Liposomal Cytarabine as Cancer Therapy: From Chemistry to Medicine
Abstract
1. Introduction
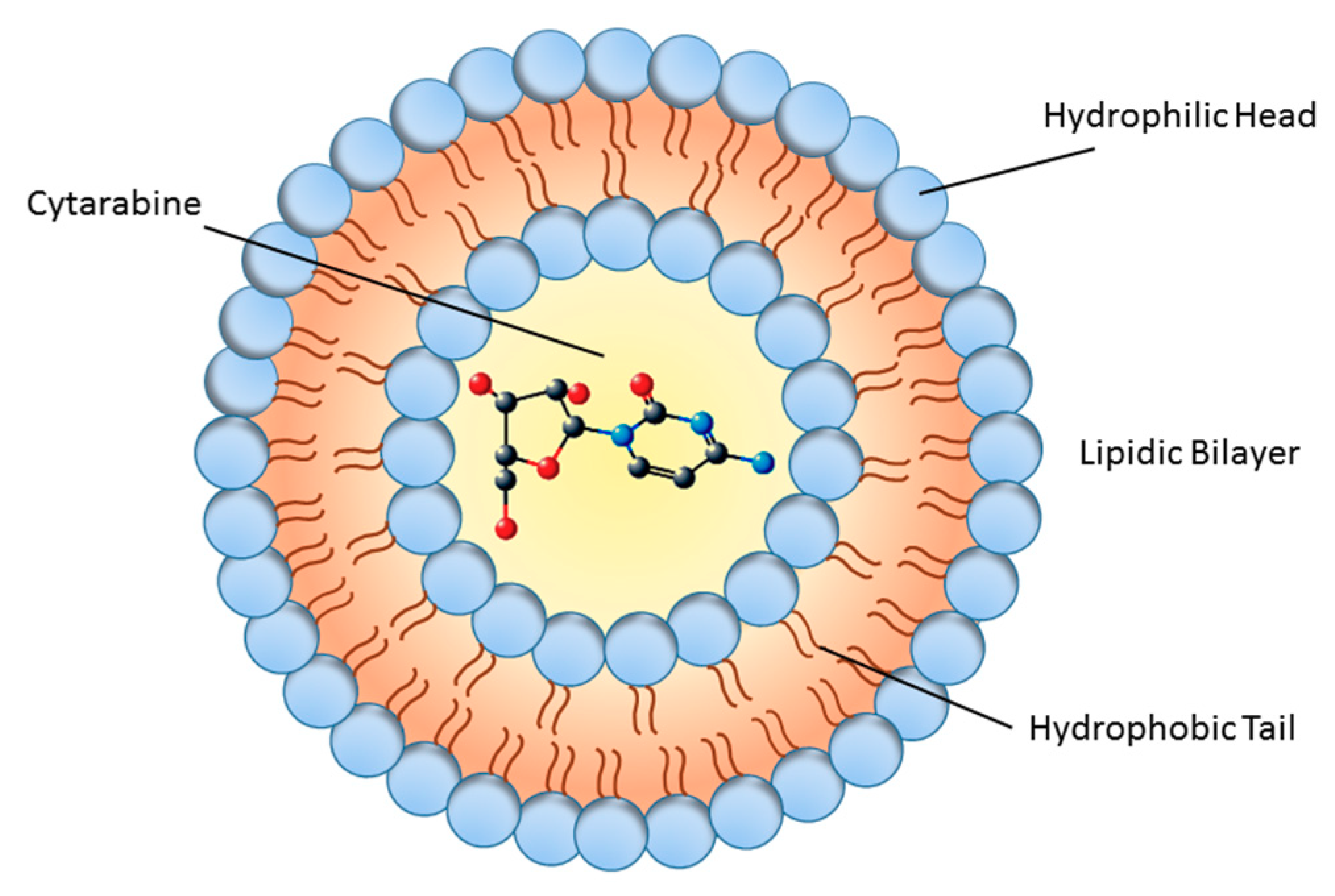
2. Chemistry of Liposomes: From Models to New Applications
3. Bioavailability and Sources
4. Cytarabine Nanoparticles in Preclinical Settings
5. Liposomal Drugs in Cancer
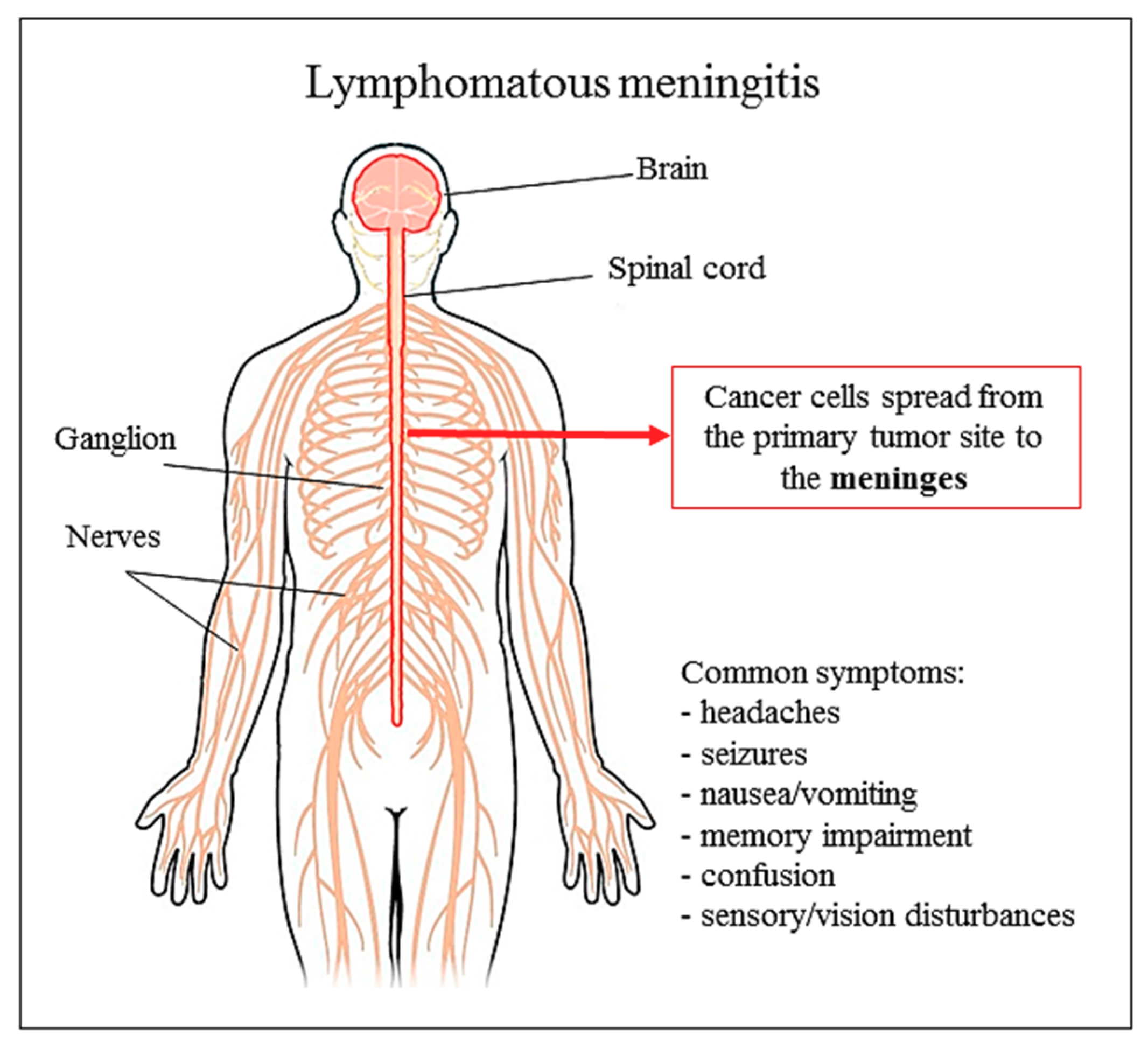
6. Liposomal Cytarabine
6.1. Preclinical Data and Research
6.2. Clinical Use
7. Future Perspectives
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Salehi, B.; Zucca, P.; Sharifi-Rad, M.; Pezzani, R.; Rajabi, S.; Setzer, W.N.; Varoni, E.M.; Iriti, M.; Kobarfard, F.; Sharifi-Rad, J. Phytotherapeutics in cancer invasion and metastasis. Phytother. Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Barabadi, H.; Alizadeh, A.; Ovais, M.; Ahmadi, A.; Shinwari, Z.; Muthupandian, S. The efficacy of green nanoparticles against cancerous and normal cell lines: A systematic review and meta-analysis. IET Nanobiotechnol. 2017, 12, 377–391. [Google Scholar] [CrossRef] [PubMed]
- Barabadi, H.; Ovais, M.; Shinwari, Z.K.; Saravanan, M. Anti-cancer green bionanomaterials: Present status and future prospects. Green Chem. Lett. Rev. 2017, 10, 285–314. [Google Scholar] [CrossRef]
- Mishra, A.P.; Salehi, B.; Sharifi-Rad, M.; Pezzani, R.; Kobarfard, F.; Sharifi-Rad, J.; Nigam, M. Programmed Cell Death, from a Cancer Perspective: An Overview. Mol. Diagn. Ther. 2018, 22, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Su, H.; Sun, W.; Cai, J.; Liu, S.; Chai, Y.; Zhang, C. Dual Chemodrug-Loaded Single-Walled Carbon Nanohorns for Multimodal Imaging-Guided Chemo-Photothermal Therapy of Tumors and Lung Metastases. Theranostics 2018, 8, 1966. [Google Scholar] [CrossRef]
- Leriche, G.; Cifelli, J.L.; Sibucao, K.C.; Patterson, J.P.; Koyanagi, T.; Gianneschi, N.C.; Yang, J. Characterization of drug encapsulation and retention in archaea-inspired tetraether liposomes. Organ. Biomol. Chem. 2017, 15, 2157–2162. [Google Scholar] [CrossRef]
- Siontorou, C.G.; Nikoleli, G.P.; Nikolelis, D.P.; Karapetis, S.K. Artificial Lipid Membranes: Past, Present, and Future. Membranes 2017, 7, 38. [Google Scholar] [CrossRef]
- Weissig, V. Liposomes Came First: The Early History of Liposomology. Methods Mol. Biol. 2017, 1522, 1–15. [Google Scholar]
- Riaz, M.K.; Riaz, M.A.; Zhang, X.; Lin, C.; Wong, K.H.; Chen, X.; Zhang, G.; Lu, A.; Yang, Z. Surface Functionalization and Targeting Strategies of Liposomes in Solid Tumor Therapy: A Review. Int. J. Mol. Sci. 2018, 19, 195. [Google Scholar] [CrossRef]
- Bally, M.B.; Mayer, L.D.; Loughrey, H.; Redelmeier, T.; Madden, T.D.; Wong, K.; Harrigan, P.R.; Hope, M.J.; Cullis, P.R. Dopamine accumulation in large unilamellar vesicle systems induced by transmembrane ion gradients. Chem. Phys. Lipids 1988, 47, 97–107. [Google Scholar] [CrossRef]
- Fatima, M.T.; Islam, Z.; Ahmad, E.; Barreto, G.E.; Md Ashraf, G. Ionic gradient liposomes: Recent advances in the stable entrapment and prolonged released of local anesthetics and anticancer drugs. Biomed. Pharmacother. 2018, 107, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Dong, X.; Swadley, C.L.; Gupte, A.; Leggas, M.; Ledebur, H.C.; Mumper, R.J. Development of idarubicin and doxorubicin solid lipid nanoparticles to overcome Pgp-mediated multiple drug resistance in leukemia. J. Biomed. Nanotechnol. 2009, 5, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Gubernator, J. Active methods of drug loading into liposomes: recent strategies for stable drug entrapment and increased in vivo activity. Expert Opin. Drug Deliv. 2011, 8, 565–580. [Google Scholar] [CrossRef] [PubMed]
- Abraham, S.A.; McKenzie, C.; Masin, D.; Ng, R.; Harasym, T.O.; Mayer, L.D.; Bally, M.B. In vitro and in vivo characterization of doxorubicin and vincristine coencapsulated within liposomes through use of transition metal ion complexation and pH gradient loading. Clin. Cancer Res. 2004, 10, 728–738. [Google Scholar] [CrossRef] [PubMed]
