Development of a Simple Pretreatment Immunoassay Based on an Organic Solvent-Tolerant Nanobody for the Detection of Carbofuran in Vegetable and Fruit Samples
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Construction of Phage Display Nanobody Library
2.3. Selection and Identification of Anti-Carbofuran Phage Clones
2.4. Expression and Purification of Nanobody Protein
2.5. Stability Analysis of Anti-Carbofuran Nanobody
2.6. Nanobody-Based Indirect Competitive ELISA
2.7. Immunoassay Validation
3. Results and Discussion
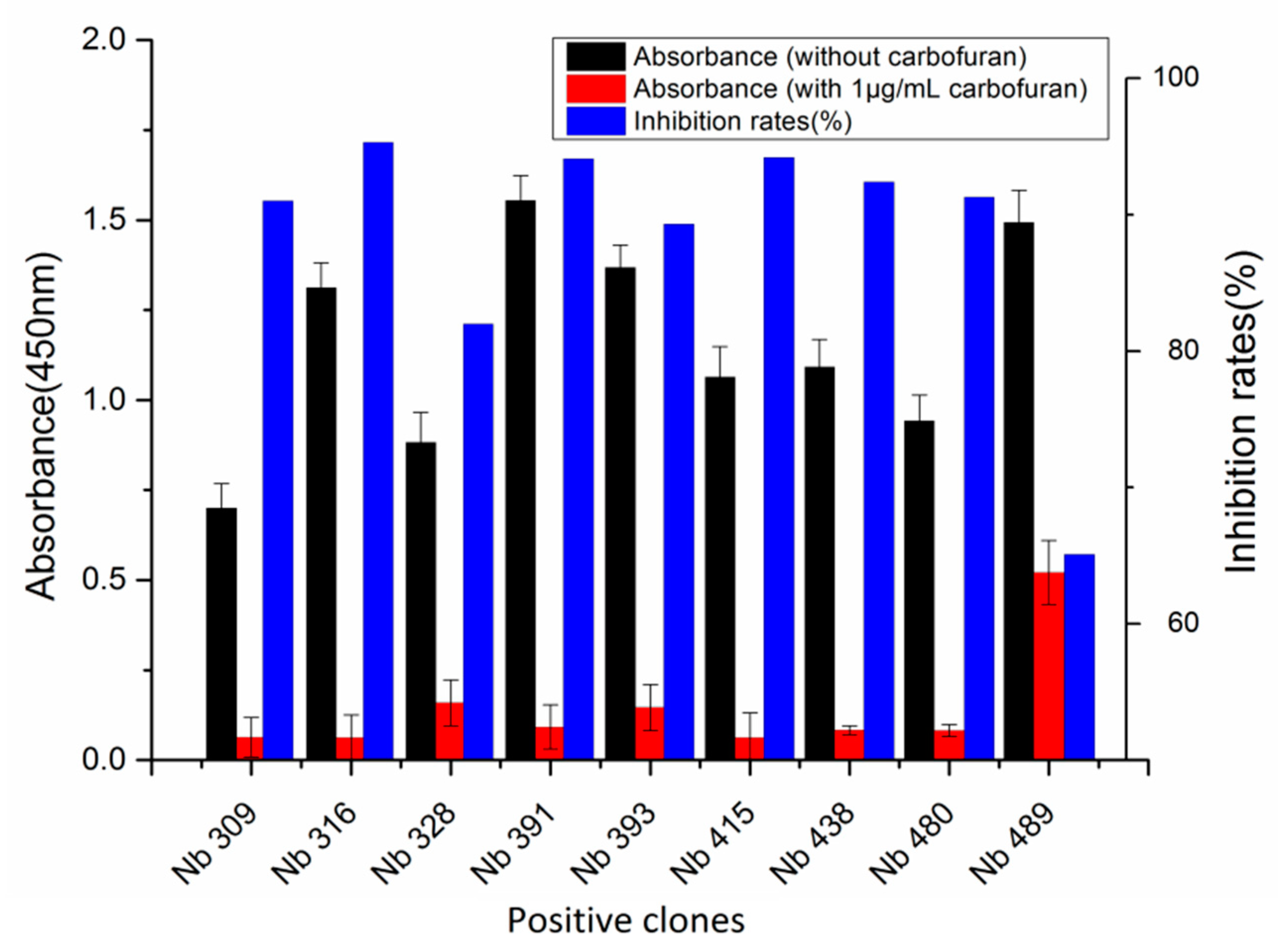
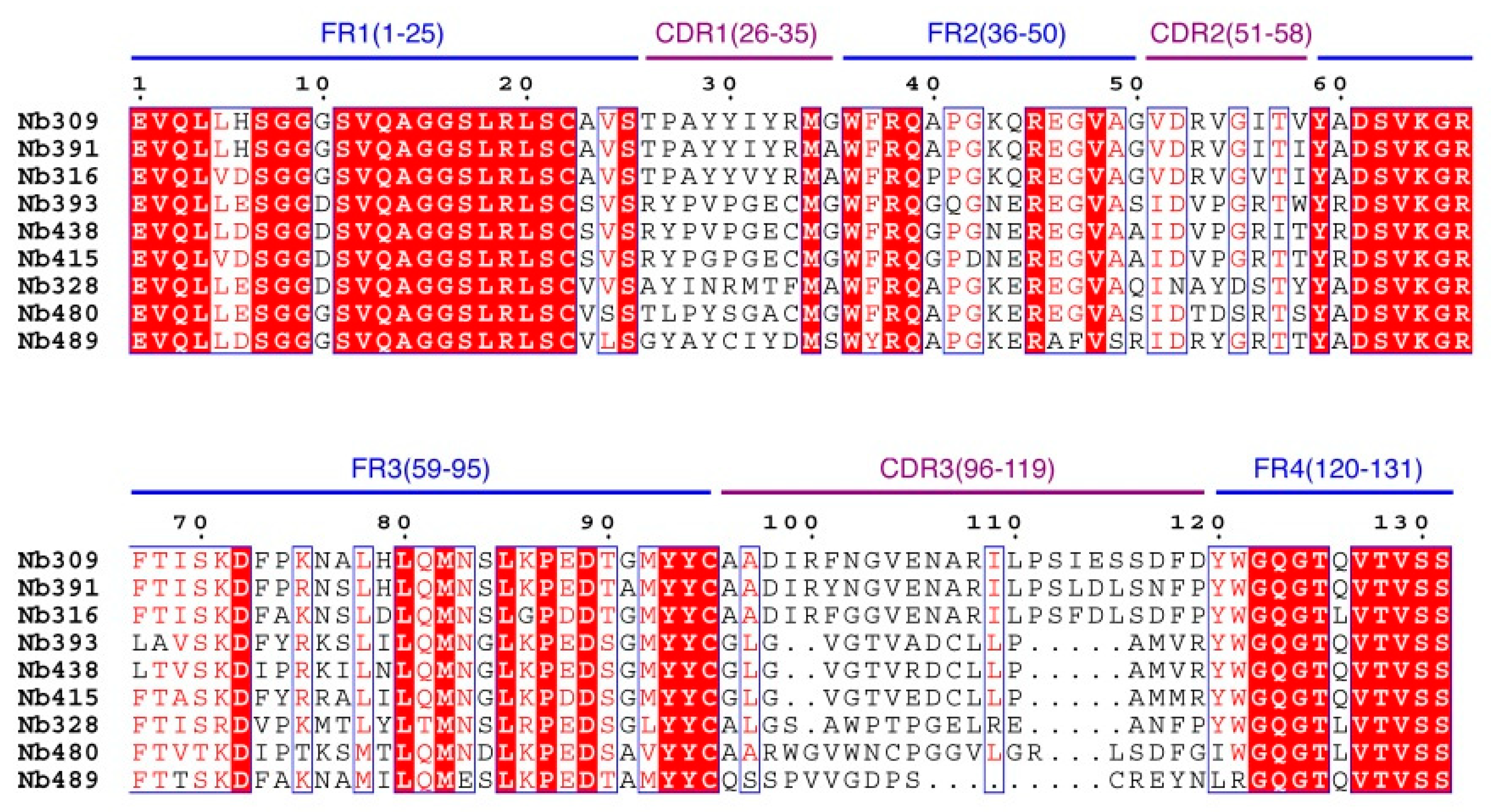
3.1. Library Construction and Selection of Anti-Carbofuran Phage Clones
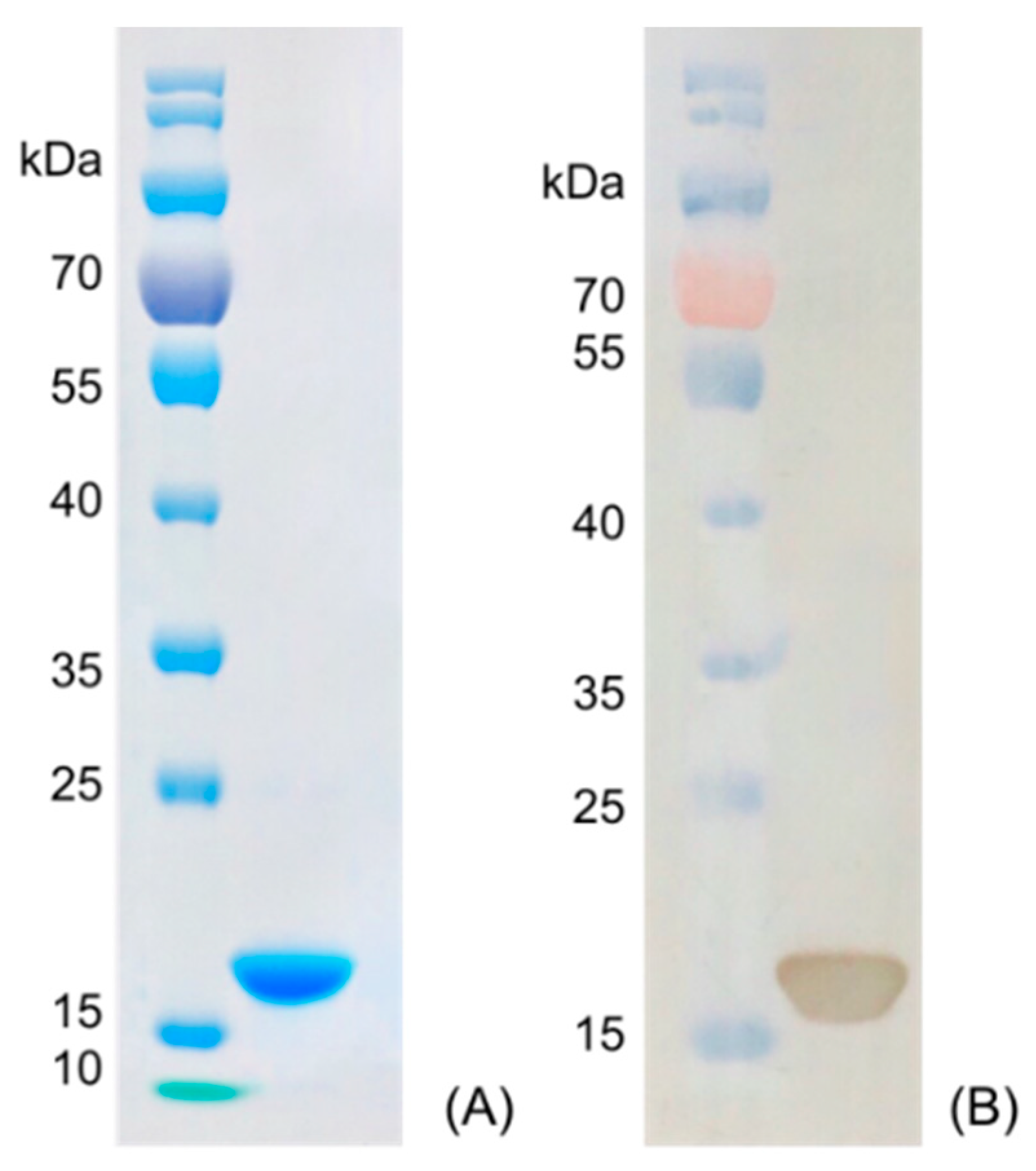
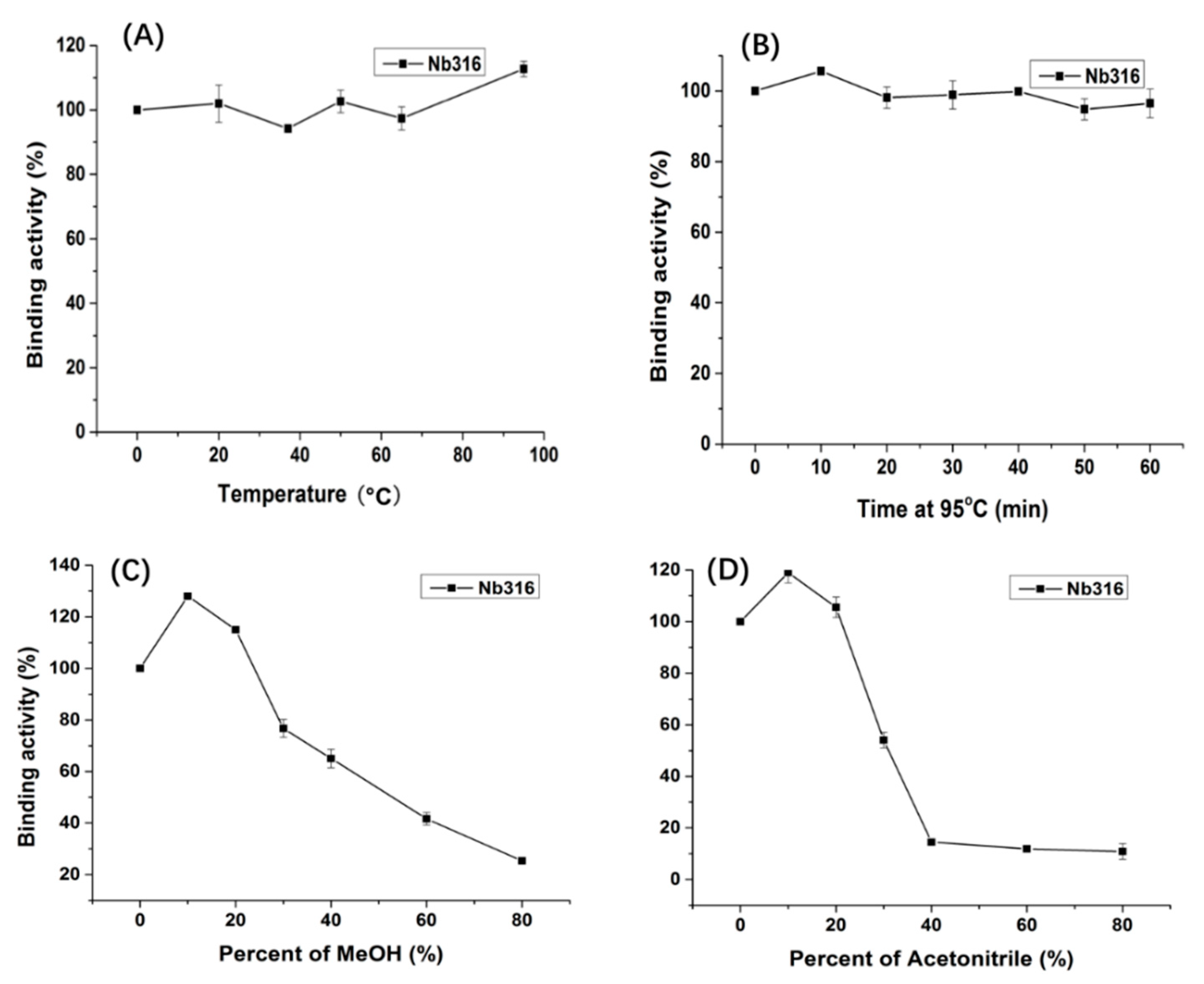
3.2. Preparation and Stability Analysis of Anti-Carbofuran Nanobody
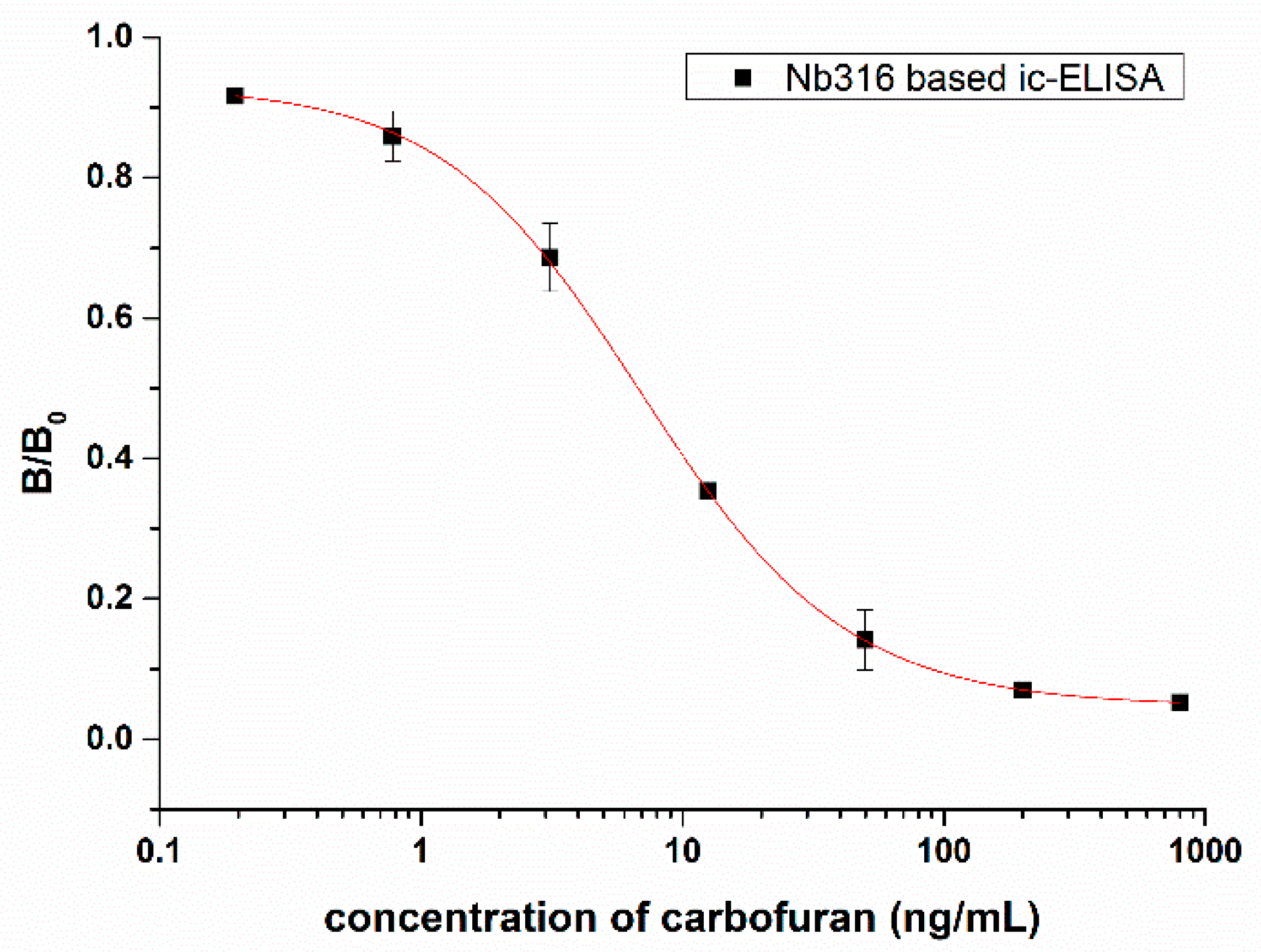
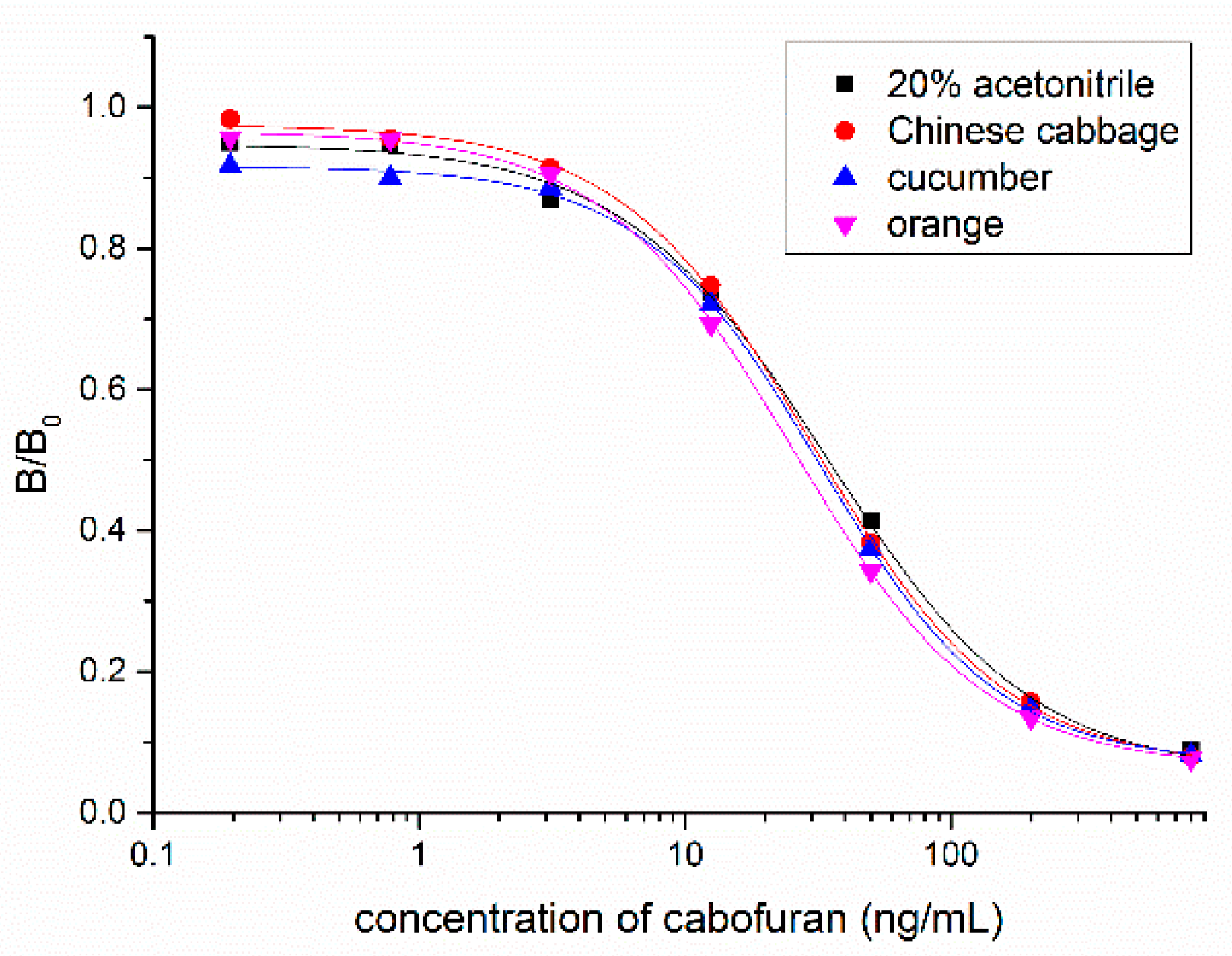
3.3. Nanobody-Based Ic-ELISA
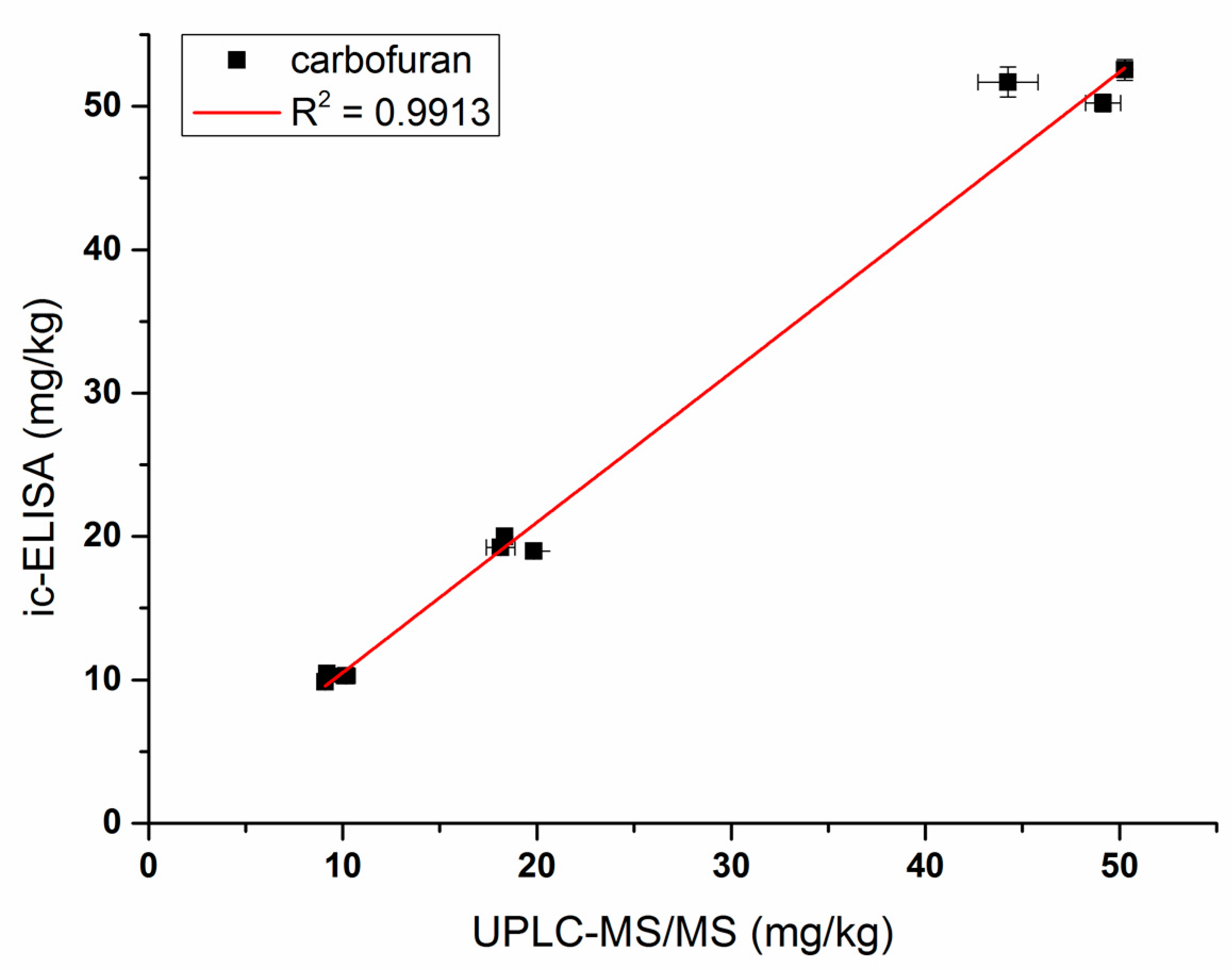
3.4. Sample Analysis by ic-ELISA and UPLC–MS/MS
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dai, Y.; Wang, T.; Hu, X.; Liu, S.; Zhang, M.; Wang, C. Highly sensitive microcantilever-based immunosensor for the detection of carbofuran in soil and vegetable samples. Food Chem. 2017, 229, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xu, D.; Hu, Y.; Liu, S.; Wei, H.; Zheng, J.; Wang, G.; Hu, X.; Wang, C. Construction of an impedimetric immunosensor for label-free detecting carbofuran residual in agricultural and environmental samples. Food Control 2015, 53, 72–80. [Google Scholar] [CrossRef]
