Mechanism of Action and Interactions between Thyroid Peroxidase and Lipoxygenase Inhibitors Derived from Plant Sources
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Material
2.3. Preparation of Pure Substance Solutions
2.4. Raw Extracts Preparation
2.5. High-Performance Liquid Chromatography-Diode-Array Detector Analysis
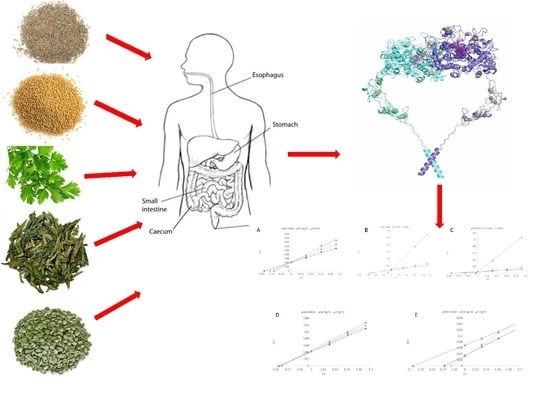
2.6. In Vitro Digestion
2.7. In Vitro Antioxidant Capacity Assay
2.8. Thyroid Peroxidase Preparation
2.9. Thyroid Peroxidase Inhibitory Assay
2.10. Inhibition of Lipoxygenase Activity
2.11. Isobolographic Analysis
2.12. Statistical Analysis
3. Results and Discussion
3.1. High-Performance Liquid Chromatography-Diode-Array Detector Phenolic Analysis
3.2. Antioxidant Analysis
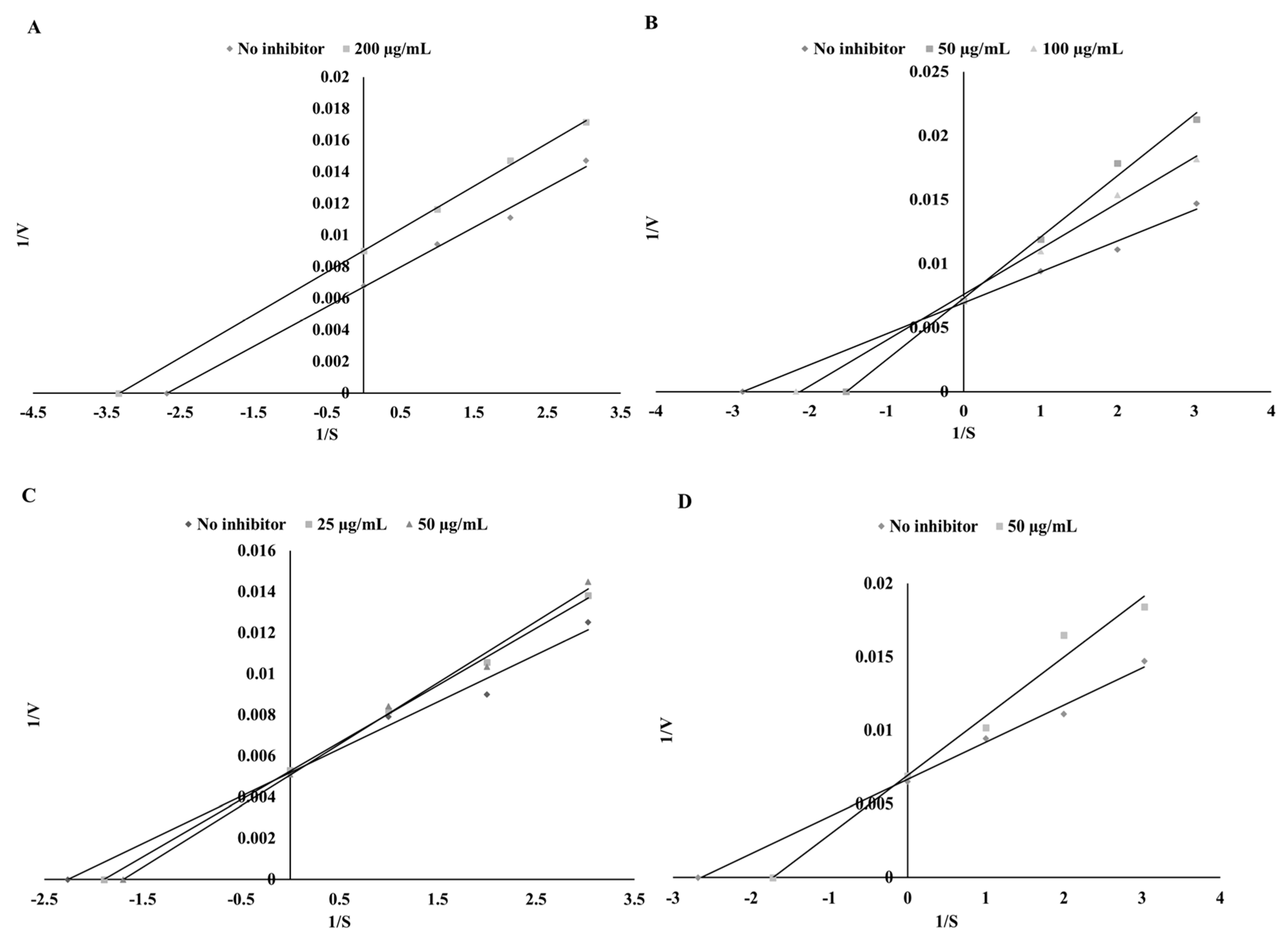
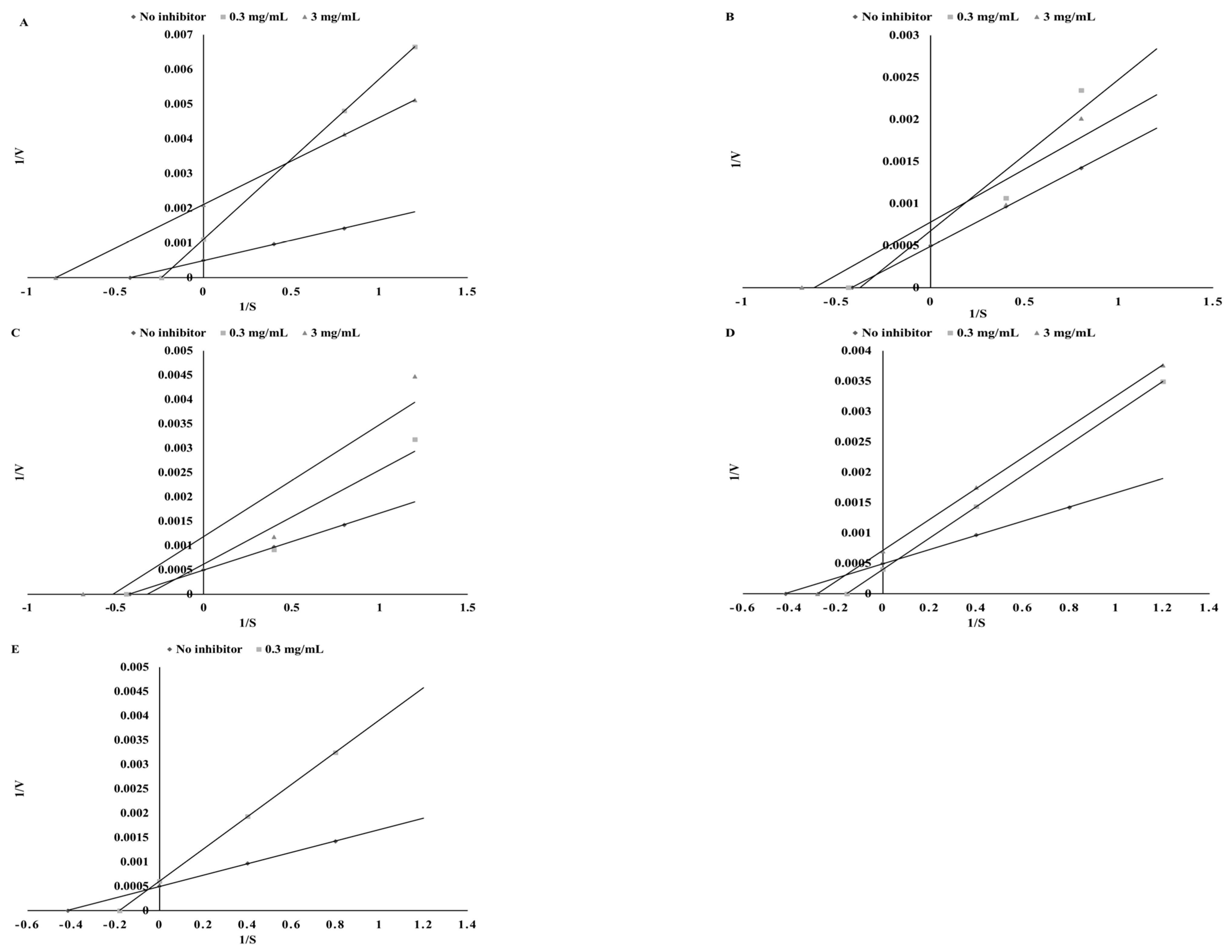
3.3. Thyroid Peroxidase Inhibitory Potential
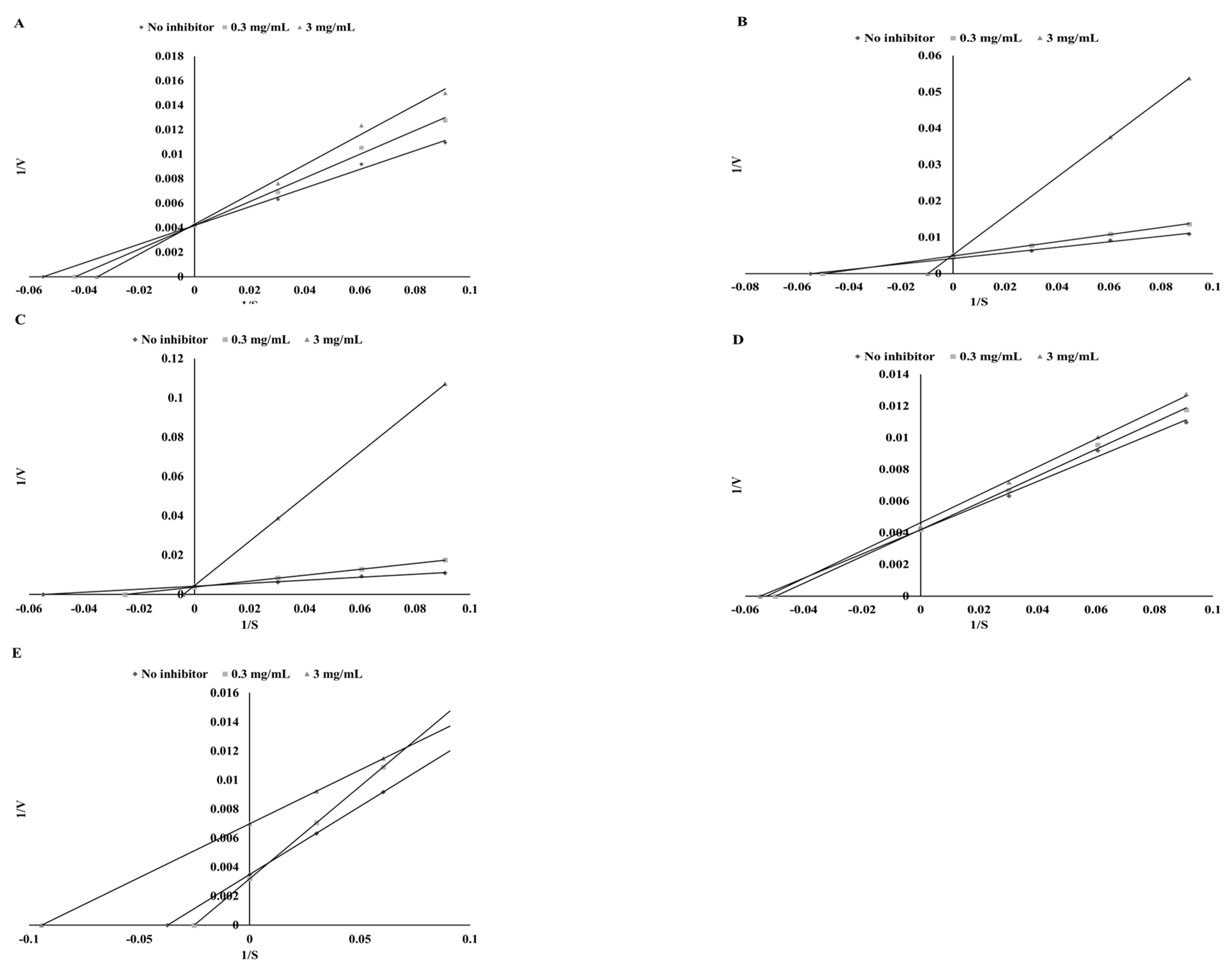
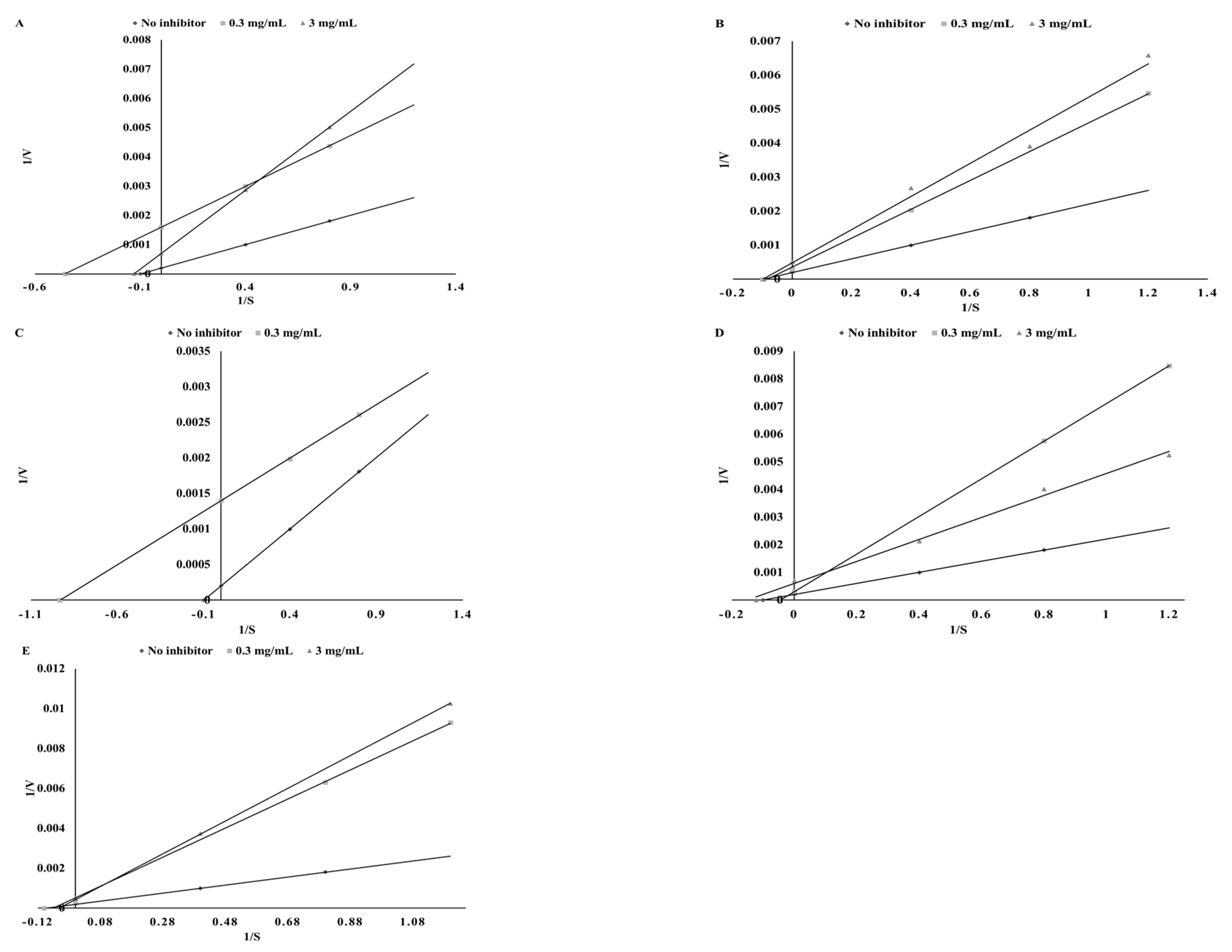
3.4. Lipoxigenase Inhibitory Potential
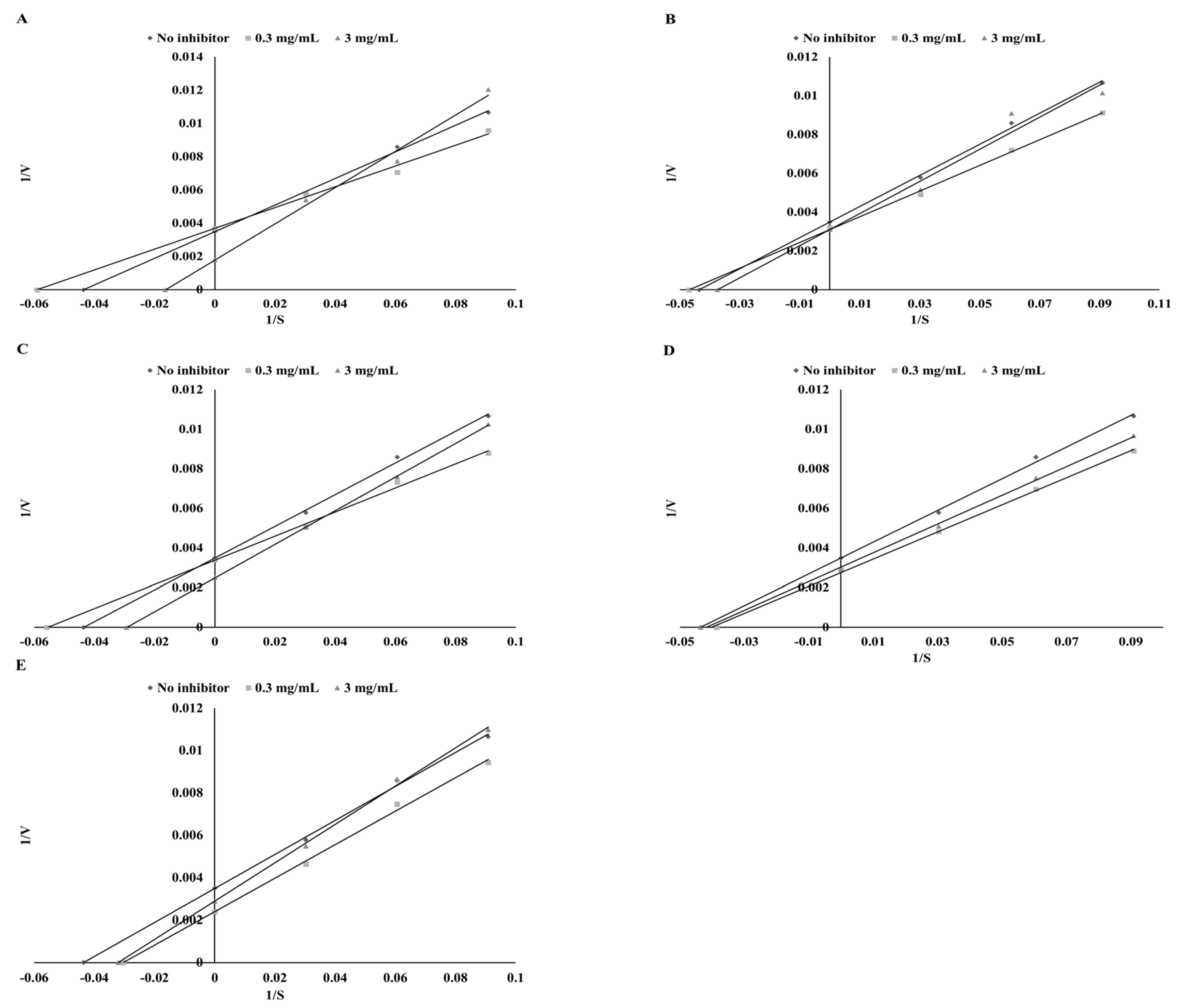
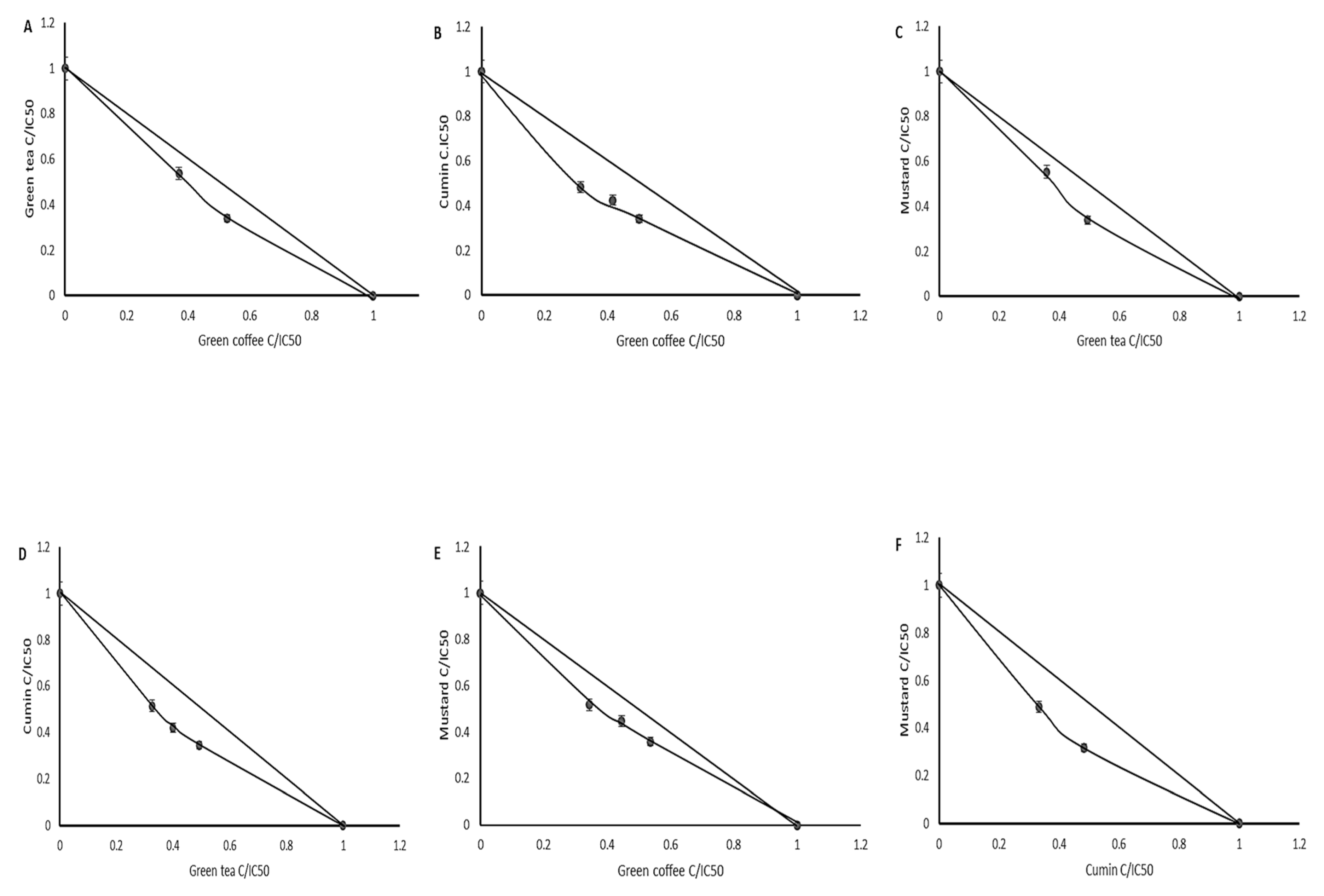
3.5. Interaction Assay
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ruf, J.; Carayon, P. Structural and functional aspects of thyroid peroxidase. Arch. Biochem. Biophys. 2006, 445, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Smith, T.J. Current concepts in the molecular pathogenesis of thyroid-associated ophthalmopathy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1735–1748. [Google Scholar] [CrossRef] [PubMed]