- Vabbilisetty, P.; Sun, X.-L. Liposome surface functionalization based on different anchoring lipids via Staudinger ligation. Org. Biomol. Cem. 2014, 12, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Nobs, L.; Buchegger, F.; Gurny, R.; Allémann, E. Current methods for attaching targeting ligands to liposomes and nanoparticles. J. Pharm. Sci. 2004, 93, 1980–1992. [Google Scholar] [CrossRef]
- Richter, R.P.; Bérat, R.; Brisson, A.R. Formation of solid-supported lipid bilayers: an integrated view. Langmuir 2006, 22, 3497–3505. [Google Scholar] [CrossRef]
- Troutier, A.-L.; Ladavière, C. An overview of lipid membrane supported by colloidal particles. Adv. Colloid Interf. Sci. 2007, 133, 1–21. [Google Scholar] [CrossRef]
- Mornet, S.; Lambert, O.; Duguet, E.; Brisson, A. The formation of supported lipid bilayers on silica nanoparticles revealed by cryoelectron microscopy. Nano Lett. 2005, 5, 281–285. [Google Scholar] [CrossRef]
- Peetla, C.; Stine, A.; Labhasetwar, V. Biophysical interactions with model lipid membranes: applications in drug discovery and drug delivery. Mol. Pharm. 2009, 6, 1264–1276. [Google Scholar] [CrossRef] [PubMed]
- Michot, J.-M.; Seral, C.; Van Bambeke, F.; Mingeot-Leclercq, M.-P.; Tulkens, P.M. Influence of efflux transporters on the accumulation and efflux of four quinolones (ciprofloxacin, levofloxacin, garenoxacin, and moxifloxacin) in J774 macrophages. Antimicrob. Agents Chemother. 2005, 49, 2429–2437. [Google Scholar] [CrossRef] [PubMed]
- Bensikaddour, H.; Snoussi, K.; Lins, L.; Van Bambeke, F.; Tulkens, P.M.; Brasseur, R.; Goormaghtigh, E.; Mingeot-Leclercq, M.-P. Interactions of ciprofloxacin with DPPC and DPPG: fluorescence anisotropy, ATR-FTIR and 31 P NMR spectroscopies and conformational analysis. Biochim. Biophys. Acta 2008, 1778, 2535–2543. [Google Scholar] [CrossRef] [PubMed]
- Fa, N.; Lins, L.; Courtoy, P.J.; Dufrêne, Y.; Van Der Smissen, P.; Brasseur, R.; Tyteca, D.; Mingeot-Leclercq, M.-P. Decrease of elastic moduli of DOPC bilayers induced by a macrolide antibiotic, azithromycin. Biochim. Biophys. Acta 2007, 1768, 1830–1838. [Google Scholar] [CrossRef] [PubMed]
- Klopman, G.; Zhu, H. Recent methodologies for the estimation of n-octanol/water partition coefficients and their use in the prediction of membrane transport properties of drugs. Mini Rev. Med. Chem. 2005, 5, 127–133. [Google Scholar] [CrossRef]
- Rodrigues, C.; Gameiro, P.; Reis, S.; Lima, J.; de Castro, B. Derivative spectrophotometry as a tool for the determination of drug partition coefficients in water/dimyristoyl-L-α-phosphatidylglycerol (DMPG) liposomes. Biophys. Chem. 2001, 94, 97–106. [Google Scholar] [CrossRef]
- Baciu, M.; Sebai, S.C.; Ces, O.; Mulet, X.; Clarke, J.A.; Shearman, G.C.; Law, R.V.; Templer, R.H.; Plisson, C.; Parker, C.A. Degradative transport of cationic amphiphilic drugs across phospholipid bilayers. Philos. Trans. Roy. Soc. Lond. A 2006, 364, 2597–2614. [Google Scholar] [CrossRef]
- Pavinatto, F.J.; Caseli, L.; Pavinatto, A.; dos Santos, D.S.; Nobre, T.M.; Zaniquelli, M.E.; Silva, H.S.; Miranda, P.B.; de Oliveira, O.N. Probing chitosan and phospholipid interactions using Langmuir and Langmuir− Blodgett films as cell membrane models. Langmuir 2007, 23, 7666–7671. [Google Scholar] [CrossRef]
- Yusupov, M.; Van der Paal, J.; Neyts, E.; Bogaerts, A. Synergistic effect of electric field and lipid oxidation on the permeability of cell membranes. Biochim. Biophys. Acta 2017, 1861, 839–847. [Google Scholar] [CrossRef]
- Phillips, M.A.; Gran, M.L.; Peppas, N.A. Targeted nanodelivery of drugs and diagnostics. Nano Today 2010, 5, 143–159. [Google Scholar] [CrossRef]
- Huynh, R.; Chaubet, F.; Jozefonvicz, J. Anticoagulant properties of dextranmethylcarboxylate benzylamide sulfate (DMCBSu); a new generation of bioactive functionalized dextran. Carbohydr. Res. 2001, 332, 75–83. [Google Scholar] [CrossRef]