- Tien, C.; Huang, H.; Chen, C.S. Accessing the Carbofuran Degradation Ability of Cultures from Natural River Biofilms in Different Environments. CLEAN Soil Air Water 2017, 45, 1600380. [Google Scholar] [CrossRef]
- Gupta, R.C. Carbofuran toxicity. J. Toxicol. Environ. Health 1994, 43, 383–418. [Google Scholar] [CrossRef] [PubMed]
- Saraji, M.; Esteki, N. Analysis of carbamate pesticides in water samples using single-drop microextraction and gas chromatography-mass spectrometry. Anal. Bioanal. Chem. 2008, 391, 1091–1100. [Google Scholar] [CrossRef]
- Carla, S.; Brett, H.; Ambrose, F.; James, K.J.; Jordi, M.E.; Yolanda, P. Liquid chromatography quadrupole time-of-flight mass spectrometry analysis of carbosulfan, carbofuran, 3-hydroxycarbofuran, and other metabolites in food. Anal. Chem. 2007, 79, 1492–1501. [Google Scholar]
- Gui, W.; Jin, M.; Sun, L.; Guo, Y.; Zhu, G. Residues determination of carbofuran in vegetables based on sensitive time-resolved fluorescence immunoassay. Food Agric. Immunol. 2009, 20, 49–56. [Google Scholar] [CrossRef]
- Bellemjid, N.; Iddar, A.; Moussaif, A.; Abbadi, N.E.; Mesfioui, A. Analysis of Carbamates Pesticides: Immunogical Technique by Local Development of Enzyme-linked Immuno-Sorbent Assay. J. Pharm. Pharmacol. 2018, 6, 395–402. [Google Scholar] [CrossRef]
- Yang, J.Y.; Zhang, Y.; Wang, H.; Xu, Z.L.; Eremin, S.A.; Shen, Y.D.; Wu, Q.; Lei, H.T.; Sun, Y.M. Development of fluorescence polarisation immunoassay for carbofuran in food and environmental water samples. Food Agric. Immunol. 2015, 26, 340–355. [Google Scholar] [CrossRef]