- Shahbaz, A.; Aziz, K.; Umair, M.; Sharifzadeh, M.; Sachmechi, I. Graves’ disease presenting as painful goiter: A case report and review of the literature. Cureus 2018, 10, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Prasek, K.; Płazińska, M.T.; Królicki, L. Diagnosis and treatment of Graves’ disease with particular emphasis on appropriate techniques in nuclear medicine. General state of knowledge. Nucl. Med. Rev. 2015, 18, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.K.; Lin, C.L.; Cheng, F.T.F.; Sung, F.C.; Kao, C.H. Cancer risk in patients with Hashimoto’s thyroiditis: A nationwide cohort study. Br. J. Cancer 2013, 109, 2496–2501. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhou, M.; Ren, G.; Yu, X.; Ma, L.; Yang, J.; Yu, F. Heterogeneous electro-Fenton using modified iron–carbon as catalyst for 2,4-dichlorophenol degradation: Influence factors, mechanism and degradation pathway. Water Res. 2015, 70, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Haynes, R.L.; Van Leyen, K. 12/15-Lipoxygenase expression is increased in oligodendrocytes and microglia of periventricular leukomalacia. Dev. Neurosci. 2013, 35, 140–154. [Google Scholar] [CrossRef]
- McGiff, J.C. Arachidonic acid metabolism. Prev. Med. 1987, 16, 503–509. [Google Scholar] [CrossRef]
- Romano, M.; Cianci, E.; Simiele, F.; Recchiuti, A. Lipoxins and aspirin-triggered lipoxins in resolution of inflammation. Eur. J. Pharmacol. 2015, 760, 49–63. [Google Scholar] [CrossRef]
- Karbownik-Lewińska, M.; Kokoszko-Bilska, A. Oxidative damage to macromolecules in the thyroid - Experimental evidence. Thyroid Res. 2012, 5, 25. [Google Scholar] [CrossRef]