- Barrera, C.; Herrera, A.P.; Rinaldi, C. Colloidal dispersions of monodisperse magnetite nanoparticles modified with poly (ethylene glycol). J. Colloid Interface Sci. 2009, 329, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, G.; Danelon, C.; Izewska, P.; Prummer, M.; Bolinger, P.Y.; Geissbühler, I.; Demurtas, D.; Dubochet, J.; Vogel, H. Multifunctional lipid/quantum dot hybrid nanocontainers for controlled targeting of live cells. Angew. Chem. 2006, 45, 5478–5483. [Google Scholar] [CrossRef] [PubMed]
- Matsuno, R.; Ishihara, K. Integrated functional nanocolloids covered with artificial cell membranes for biomedical applications. Nano Today 2011, 6, 61–74. [Google Scholar] [CrossRef]
- Thanh, N.T.; Green, L.A. Functionalisation of nanoparticles for biomedical applications. Nano Today 2010, 5, 213–230. [Google Scholar] [CrossRef]
- Dubertret, B.; Skourides, P.; Norris, D.J.; Noireaux, V.; Brivanlou, A.H.; Libchaber, A. In vivo imaging of quantum dots encapsulated in phospholipid micelles. Science 2002, 298, 1759–1762. [Google Scholar] [CrossRef]
- Hyodo, K.; Yamamoto, E.; Suzuki, T.; Kikuchi, H.; Asano, M.; Ishihara, H. Development of liposomal anticancer drugs. Biol. Pharm. Bull. 2013, 36, 703–707. [Google Scholar] [CrossRef]
- Dhoot, N.O.; Wheatley, M.A. Microencapsulated liposomes in controlled drug delivery: strategies to modulate drug release and eliminate the burst effect. J. Pharm. Sci. 2003, 92, 679–689. [Google Scholar] [CrossRef]
- Kim, S. DepoFoam-mediated drug delivery into cerebrospinal fluid. Methods Neurosci. 1994, 21, 118–131. [Google Scholar]
- Kim, S.; Turker, M.S.; Chi, E.Y.; Sela, S.; Martin, G.M. Preparation of multivesicular liposomes. Biochim. Biophys. Acta 1983, 728, 339–348. [Google Scholar] [CrossRef]
- Liu, L.; Ye, Q.; Lu, M.; Chen, S.-T.; Tseng, H.-W.; Lo, Y.-C.; Ho, C. A New Approach to Deliver Anti-cancer Nanodrugs with Reduced Off-target Toxicities and Improved Efficiency by Temporarily Blunting the Reticuloendothelial System with Intralipid. Sci. Rep. 2017, 7, 16106. [Google Scholar] [CrossRef] [PubMed]
- Galmarini, C.M.; Mackey, J.R.; Dumontet, C. Nucleoside analogues and nucleobases in cancer treatment. Lancet Oncol. 2002, 3, 415–424. [Google Scholar] [CrossRef]
- Eloy, J.O.; de Souza, M.C.; Petrilli, R.; Barcellos, J.P.A.; Lee, R.J.; Marchetti, J.M. Liposomes as carriers of hydrophilic small molecule drugs: strategies to enhance encapsulation and delivery. Colloids Surf. B 2014, 123, 345–363. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Hu, X.; Jiang, W.; Liu, R.; Zhang, D.; Zhang, J.; Li, Z.; Luan, Y. Rational Design of a New Self-Codelivery System from Redox-Sensitive Camptothecin–Cytarabine Conjugate Assembly for Effectively Synergistic Anticancer Therapy. Adv. Healthc. Mater. 2017, 6, 1700829. [Google Scholar] [CrossRef]
- Chhikara, B.S.; Parang, K. Development of cytarabine prodrugs and delivery systems for leukemia treatment. Expert Opin. Drug Deliv. 2010, 7, 1399–1414. [Google Scholar] [CrossRef]
- Benesch, M.; Urban, C. Liposomal cytarabine for leukemic and lymphomatous meningitis: recent developments. Expert Opin. Pharm. 2008, 9, 301–309. [Google Scholar] [CrossRef]
- Phuphanich, S.; Maria, B.; Braeckman, R.; Chamberlain, M. A pharmacokinetic study of intra-CSF administered encapsulated cytarabine (DepoCyt®) for the treatment of neoplastic meningitis in patients with leukemia, lymphoma, or solid tumors as part of a phase III study. J. Neurooncol. 2007, 81, 201–208. [Google Scholar] [CrossRef]
- Craig, C. Current treatment approaches for neoplastic meningitis: Nursing management of patients receiving intrathecal DepoCyt. Oncol. Nurs. Forum 2000, 27, 1225–1230. [Google Scholar]
- Goldberg, M.S. Improving cancer immunotherapy through nanotechnology. Nat. Rev. Cancer 2019, 19, 587–602. [Google Scholar] [CrossRef]