- Liu, A.; Anfossi, L.; Shen, L.; Li, C.; Wang, X. Non-competitive immunoassay for low-molecular-weight contaminant detection in food, feed and agricultural products: A mini-review. Trends Food Sci. Tech. 2018, 71, 181–187. [Google Scholar] [CrossRef]
- Kim, H.; McCoy, M.R.; Majkova, Z.; Dechant, J.E.; Gee, S.J.; Tabares-da Rosa, S.; González-Sapienza, G.G.; Hammock, B.D. Isolation of Alpaca Anti-Hapten Heavy Chain Single Domain Antibodies for Development of Sensitive Immunoassay. Anal. Chem. 2012, 84, 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Hamers-Casterman, C.; Atarhouch, T.; Muyldermans, S.; Robinson, G.; Hammers, C.; Songa, E.B.; Bendahman, N.; Hammers, R. Naturally occurring antibodies devoid of light chains. Nature 1993, 363, 446–448. [Google Scholar] [CrossRef] [PubMed]
- Rossotti, M.A.; Pirez, M.; Gonzalez-Techera, A.; Cui, Y.; Bever, C.S.; Lee, K.S.S.; Morisseau, C.; Leizagoyen, C.; Gee, S.; Hammock, B.D.; et al. Method for Sorting and Pairwise Selection of Nanobodies for the Development of Highly Sensitive Sandwich Immunoassays. Anal. Chem. 2015, 87, 11907–11914. [Google Scholar] [CrossRef] [PubMed]
- Bever, C.S.; Dong, J.; Vasylieva, N.; Barnych, B.; Cui, Y.; Xu, Z.; Hammock, B.D.; Gee, S.J. VHH antibodies: Emerging reagents for the analysis of environmental chemicals. Anal. Bioanal. Chem. 2016, 408, 5985–6002. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Duan, Z.; Liu, X.; Deng, X.; Tang, Z. Development of a Nanobody-Based Competitive Dot ELISA for Visual Screening of Ochratoxin A in Cereals. Food Anal. Methods 2017, 10, 3558–3564. [Google Scholar] [CrossRef]