- Mancini, A.; Di Segni, C.; Raimondo, S.; Olivieri, G.; Silvestrini, A.; Meucci, E.; Currò, D. Thyroid hormones, oxidative stress, and inflammation. Mediators Inflamm. 2016, 2016, 6757154. [Google Scholar] [CrossRef] [PubMed]
- Saura-Calixto, F.; Serrano, J.; Goñi, I. Intake and bioaccessibility of total polyphenols in a whole diet. Food Chem. 2007, 101, 492–501. [Google Scholar] [CrossRef]
- Oomen, A.G.; Hack, A.; Minekus, M.; Zeijdner, E.; Cornelis, C.; Schoeters, G.; Verstraete, W.; Van De Wiele, T.; Wragg, J.; Rompelberg, C.J.M.; et al. Comparison of five in vitro digestion models to study the bioaccessibility of soil contaminants. Environ. Sci. Technol. 2002, 36, 3326–3334. [Google Scholar] [CrossRef] [PubMed]
- Williamson, E.M. Synergy and other interactions in phytomedicines. Phytomedicine 2001, 8, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Chou, T. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef]
- Rothwell, J.A.; Pérez-Jiménez, J.; Neveu, V.; Medina-Ramon, A.; M’Hiri, N.; Garcia Lobato, P.; Manach, C.; Knox, K.; Eisner, R.; Wishart, D.; et al. Phenol-Explorer 3.0: A major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database 2013. [Google Scholar] [CrossRef]
- Świeca, M.; Gawlik-Dziki, U.; Dziki, D.; Baraniak, B.; Czyz, J. The influence of protein-flavonoid interactions on protein digestibility in vitro and the antioxidant quality of breads enriched with onion skin. Food Chem. 2013, 141, 451–458. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food-an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Jomaa, B. Simple and rapid in vitro assay for detecting human thyroid peroxidase disruption. ALTEX 2015, 32, 191–200. [Google Scholar]