- Cao, W.M.; Gao, Y.; Yang, H.J.; Xie, S.N.; Ding, X.W.; Pan, Z.W.; Ye, W.W.; Wang, X.J. Novel germline mutations and unclassified variants of BRCA1 and BRCA2 genes in Chinese women with familial breast/ovarian cancer. BMC Cancer 2016, 16, 64. [Google Scholar] [CrossRef]
- Wang, J.; Yin, C.; Tang, G.; Lin, X.; Wu, Q. Glucose-functionalized multidrug-conjugating nanoparticles based on amphiphilic terpolymer with enhanced anti-tumorous cell cytotoxicity. Int. J. Pharm. 2013, 441, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Dube, B.; Sawant, K. Synthesis of cytarabine lipid drug conjugate for treatment of meningeal leukemia: Development, characterization and in vitro cell line studies. J. Biomed. Nanotechnol. 2012, 8, 928–937. [Google Scholar] [CrossRef] [PubMed]
- Pentak, D.; Maciazek-Jurczyk, M.; Zawada, Z.H. The role of nanoparticles in the albumin-cytarabine and albumin-methotrexate interactions. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 73, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Li, X.; Wu, Q.; Wang, J.L.; Lin, X.F. Multidrug nanoparticles based on novel random copolymer containing cytarabine and fluorodeoxyuridine. J. Colloid Interface Sci. 2010, 349, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Shukla, R.N.; Bajpai, A.K. Genipin-modified gelatin nanocarriers as swelling controlled drug delivery system for in vitro release of cytarabine. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 457–465. [Google Scholar] [CrossRef]
- Yadav, K.S.; Sawant, K.K. Modified nanoprecipitation method for preparation of cytarabine-loaded PLGA nanoparticles. AAPS PharmSciTech 2010, 11, 1456–1465. [Google Scholar] [CrossRef]
- Yadav, K.S.; Jacob, S.; Sachdeva, G.; Chuttani, K.; Mishra, A.K.; Sawant, K.K. Long circulating PEGylated PLGA nanoparticles of cytarabine for targeting leukemia. J. Microencapsul. 2011, 28, 729–742. [Google Scholar] [CrossRef]
- Pawar, H.R.; Bhosale, S.S.; Derle, N.D. Use of liposomes in cancer therapy: A review. Int. J. Pharm. Sci. Res. 2012, 3, 3585. [Google Scholar]
- Kang, L.; Gao, Z.; Huang, W.; Jin, M.; Wang, Q. Nanocarrier-mediated co-delivery of chemotherapeutic drugs and gene agents for cancer treatment. Acta Pharm. Sin. B 2015, 5, 169–175. [Google Scholar] [CrossRef]
- Aghebati-maleki, L.; Salehi, B.; Behfar, R.; Saeidmanesh, H.; Ahmadian, F.; Sarebanhassanabadi, M.; Negahdary, M. Designing a hydrogen peroxide biosensor using catalase and modified electrode with magnesium oxide nanoparticles. Int. J. Electrochem. Sci 2014, 9, 257–271. [Google Scholar]
- Sistani, P.; Sofimaryo, L.; Masoudi, Z.R.; Sayad, A.; Rahimzadeh, R.; Salehi, B. A penicillin biosensor by using silver nanoparticles. Int. J. Electrochem. Sci. 2014, 9, 6201–6212. [Google Scholar]
- Salehi, B.; Mehrabian, S.; Ahmadi, M. Investigation of antibacterial effect of Cadmium Oxide nanoparticles on Staphylococcus Aureus bacteria. J. nanobiotechnol. 2014, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Noble, G.T.; Stefanick, J.F.; Ashley, J.D.; Kiziltepe, T.; Bilgicer, B. Ligand-targeted liposome design: challenges and fundamental considerations. Trends Biotechnol. 2014, 32, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Slingerland, M.; Guchelaar, H.-J.; Gelderblom, H. Liposomal drug formulations in cancer therapy: 15 years along the road. Drug Discov. Today 2012, 17, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Olusanya, T.O.B.; Haj Ahmad, R.R.; Ibegbu, D.M.; Smith, J.R.; Elkordy, A.A. Liposomal Drug Delivery Systems and Anticancer Drugs. Molecules 2018, 23, 907. [Google Scholar] [CrossRef] [PubMed]
- Jha, S.; Sharma, P.K.; Malviya, R. Liposomal Drug Delivery System for Cancer Therapy: Advancement and Patents. Recent Patents Drug Deliv. Formul. 2016, 10, 177–183. [Google Scholar]
- Petre, C.E.; Dittmer, D.P. Liposomal daunorubicin as treatment for Kaposi’s sarcoma. Int. J. Nanomed. 2007, 2, 277–288. [Google Scholar]