- Ren, W.; Li, Z.; Xu, Y.; Wan, D.; Barnych, B.; Li, Y.; Tu, Z.; He, Q.; Fu, J.; Hammock, B.D. One-Step Ultrasensitive Bioluminescent Enzyme Immunoassay Based on Nanobody/Nanoluciferase Fusion for Detection of Aflatoxin B1 in Cereal. J. Agric. Food Chem. 2019, 67, 5221–5229. [Google Scholar] [CrossRef]
- Xu, C.; Yang, Y.; Liu, L.; Li, J.; Liu, X.; Zhang, X.; Liu, Y.; Zhang, C.; Liu, X. Microcystin-LR nanobody screening from an alpaca phage display nanobody library and its expression and application. Ecotoxicol. Environ. Saf. 2018, 151, 220–227. [Google Scholar] [CrossRef]
- Wang, K.; Liu, Z.; Ding, G.; Li, J.; Vasylieva, N.; Li, Q.X.; Li, D.; Gee, S.J.; Hammock, B.D.; Xu, T. Development of a one-step immunoassay for triazophos using camel single-domain antibody-alkaline phosphatase fusion protein. Anal. Bioanal. Chem. 2019, 411, 1287–1295. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Z.; Wang, F.; Cai, J.; Dong, J.; Zhang, J.; Si, R.; Wang, C.; Wang, Y.; Shen, Y.; et al. Isolation of Bactrian Camel Single Domain Antibody for Parathion and Development of One-Step dc-FEIA Method Using VHH-Alkaline Phosphatase Fusion Protein. Anal. Chem. 2018, 90, 12886–12892. [Google Scholar] [CrossRef]
- Wang, K.; Vasylieva, N.; Wan, D.; Eads, D.A.; Yang, J.; Tretten, T.; Barnych, B.; Li, J.; Li, Q.X.; Gee, S.J.; et al. Quantitative Detection of Fipronil and Fipronil-Sulfone in Sera of Black-Tailed Prairie Dogs and Rats after Oral Exposure to Fipronil by Camel Single-Domain Antibody-Based Immunoassays. Anal. Chem. 2019, 91, 1532–1540. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, K.; Wu, S.; Wang, Z.; Ding, G.; Hao, X.; Li, Q.X.; Li, J.; Gee, S.J.; Hammock, B.D. Development of a camelid variable domain of heavy chain antibody-based immunoassay for the detection of carbaryl in cereals. J. Sci. Food Agric. 2019, 99, 4383–4390. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Y.; Wang, H.; Jiang, J.M.; Sun, Y.M.; Pan, K.; Lei, H.T.; Wu, Q.; Shen, Y.D.; Xiao, Z.L.; Xu, Z.L. Development of an enzyme-linked immuno-sorbent assay (ELISA) method for carbofuran residues. Molecules 2008, 13, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimizadeh, W.; Mousavi Gargari, S.; Rajabibazl, M.; Safaee Ardekani, L.; Zare, H.; Bakherad, H. Isolation and characterization of protective anti-LPS nanobody against V. cholerae O1 recognizing Inaba and Ogawa serotypes. Appl. Microbiol. Biot. 2013, 97, 4457–4466. [Google Scholar] [CrossRef] [PubMed]
- Olichon, A.; Schweizer, D.; Muyldermans, S.; Marco, A.D. Heating as a rapid purification method for recovering correctly-folded thermotolerant VH and VHH domains. BMC Biotechnol. 2007, 7, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Cieślik, E.; Sadowska-Rociek, A.; Ruiz, J.M.M.; Surma-Zadora, M. Evaluation of QuEChERS method for the determination of organochlorine pesticide residues in selected groups of fruits. Food Chem. 2011, 125, 773–778. [Google Scholar] [CrossRef]
- Cécile, V.; Remy, L.; Dirk, S.; Sergio, M.R.; Serge, M.; Katja, C. General strategy to humanize a camelid single-domain antibody and identification of a universal humanized nanobody scaffold. J. Biol. Chem. 2009, 284, 3273–3284. [Google Scholar]