- Axelrod, B.; Cheesbrough, T.M.; Laakso, S. Lipoxygenase from Soybeans: EC 1.13.11.12 Linoleate: Oxygen oxidoreductase. Methods Enzymol. 1981, 71, 441–451. [Google Scholar]
- Shan, B.; Cai, Y.Z.; Sun, M.; Corke, H. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J. Agric. Food Chem. 2005, 53, 7749–7759. [Google Scholar] [CrossRef] [PubMed]
- Ani, V.; Varadaraj, M.C.; Naidu, K.A. Antioxidant and antibacterial activities of polyphenolic compounds from bitter cumin (Cuminum nigrum L.). Eur. Food Res. Technol. 2006, 224, 109–115. [Google Scholar] [CrossRef]
- Głowacki, R.; Furmaniak, P.; Kubalczyk, P.; Borowczyk, K. Determination of total apigenin in herbs by micellar electrokinetic chromatography with UV detection. J. Anal. Methods Chem. 2016, 2016, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yashin, A.; Yashin, Y.; Xia, X.; Nemzer, B. Antioxidant activity of spices and their impact on human health: A review. Antioxidants 2017, 6, 70. [Google Scholar] [CrossRef] [PubMed]
- Engels, C.; Schieber, A.; Gänzle, M.G. Sinapic acid derivatives in defatted Oriental mustard (Brassica juncea L.) seed meal extracts using UHPLC-DAD-ESI-MS n and identification of compounds with antibacterial activity. Eur. Food Res. Technol. 2012, 234, 535–542. [Google Scholar] [CrossRef]
- Henning, S.M.; Fajardo-Lira, C.; Lee, H.W.; Youssefian, A.A.; Go, V.L.W.; Heber, D. Catechin content of 18 teas and a green tea extract supplement correlates with the antioxidant capacity. Nutr. Cancer 2003, 45, 226–235. [Google Scholar] [CrossRef]