- Hardiansyah, A.; Huang, L.Y.; Yang, M.C.; Liu, T.Y.; Tsai, S.C.; Yang, C.Y.; Kuo, C.Y.; Chan, T.Y.; Zou, H.M.; Lian, W.N.; et al. Magnetic liposomes for colorectal cancer cells therapy by high-frequency magnetic field treatment. Nanoscale Res. Lett. 2014, 9, 497. [Google Scholar] [CrossRef]
- Mock, J.N.; Costyn, L.J.; Wilding, S.L.; Arnold, R.D.; Cummings, B.S. Evidence for distinct mechanisms of uptake and antitumor activity of secretory phospholipase A2 responsive liposome in prostate cancer. Integr. Biol. 2013, 5, 172–182. [Google Scholar] [CrossRef]
- Berlin Grace, V.M.; Viswanathan, S. Pharmacokinetics and therapeutic efficiency of a novel cationic liposome nano-formulated all trans retinoic acid in lung cancer mice model. J. Drug Deliv. Sci. Technol. 2017, 39, 223–236. [Google Scholar] [CrossRef]
- Legut, M.; Lipka, D.; Filipczak, N.; Piwoni, A.; Kozubek, A.; Gubernator, J. Anacardic acid enhances the anticancer activity of liposomal mitoxantrone towards melanoma cell lines—in vitro studies. Int. J. Nanomed. 2014, 9, 653–668. [Google Scholar] [PubMed]
- Wang-Gillam, A.; Li, C.P.; Bodoky, G.; Dean, A.; Shan, Y.S.; Jameson, G.; Macarulla, T.; Lee, K.H.; Cunningham, D.; Blanc, J.F.; et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): A global, randomised, open-label, phase 3 trial. Lancet 2016, 387, 545–557. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, W.Y.; Ma, X.; Ju, R.J.; Li, X.Y.; Li, N.; Sun, M.G.; Shi, J.F.; Zhang, C.X.; Lu, W.L. The anticancer efficacy of paclitaxel liposomes modified with mitochondrial targeting conjugate in resistant lung cancer. Biomaterials 2013, 34, 3626–3638. [Google Scholar] [CrossRef] [PubMed]
- Gabizon, A.A.; Shmeeda, H.; Zalipsky, S. Pros and cons of the liposome platform in cancer drug targeting. J. Liposome Res. 2006, 16, 175–183. [Google Scholar] [CrossRef]
- Gratton, S.E. In Vitro and In Vivo Studies of Nanomolded PRINT Particles of Precisely Controlled Size, Shape, and Surface Chemistry. Ph.D. Thesis, The University of North Carolina, Chapel Hill, NC, USA, 2008. [Google Scholar]
- Hamada, A.; Kawaguchi, T.; Nakano, M. Clinical pharmacokinetics of cytarabine formulations. Clin. Pharm. 2002, 41, 705–718. [Google Scholar] [CrossRef]
- Evans, J.S.; Musser, E.A.; Mengel, G.D.; Forsblad, K.R.; Hunter, J.H. Antitumor activity of 1-beta-D-arainofuranosylcytosine hydrochloride. Proc. Soc. Exp. Biol. Med. 1961, 106, 350–353. [Google Scholar] [CrossRef]
- Talley, R.W.; Vaitkevicius, V.K. Megaloblastosis produced by a cytosine antagonist, 1-beta-D-arabinofuranosylcytosine. Blood 1963, 21, 352–362. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M.; Battershill, C.N. Therapeutic Agents from the Sea: Biodiversity, Chemo-Evolutionary Insight and Advances To the End of Darwin’s 200th Year; National Library of Medicine: Bethesda, MD, USA, 2009. [Google Scholar]
- Tyner, J.W.; Tardi, P.; Mayer, L.; Fletcher, L.B.; Spurgeon, S.; Kovacsovics, T.; Loriaux, M.M. Evaluation of CPX-351 (cytarabine: Daunorubicin) liposome injection anti-Leukemic activity against primary patient leukemia cells. Am. Soc. Hematol. 2010. [Google Scholar]
- Hare, J.I.; Lammers, T.; Ashford, M.B.; Puri, S.; Storm, G.; Barry, S.T. Challenges and strategies in anti-cancer nanomedicine development: An industry perspective. Adv. Drug Deliv. Rev. 2017, 108, 25–38. [Google Scholar] [CrossRef]
- Lammers, T.; Kiessling, F.; Hennink, W.E.; Storm, G. Drug targeting to tumors: Principles, pitfalls and (pre-) clinical progress. J. Control. Rel. 2012, 161, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Juzenas, P.; Chen, W.; Sun, Y.-P.; Coelho, M.A.N.; Generalov, R.; Generalova, N.; Christensen, I.L. Quantum dots and nanoparticles for photodynamic and radiation therapies of cancer. Adv. Drug Deliv. Rev. 2008, 60, 1600–1614. [Google Scholar] [CrossRef] [PubMed]
- Bhojwani, D.; Pui, C.-h. Intrathecal liposomal cytarabine: More friend than foe? Leuk. Lymphoma 2008, 49, 1427–1430. [Google Scholar] [CrossRef] [PubMed]