- Muyldermans, S.; Atarhouch, T.; Saldanha, J.; Barbosa, J.A.R.G.; Hamers, R. Sequence and structure of VH domain from naturally occurring camel heavy chain immunoglobulins lacking light chains. Protein Eng. 1994, 7, 1129–1135. [Google Scholar] [CrossRef]
- Pérez, J.M.J.; Renisio, J.G.; Prompers, J.J.; van Platerink, C.J.; Cambillau, C.; Darbon, H.; Frenken, L.G.J. Thermal Unfolding of a Llama Antibody Fragment: A Two-State Reversible Process. Biochemistry 2001, 40, 74–83. [Google Scholar] [CrossRef]
- Akazawaogawa, Y.; Uegaki, K.; Hagihara, Y. The role of intra-domain disulfide bonds in heat-induced irreversible denaturation of camelid single domain VHH antibodies. J. Biochem. 2016, 159, 111–121. [Google Scholar] [CrossRef]
- Turner, K.B.; Zabetakis, D.; Goldman, E.R.; Anderson, G.P. Enhanced stabilization of a stable single domain antibody for SEB toxin by random mutagenesis and stringent selection. Protein Eng. Des. Sel. 2014, 27, 89–95. [Google Scholar] [CrossRef]
- Zabetakis, D.; Olson, M.A.; Anderson, G.P.; Legler, P.M.; Goldman, E.R. Evaluation of Disulfide Bond Position to Enhance the Thermal Stability of a Highly Stable Single Domain Antibody. PLoS ONE 2014, 9, e115405. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Jin, M.; Gui, W.; Guo, Y.; Jin, R.; Wang, C.; Liang, C.; Liu, Y.; Wang, T. Development of a direct competitive enzyme-linked immunoassay for carbofuran in vegetables. Food Chem. 2008, 107, 1737–1742. [Google Scholar] [CrossRef]
