassalli, M.; Carbone, M.; Ciavatta, M.L.; Mollo, E.; et al. In Silico Identification and Experimental Validation of Novel Anti-Alzheimer′s Multitargeted Ligands from a Marine Source Featuring a “2-Aminoimidazole plus Aromatic Group′′ Scaffold. ACS Chem. Neurosci. 2018, 9, 1290–1303. [Google Scholar] [CrossRef] [PubMed]
- Marston, A.; Kissling, J.; Hostettmann, K. A rapid TLC bioautographic method for the detection of acetylcholinesterase and butyrylcholinesterase inhibitors in plants. Phytochem. Anal. 2002, 13, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Daskiewicz, J.B.; Depeint, F.; Viornery, L.; Bayet, C.; Comte-Sarrazin, G.; Comte, G.; Gee, J.M.; Johnson, I.T.; Ndjoko, K.; Hostettmann, K.; et al. Effects of flavonoids on cell pro- liferation and caspase activation in a human colonic cell line HT29: An SAR study. J. Med. Chem. 2005, 48, 2790–2804. [Google Scholar] [CrossRef] [PubMed]
- Nichenametla, S.N.; Taruscio, T.G.; Barney, D.L.; Exon, J.H. A review of the effects and mechanisms of polyphenolics in cancer. Crit. Rev. Food Sci. Nutr. 2006, 46, 161–183. [Google Scholar] [CrossRef]
- Vizzotto, M.; Porter, W.; Byrne, D.; Cisneros-Zevallos, L. Polyphenols of selected peach and plum genotypes reduce cell viability and inhibit proliferation of breast cancer cells while not affecting normal cells. Food Chem. 2014, 164, 363–370. [Google Scholar] [CrossRef]
- Amiot, M.J.; Riva, C.; Vinet, A. Effects of dietary polyphenols on metabolic syndrome features in humans: A systematic review. Obes. Rev. 2016, 17, 573–586. [Google Scholar] [CrossRef]
- Chiva-Blanch, G.; Badimon, L. Effects of Polyphenol Intake on Metabolic Syndrome: Current Evidences from Human Trials. Oxid. Med. Cell. Longev. 2017, 5812401. [Google Scholar] [CrossRef]
- LaFoya, B.; Munroe, J.A.; Albig, A.R. A comparison of resveratrol and other polyphenolic compounds on Notch activation and endothelial cell activity. PLoS ONE 2019, 14, e0210607. [Google Scholar] [CrossRef]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
- Putnik, P.; Bursać Kovačević, D.; Režek Jambrak, A.; Barba, F.J.; Cravotto, G.; Binello, A.; Lorenzo, J.M.; Shpigelman, A. Innovative “Green′′ and Novel Strategies for the Extraction of Bioactive Added Value Compounds from Citrus Wastes—A Review. Molecules 2017, 22, 680. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pagliara, V.; Nasso, R.; Di Donato, P.; Finore, I.; Poli, A.; Masullo, M.; Arcone, R. Lemon Peel Polyphenol Extract Reduces Interleukin-6-Induced Cell Migration, Invasiveness, and Matrix Metalloproteinase-9/2 Expression in Human Gastric Adenocarcinoma MKN-28 and AGS Cell Lines. Biomolecules 2019, 9, 833. https://doi.org/10.3390/biom9120833
Pagliara V, Nasso R, Di Donato P, Finore I, Poli A, Masullo M, Arcone R. Lemon Peel Polyphenol Extract Reduces Interleukin-6-Induced Cell Migration, Invasiveness, and Matrix Metalloproteinase-9/2 Expression in Human Gastric Adenocarcinoma MKN-28 and AGS Cell Lines. Biomolecules. 2019; 9(12):833. https://doi.org/10.3390/biom9120833
Chicago/Turabian StylePagliara, Valentina, Rosarita Nasso, Paola Di Donato, Ilaria Finore, Annarita Poli, Mariorosario Masullo, and Rosaria Arcone. 2019. "Lemon Peel Polyphenol Extract Reduces Interleukin-6-Induced Cell Migration, Invasiveness, and Matrix Metalloproteinase-9/2 Expression in Human Gastric Adenocarcinoma MKN-28 and AGS Cell Lines" Biomolecules 9, no. 12: 833. https://doi.org/10.3390/biom9120833
APA StylePagliara, V., Nasso, R., Di Donato, P., Finore, I., Poli, A., Masullo, M., & Arcone, R. (2019). Lemon Peel Polyphenol Extract Reduces Interleukin-6-Induced Cell Migration, Invasiveness, and Matrix Metalloproteinase-9/2 Expression in Human Gastric Adenocarcinoma MKN-28 and AGS Cell Lines. Biomolecules, 9(12), 833. https://doi.org/10.3390/biom9120833