- Kratz, F.; Senter, P.; Steinhagen, H. Drug Delivery in Oncology: From Basic Research to Cancer Therapy; John Wiley & Sons: Hoboken, NJ, USA, 2013; Volume 3. [Google Scholar]
- Bassan, R.; Masciulli, A.; Intermesoli, T.; Audisio, E.; Rossi, G.; Pogliani, E.M.; Cassibba, V.; Mattei, D.; Romani, C.; Cortelezzi, A.; et al. Randomized trial of radiation-free central nervous system prophylaxis comparing intrathecal triple therapy with liposomal cytarabine in acute lymphoblastic leukemia. Haematologica 2015, 100, 786–793. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Egusquiaguirre, S.P.; Igartua, M.; Hernández, R.M.; Pedraz, J.L. Nanoparticle delivery systems for cancer therapy: Advances in clinical and preclinical research. Clin. Transl. Oncol. 2012, 14, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, M.C. Neurotoxicity of intra-CSF liposomal cytarabine (DepoCyt) administered for the treatment of leptomeningeal metastases: A retrospective case series. J. Neurooncol. 2012, 109, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chatelut, E.; Kim, J.C.; Howell, S.B.; Cates, C.; Kormanik, P.A.; Chamberlain, M.C. Extended CSF cytarabine exposure following intrathecal administration of DTC 101. J. Clin. Oncol. 1993, 11, 2186–2193. [Google Scholar] [CrossRef]
- Kohn, F.R.; Malkmus, S.A.; Brownson, E.A.; Rossi, S.S.; Yaksh, T.L. Fate of the predominant phospholipid component of DepoFoamTM drug delivery matrix after intrathecal administration of sustained-release encapsulated cytarabine in rats. Drug Deliv. 1998, 5, 143–151. [Google Scholar] [CrossRef][Green Version]
- Wan, S.H.; Huffman, D.H.; Azarnoff, D.L.; Hoogstraten, B.; Larsen, W.E. Pharmacokinetics of 1-β-D-arabinofuranosylcytosine in humans. Cancer Res. 1974, 34, 392–397. [Google Scholar]
- Groothuis, D.R.; Benalcazar, H.; Allen, C.V.; Wise, R.M.; Dills, C.; Dobrescu, C.; Rothholtz, V.; Levy, R.M. Comparison of cytosine arabinoside delivery to rat brain by intravenous, intrathecal, intraventricular and intraparenchymal routes of administration. Brain Res. 2000, 856, 281–290. [Google Scholar] [CrossRef]
- Scott-Moncrieff, J.C.R.; Chan, T.C.; Samuels, M.L.; Cook, J.R.; Coppoc, G.L.; DeNicola, D.B.; Richardson, R.C. Plasma and cerebrospinal fluid pharmacokinetics of cytosine arabinoside in dogs. Cancer Chemother. Pharmacol. 1991, 29, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Tardi, P.; Johnstone, S.; Harasym, N.; Xie, S.; Harasym, T.; Zisman, N.; Harvie, P.; Bermudes, D.; Mayer, L. In vivo maintenance of synergistic cytarabine: Daunorubicin ratios greatly enhances therapeutic efficacy. Leuk. Res. 2009, 33, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Bayne, W.F.; Mayer, L.D.; Swenson, C.E. Pharmacokinetics of CPX-351 (cytarabine/daunorubicin HCl) liposome injection in the mouse. J. Pharma. Sci. 2009, 98, 2540–2548. [Google Scholar] [CrossRef] [PubMed]
- Carol, H.; Fan, M.M.; Harasym, T.O.; Boehm, I.; Mayer, L.D.; Houghton, P.; Smith, M.A.; Lock, R.B. Efficacy of CPX-351,(cytarabine: Daunorubicin) liposome injection, against acute lymphoblastic leukemia (ALL) xenograft models of the Pediatric Preclinical Testing Program. Pediatric Blood Cancer 2015, 62, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Dicko, A.; Kwak, S.; Frazier, A.A.; Mayer, L.D.; Liboiron, B.D. Biophysical characterization of a liposomal formulation of cytarabine and daunorubicin. Int. J. Pharma. 2010, 391, 248–259. [Google Scholar] [CrossRef]
- Jabbour, E.; Cortes, J.E.; Giles, F.J.; O’Brien, S.; Kantarjian, H.M. Current and emerging treatment options in chronic myeloid leukemia. Cancer 2007, 109, 2171–2181. [Google Scholar] [CrossRef]
- Shah, M.; Agarwal, B. Recent advances in management of acute myeloid leukemia (AML). Ind. J. Pediatrics 2008, 75, 831–837. [Google Scholar] [CrossRef]
- Drugs.com. Depocyt. Available online: https://www.drugs.com/pro/depocyt.html (accessed on 1 June 2018).