- Moreno, M.; Abad, A.; Pelegrí, P.; Martínez, M.; Sáez, A.; Gamón, M.; Montoya, A. Validation of a Monoclonal Enzyme Immunoassay for the Determination of Carbofuran in Fruits and Vegetables. J. Agric. Food Chem. 2001, 49, 1713–1719. [Google Scholar] [CrossRef] [PubMed]
| Analogues | Molecular Structural | IC50 (ng/mL) | Cross-Reactivity (%) |
|---|---|---|---|
| Carbofuran | 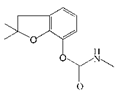 | 7.27 | 100 |
| Benfuracarb | 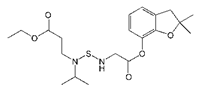 | 142.51 | 5.1 |
| Fenobucarb | 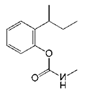 | 204.6 | 3.5 |
| Carbosulfan | 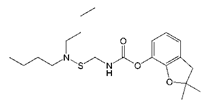 | 280.93 | 2.6 |
| 3-Hydroxycarbofuran |  | 366.05 | 2.0 |
| Isoprocarb | 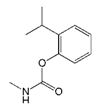 | 1351.82 | 0.5 |
| Carbaryl | 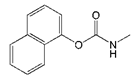 | >2000 | <0.1 |
| Aldicarb | 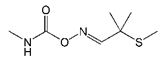 | >2000 | <0.1 |
| Methomyl | 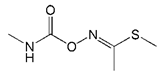 | >2000 | <0.1 |
| Pirimicarb | 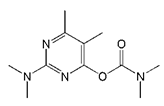 | >2000 | <0.1 |
| Mercaptodimethur | 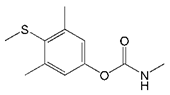 | >2000 | <0.1 |
| Tsumacide | 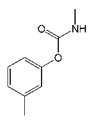 | >2000 | <0.1 |
| Added (mg/kg) | Chinese Cabbage | Cucumber | Orange | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Found ± SD (mg/kg) | Recovery (%) | CV (%) | Found ± SD (mg/kg) | Recovery (%) | CV (%) | Found ± SD (mg/kg) | Recovery (%) | CV (%) | |
| 10 | 10.27 ± 0.34 | 102.7 | 3.33 | 9.73 ± 0.24 | 97.35 | 2.52 | 8.47 ± 0.2 | 84.74 | 2.35 |
| 20 | 18.44 ± 0.54 | 92.25 | 2.61 | 20.45 ± 0.31 | 102.26 | 1.50 | 16.46 ± 0.22 | 82.31 | 1.21 |