- Stan, M.; Soran, M.L.; Varodi, C.; Lung, I. Extraction and identification of flavonoids from parsley extracts by HPLC analysis. AIP Conf. Proc. 2012, 1425, 50–52. [Google Scholar]
- Mayengbam, S.; Aachary, A.; Thiyam-Holländer, U. Endogenous phenolics in hulls and cotyledons of mustard and canola: A comparative study on its sinapates and antioxidant capacity. Antioxidants 2014, 3, 544–558. [Google Scholar] [CrossRef]
- Acimovic, M.; Tesevic, V.; Mara, D.; Cvetkovic, M.; Stankovic, J.; Filipovic, V. The analysis of cumin seeds essential oil and total polyphenols from postdestillation waste material. Adv. Technol. 2016, 5, 23–30. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Munekata, P.E.S. Phenolic compounds of green tea: Health benefits and technological application in food. Asian Pac. J. Trop. Biomed. 2016, 6, 709–719. [Google Scholar] [CrossRef]
- Hinneburg, I.; Damien Dorman, H.J.; Hiltunen, R. Antioxidant activities of extracts from selected culinary herbs and spices. Food Chem. 2006, 97, 122–129. [Google Scholar] [CrossRef]
- Tang, E.L.H.; Rajarajeswaran, J.; Fung, S.; Kanthimathi, M.S. Petroselinum crispum has antioxidant properties, protects against DNA damage and inhibits proliferation and migration of cancer cells. J. Sci. Food Agric. 2015, 95, 2763–2771. [Google Scholar] [CrossRef] [PubMed]
- Fawole, O.A.; Opara, U.L. Stability of total phenolic concentration and antioxidant capacity of extracts from pomegranate co-products subjectes to in vitro digestion. BMC Complement. Altern. Med. 2016, 16, 358. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U.; Bryda, J.; Dziki, D.; Áwieca, M.; Habza-Kowalska, E.; Złotek, U. Impact of interactions between ferulic and chlorogenic acids on enzymatic and non-enzymatic lipids oxidation: An example of bread enriched with green coffee flour. Appl. Sci. 2019, 9, 568. [Google Scholar] [CrossRef]
- Leonard, J.A.; Tan, Y.; Gilbert, M.; Isaacs, K.; El-masri, H. Estimating margin of exposure to thyroid peroxidase inhibitors using high-throughput in vitro data, high-throughput exposure modeling, and physiologically based pharmacokinetic/pharmacodynamic modeling. Toxicol. Sci. 2016, 151, 57–70. [Google Scholar] [CrossRef]
- Lau, K.; McLean, W.G.; Williams, D.P.; Howard, C.V. Synergistic interactions between commonly used food additives in a developmental neurotoxicity test. Toxicol. Sci. 2006, 90, 178–187. [Google Scholar] [CrossRef]
- Divi, R.L.; Doerge, D.R. Inhibition of thyroid peroxidase by dietary flavonoids. Chem. Res. Toxicol. 1996, 9, 16–23. [Google Scholar] [CrossRef]
- Durak, A.; Gawlik-Dziki, U.; Kowalska, I. Evaluation of interactions between coffee and cardamom, their type, and strength in relation to interactions in a model system. CyTA J. Food 2017, 15, 266–276. [Google Scholar] [CrossRef][Green Version]
| Plant Source | Polyphenol | Concentration (mg/g DW) |
|---|---|---|
| Green coffee | Catechin | 47.7 ± 0.23 a |
| Green tea | Catechin | 11.5 ± 0.58 b |
| Parsley | Apigenin | 0.69 ± 0.034 |
| Cumin | Kaempferol | 0.09 ± 0.004 |
| Mustard | Sinapinic acid | 1.25 ± 0.627 |
| Pure chemical standards | IC50 (mg/mL) | |
| Sinapinic acid | 0.084 ± 0. 001 a | |
| Apigenin | 0.406 ± 0.003 c | |
| Catechin | 0.078 ± 0.008 a | |