- Chamberlain, M.C.; Khatibi, S.; Kim, J.C.; Howell, S.B.; Chatelut, E.; Kim, S. Treatment of leptomeningeal metastasis with intraventricular administration of depot cytarabine (DTC 101). A phase I study. Arch. Neurol. 1993, 50, 261–264. [Google Scholar] [CrossRef]
- Chamberlain, M.C.; Kormanik, P.; Howell, S.B.; Kim, S. Pharmacokinetics of intralumbar DTC-101 for the treatment of leptomeningeal metastases. Arch. Neurol. 1995, 52, 912–917. [Google Scholar] [CrossRef]
- Mrugala, M.M.; Kim, B.; Sharma, A.; Johnson, N.; Graham, C.; Kurland, B.F.; Gralow, J. Phase II Study of Systemic High-dose Methotrexate and Intrathecal Liposomal Cytarabine for Treatment of Leptomeningeal Carcinomatosis From Breast Cancer. Clin. Breast Cancer 2019. [Google Scholar] [CrossRef]
- Glantz, M.J.; Jaeckle, K.A.; Chamberlain, M.C.; Phuphanich, S.; Recht, L.; Swinnen, L.J.; Maria, B.; LaFollette, S.; Schumann, G.B.; Cole, B.F.; et al. A randomized controlled trial comparing intrathecal sustained-release cytarabine (DepoCyt) to intrathecal methotrexate in patients with neoplastic meningitis from solid tumors. Clin. Cancer Res. 1999, 5, 3394–3402. [Google Scholar] [PubMed]
- Beauchesne, P.; Blonski, M.; Brissart, H. Response to intrathecal infusions of Depocyt(R) in secondary diffuse leptomeningeal gliomatosis. A case report. In vivo 2011, 25, 991–993. [Google Scholar] [PubMed]
- Zimm, S.; Collins, J.M.; Miser, J.; Chatterji, D.; Poplack, D.G. Cytosine arabinoside cerebrospinal fluid kinetics. Clin. Pharmacol. Ther. 1984, 35, 826–830. [Google Scholar] [CrossRef] [PubMed]
- Glantz, M.J.; LaFollette, S.; Jaeckle, K.A.; Shapiro, W.; Swinnen, L.; Rozental, J.R.; Phuphanich, S.; Rogers, L.R.; Gutheil, J.C.; Batchelor, T.; et al. Randomized trial of a slow-release versus a standard formulation of cytarabine for the intrathecal treatment of lymphomatous meningitis. J. Clin. Oncol. 1999, 17, 3110–3116. [Google Scholar] [CrossRef] [PubMed]
- Lassaletta, A.; Lopez-Ibor, B.; Mateos, E.; Gonzalez-Vicent, M.; Perez-Martinez, A.; Sevilla, J.; Diaz, M.A.; Madero, L. Intrathecal liposomal cytarabine in children under 4 years with malignant brain tumors. J. Neurooncol. 2009, 95, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Persons, S. The decline of homeopathy—the University of Iowa, 1876–1919. Bull. Hist. Med. 1991, 65, 74–87. [Google Scholar] [PubMed]
- Fleischhack, G.; Jaehde, U.; Bode, U. Pharmacokinetics following intraventricular administration of chemotherapy in patients with neoplastic meningitis. Clin. Pharm. 2005, 44, 1–31. [Google Scholar] [CrossRef]
- Angst, M.S.; Drover, D.R. Pharmacology of drugs formulated with DepoFoam: A sustained release drug delivery system for parenteral administration using multivesicular liposome technology. Clin. Pharm. 2006, 45, 1153–1176. [Google Scholar] [CrossRef]
- Bohn, J.P.; Reinstadler, V.; Pall, G.; Stockhammer, G.; Steurer, M.; Oberacher, H.; Wolf, D. Cerebrospinal Fluid Drug Concentrations and Clinical Outcome of Patients with Neoplastic Meningitis Treated with Liposomal Cytarabine. Eur. J. Drug. Metab. Pharm. 2019. [Google Scholar] [CrossRef]
- Chen, K.T.J.; Gilabert-Oriol, R.; Bally, M.B.; Leung, A.W.Y. Recent Treatment Advances and the Role of Nanotechnology, Combination Products, and Immunotherapy in Changing the Therapeutic Landscape of Acute Myeloid Leukemia. Pharm. Res. 2019, 36, 125. [Google Scholar] [CrossRef]
- Mayer, L.D.; Tardi, P.; Louie, A.C. CPX-351: A nanoscale liposomal co-formulation of daunorubicin and cytarabine with unique biodistribution and tumor cell uptake properties. Int. J. Nanomed. 2019, 14, 3819–3830. [Google Scholar] [CrossRef] [PubMed]
- Tolcher, A.W.; Mayer, L.D. Improving combination cancer therapy: The CombiPlex((R)) development platform. Future Oncol. 2018, 14, 1317–1332. [Google Scholar] [CrossRef] [PubMed]
| Phase | Treatment | Disease | Enrollment | Identifier |
|---|---|---|---|---|
| Phase 2 | DepoCyt, methotrexate | Leptomeningeal metastasis of breast cancer | 3 | NCT00992602 |
| Phase 2 | DepoCyt | Lymphomatous or leukemic meningitis | 4 | NCT00523939 |