| 50 | 51.96 ± 0.75 | 103.92 | 1.44 | 46.24 ± 0.51 | 92.48 | 1.11 | 43.77 ± 0.91 | 87.54 | 20.76 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.-r.; Wang, Y.; Dong, J.-x.; Yang, J.-y.; Zhang, Y.-q.; Wang, F.; Si, R.; Xu, Z.-l.; Wang, H.; Xiao, Z.-l.; et al. Development of a Simple Pretreatment Immunoassay Based on an Organic Solvent-Tolerant Nanobody for the Detection of Carbofuran in Vegetable and Fruit Samples. Biomolecules 2019, 9, 576. https://doi.org/10.3390/biom9100576
Zhang J-r, Wang Y, Dong J-x, Yang J-y, Zhang Y-q, Wang F, Si R, Xu Z-l, Wang H, Xiao Z-l, et al. Development of a Simple Pretreatment Immunoassay Based on an Organic Solvent-Tolerant Nanobody for the Detection of Carbofuran in Vegetable and Fruit Samples. Biomolecules. 2019; 9(10):576. https://doi.org/10.3390/biom9100576
Chicago/Turabian StyleZhang, Jin-ru, Yu Wang, Jie-xian Dong, Jin-yi Yang, Yu-qi Zhang, Feng Wang, Rui Si, Zhen-lin Xu, Hong Wang, Zhi-li Xiao, and et al. 2019. "Development of a Simple Pretreatment Immunoassay Based on an Organic Solvent-Tolerant Nanobody for the Detection of Carbofuran in Vegetable and Fruit Samples" Biomolecules 9, no. 10: 576. https://doi.org/10.3390/biom9100576
APA StyleZhang, J.-r., Wang, Y., Dong, J.-x., Yang, J.-y., Zhang, Y.-q., Wang, F., Si, R., Xu, Z.-l., Wang, H., Xiao, Z.-l., & Shen, Y.-d. (2019). Development of a Simple Pretreatment Immunoassay Based on an Organic Solvent-Tolerant Nanobody for the Detection of Carbofuran in Vegetable and Fruit Samples. Biomolecules, 9(10), 576. https://doi.org/10.3390/biom9100576