| Kaempferol | 0.337 ± 0.002 b | |
| Plant extracts | IC50 (mg DW/mL) | |
| Plant | EE | DE |
| Parsley | 3.47 ± 0.17 e | 1.09 ± 0.05 c |
| Green coffee | 0.78 ± 0.04 d | 0.87 ± 0.04 b |
| Green tea | 0.06 ± 0.0003 a | 1.33 ± 0.06 d |
| Cumin | 0.32 ± 0.01 b | 6.52 ± 0.32 e |
| Mustard | 0.41 ± 0.02 c | 0.42 ± 0.02 a |
| Substance | Mode of Inhibition | EFC50 (µg/mL) | Ki (µg/mL) | Vmax (ΔAU/min) |
|---|---|---|---|---|
| Sinapinic acid | Competitive | 25.42 ± 1.13 a | 45.92 ± 3.22 b | 149.25 ± 6.26 a |
| Apigenin | Uncompetitive | 116.28 ± 5.38 d | 338.73 ± 12.31 d | 149.25 ± 5.12 a |
| Catechin | Competitive | 29.76 ± 2.05 b | 33.42 ± 1.35 a | 144.93 ± 4.33 a |
| Kaempferol | Competitive | 61.68 ± 3.12 c | 186.95 ± 6.89 c | 192.31 ± 8.34 b |
| Plant | Ethanol Extracts | Extracts after Digestion in Vitro | ||||
|---|---|---|---|---|---|---|
| Mode of Inhibition | IC50 (mg DW/mL) | Ki (mg DW/mL) | Vmax (Δ AU/min) | Action | % of Activation | |
| Parsley | competitive | 103.45 ± 3.12 ab | 387.42 ± 11.53 c | 238.1 | activation | 0.39 ± 0.06 a |
| Green coffee | competitive | 185.01 ± 6.32 d | 1971.0 ± 53.5 d | 238.1 | activation | 17.73 ± 1.12 c |
| Green tea | competitive | 126.26 ± 4.15 c | 107.12 ± 3.15 a | 238.1 | activation | 14.07 ± 0.92 b |
| Cumin | competitive | 100.00 ± 3.21 a | 1891.5 ± 42.6 d | 238.1 | activation | 19.85 ± 1.30 c |
| Mustard | competitive | 106.13 ± 4.15 b | 218.4 ± 5.39 b | 285.7 | activation | 24.66 ± 1.42 d |
| Plant | Ethanol Extracts | Extracts after Digestion in Vitro | ||||
|---|---|---|---|---|---|---|