| Not Applicable | DepoCyt, sorafenib | Neoplastic meningitis | 2 | NCT00964743 |
| Phase 3 | DepoCyt | Leptomeningeal metastasis of breast cancer | 74 | NCT01645839 |
| Not Applicable | Vyxenos, liposomal cytarabine, and daunorubicin | Untreated myelodysplastic syndrome, acute myeloid leukemia, acute biphenotypic leukemia, myelodysplastic syndrome | 48 | NCT01804101 |
| Phase 3 | Vyxenos, 7+3 (liposomal cytarabine and daunorubicin) | High risk of acute myeloid leukemia | 309 | NCT01696084 |
| Study Type | Treatment | Disease | Results | Ref. |
|---|---|---|---|---|
| Phase I | Intraventricular | Leptomeningeal metastasis | Well-tolerated toxicity, duration of response with a median of over 11 weeks | [102] |
| Phase I | Intrathecal | Neoplastic meningitis | The therapeutic intra-lumbar concentration of free Ara-C was maintained for up to 14 days | [90] |
| Phase I | Intra-lumbar | Leptomeningeal metastasis | Extended free Ara-C concentrations | [103] |
| Phase II | Intrathecal | Leptomeningeal metastasis | Well-tolerated toxicity, systemic high-dose methotrexate + liposomal cytarabine | [104] |
| Randomized controlled trial | Intrathecal | Neoplastic meningitis | Increased time to neurological progression. Median survival was 105 days with DepoCyt and 78 days with methotrexate | [105] |
| Open-label study | Intraventricular or lumbar puncture | Leukemia, lymphoma, or solid tumors as part of a phase III study | Extended exposure compared with standard Ara-C | [47] |
| Case-report | Intrathecal | Secondary diffuse leptomeningeal gliomatosis | Improvement of the clinical status | [106] |
| Retrospective case series | Intraventricular | Leptomeningeal metastasis | Well tolerate toxicity, in general | [89] |
| Study Type | No. of Patients | Results | Reference |
|---|---|---|---|
| Phase I | 9 | Duration of response: 2–14 weeks, median 11 | [102] |
| Phase I | 12 | Therapeutic intralumbar concentration of free cytarabine maintained for 14 days | [90] |
| Phase I | 8 | Lumbar and intraventricular max concentration of free cytarabine: 226 and 6.06 mg/L; half-life, 277 and 130 h, respectively | [103] |
| Phase I | 18 children (3–21 years) | Prolonged disease stabilization or response: 14 patients Maximum-tolerated dose: 35 mg | [109] |
| Randomized controlled trial | 31 treated with D, 30 with M | Median survival: 105 days (D), 78 days (M) Median time to neurological progression: 58 (D) vs 30 (M) days Neoplastic meningitis-specific survival: 343 (D) versus 98 (M) days Adverse events: comparable D vs. M | [105] |
| Open-label study | 8 | Concentration of free and encapsulated cytarabine in the ventricular and lumbar CSF: 0.01 to 1500 µg/mL | [47] |
| Case-report | 1 | Duration of response with D: 6 months | [110] |
| Retrospective case series | 120 | D well tolerated, but 12.5% had serious treatment-related neurological complications | [89] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salehi, B.; Selamoglu, Z.; S. Mileski, K.; Pezzani, R.; Redaelli, M.; C. Cho, W.; Kobarfard, F.; Rajabi, S.; Martorell, M.; Kumar, P.; et al. Liposomal Cytarabine as Cancer Therapy: From Chemistry to Medicine. Biomolecules 2019, 9, 773. https://doi.org/10.3390/biom9120773
Salehi B, Selamoglu Z, S. Mileski K, Pezzani R, Redaelli M, C. Cho W, Kobarfard F, Rajabi S, Martorell M, Kumar P, et al. Liposomal Cytarabine as Cancer Therapy: From Chemistry to Medicine. Biomolecules. 2019; 9(12):773. https://doi.org/10.3390/biom9120773
Chicago/Turabian StyleSalehi, Bahare, Zeliha Selamoglu, Ksenija S. Mileski, Raffaele Pezzani, Marco Redaelli, William C. Cho, Farzad Kobarfard, Sadegh Rajabi, Miquel Martorell, Pradeep Kumar, and et al. 2019. "Liposomal Cytarabine as Cancer Therapy: From Chemistry to Medicine" Biomolecules 9, no. 12: 773. https://doi.org/10.3390/biom9120773
APA StyleSalehi, B., Selamoglu, Z., S. Mileski, K., Pezzani, R., Redaelli, M., C. Cho, W., Kobarfard, F., Rajabi, S., Martorell, M., Kumar, P., Martins, N., Subhra Santra, T., & Sharifi-Rad, J. (2019). Liposomal Cytarabine as Cancer Therapy: From Chemistry to Medicine. Biomolecules, 9(12), 773. https://doi.org/10.3390/biom9120773