| Mode of Inhibition | IC50 (mg DW/mL) | Ki (mg DW/mL) | Mode of Inhibition | IC50 (mg DW/mL) | Ki (mg DW/mL) | |
| Parsley | mixed | 24.39 ± 1.18b | 32.85 ± 1.43d | uncompetitive | 45.05 ± 1.98c | 6.44 ± 0.53b |
| Green coffee | mixed | 15.96 ± 1.12a | 2.82 ± 0.16a | noncompetitive | 30.43 ± 1.52a | 30.43 ± 1.16d |
| Green tea | mixed | 13.74 ± 0.92a | 2.84 ± 0.21a | uncompetitive | 29.73 ± 1.12a | 4.96 ± 0.19a |
| Cumin | mixed | 21.54 ± 1.23b | 12.61 ± 0.96b | competitive | 34.13 ± 0.85b | 26.94 ± 0.97c |
| Mustard | competitive | 29.01 ± 1.41c | 22.46 ± 1.18c | competitive | 32.01 ± 1.02ab | 72.74 ± 2.16e |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habza-Kowalska, E.; Gawlik-Dziki, U.; Dziki, D. Mechanism of Action and Interactions between Thyroid Peroxidase and Lipoxygenase Inhibitors Derived from Plant Sources. Biomolecules 2019, 9, 663. https://doi.org/10.3390/biom9110663
Habza-Kowalska E, Gawlik-Dziki U, Dziki D. Mechanism of Action and Interactions between Thyroid Peroxidase and Lipoxygenase Inhibitors Derived from Plant Sources. Biomolecules. 2019; 9(11):663. https://doi.org/10.3390/biom9110663
Chicago/Turabian StyleHabza-Kowalska, Ewa, Urszula Gawlik-Dziki, and Dariusz Dziki. 2019. "Mechanism of Action and Interactions between Thyroid Peroxidase and Lipoxygenase Inhibitors Derived from Plant Sources" Biomolecules 9, no. 11: 663. https://doi.org/10.3390/biom9110663
APA StyleHabza-Kowalska, E., Gawlik-Dziki, U., & Dziki, D. (2019). Mechanism of Action and Interactions between Thyroid Peroxidase and Lipoxygenase Inhibitors Derived from Plant Sources. Biomolecules, 9(11), 663. https://doi.org/10.3390/biom9110663